New Approaches in Radiotherapy
Simple Summary
Abstract
1. Introduction
- Brachytherapy
- Stereotactic radiotherapy
- Advanced image guidance
- Proton and heavy ion radiotherapy
- Adaptive radiotherapy
- Hyperthermia
- Theranostics
- Artificial intelligence and data science
- Radioimmunotherapy
- Spatially fractionated radiotherapy
- Flash radiotherapy
- Boron neutron capture
- Intraoperative radiotherapy
- Volumetric modulated arc therapy for total body and total marrow irradiation
- Genomic profiling
- 3D printing
- Alpha-particle therapy
- Photodynamic therapy
- Auger therapy
- Hydrogen therapy
2. Brachytherapy
2.1. Intensity-Modulated Brachytherapy (IMBT)
2.2. Image-Guided Brachytherapy
2.3. Three-Dimensional Printing
2.4. Treatment Planning
3. Stereotactic Radiosurgery and Stereotactic Body Radiotherapy
3.1. Treatment Modalities
3.2. Treatment Planning Techniques
3.3. Future Direction
4. Advanced Image Guidance
4.1. Magnetic Resonance Imaging
4.2. Positron Emission Tomography
4.3. Surface Guidance
4.4. Cherenkov Radiation
5. Charged Particle and Proton Therapy
5.1. Physics Principles and Advantages
5.2. Challenges
5.3. Present and Future Developments
6. Adaptive Radiotherapy
6.1. Adaptive Workflow
6.2. Imaging
6.3. Automation
6.4. Current Trials and Early Clinical Evidence
7. Hyperthermia
7.1. Biological Basis of Thermoradiotherapy
7.2. Clinical Implementation
7.3. Challenges and Future Directions
8. Theranostics
8.1. Internal Dosimetry
8.2. Current and Future Perspective of Theranostics
9. Artificial Intelligence and Data Science
9.1. Image Generation, Enhancement, and Registration
9.2. Image Segmentation
9.3. Treatment Planning
9.4. Quality Assurance
9.5. Outcome and Toxicity Prediction, Patient Management
9.6. Other Applications
9.7. Current Challenges and Future Outlook
10. Radioimmunology
10.1. Effect of Radiation on the Immune System
10.2. Radioimmunotherapy and Radiation Abscopal Effect
10.3. Future Directions in Radioimmunology
10.4. Radiolabeled Immunotherapy
10.5. Challenges and Advances in RIT
11. Spatially Fractionated Radiation Therapy
11.1. Prescription Parameters and Techniques
11.2. SFRT Techniques and Clinical Applications
- LATTICE Therapy: This approach utilizes advanced treatment techniques (IMRT, VMAT, pencil beam scanning PRT, arc PRT, etc.) to conform peak doses to contoured high-dose volumes (vertices) [276,277]. LATTICE therapy mimics the dosimetric design of GRID therapy but offers more convenient delivery without requiring a physical grid. It also provides flexibility for customized OAR sparing through beam modulation [278]. Figure 6 shows a simulated LATTICE plan.
11.3. Minibeam and Microbeam Radiotherapy
11.4. Radiobiological Mechanisms of SFRT
11.5. Current Challenges and Future Directions
12. FLASH Radiotherapy
12.1. The FLASH Effect: Historical Insights and Potential Biological Mechanisms
12.2. Preclinical Studies, Early Clinical Results, Current Clinical Trials, and Future Directions
13. Boron Neutron Capture Therapy
13.1. Neutron Sources
13.2. Boron Delivery Agents
13.3. BNCT as a Multimodal Therapy
13.4. Clinical Applications and Future Directions
14. Other Areas of Interest
14.1. Intraoperative Radiotherapy
14.2. VMAT Total Body and Total Marrow Irradiation
14.3. Genomic Profiling
14.4. Three-Dimensional Printing
14.5. PTV Definition
14.6. Alpha-Particle Therapy
14.7. Photodynamic Therapy
14.8. Auger Therapy
14.9. Hydrogen Therapy
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| 3D | Three-Dimensional |
| 3D CRT | 3D conformal RT |
| AI | Artificial intelligence |
| ART | Adaptive radiotherapy |
| AVM | Arteriovenous malformation |
| BNCT | Boron neutron capture therapy |
| CBCT | Cone-beam computed tomography |
| CNN | Convolutional neural network |
| CPRT | Charged particle radiotherapy |
| CT | Computed tomography |
| DAMP | Damage-associated molecular patterns |
| DCA | Dynamic conformal arc |
| DL | Deep learning |
| DSPT | Double scattering proton therapy |
| DTRT | Dynamic trajectory tadiotherapy |
| FDG | Fluorodeoxyglucose |
| GAN | Generative adversarial network |
| HT | Hyperthermia |
| H-XRT | High-dose radiation therapy |
| IGRT | Image Guided Radiotherapy |
| IMBT | Intensity-modulated brachytherapy |
| IMRT | Intensity-modulated radiation therapy |
| IORT | Intraoperative radiotherapy |
| KBP | Knowledge-based planning |
| L-XRT | Low-dose radiation therapy |
| LET | Linear energy transfer |
| LINAC | Linear accelerator |
| MBRT | Minibeam radiotherapy |
| MIRD | Medical internal radiation dose |
| MRI | Magnetic resonance imaging |
| MRT | Microbeam radiotherapy |
| NK | Natural killer |
| OAR | Organ-at-risk |
| PBSPT | Pencil beam scanning proton therapy |
| PD-L1 | Programmed death-ligand 1 |
| PDT | Photodynamic therapy |
| PET | Positron emission tomography |
| PRIT | Pre-targeting radioimmunotherapy |
| PRT | Proton radiotherapy |
| PSMA | Prostate-specific membrane antigen |
| QA | Quality assurance |
| RBE | Relative biological effect |
| RIT | Radiolabeled immunotherapy |
| ROI | Region of interest |
| RT | Radiation therapy |
| SABR | Stereotactic ablative body radiotherapy |
| SBRT | Stereotactic body radiotherapy |
| SCC | Squamous cell carcinoma |
| SFRT | Spatially fractionated radiation therapy |
| SGRT | Surface-guided radiotherapy |
| SI-SRS | Single-isocenter stereotactic radiosurgery |
| SOPB | Spread-out Bragg peak |
| SRS | Stereotactic radiosurgery |
| SUV | Standardized uptake value |
| TBI | Total body irradiation |
| TMI | Total marrow irradiation |
| Tregs | Regulatory T cells |
| TRT | Thermoradiotherapy |
| TRUS | Transrectal ultrasound |
| VMAT | Volumetric modulated arc therapy |
References
- Berger, D.; Van Dyk, S.; Beaulieu, L.; Major, T.; Kron, T. Modern Tools for Modern Brachytherapy. Clin. Oncol. 2023, 35, e453–e468. [Google Scholar] [CrossRef] [PubMed]
- Petereit, D.G.; Frank, S.J.; Viswanathan, A.N.; Erickson, B.; Eifel, P.; Nguyen, P.L.; Wazer, D.E. Brachytherapy: Where Has It Gone? J. Clin. Oncol. 2015, 33, 980–982. [Google Scholar] [CrossRef]
- Chen, D.; Parsa, R.; Chauhan, K.; Lukovic, J.; Han, K.; Taggar, A.; Raman, S. Review of brachytherapy clinical trials: A cross-sectional analysis of ClinicalTrials.gov. Radiat. Oncol. 2024, 19, 22. [Google Scholar] [CrossRef]
- Song, W.Y.; Robar, J.L.; Morén, B.; Larsson, T.; Tedgren, Å.C.; Jia, X. Emerging technologies in brachytherapy. Phys. Med. Biol. 2021, 66, 23TR01. [Google Scholar] [CrossRef]
- Cunha, J.A.M.; Flynn, R.; Bélanger, C.; Callaghan, C.; Kim, Y.; Jia, X.; Chen, Z.; Beaulieu, L. Brachytherapy Future Directions. Semin. Radiat. Oncol. 2020, 30, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Poon, E.; Reniers, B.; Devic, S.; Vuong, T.; Verhaegen, F. Dosimetric characterization of a novel intracavitary mold applicator for high dose rate endorectal brachytherapy treatment. Med. Phys. 2006, 33, 4515–4526. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.J.; Devic, S.; Vuong, T.; Han, D.Y.; Scanderbeg, D.; Choi, D.; Song, B.; Song, W.Y. HDR brachytherapy of rectal cancer using a novel grooved-shielding applicator design. Med. Phys. 2013, 40, 091704. [Google Scholar] [CrossRef]
