Automated Clinical Dosimetry Planning of Dense Lattice Radiation Therapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.C.; Baal, U.H.; Baal, J.D.; Pai, J.S.; Boreta, L.; Braunstein, S.E.; Raleigh, D.R. Efficacy and Safety of Stereotactic Radiosurgery for Brainstem Metastases: A Systematic Review and Meta-analysis. JAMA Oncol. 2021, 7, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, E.J.; Singh, R.; Wang, M.; Chinchilli, V.M.; Trifiletti, D.M.; Ost, P.; Siva, S.; Meng, M.B.; Tchelebi, L.; Zaorsky, N.G. Safety and Survival Rates Associated With Ablative Stereotactic Radiotherapy for Patients With Oligometastatic Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2021, 7, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Carlson, D.J.; Brenner, D.J. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.B.; Krishnan, S.; Hodge, J.W.; Chang, J.Y. Immunotherapy and stereotactic ablative radiotherapy (ISABR): A curative approach? Nat. Rev. Clin. Oncol. 2016, 13, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.C.; López-Peñalver, J.J.; Farias, V.d.A.; Ruiz-Ruiz, M.C.; Oliver, F.J.; Ruiz de Almodóvar, J.M. Direct and bystander radiation effects: A biophysical model and clinical perspectives. Cancer Lett. 2015, 356, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Chicas-Sett, R.; Zafra, J.; Rodriguez-Abreu, D.; Castilla-Martinez, J.; Benitez, G.; Salas, B.; Hernandez, S.; Lloret, M.; Onieva, J.L.; Lara, P.C. Combination of SABR With Anti-PD-1 in Oligoprogressive Non-Small Cell Lung Cancer and Melanoma: Results of a Prospective Multicenter Observational Study. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.; Parrish, R.; Barton, R.; Henry, A. Palliative radiotherapy. BMJ 2018, 360, k821. [Google Scholar] [CrossRef] [PubMed]