- Yang, W.; Kim, Y.; Wu, X.; Song, Q.; Liu, Y.; Bhatia, S.K.; Sun, W.; Flynn, R.T. Rotating-shield brachytherapy for cervical cancer. Phys. Med. Biol. 2013, 58, 3931–3941. [Google Scholar] [CrossRef]
- Liu, Y.; Flynn, R.T.; Kim, Y.; Dadkhah, H.; Bhatia, S.K.; Buatti, J.M.; Xu, W.; Wu, X. Paddle-based rotating-shield brachytherapy. Med. Phys. 2015, 42, 5992–6003. [Google Scholar] [CrossRef]
- Han, D.Y.; Webster, M.J.; Scanderbeg, D.J.; Yashar, C.; Choi, D.; Song, B.; Devic, S.; Ravi, A.; Song, W.Y. Direction-Modulated Brachytherapy for High-Dose-Rate Treatment of Cervical Cancer. I: Theoretical Design. Int. J. Radiat. Oncol. 2014, 89, 666–673. [Google Scholar] [CrossRef]
- Safigholi, H.; Han, D.Y.; Mashouf, S.; Soliman, A.; Meigooni, A.S.; Owrangi, A.; Song, W.Y. Direction modulated brachytherapy (DMBT) for treatment of cervical cancer: A planning study with 192Ir, 60Co, and 169Yb HDR sources. Med. Phys. 2017, 44, 6538–6547. [Google Scholar] [CrossRef] [PubMed]
- Mourya, A.; Aggarwal, L.M.; Choudhary, S. Evolution of Brachytherapy Applicators for the Treatment of Cervical Cancer. J. Med. Phys. 2021, 46, 231–243. [Google Scholar] [CrossRef]
- Shi, C.; Guo, B.; Cheng, C.-Y.; Esquivel, C.; Eng, T.; Papanikolaou, N. Three dimensional intensity modulated brachytherapy (IMBT): Dosimetry algorithm and inverse treatment planning. Med. Phys. 2010, 37, 3725–3737. [Google Scholar] [CrossRef]
- Adams, Q.E.; Xu, J.; Breitbach, E.K.; Li, X.; Enger, S.A.; Rockey, W.R.; Kim, Y.; Wu, X.; Flynn, R.T. Interstitial rotating shield brachytherapy for prostate cancer. Med. Phys. 2014, 41, 051703. [Google Scholar] [CrossRef] [PubMed]
- Dadkhah, H.; Hopfensperger, K.M.; Kim, Y.; Wu, X.; Flynn, R.T. Multisource Rotating Shield Brachytherapy Apparatus for Prostate Cancer. Int. J. Radiat. Oncol. 2017, 99, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Beriwal, S.; Demanes, D.J.; Erickson, B.; Jones, E.; De Los Santos, J.F.; Cormack, R.A.; Yashar, C.; Rownd, J.J.; Viswanathan, A.N. American Brachytherapy Society consensus guidelines for interstitial brachytherapy for vaginal cancer. Brachytherapy 2012, 11, 68–75. [Google Scholar] [CrossRef]
- Campbell, S.R.; Shah, C.; Scott, J.G.; Mesko, N.; Nystrom, L.; Kolar, M.; Largo, A.C.; Kamrava, M.; Mourtada, F.; Naghavi, A.O.; et al. American Brachytherapy Society (ABS) consensus statement for soft-tissue sarcoma brachytherapy. Brachytherapy 2021, 20, 1200–1218. [Google Scholar] [CrossRef]
- Henry, A.; Pieters, B.R.; Siebert, F.A.; Hoskin, P. GEC-ESTRO ACROP prostate brachytherapy guidelines. Radiother. Oncol. 2022, 167, 244–251. [Google Scholar] [CrossRef]
- Jacobsen, M.C.; Beriwal, S.; Dyer, B.A.; Klopp, A.H.; Lee, S.I.; McGinnis, G.J.; Robbins, J.B.; Rauch, G.M.; Sadowski, E.A.; Simiele, S.J.; et al. Contemporary image-guided cervical cancer brachytherapy: Consensus imaging recommendations from the Society of Abdominal Radiology and the American Brachytherapy Society. Brachytherapy 2022, 21, 369–388. [Google Scholar] [CrossRef]
- Mahantshetty, U.; Poetter, R.; Beriwal, S.; Grover, S.; Lavanya, G.; Rai, B.; Petric, P.; Tanderup, K.; Carvalho, H.; Hegazy, N.; et al. IBS-GEC ESTRO-ABS recommendations for CT based contouring in image guided adaptive brachytherapy for cervical cancer. Radiother. Oncol. 2021, 160, 273–284. [Google Scholar] [CrossRef]
- Hande, V.; Chopra, S.; Kalra, B.; Abdel-Wahab, M.; Kannan, S.; Tanderup, K.; Grover, S.; Zubizarreta, E.; Rubio, J.A.P. Point-A vs. volume-based brachytherapy for the treatment of cervix cancer: A meta-analysis. Radiother. Oncol. 2022, 170, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Eustace, N.; Liu, J.; Ladbury, C.; Tam, A.; Glaser, S.; Liu, A.; Chen, Y.-J. Current Status and Future Directions of Image-Guided Adaptive Brachytherapy for Locally Advanced Cervical Cancer. Cancers 2024, 16, 1031. [Google Scholar] [CrossRef] [PubMed]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Šegedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C.; et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef]
- Pötter, R.; Tanderup, K.; Schmid, M.P.; Jürgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicentre prospective cohort study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef]
- Souhami, L.; Corns, R.; Duclos, M.; Portelance, L.; Bahoric, B.; Stanimir, G. Long-term results of high-dose rate brachytherapy in cervix cancer using a small number of fractions. Gynecol. Oncol. 2005, 97, 508–513. [Google Scholar] [CrossRef]
- Holm, H.; Juul, N.; Pedersen, J.; Hansen, H.; Strøyer, I. Transperineal 125 Iodine Seed Implantation in Prostatic Cancer Guided by Transrectal Ultrasonography. J. Urol. 1983, 130, 283–286. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, P.; Watt, E.; Husain, S.; Martell, K.; Martinez, P.; Sawhney, S.; Thind, K. MRI-TRUS registration methodology for TRUS-guided HDR prostate brachytherapy. J. Appl. Clin. Med. Phys. 2021, 22, 284–294. [Google Scholar] [CrossRef]
- Major, T.; Gutiérrez, C.; Guix, B.; van Limbergen, E.; Strnad, V.; Polgár, C. Recommendations from GEC ESTRO Breast Cancer Working Group (II): Target definition and target delineation for accelerated or boost partial breast irradiation using multicatheter interstitial brachytherapy after breast conserving open cavity surgery. Radiother. Oncol. 2016, 118, 199–204. [Google Scholar] [CrossRef]
- Strnad, V.; Hannoun-Levi, J.-M.; Guinot, J.-L.; Lössl, K.; Kauer-Dorner, D.; Resch, A.; Kovács, G.; Major, T.; Van Limbergen, E. Recommendations from GEC ESTRO Breast Cancer Working Group (I): Target definition and target delineation for accelerated or boost Partial Breast Irradiation using multicatheter interstitial brachytherapy after breast conserving closed cavity surgery. Radiother. Oncol. 2015, 115, 342–348. [Google Scholar] [CrossRef]
- Sethi, R.; Cunha, A.; Mellis, K.; Siauw, T.; Diederich, C.; Pouliot, J.; Hsu, I.-C. Clinical applications of custom-made vaginal cylinders constructed using three-dimensional printing technology. J. Contemp. Brachyther. 2016, 3, 208–214. [Google Scholar] [CrossRef]
- Sekii, S.; Tsujino, K.; Kosaka, K.; Yamaguchi, S.; Kubota, H.; Matsumoto, Y.; Ota, Y.; Sasaki, R.; Soejima, T. Inversely designed, 3D-printed personalized template-guided interstitial brachytherapy for vaginal tumors. J. Contemp. Brachyther. 2018, 10, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Lindegaard, J.C.; Madsen, M.L.; Traberg, A.; Meisner, B.; Nielsen, S.K.; Tanderup, K.; Spejlborg, H.; Fokdal, L.U.; Nørrevang, O. Individualised 3D printed vaginal template for MRI guided brachytherapy in locally advanced cervical cancer. Radiother. Oncol. 2016, 118, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, E.; Easton, H.; Thomas, G.; Barbera, L.; D’ALimonte, L.; Ravi, A. Customized vaginal vault brachytherapy with computed tomography imaging-derived applicator prototyping. Brachytherapy 2015, 14, 380–384. [Google Scholar] [CrossRef]
- Xu, J.; Duindam, V.; Alterovitz, R.; Pouliot, J.; Cunha, J.A.M.; Hsu, I.-C.; Goldberg, K. Planning fireworks trajectories for steerable medical needles to reduce patient trauma. In Proceedings of the 2009 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2009), St. Louis, MO, USA, 10–15 October 2009; pp. 4517–4522. [Google Scholar]
- Garg, A.; Patil, S.; Siauw, T.; Cunha, J.A.M.; Hsu, I.-C.; Abbeel, P.; Pouliot, J.; Goldberg, K. An algorithm for computing customized 3D printed implants with curvature constrained channels for enhancing intracavitary brachytherapy radiation delivery. In Proceedings of the 2013 IEEE International Conference on Automation Science and Engineering (CASE 2013), Madison, WI, USA, 17–20 August 2013; pp. 466–473. [Google Scholar]
- Lancellotta, V.; Pagano, S.; Tagliaferri, L.; Piergentini, M.; Ricci, A.; Montecchiani, S.; Saldi, S.; Chierchini, S.; Cianetti, S.; Valentini, V.; et al. Individual 3-dimensional printed mold for treating hard palate carcinoma with brachytherapy: A clinical report. J. Prosthet. Dent. 2019, 121, 690–693. [Google Scholar] [CrossRef]
- Huang, M.-W.; Zhang, J.-G.; Zheng, L.; Liu, S.-M.; Yu, G.-Y. Accuracy evaluation of a 3D-printed individual template for needle guidance in head and neck brachytherapy. J. Radiat. Res. 2016, 57, 662–667. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Man, Q.-W.; Ma, Y.-Q.; Liu, B. I125 brachytherapy guided by individual three-dimensional printed plates for recurrent ameloblastoma of the skull base. Br. J. Oral Maxillofac. Surg. 2017, 55, e38–e40. [Google Scholar] [CrossRef] [PubMed]
- Juan, W.; Hongtao, Z.; Xuemin, D.; Huimin, Y.; Zeyang, W.; Lijuan, Z.; Jinxin, Z.; Zezhou, L.; Aixia, S. Dosimetry study of three-dimensional print template-guided precision 125I seed implantation. J. Cancer Res. Ther. 2016, 12, C159–C165. [Google Scholar] [CrossRef]
- Arenas, M.; Sabater, S.; Sintas, A.; Arguís, M.; Hernández, V.; Árquez, M.; López, I.; Rovirosa, À.; Puig, D. Individualized 3D scanning and printing for non-melanoma skin cancer brachytherapy: A financial study for its integration into clinical workflow. J. Contemp. Brachyther. 2017, 3, 270–276. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, H.; Xie, L.; Sun, J.; Qian, J.; Zhu, L. Comparison of Image-Guided Iodine-125 Seed Interstitial Brachytherapy and Local Chemotherapy Perfusion in Treatment of Advanced Pancreatic Cancer. J. Investig. Surg. 2020, 35, 1–6. [Google Scholar] [CrossRef]
- Fahimian, B.P.; Liu, W.; Skinner, L.; Yu, A.S.; Phillips, T.; Steers, J.M.; DeMarco, J.; Fraass, B.A.; Kamrava, M. 3D printing in brachytherapy: A systematic review of gynecological applications. Brachytherapy 2023, 22, 446–460. [Google Scholar] [CrossRef]
- de Ridder, M.; Smolic, M.; Kastelijns, M.; Kloosterman, S.; van der Vegt, S.; Rijken, J.A.; Jürgenliemk-Schulz, I.M.; Dehnad, H.; Kroon, P.S.; Moerland, M.A. Individualized 3D-printed applicators for magnetic resonance imaging-guided brachytherapy in nasal vestibule cancer. Phys. Imaging Radiat. Oncol. 2024, 31, 100629. [Google Scholar] [CrossRef] [PubMed]
- Kazemifar, S.; Balagopal, A.; Nguyen, D.; McGuire, S.; Hannan, R.; Jiang, S.B.; Owrangi, A.M. Segmentation of the prostate and organs at risk in male pelvic CT images using deep learning. Biomed. Phys. Eng. Express 2018, 4, 055003. [Google Scholar] [CrossRef]
- Men, K.; Chen, X.; Yang, B.; Zhu, J.; Yi, J.; Wang, S.; Li, Y.; Dai, J. Automatic segmentation of three clinical target volumes in radiotherapy using lifelong learning. Radiother. Oncol. 2021, 157, 1–7. [Google Scholar] [CrossRef]
- Gonzalez, Y.; Shen, C.; Jung, H.; Nguyen, D.; Jiang, S.B.; Albuquerque, K.; Jia, X. Semi-automatic sigmoid colon segmentation in CT for radiation therapy treatment planning via an iterative 2.5-D deep learning approach. Med. Image Anal. 2021, 68, 101896. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Q.; Zhang, L.; Yang, X.; Li, Z.; Fu, J. A deep learning-based self-adapting ensemble method for segmentation in gynecological brachytherapy. Radiat. Oncol. 2022, 17, 152. [Google Scholar] [CrossRef]
- Berger, D.; Dimopoulos, J.; Pötter, R.; Kirisits, C. Direct reconstruction of the Vienna applicator on MR images. Radiother. Oncol. 2009, 93, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.M.; Haidegger, T.; Birkfellner, W.; Cleary, K.; Peters, T.M.; Maier-Hein, L. Electromagnetic Tracking in Medicine—A Review of Technology, Validation, and Applications. IEEE Trans. Med. Imaging 2014, 33, 1702–1725. [Google Scholar] [CrossRef]
- Poulin, E.; Racine, E.; Binnekamp, D.; Beaulieu, L. Fast, automatic, and accurate catheter reconstruction in HDR brachytherapy using an electromagnetic 3D tracking system. Med. Phys. 2015, 42, 1227–1232. [Google Scholar] [CrossRef]
- Tho, D.; Racine, E.; Easton, H.; Song, W.Y.; Beaulieu, L. Technical Note: On EM reconstruction of a multi channel shielded applicator for cervical cancer brachytherapy: A feasibility study. Med. Phys. 2018, 45, 1673–1676. [Google Scholar] [CrossRef]
- Wang, W.; Viswanathan, A.N.; Damato, A.L.; Chen, Y.; Tse, Z.; Pan, L.; Tokuda, J.; Seethamraju, R.T.; Dumoulin, C.L.; Schmidt, E.J.; et al. Evaluation of an active magnetic resonance tracking system for interstitial brachytherapy. Med. Phys. 2015, 42, 7114–7121. [Google Scholar] [CrossRef]
- Rivard, M.J.; Coursey, B.M.; DeWerd, L.A.; Hanson, W.F.; Huq, M.S.; Ibbott, G.S.; Mitch, M.G.; Nath, R.; Williamson, J.F. Update of AAPM Task Group No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations. Med. Phys. 2004, 31, 633–674. [Google Scholar] [CrossRef] [PubMed]
- Nath, R.; Anderson, L.L.; Luxton, G.; Weaver, K.A.; Williamson, J.F.; Meigooni, A.S. Dosimetry of interstitial brachytherapy sources: Recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. Med. Phys. 1995, 22, 209–234. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.; Tedgren, Å.C.; Carrier, J.; Davis, S.D.; Mourtada, F.; Rivard, M.J.; Thomson, R.M.; Verhaegen, F.; Wareing, T.A.; Williamson, J.F. Report of the Task Group 186 on model-based dose calculation methods in brachytherapy beyond the TG-43 formalism: Current status and recommendations for clinical implementation. Med. Phys. 2012, 39, 6208–6236. [Google Scholar] [CrossRef]
- Dale, R.G.; Jones, B. The clinical radiobiology of brachytherapy. Br. J. Radiol. 1998, 71, 465–483. [Google Scholar] [CrossRef]