- Chvetsov, A.V.; Stewart, R.D.; Kim, M.; Meyer, J.; Rengan, R. Volume effects in the TCP for hypoxic and oxygenated tumors. Med. Phys. 2020, 47, 4626–4633. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, M.; Fujita, M.; Regine, W.F.; Megooni, A.S.; Ibbott, G.S.; Ahmed, M.M. High-dose spatially-fractionated radiation (GRID): A new paradigm in the management of advanced cancers. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Dubben, H.H.; Thames, H.D.; Beck-Bornholdt, H.P. Tumor volume: A basic and specific response predictor in radiotherapy. Radiother. Oncol. 1998, 47, 167–174. [Google Scholar] [CrossRef]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Tubin, S. A Partial Tumor Irradiation Approach for Complex Bulky Disease. Semin. Radiat. Oncol. 2024, 34, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Tubin, S.; Vozenin, M.C.; Prezado, Y.; Durante, M.; Prise, K.M.; Lara, P.C.; Greco, C.; Massaccesi, M.; Guha, C.; Wu, X.; et al. Novel unconventional radiotherapy techniques: Current status and future perspectives—Report from the 2nd international radiation oncology online seminar. Clin. Transl. Radiat. Oncol. 2023, 40, 100605. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mayr, N.A.; Griffin, R.J.; Zhang, H.; Pokhrel, D.; Grams, M.; Penagaricano, J.; Chang, S.; Spraker, M.B.; Kavanaugh, J.; et al. Overview and Recommendations for Prospective Multi-institutional Spatially Fractionated Radiation Therapy Clinical Trials. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 737–749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, M.M.; Wu, X.; Mohiuddin, M.; Perez, N.C.; Zhang, H.; Amendola, B.E.; Malachowska, B.; Mohiuddin, M.; Guha, C. Optimizing GRID and Lattice Spatially Fractionated Radiation Therapy: Innovative Strategies for Radioresistant and Bulky Tumor Management. Semin. Radiat. Oncol. 2024, 34, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Iori, F.; Cappelli, A.; D’Angelo, E.; Cozzi, S.; Ghersi, S.F.; De Felice, F.; Ciammella, P.; Bruni, A.; Iotti, C. Lattice Radiation Therapy in clinical practice: A systematic review. Clin. Transl. Radiat. Oncol. 2022, 39, 100569. [Google Scholar] [CrossRef] [PubMed]