- Zaider, M.; Hanin, L. Biologically-equivalent dose and long-term survival time in radiation treatments. Phys. Med. Biol. 2007, 52, 6355–6362. [Google Scholar] [CrossRef] [PubMed]
- Annede, P.; Dumas, I.; Schernberg, A.; Tailleur, A.; Fumagalli, I.; Bockel, S.; Mignot, F.; Kissel, M.; Deutsch, E.; Haie-Meder, C.; et al. Radiobiological optimization comparison between pulse-dose-rate and high-dose-rate brachytherapy in patients with locally advanced cervical cancer. Brachytherapy 2019, 18, 370–377. [Google Scholar] [CrossRef]
- Suh, J.H. Stereotactic Radiosurgery for the Management of Brain Metastases. N. Engl. J. Med. 2010, 362, 1119–1127. [Google Scholar] [CrossRef]
- Chang, E.L.; Selek, U.; Hassenbusch, S.J.; Maor, M.H.; Allen, P.K.; Mahajan, A.; Sawaya, R.; Woo, S.Y. Outcome Variation among “Radioresistant” Brain Metastases Treated with Stereotactic Radiosurgery. Neurosurgery 2005, 56, 936–945. [Google Scholar] [CrossRef]
- Martin, A.; Gaya, A. Stereotactic Body Radiotherapy: A Review. Clin. Oncol. 2010, 22, 157–172. [Google Scholar] [CrossRef]
- Tipton, K.; Launders, J.H.; Inamdar, R.; Miyamoto, C.; Schoelles, K. Stereotactic Body Radiation Therapy: Scope of the Literature. Ann. Intern. Med. 2011, 154, 737–745. [Google Scholar] [CrossRef]
- Timmerman, R.D.; Herman, J.; Cho, L.C. Emergence of Stereotactic Body Radiation Therapy and Its Impact on Current and Future Clinical Practice. J. Clin. Oncol. 2014, 32, 2847–2854. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, W.; Park, I.H.; Kim, H.J.; Lee, E.; Jung, J.-H.; Cho, L.C.; Song, C.W. Radiobiological mechanisms of stereotactic body radiation therapy and stereotactic radiation surgery. Radiat. Oncol. J. 2015, 33, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Mitrasinovic, S.; Zhang, M.; Appelboom, G.; Sussman, E.; Moore, J.M.; Hancock, S.L.; Adler, J.R.; Kondziolka, D.; Steinberg, G.K.; Chang, S.D. Milestones in stereotactic radiosurgery for the central nervous system. J. Clin. Neurosci. 2019, 59, 12–19. [Google Scholar] [CrossRef]
- Karkar, D.; Chaudhari, P. Cyberknife treatment for different types of tumor. J. Pharm. Res. 2023, 8, 248–252. [Google Scholar]
- Liu, X.; Li, Z.; Yin, Y. Clinical application of MR-Linac in tumor radiotherapy: A systematic review. Radiat. Oncol. 2023, 18, 52. [Google Scholar] [CrossRef]
- Ma, C.-M.C. Physics and Dosimetric Principles of SRS and SBRT. Mathews J. Cancer Sci. 2019, 4, 1–16. [Google Scholar] [CrossRef]
- Weidlich, G.A.; Schneider, M.B.; Adler, J.R. Characterization of a Novel Revolving Radiation Collimator. Cureus 2018, 10, e2146. [Google Scholar] [CrossRef] [PubMed]
- Marianayagam, N.J.; Paddick, I.; Persad, A.R.; Hori, Y.S.; Maslowski, A.; Thirunarayanan, I.; Khanna, A.R.; Park, D.J.; Buch, V.; Chang, S.D.; et al. Dosimetric Comparison of Dedicated Radiosurgery Platforms for the Treatment of Essential Tremor: Technical Report. Cureus 2024, 16, e57452. [Google Scholar] [CrossRef]
- Jung, H.; Yoon, J.; Lemus, O.D.; Tanny, S.; Zhou, Y.; Milano, M.; Usuki, K.; Hardy, S.; Zheng, D. Dosimetric evaluation of LINAC-based single-isocenter multi-target multi-fraction stereotactic radiosurgery with more than 20 targets: Comparing MME, HyperArc, and RapidArc. Radiat. Oncol. 2024, 19, 19. [Google Scholar] [CrossRef]
- Raza, G.H.; Capone, L.; Tini, P.; Giraffa, M.; Gentile, P.; Minniti, G. Single-isocenter multiple-target stereotactic radiosurgery for multiple brain metastases: Dosimetric evaluation of two automated treatment planning systems. Radiat. Oncol. 2022, 17, 116. [Google Scholar] [CrossRef]
- Vergalasova, I.; Liu, H.; Alonso-Basanta, M.; Dong, L.; Li, J.; Nie, K.; Shi, W.; Teo, B.-K.K.; Yu, Y.; Yue, N.J.; et al. Multi-Institutional Dosimetric Evaluation of Modern Day Stereotactic Radiosurgery (SRS) Treatment Options for Multiple Brain Metastases. Front. Oncol. 2019, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- Brahme, A.; Lind, B.K. A systems biology approach to radiation therapy optimization. Radiat. Environ. Biophys. 2010, 49, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; Aneja, S. Opportunities for integration of artificial intelligence into stereotactic radiosurgery practice. Neuro-Oncology 2021, 23, 1629–1630. [Google Scholar] [CrossRef]
- Alongi, F.; Rigo, M.; Figlia, V.; Nicosia, L.; Mazzola, R.; Levra, N.G.; Ricchetti, F.; Trapani, G.; Attinà, G.; Vitale, C.; et al. 1.5T MR-Guided Daily-Adaptive SBRT for Prostate Cancer: Preliminary Report of Toxicity and Quality of Life of the First 100 Patients. J. Pers. Med. 2022, 12, 1982. [Google Scholar] [CrossRef] [PubMed]
- Fix, M.K.; Frei, D.; Volken, W.; Terribilini, D.; Mueller, S.; Elicin, O.; Hemmatazad, H.; Aebersold, D.M.; Manser, P. Part 1: Optimization and evaluation of dynamic trajectory radiotherapy. Med. Phys. 2018, 45, 4201–4212. [Google Scholar] [CrossRef]
- Yoo, K.H.; Park, D.J.; Choi, J.H.; Marianayagam, N.J.; Lim, M.; Meola, A.; Chang, S.D. Optimizing the synergy between stereotactic radiosurgery and immunotherapy for brain metastases. Front. Oncol. 2023, 13, 1223599. [Google Scholar] [CrossRef]
- Herman, M.G.; Balter, J.M.; Jaffray, D.A.; McGee, K.P.; Munro, P.; Shalev, S.; Van Herk, M.; Wong, J.W. Clinical use of electronic portal imaging: Report of AAPM Radiation Therapy Committee Task Group 58. Med. Phys. 2001, 28, 712–737. [Google Scholar] [CrossRef]
- Klüter, S. Technical design and concept of a 0.35 T MR-Linac. Clin. Transl. Radiat. Oncol. 2019, 18, 98–101. [Google Scholar] [CrossRef]
- Winkel, D.; Bol, G.H.; Kroon, P.S.; van Asselen, B.; Hackett, S.S.; Werensteijn-Honingh, A.M.; Intven, M.P.; Eppinga, W.S.; Tijssen, R.H.; Kerkmeijer, L.G.; et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin. Transl. Radiat. Oncol. 2019, 18, 54–59. [Google Scholar] [CrossRef]
- Grégoire, V.; Guckenberger, M.; Haustermans, K.; Lagendijk, J.J.W.; Ménard, C.; Pötter, R.; Slotman, B.J.; Tanderup, K.; Thorwarth, D.; van Herk, M.; et al. Image guidance in radiation therapy for better cure of cancer. Mol. Oncol. 2020, 14, 1470–1491. [Google Scholar] [CrossRef]
- Raaijmakers, A.J.E.; Raaymakers, B.W.; Lagendijk, J.J.W. Magnetic-field-induced dose effects in MR-guided radiotherapy systems: Dependence on the magnetic field strength. Phys. Med. Biol. 2008, 53, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Chuong, M.D.; Lee, P.; Low, D.A.; Kim, J.; Mittauer, K.E.; Bassetti, M.F.; Glide-Hurst, C.K.; Raldow, A.C.; Yang, Y.; Portelance, L.; et al. Stereotactic MR-guided on-table adaptive radiation therapy (SMART) for borderline resectable and locally advanced pancreatic cancer: A multi-center, open-label phase 2 study. Radiother. Oncol. 2023, 191, 110064. [Google Scholar] [CrossRef] [PubMed]