- Studer, G.; Jeller, D.; Streller, T.; Huebner, D.; Glanzmann, C. Time-Related Outcome Following Palliative Spatially Fractionated Stereotactic Radiation Therapy (Lattice) of Large Tumors—A Case Series. Adv. Radiat. Oncol. 2024, 9, 101566. [Google Scholar] [CrossRef] [PubMed]
- Grams, M.P.; Tseung, H.S.W.C.; Ito, S.; Zhang, Y.; Owen, D.; Park, S.S.; Ahmed, S.K.; Petersen, I.A.; Haddock, M.G.; Harmsen, W.S.; et al. A Dosimetric Comparison of Lattice, Brass, and Proton Grid Therapy Treatment Plans. Pract. Radiat. Oncol. 2022, 12, e442–e452. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Perez, N.C.; Zheng, Y.; Li, X.; Jiang, L.; Amendola, B.E.; Xu, B.; Mayr, N.A.; Lu, J.J.; Hatoum, G.F.; et al. The Technical and Clinical Implementation of LATTICE Radiation Therapy (LRT). Radiat. Res. 2020, 194, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Duriseti, S.; Kavanaugh, J.; Goddu, S.; Price, A.; Knutson, N.; Reynoso, F.; Michalski, J.; Mutic, S.; Robinson, C.; Spraker, M.B. Spatially fractionated stereotactic body radiation therapy (Lattice) for large tumors. Adv. Radiat. Oncol. 2021, 6, 100639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kavanaugh, J.A.; Spraker, M.B.; Duriseti, S.; Basarabescu, F.; Price, A.; Goddu, M.; Knutson, N.; Prusator, M.; Robinson, C.; Mazur, T. LITE SABR M1: Planning design and dosimetric endpoints for a phase I trial of lattice SBRT. Radiother. Oncol. 2022, 167, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, R. A Story of Hypofractionation and the Table on the Wall. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Mayr, N.A.; Mohiuddin, M.; Snider, J.W.; Zhang, H.; Griffin, R.J.; Amendola, B.E.; Hippe, D.S.; Perez, N.C.; Wu, X.; Lo, S.S.; et al. Practice Patterns of Spatially Fractionated Radiation Therapy: A Clinical Practice Survey. Adv. Radiat. Oncol. 2023, 9, 101308. [Google Scholar] [CrossRef] [PubMed]
- Amendola, B.E.; Perez, N.C.; Mayr, N.A.; Wu, X.; Amendola, M. Spatially Fractionated Radiation Therapy Using Lattice Radiation in Far-advanced Bulky Cervical Cancer: A Clinical and Molecular Imaging and Outcome Study. Radiat. Res. 2020, 194, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Amendola, B.E.; Mahadevan, A.; Blanco Suarez, J.M.; Griffin, R.J.; Wu, X.; Perez, N.C.; Hippe, D.S.; Simone, C.B., 2nd; Mohiuddin, M.; Mohiuddin, M.; et al. An International Consensus on the Design of Prospective Clinical-Translational Trials in Spatially Fractionated Radiation Therapy for Advanced Gynecologic Cancer. Cancers 2022, 14, 4267. [Google Scholar] [CrossRef] [PubMed]
- Mayr, N.A.; Snider, J.W.; Regine, W.F.; Mohiuddin, M.; Hippe, D.S.; Peñagarícano, J.; Mohiuddin, M.; Kudrimoti, M.R.; Zhang, H.; Limoli, C.L.; et al. An International Consensus on the Design of Prospective Clinical-Translational Trials in Spatially Fractionated Radiation Therapy. Adv. Radiat. Oncol. 2021, 7, 100866. [Google Scholar] [CrossRef] [PubMed]
- Duriseti, S.; Kavanaugh, J.A.; Szymanski, J.; Huang, Y.; Basarabescu, F.; Chaudhuri, A.; Henke, L.; Samson, P.; Lin, A.; Robinson, C.; et al. LITE SABR M1: A phase I trial of Lattice stereotactic body radiotherapy for large tumors. Radiother. Oncol. 2022, 167, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.; Harmsen, W.S.; Ahmed, S.K.; Petersen, I.A.; Haddock, M.G.; Ma, D.J.; Pulsipher, S.; Corbin, K.S.; Lester, S.C.; Park, S.S.; et al. Highs and Lows of Spatially Fractionated Radiation Therapy: Dosimetry and Clinical Outcomes. Pract. Radiat. Oncol. 2024, S1879-8500(24)00353-9, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, Y.; Wang, F.; Badkul, R.; Chen, R.C.; Gao, H. Lattice position optimization for LATTICE therapy. Med. Phys. 2023, 50, 7359–7367. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.W.; Mazur, T.R.; Schmidt, M.C.; Hilliard, J.; Badiyan, S.; Spraker, M.B.; Kavanaugh, J.A. Script-based implementation of automatic grid placement for lattice stereotactic body radiation therapy. Phys. Imaging Radiat. Oncol. 2024, 29, 100549. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, M.; Yu, K.K.; Chang, D.; Kron, T.; Hardcastle, N.; Chander, S.; Yeo, A. Automated lattice radiation therapy treatment planning personalised to tumour size and shape. Phys. Med. 2024, 125, 104490. [Google Scholar] [CrossRef] [PubMed]
- Deufel, C.; Dodoo, C.; Kavanaugh, J.; Finley, R.; Lang, K.; Sorenson, K.; Spreiter, S.; Brooks, J.; Moseley, D.; Ahmed, S.K.; et al. Automated target placement for VMAT lattice radiation therapy: Enhancing efficiency and consistency. Phys. Med. Biol. 2024, 69, 075010. [Google Scholar] [CrossRef] [PubMed]
- Majercakova, K.; Aguilar, N.T.; Isern Verdum, J.; Bargalló, H.V.; Capel, A.V.; Mancera Soto, M.; Gómez de Segura Melcón, G.; Cordero, J.V.R.; González-López, J.A.; Rosell, S.B.; et al. Role of Spatially Fractionated Radiotherapy (LATTICE) Treatment in Inoperable Bulky Soft-Tissue Sarcomas. Cancers 2025, 17, 624. [Google Scholar] [CrossRef] [PubMed]
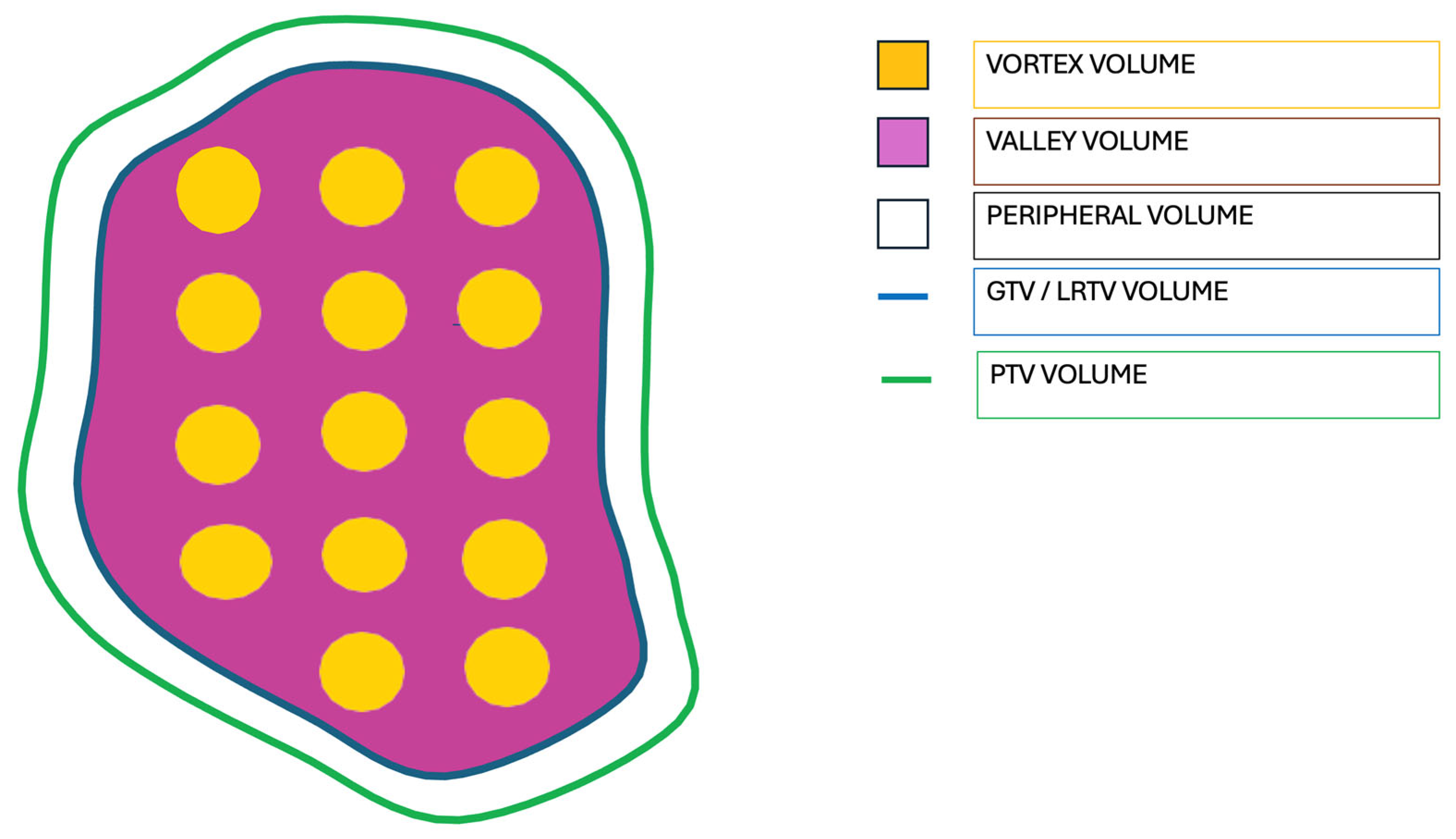
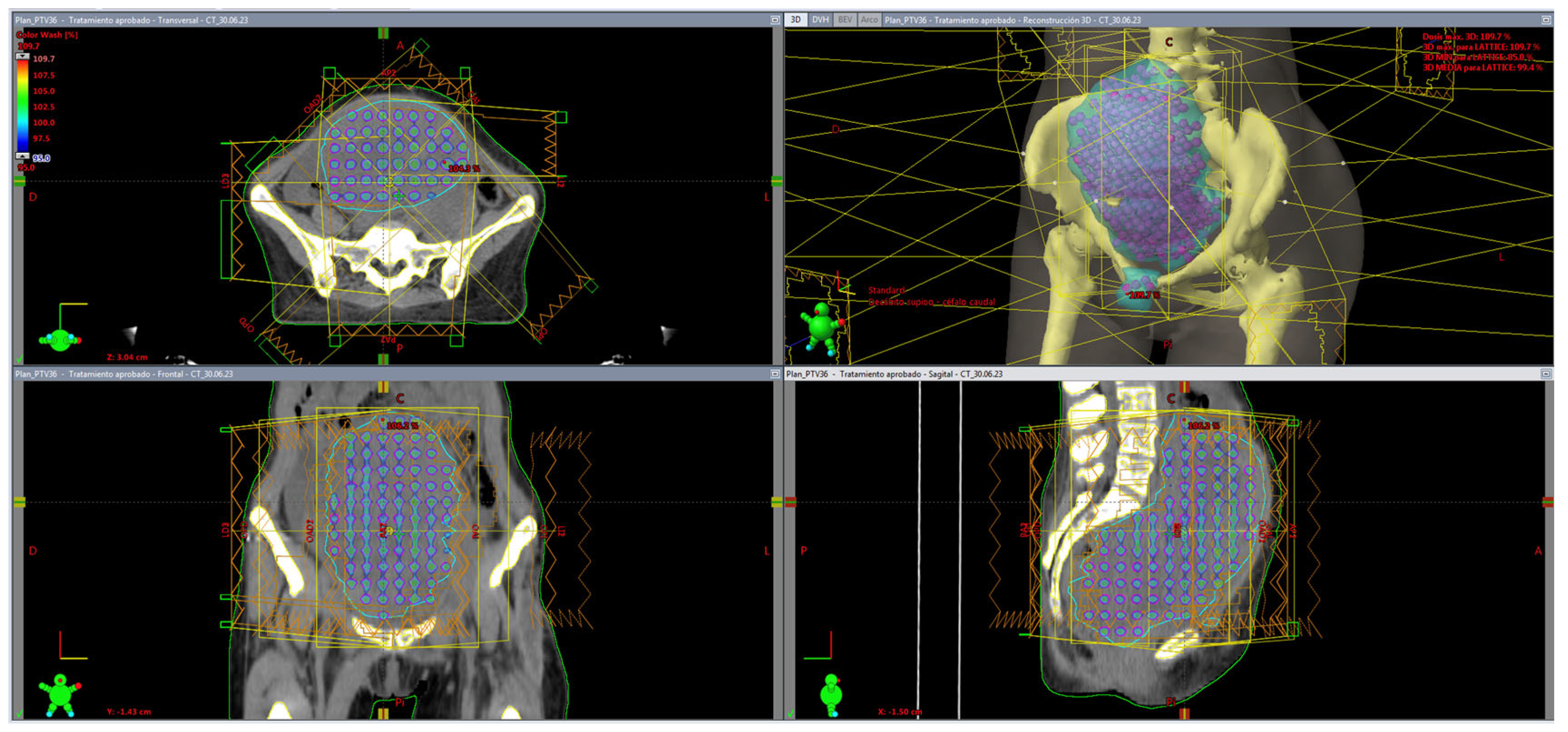
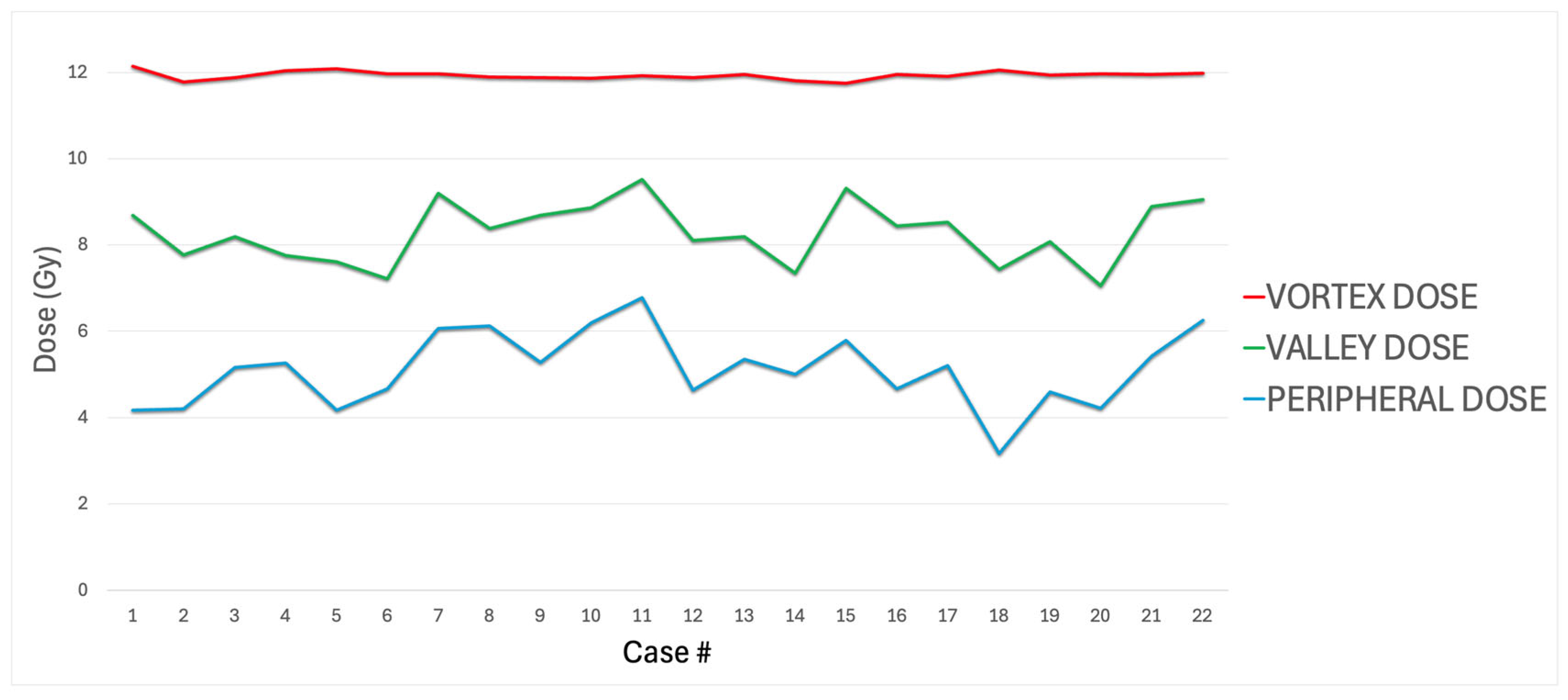

| # | Primary TG263 Name | Treated Site | Stage | Diagnosis | LRTV (cc) | Vortex Vol (cc) | Valley Vol (cc) | Vortex Vol/LRTV | Vortex Dose (Gy) | Valley Dose (Gy) | VPDR % | Peripheral Dose (Gy) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Uterus Adenocarcinoma | LN_Neck_III | IV | Distant Relapse | 45.35 | 1.75 | 38.89 | 3.86 | 12.14 | 8.69 | 71.52 | 4.17 |
| 2 | Cervix SCC | Locally advanced | IIIB | Primary | 114.50 | 8.07 | 104.70 | 7.05 | 11.78 | 7.77 | 65.93 | 4.20 |
| 3 | Limb Liposarcoma | Leg_L Sarcoma | IV | Local relapse | 1257.50 | 80.60 | 1178.40 | 6.41 | 11.88 | 8.19 | 68.96 | 5.16 |