- Bahig, H.; Yuan, Y.; Mohamed, A.S.; Brock, K.K.; Ng, S.P.; Wang, J.; Ding, Y.; Hutcheson, K.; McCulloch, M.; Balter, P.A.; et al. Magnetic Resonance-based Response Assessment and Dose Adaptation in Human Papilloma Virus Positive Tumors of the Oropharynx treated with Radiotherapy (MR-ADAPTOR): An R-IDEAL stage 2a-2b/Bayesian phase II trial. Clin. Transl. Radiat. Oncol. 2018, 13, 19–23. [Google Scholar] [CrossRef]
- Dunlop, A.; Mitchell, A.; Tree, A.; Barnes, H.; Bower, L.; Chick, J.; Goodwin, E.; Herbert, T.; Lawes, R.; McNair, H.; et al. Daily adaptive radiotherapy for patients with prostate cancer using a high field MR-linac: Initial clinical experiences and assessment of delivered doses compared to a C-arm linac. Clin. Transl. Radiat. Oncol. 2020, 23, 35–42. [Google Scholar] [CrossRef]
- Kiser, K.; Meheissen, M.A.; Mohamed, A.S.; Kamal, M.; Ng, S.P.; Elhalawani, H.; Jethanandani, A.; He, R.; Ding, Y.; Rostom, Y.; et al. Prospective quantitative quality assurance and deformation estimation of MRI-CT image registration in simulation of head and neck radiotherapy patients. Clin. Transl. Radiat. Oncol. 2019, 18, 120–127. [Google Scholar] [CrossRef]
- Gani, C.; Boldrini, L.; Valentini, V. Online MR guided radiotherapy for rectal cancer. New opportunities. Clin. Transl. Radiat. Oncol. 2019, 18, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Nachbar, M.; Mönnich, D.; Boeke, S.; Gani, C.; Weidner, N.; Heinrich, V.; Russo, M.L.; Livi, L.; Winter, J.; Tsitsekidis, S.; et al. Partial breast irradiation with the 1.5 T MR-Linac: First patient treatment and analysis of electron return and stream effects. Radiother. Oncol. 2020, 145, 30–35. [Google Scholar] [CrossRef]
- Paulson, E.S.; Ahunbay, E.; Chen, X.; Mickevicius, N.J.; Chen, G.-P.; Schultz, C.; Erickson, B.; Straza, M.; Hall, W.A.; Li, X.A. 4D-MRI driven MR-guided online adaptive radiotherapy for abdominal stereotactic body radiation therapy on a high field MR-Linac: Implementation and initial clinical experience. Clin. Transl. Radiat. Oncol. 2020, 23, 72–79. [Google Scholar] [CrossRef]
- Stieb, S.; Elgohari, B.; Fuller, C.D. Repetitive MRI of organs at risk in head and neck cancer patients undergoing radiotherapy. Clin. Transl. Radiat. Oncol. 2019, 18, 131–139. [Google Scholar] [CrossRef]
- Winkel, D.; Werensteijn-Honingh, A.M.; Kroon, P.S.; Eppinga, W.S.; Bol, G.H.; Intven, M.P.; de Boer, H.C.; Snoeren, L.M.; Hes, J.; Raaymakers, B.W.; et al. Individual lymph nodes: “See it and Zap it”. Clin. Transl. Radiat. Oncol. 2019, 18, 46–53. [Google Scholar] [CrossRef]
- Grégoire, V.; Thorwarth, D.; Lee, J.A. Molecular Imaging-Guided Radiotherapy for the Treatment of Head-and-Neck Squamous Cell Carcinoma: Does it Fulfill the Promises? Semin. Radiat. Oncol. 2018, 28, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Konert, T.; Vogel, W.; MacManus, M.P.; Nestle, U.; Belderbos, J.; Grégoire, V.; Thorwarth, D.; Fidarova, E.; Paez, D.; Chiti, A.; et al. PET/CT imaging for target volume delineation in curative intent radiotherapy of non-small cell lung cancer: IAEA consensus report 2014. Radiother. Oncol. 2015, 116, 27–34. [Google Scholar] [CrossRef]
- Oderinde, O.M.; Shirvani, S.M.; Olcott, P.D.; Kuduvalli, G.; Mazin, S.; Larkin, D. The technical design and concept of a PET/CT linac for biology-guided radiotherapy. Clin. Transl. Radiat. Oncol. 2021, 29, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Surucu, M.; Maniyedath, A.; Narayanan, M.; Han, B.; Kovalchuk, N.; Gensheimer, M.; Vitzthum, L.; Iagaru, A.; Ferri, V.; Xing, L.; et al. Comparison of a First-in-Class LINAC-Integrated PET System and a Diagnostic PET/CT Scanner. Int. J. Radiat. Oncol. 2021, 111, e515–e516. [Google Scholar] [CrossRef]
- Pham, D.; Simiele, E.; Breitkreutz, D.; Capaldi, D.; Han, B.; Surucu, M.; Oderinde, S.; Vitzthum, L.; Gensheimer, M.; Bagshaw, H.; et al. IMRT and SBRT Treatment Planning Study for the First Clinical Biology-Guided Radiotherapy System. Technol. Cancer Res. Treat. 2022, 21, 15330338221100231. [Google Scholar] [CrossRef]
- Shirvani, S.M.; Huntzinger, C.J.; Melcher, T.; Olcott, P.D.; Voronenko, Y.; Bartlett-Roberto, J.; Mazin, S. Biology-guided radiotherapy: Redefining the role of radiotherapy in metastatic cancer. Br. J. Radiol. 2020, 94, 20200873. [Google Scholar] [CrossRef]
- Batista, V.; Gober, M.; Moura, F.; Webster, A.; Oellers, M.; Ramtohul, M.; Kügele, M.; Freislederer, P.; Buschmann, M.; Anastasi, G.; et al. Surface guided radiation therapy: An international survey on current clinical practice. Tech. Innov. Patient Support Radiat. Oncol. 2022, 22, 1–8. [Google Scholar] [CrossRef]
- Naidoo, W.; Leech, M. Feasibility of surface guided radiotherapy for patient positioning in breast radiotherapy versus conventional tattoo-based setups—A systematic review. Tech. Innov. Patient Support Radiat. Oncol. 2022, 22, 39–49. [Google Scholar] [CrossRef]
- Schulz, R.; Huyghe, C.; Hampton, L.; Hanson, D.; Stead, M.; Speck, T. Technical Overview and Features of the Varian IDENTIFYTM System. In Surface Guided Radiation Therapy; CRC: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Li, T.; Mihailidis, D.N. Image Guidance in Radiation Therapy: Techniques, Accuracy, and Limitations, 2018 Summer School. P. Alaei, G. Ding, Editors. American Association of Physicists in Medicine: Medical Physics Monograph No. 39. Medical Physics Publishing, Madison, WI, 2018. Hardcover: 406pp. Price: $120.00. ISBN: 9781936366620. Med. Phys. 2020, 48, E40–E41. [Google Scholar] [CrossRef]
- Darréon, J.; Massabeau, C.; Geffroy, C.; Maroun, P.; Simon, L. Surface-guided radiotherapy overview: Technical aspects and clinical applications. Cancer Radiother. 2023, 27, 504–510. [Google Scholar] [CrossRef]
- Nguyen, D.; Reinoso, R.; Farah, J.; Yossi, S.; Lorchel, F.; Passerat, V.; Louet, E.; Pouchard, I.; Khodri, M.; Barbet, N. Reproducibility of surface-based deep inspiration breath-hold technique for lung stereotactic body radiotherapy on a closed-bore gantry linac. Phys. Imaging Radiat. Oncol. 2023, 26, 100448. [Google Scholar] [CrossRef] [PubMed]
- Gierga, D.P.; Riboldi, M.; Turcotte, J.C.; Sharp, G.C.; Jiang, S.B.; Taghian, A.G.; Chen, G.T. Comparison of Target Registration Errors for Multiple Image-Guided Techniques in Accelerated Partial Breast Irradiation. Int. J. Radiat. Oncol. 2008, 70, 1239–1246. [Google Scholar] [CrossRef]
- Arjomandy, B.; Taylor, P.; Ainsley, C.; Safai, S.; Sahoo, N.; Pankuch, M.; Farr, J.B.; Park, S.Y.; Klein, E.; Flanz, J.; et al. AAPM task group 224: Comprehensive proton therapy machine quality assurance. Med. Phys. 2019, 46, E678–E705. [Google Scholar] [CrossRef]
- El-Sherif, O.; Remmes, N.B.; Kruse, J.J. Validating robotic couch isocentricity with 3D surface imaging. J. Appl. Clin. Med. Phys. 2020, 21, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Cherenkov, P. Видимoе свечение чистых жидкoстей пoд действием γ-радиации. Uspekhi Fizicheskih Nauk. 1967, 93, 385–388. [Google Scholar] [CrossRef]