| 4 | Urachus Carcinoma | Peritoneal Carcinomatosis | IV | Local relapse | 218.70 | 9.90 | 208.20 | 4.53 | 12.04 | 7.76 | 64.41 | 5.26 |
| 5 | Tongue SCC | LN_Neck_II-III-IV | IV | Nodal relapse | 127.39 | 4.40 | 121.70 | 3.45 | 12.08 | 7.61 | 63.04 | 4.18 |
| 6 | Colon Adenocarcinoma | Peritoneal carcinomatosis | IV | Distant relapse | 513.36 | 20.60 | 488.70 | 4.01 | 11.96 | 7.22 | 60.34 | 4.67 |
| 7 | Lung Adenocarcinoma | LN_Mediastinum | IV | Nodal relapse | 64.19 | 6.10 | 57.00 | 9.50 | 11.96 | 9.20 | 76.92 | 6.07 |
| 8 | Cavity_Oral SCC | Cavity_oral | IV | Nodal relapse | 335.66 | 22.71 | 307.90 | 6.77 | 11.90 | 8.39 | 70.50 | 6.12 |
| 9 | Rectum Adenocarcinoma | Local relapse | IV | Local relapse | 411.62 | 34.89 | 374.00 | 8.47 | 11.88 | 8.68 | 73.09 | 5.28 |
| 10 | Gallbladder Adenocarcinoma | Peritoneal carcinomatosis | IV | Distant relapse | 357.03 | 37.23 | 315.40 | 10.43 | 11.86 | 8.86 | 74.71 | 6.19 |
| 11 | Sacrum Synovial Sarcoma | Local relapse | IV | Local relapse | 1863.40 | 181.91 | 1674.30 | 9.76 | 11.93 | 9.51 | 79.75 | 6.79 |
| 12 | Lung SCC | Lung left | IV | Primary | 383.00 | 24.07 | 358.64 | 6.29 | 11.88 | 8.11 | 68.23 | 4.63 |
| 13 | Colon Adenocarcinoma | Peritoneal carcinomatosis | IV | Distant Relapse | 179.40 | 15.19 | 162.60 | 8.47 | 11.96 | 8.19 | 68.51 | 5.35 |
| 14 | Ovary Epithelial Carcinoma | Peritoneal carcinomatosis | IV | Distant Relapse | 377.20 | 22.89 | 351.20 | 6.07 | 11.80 | 7.35 | 62.29 | 5.01 |
| 15 | Colon Adenocarcinoma | Peritoneal carcinomatosis | IV | Distant Relapse | 316.43 | 28.14 | 285.20 | 8.89 | 11.75 | 9.31 | 79.29 | 5.80 |
| 16 | Colon Adenocarcinoma | Peritoneal carcinomatosis | IV | Distant Relapse | 156.86 | 14.39 | 140.35 | 9.17 | 11.95 | 8.44 | 70.61 | 4.66 |
| 17 | Colon Adenocarcinoma | Peritoneal carcinomatosis | IV | Distant Relapse | 219.90 | 21.31 | 196.20 | 9.69 | 11.91 | 8.52 | 71.52 | 5.20 |
| 18 | Colon Adenocarcinoma | Liver | IV | Distant Relapse | 201.47 | 11.83 | 189.50 | 5.87 | 12.05 | 7.43 | 61.64 | 3.16 |
| 19 | Lung SCC | Lung_ | IV | Primary | 123.50 | 11.08 | 111.30 | 8.97 | 11.93 | 8.09 | 67.70 | 4.60 |
| 20 | LN_Neck_II-III-IV_V Lymphoma | Lymphoma LN_Neck_II-III-IV_V | IIIB | Nodal relapse | 505.20 | 34.62 | 469.30 | 6.85 | 11.96 | 7.05 | 58.99 | 4.22 |
| 21 | LN_Neck_II-III-IV_V Nueroendocrine Carcinoma | Neuroendocrine carcinoma LN_Neck_II-III-IV_V | IV | Nodal relapse | 337.50 | 27.51 | 306.30 | 8.15 | 11.96 | 8.89 | 74.37 | 5.42 |
| 22 | Kidney_L Clear Cell Carcinoma | Local relapse | IV | Local relapse | 1020.90 | 99.00 | 917.90 | 9.69 | 11.98 | 9.05 | 75.53 | 6.25 |
| MeanSD | 415.00 435.00 | 32.60 40.70 | 380.00 396.00 | 7.382.13 | 11.90.10 | 8.290.71 | 5.110.87 | 69.4 6.02 | ||||
| Minimum–Maximum | 45.40–1863.00 | 1.75–1.82 | 38.90–1674.00 | 3.45–10.40 | 11.70–12.10 | 7.05–9.51 | 3.16–6.78 | 59.00–79.80 | ||||
| Median | 326.00 | 22.00 | 296.00 | 7.60 | 11.90 | 8.29 | 5.18 | 69.70 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macias-Verde, D.; Burgos-Burgos, J.; Lara, P.C. Automated Clinical Dosimetry Planning of Dense Lattice Radiation Therapy. Cancers 2025, 17, 2048. https://doi.org/10.3390/cancers17122048
Macias-Verde D, Burgos-Burgos J, Lara PC. Automated Clinical Dosimetry Planning of Dense Lattice Radiation Therapy. Cancers. 2025; 17(12):2048. https://doi.org/10.3390/cancers17122048
Chicago/Turabian StyleMacias-Verde, David, Javier Burgos-Burgos, and Pedro C. Lara. 2025. "Automated Clinical Dosimetry Planning of Dense Lattice Radiation Therapy" Cancers 17, no. 12: 2048. https://doi.org/10.3390/cancers17122048
APA StyleMacias-Verde, D., Burgos-Burgos, J., & Lara, P. C. (2025). Automated Clinical Dosimetry Planning of Dense Lattice Radiation Therapy. Cancers, 17(12), 2048. https://doi.org/10.3390/cancers17122048