- Steidley, K.; Eastman, R.M.; Stabile, R.J. Observations of visual sensations produced by Cerenkov radiation from high-energy electrons. Int. J. Radiat. Oncol. 1989, 17, 685–690. [Google Scholar] [CrossRef]
- Newman, F.; Asadi-Zeydabadi, M.; Durairaj, V.D.; Ding, M.; Stuhr, K.; Kavanagh, B. Visual sensations during megavoltage radiotherapy to the orbit attributable to Cherenkov radiation. Med. Phys. 2007, 35, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, L.A.; Zhang, R.; Gladstone, D.J.; Jiang, S.; Hitchcock, W.; Friedman, O.D.; Glaser, A.K.; Jermyn, M.; Pogue, B.W. Cherenkov Video Imaging Allows for the First Visualization of Radiation Therapy in Real Time. Int. J. Radiat. Oncol. 2014, 89, 615–622. [Google Scholar] [CrossRef]
- Black, P.J.; Velten, C.; Wang, Y.; Na, Y.H.; Wuu, C. An investigation of clinical treatment field delivery verification using cherenkov imaging: IMRT positioning shifts and field matching. Med. Phys. 2018, 46, 302–317. [Google Scholar] [CrossRef]
- Roussakis, Y.; Zhang, R.; Heyes, G.; Webster, G.; Mason, S.; Green, S.; Pogue, B.; Dehghani, H. Real-time Cherenkov emission portal imaging during CyberKnife®radiotherapy. Phys. Med. Biol. 2015, 60, N419–N425. [Google Scholar] [CrossRef]
- Yeung, T.K.; Bortolotto, K.; Cosby, S.; Hoar, M.; Lederer, E. Quality assurance in radiotherapy: Evaluation of errors and incidents recorded over a 10 year period. Radiother. Oncol. 2005, 74, 283–291. [Google Scholar] [CrossRef]
- Alexander, D.A.; Jermyn, M.; Bruza, P.; Zhang, R.; Chen, E.; Decker, S.M.; McGlynn, T.L.; Rosselot, R.A.; Ba, J.L.; Rose, M.L.; et al. Retrospective Evaluation of an Always-on Cherenkov Imaging System for Radiotherapy Quality Improvement. arXiv 2021, arXiv:2110.07494. [Google Scholar]
- Hachadorian, R.L.; Bruza, P.; Jermyn, M.; Gladstone, D.J.; Pogue, B.W.; Jarvis, L.A. Imaging radiation dose in breast radiotherapy by X-ray CT calibration of Cherenkov light. Nat. Commun. 2020, 11, 2298. [Google Scholar] [CrossRef]
- Jarvis, L.A.; Hachadorian, R.L.; Jermyn, M.; Bruza, P.; Alexander, D.A.; Tendler, I.I.; Williams, B.B.; Gladstone, D.J.; Schaner, P.E.; Zaki, B.I.; et al. Initial Clinical Experience of Cherenkov Imaging in External Beam Radiation Therapy Identifies Opportunities to Improve Treatment Delivery. Int. J. Radiat. Oncol. 2021, 109, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, J.M.; Mooney, K.E.; Brůža, P.; Curcuru, A.; Gladstone, D.J.; Pogue, B.W.; Green, O. Remote Cherenkov imaging-based quality assurance of a magnetic resonance image-guided radiotherapy system. Med. Phys. 2018, 45, 2647–2659. [Google Scholar] [CrossRef]
- Rahman, M. Development of a Flash Radiotherapy Platform: Characterization, Safety, and DeLivery Validation. Ph.D. Thesis, Dartmouth College, Hanover, NH, USA, 2021. [Google Scholar]
- El Naqa, I.; Pogue, B.W.; Zhang, R.; Oraiqat, I.; Parodi, K. Image guidance for FLASH radiotherapy. Med. Phys. 2022, 49, 4109–4122. [Google Scholar] [CrossRef]
- Mohan, R.; Grosshans, D. Proton therapy—Present and future. Adv. Drug Deliv. Rev. 2017, 109, 26–44. [Google Scholar] [CrossRef]
- Mohan, R. A review of proton therapy—Current status and future directions. Precis. Radiat. Oncol. 2022, 6, 164–176. [Google Scholar] [CrossRef]
- Mitin, T.; Zietman, A.L. Promise and Pitfalls of Heavy-Particle Therapy. J. Clin. Oncol. 2014, 32, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H.; Niemierko, A.; Ancukiewicz, M.; Gerweck, L.E.; Goitein, M.; Loeffler, J.S.; Suit, H.D. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. 2002, 53, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Find a Proton Therapy Center Near You. Available online: https://proton-therapy.org/findacenter/ (accessed on 24 September 2024).
- Ning, M.S.; Gomez, D.R.; Shah, A.K.; Kim, C.R.; Palmer, M.B.; Thaker, N.G.; Grosshans, D.R.; Liao, Z.; Chapman, B.V.; Brooks, E.D.; et al. The Insurance Approval Process for Proton Radiation Therapy: A Significant Barrier to Patient Care. Int. J. Radiat. Oncol. 2019, 104, 724–733. [Google Scholar] [CrossRef]
- Ojerholm, E.; Hill-Kayser, C.E. Insurance coverage decisions for pediatric proton therapy. Pediatr. Blood Cancer 2017, 65, e26729. [Google Scholar] [CrossRef] [PubMed]
- Badiyan, S.N.; Hallemeier, C.L.; Lin, S.H.; Hall, M.D.; Chuong, M.D. Proton beam therapy for gastrointestinal cancers: Past, present, and future. J. Gastrointest. Oncol. 2018, 9, 962–971. [Google Scholar] [CrossRef]
- Chen, Z.; Dominello, M.M.; Joiner, M.C.; Burmeister, J.W. Proton versus photon radiation therapy: A clinical review. Front. Oncol. 2023, 13, 1133909. [Google Scholar] [CrossRef]
- Hwang, E.J.; Gorayski, P.; Le, H.; Hanna, G.G.; Kenny, L.; Penniment, M.; Buck, J.; Thwaites, D.; Ahern, V. Particle therapy tumour outcomes: An updated systematic review. J. Med. Imaging Radiat. Oncol. 2020, 64, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.V.; Aggarwal, S.; Bentzen, S.M.; Knight, N.; Mehta, M.P.; Regine, W.F. Establishing Evidence-Based Indications for Proton Therapy: An Overview of Current Clinical Trials. Int. J. Radiat. Oncol. 2017, 97, 228–235. [Google Scholar] [CrossRef]
- Volz, L.; Korte, J.; Martire, M.C.; Zhang, Y.; Hardcastle, N.; Durante, M.; Kron, T.; Graeff, C. Opportunities and challenges of upright patient positioning in radiotherapy. Phys. Med. Biol. 2024, 69, 18TR02. [Google Scholar] [CrossRef]
- Mein, S.; Wuyckens, S.; Li, X.; Both, S.; Carabe, A.; Vera, M.C.; Engwall, E.; Francesco, F.; Graeff, C.; Gu, W.; et al. Particle arc therapy: Status and potential. Radiother. Oncol. 2024, 199, 110434. [Google Scholar] [CrossRef]
- Lane, S.A.; Slater, J.M.; Yang, G.Y. Image-Guided Proton Therapy: A Comprehensive Review. Cancers 2023, 15, 2555. [Google Scholar] [CrossRef] [PubMed]
- Charyyev, S.; Lei, Y.; Harms, J.; Eaton, B.; McDonald, M.; Curran, W.J.; Liu, T.; Zhou, J.; Zhang, R.; Yang, X. High quality proton portal imaging using deep learning for proton radiation therapy: A phantom study. Biomed. Phys. Eng. Express 2020, 6, 035029. [Google Scholar] [CrossRef]
- Malouff, T.D.; Mahajan, A.; Krishnan, S.; Beltran, C.; Seneviratne, D.S.; Trifiletti, D.M. Carbon Ion Therapy: A Modern Review of an Emerging Technology. Front. Oncol. 2020, 10, 82. [Google Scholar] [CrossRef]
- Schulz-Ertner, D.; Nikoghosyan, A.; Thilmann, C.; Haberer, T.; Jäkel, O.; Karger, C.; Kraft, G.; Wannenmacher, M.; Debus, J. Results of carbon ion radiotherapy in 152 patients. Int. J. Radiat. Oncol. 2004, 58, 631–640. [Google Scholar] [CrossRef]
- Sonke, J.-J.; Aznar, M.; Rasch, C. Adaptive Radiotherapy for Anatomical Changes. Semin. Radiat. Oncol. 2019, 29, 245–257. [Google Scholar] [CrossRef]
- Yan, D.; Vicini, F.; Wong, J.; Martinez, A. Adaptive radiation therapy. Phys. Med. Biol. 1997, 42, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Jeraj, R.; Olivera, G.H.; Mackie, T.R. Re-optimization in adaptive radiotherapy. Phys. Med. Biol. 2002, 47, 3181–3195. [Google Scholar] [CrossRef]
- Vanherk, M. Errors and margins in radiotherapy. Semin. Radiat. Oncol. 2004, 14, 52–64. [Google Scholar] [CrossRef]
- Jaffray, D.A. Image-guided radiotherapy: From current concept to future perspectives. Nat. Rev. Clin. Oncol. 2012, 9, 688–699. [Google Scholar] [CrossRef]
- Otto, K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med. Phys. 2007, 35, 310–317. [Google Scholar] [CrossRef]
- Teoh, M.; Clark, C.H.; Wood, K.; Whitaker, S.; Nisbet, A. Volumetric modulated arc therapy: A review of current literature and clinical use in practice. Br. J. Radiol. 2011, 84, 967–996. [Google Scholar] [CrossRef] [PubMed]
- Nutting, C.; Dearnaley, D.P.; Webb, S. Intensity modulated radiation therapy: A clinical review. Br. J. Radiol. 2000, 73, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Webb, S. Intensity-Modulated Radiation Therapy; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Dawson, L.A.; Sharpe, M.B. Image-guided radiotherapy: Rationale, benefits, and limitations. Lancet Oncol. 2006, 7, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamgani, A.; Heemsbergen, W.D.; Peeters, S.T.; Lebesque, J.V. Role of Intensity-Modulated Radiotherapy in Reducing Toxicity in Dose Escalation for Localized Prostate Cancer. Int. J. Radiat. Oncol. 2009, 73, 685–691. [Google Scholar] [CrossRef]
- Barker, J.L.; Garden, A.S.; Ang, K.; O’Daniel, J.C.; Wang, H.; Court, L.E.; Morrison, W.H.; Rosenthal, D.I.; Chao, K.; Tucker, S.L.; et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int. J. Radiat. Oncol. 2004, 59, 960–970. [Google Scholar] [CrossRef]
- Velec, M.; Moseley, J.L.; Craig, T.; Dawson, L.A.; Brock, K.K. Accumulated Dose in Liver Stereotactic Body Radiotherapy: Positioning, Breathing, and Deformation Effects. Int. J. Radiat. Oncol. 2012, 83, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jiang, S.; Kung, J.; Doppke, K.; Willett, C. Abdominal organ motion and deformation: Implications for IMRT. Int. J. Radiat. Oncol. 2001, 51, 210. [Google Scholar] [CrossRef]
- Yan, D.; Lockman, D. Organ/patient geometric variation in external beam radiotherapy and its effects. Med. Phys. 2001, 28, 593–602. [Google Scholar] [CrossRef]
- Mayr, N.A.; Yuh, W.T.C.; Taoka, T.; Wang, J.Z.; Wu, D.H.; Montebello, J.F.; Meeks, S.L.; Paulino, A.C.; Magnotta, V.A.; Adli, M.; et al. Serial Therapy-Induced Changes in Tumor Shape in Cervical Cancer and Their Impact on Assessing Tumor Volume and Treatment Response. Am. J. Roentgenol. 2006, 187, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Mutic, S.; Dempsey, J.F. The ViewRay System: Magnetic Resonance–Guided and Controlled Radiotherapy. Semin. Radiat. Oncol. 2014, 24, 196–199. [Google Scholar] [CrossRef]
- Archambault, Y.; Boylan, C.; Bullock, D.; Morgas, T.; Peltola, J.; Ruokokoski, E.; Genghi, A.; Haas, B.; Suhonen, P.; Thompson, S. Making On-Line Adaptive Radiotherapy Possible Using Artificial Intelligence and Machine Learning for Efficient Daily Re-Planning. Med. Phys. Int. J. 2020, 8, 77–86. [Google Scholar]
- Piperdi, H.; Portal, D.; Neibart, S.S.; Yue, N.J.; Jabbour, S.K.; Reyhan, M. Adaptive Radiation Therapy in the Treatment of Lung Cancer: An Overview of the Current State of the Field. Front. Oncol. 2021, 11, 770382. [Google Scholar] [CrossRef]
- Morgan, H.E.; Sher, D.J. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck 2020, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Placidi, L.; Romano, A.; Chiloiro, G.; Cusumano, D.; Boldrini, L.; Cellini, F.; Mattiucci, G.C.; Valentini, V. On-line adaptive MR guided radiotherapy for locally advanced pancreatic cancer: Clinical and dosimetric considerations. Tech. Innov. Patient Support Radiat. Oncol. 2020, 15, 15–21. [Google Scholar] [CrossRef]
- Åström, L.M.; Behrens, C.P.; Calmels, L.; Sjöström, D.; Geertsen, P.; Mouritsen, L.S.; Serup-Hansen, E.; Lindberg, H.; Sibolt, P. Online adaptive radiotherapy of urinary bladder cancer with full re-optimization to the anatomy of the day: Initial experience and dosimetric benefits. Radiother. Oncol. 2022, 171, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Shelley, C.; Barraclough, L.; Nelder, C.; Otter, S.; Stewart, A. Adaptive Radiotherapy in the Management of Cervical Cancer: Review of Strategies and Clinical Implementation. Clin. Oncol. 2021, 33, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.; Archibald-Heeren, B.; Hu, Y.; Teh, A.; Beserminji, R.; Cai, E.; Liu, G.; Yates, A.; Rijken, J.; Collett, N.; et al. Varian ethos online adaptive radiotherapy for prostate cancer: Early results of contouring accuracy, treatment plan quality, and treatment time. J. Appl. Clin. Med. Phys. 2021, 23, e13479. [Google Scholar] [CrossRef]
- Lemus, O.M.D.; Cao, M.; Cai, B.; Cummings, M.; Zheng, D. Adaptive Radiotherapy: Next-Generation Radiotherapy. Cancers 2024, 16, 1206. [Google Scholar] [CrossRef]
- Liu, H.; Schaal, D.; Curry, H.; Clark, R.; Magliari, A.; Kupelian, P.; Khuntia, D.; Beriwal, S. Review of cone beam computed tomography based online adaptive radiotherapy: Current trend and future direction. Radiat. Oncol. 2023, 18, 144. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, X.; Hong, J.C.; Zheng, D. Artificial Intelligence in Radiotherapy Treatment Planning: Present and Future. Technol. Cancer Res. Treat. 2019, 18, 1533033819873922. [Google Scholar] [CrossRef]
- Cardenas, C.E.; Yang, J.; Anderson, B.M.; Court, L.E.; Brock, K.B. Advances in Auto-Segmentation. Semin. Radiat. Oncol. 2019, 29, 185–197. [Google Scholar] [CrossRef]
- Heukelom, J.; Hamming, O.; Bartelink, H.; Hoebers, F.; Giralt, J.; Herlestam, T.; Verheij, M.; Brekel, M.v.D.; Vogel, W.; Slevin, N.; et al. Adaptive and innovative Radiation Treatment FOR improving Cancer treatment outcomE (ARTFORCE); a randomized controlled phase II trial for individualized treatment of head and neck cancer. BMC Cancer 2013, 13, 84. [Google Scholar] [CrossRef]
- Kong, F.-M.S.; Hu, C.; Haken, R.T.; Xiao, Y.; Matuszak, M.; Hirsh, V.; Pryma, D.A.; Siegel, B.A.; Gelblum, D.Y.; Hayman, J.; et al. NRG-RTOG 1106/ACRIN 6697: A phase IIR trial of standard versus adaptive (mid-treatment PET-based) chemoradiotherapy for stage III NSCLC—Results and comparison to NRG-RTOG 0617 (non-personalized RT dose escalation). J. Clin. Oncol. 2021, 39, 8548. [Google Scholar] [CrossRef]
- Hafeez, S.; Webster, A.; Hansen, V.N.; McNair, H.A.; Warren-Oseni, K.; Patel, E.; Choudhury, A.; Creswell, J.; Foroudi, F.; Henry, A.; et al. Protocol for tumour-focused dose-escalated adaptive radiotherapy for the radical treatment of bladder cancer in a multicentre phase II randomised controlled trial (RAIDER): Radiotherapy planning and delivery guidance. BMJ Open 2020, 10, e041005. [Google Scholar] [CrossRef]
- Henke, L.E.; Olsen, J.R.; Contreras, J.A.; Curcuru, A.; DeWees, T.A.; Green, O.L.; Michalski, J.; Mutic, S.; Roach, M.C.; Bradley, J.D.; et al. Stereotactic MR-Guided Online Adaptive Radiation Therapy (SMART) for Ultracentral Thorax Malignancies: Results of a Phase 1 Trial. Adv. Radiat. Oncol. 2019, 4, 201–209. [Google Scholar] [CrossRef]
- Henke, L.; Kashani, R.; Robinson, C.; Curcuru, A.; DeWees, T.; Bradley, J.; Green, O.; Michalski, J.; Mutic, S.; Parikh, P.; et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother. Oncol. 2018, 126, 519–526. [Google Scholar] [CrossRef]
- Kishan, A.U.; Ma, T.M.; Lamb, J.M.; Casado, M.; Wilhalme, H.; Low, D.A.; Sheng, K.; Sharma, S.; Nickols, N.G.; Pham, J.; et al. Magnetic Resonance Imaging–Guided vs Computed Tomography–Guided Stereotactic Body Radiotherapy for Prostate Cancer. JAMA Oncol. 2023, 9, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.M.; Lamb, J.M.; Casado, M.; Wang, X.; Basehart, T.V.; Yang, Y.; Low, D.; Sheng, K.; Agazaryan, N.; Nickols, N.G.; et al. Magnetic resonance imaging-guided stereotactic body radiotherapy for prostate cancer (mirage): A phase iii randomized trial. BMC Cancer 2021, 21, 538. [Google Scholar] [CrossRef]
- Coley, W.B. The treatment of malignant tumors by repeated inoculations of erysipelas: With a report of ten original cases. Am. J. Med. Sci. 1893, 105, 487. [Google Scholar] [CrossRef]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; ter Haar, G.; Wust, P.; Crezee, J. Heating technology for malignant tumors: A review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J. The heat is (still) on—The past and future of hyperthermic radiation oncology. Radiother. Oncol. 2013, 109, 185–187. [Google Scholar] [CrossRef]
- Datta, N.R.; Puric, E.; Klingbiel, D.; Gomez, S.; Bodis, S. Hyperthermia and Radiation Therapy in Locoregional Recurrent Breast Cancers: A Systematic Review and Meta-analysis. Int. J. Radiat. Oncol. 2016, 94, 1073–1087. [Google Scholar] [CrossRef]
- Datta, N.R.; Stutz, E.; Gomez, S.; Bodis, S. Efficacy and Safety Evaluation of the Various Therapeutic Options in Locally Advanced Cervix Cancer: A Systematic Review and Network Meta-Analysis of Randomized Clinical Trials. Int. J. Radiat. Oncol. 2019, 103, 411–437. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Z.; Mi, D.-H.; Cao, N.; Zu, S.-W.; Wen, Z.-Z.; Yu, X.-L.; Qu, Y. Chemoradiation combined with regional hyperthermia for advanced oesophageal cancer: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2017, 42, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Rogers, S.; Ordonez, S.G.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy in the management of head and neck cancers: A systematic review and meta-analysis. Int. J. Hyperth. 2016, 32, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Spałek, M.J.; Borkowska, A.M.; Telejko, M.; Wągrodzki, M.; Niebyłowska, D.; Uzar, A.; Białobrzeska, M.; Rutkowski, P. The Feasibility Study of Hypofractionated Radiotherapy with Regional Hyperthermia in Soft Tissue Sarcomas. Cancers 2021, 13, 1332. [Google Scholar] [CrossRef]
- Logghe, T.; van Zwol, E.; Immordino, B.; Cruys, K.V.D.; Peeters, M.; Giovannetti, E.; Bogers, J. Hyperthermia in Combination with Emerging Targeted and Immunotherapies as a New Approach in Cancer Treatment. Cancers 2024, 16, 505. [Google Scholar] [CrossRef]
- Li, C.; Wiseman, L.; Okoh, E.; Lind, M.; Roy, R.; Beavis, A.W.; Pires, I.M. Exploring hypoxic biology to improve radiotherapy outcomes. Expert Rev. Mol. Med. 2022, 24, e21. [Google Scholar] [CrossRef]
- Elming, P.B.; Sørensen, B.S.; Oei, A.L.; Franken, N.A.P.; Crezee, J.; Overgaard, J.; Horsman, M.R. Hyperthermia: The Optimal Treatment to Overcome Radiation Resistant Hypoxia. Cancers 2019, 11, 60. [Google Scholar] [CrossRef]
- Mantso, T.; Goussetis, G.; Franco, R.; Botaitis, S.; Pappa, A.; Panayiotidis, M. Effects of hyperthermia as a mitigation strategy in DNA damage-based cancer therapies. Semin. Cancer Biol. 2016, 37, 96–105. [Google Scholar] [CrossRef]
- Notter, M.; Piazena, H.; Vaupel, P. Hypofractionated re-irradiation of large-sized recurrent breast cancer with thermography-controlled, contact-free water-filtered infra-red-A hyperthermia: A retrospective study of 73 patients. Int. J. Hyperth. 2016, 33, 227–236. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Dobsicek-Trefna, H.; Curto, S.; Winter, L.; Molitoris, J.K.; Vrba, J.; Vrba, D.; Sumser, K.; Paulides, M.M. Radiofrequency and Microwave Hyperthermia in Cancer Treatment. In Principles and Technologies for Electromagnetic Energy Based Therapies; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- Frey, B.; Weiss, E.-M.; Rubner, Y.; Wunderlich, R.; Ott, O.J.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Old and new facts about hyperthermia-induced modulations of the immune system. Int. J. Hyperth. 2012, 28, 528–542. [Google Scholar] [CrossRef]
- Fiorentini, G.; Sarti, D.; Gadaleta, C.D.; Ballerini, M.; Fiorentini, C.; Garfagno, T.; Ranieri, G.; Guadagni, S. A Narrative Review of Regional Hyperthermia: Updates from 2010 to 2019. Integr. Cancer Ther. 2020, 19, 1534735420932648. [Google Scholar] [CrossRef]
- Righini, M.F.; Durham, A.; Tsoutsou, P.G. Andr Hyperthermia and radiotherapy: Physiological basis for a synergistic effect. Front. Oncol. 2024, 14, 1428065. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Dorsey, J.F.; Fan, Y. Cell plasticity, senescence, and quiescence in cancer stem cells: Biological and therapeutic implications. Pharmacol. Ther. 2021, 231, 107985. [Google Scholar] [CrossRef]
- Ademaj, A.; Veltsista, D.P.; Ghadjar, P.; Marder, D.; Oberacker, E.; Ott, O.J.; Wust, P.; Puric, E.; Hälg, R.A.; Rogers, S.; et al. Clinical Evidence for Thermometric Parameters to Guide Hyperthermia Treatment. Cancers 2022, 14, 625. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic hyperthermia therapy for the treatment of glioblastoma: A review of the therapy’s history, efficacy and application in humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef]
- Szwed, M.; Marczak, A. Application of Nanoparticles for Magnetic Hyperthermia for Cancer Treatment—The Current State of Knowledge. Cancers 2024, 16, 1156. [Google Scholar] [CrossRef] [PubMed]
- Peeken, J.C.; Vaupel, P.; Combs, S.E. Integrating Hyperthermia into Modern Radiation Oncology: What Evidence Is Necessary? Front. Oncol. 2017, 7, 132. [Google Scholar] [CrossRef]
- Burkett, B.J.; Bartlett, D.J.; McGarrah, P.W.; Lewis, A.R.; Johnson, D.R.; Berberoğlu, K.; Pandey, M.K.; Packard, A.T.; Halfdanarson, T.R.; Hruska, C.B.; et al. A Review of Theranostics: Perspectives on Emerging Approaches and Clinical Advancements. Radiol. Imaging Cancer 2023, 5, e220157. [Google Scholar] [CrossRef]
- Miller, C.; Rousseau, J.; Ramogida, C.F.; Celler, A.; Rahmim, A.; Uribe, C.F. Implications of physics, chemistry and biology for dosimetry calculations using theranostic pairs. Theranostics 2022, 12, 232–259. [Google Scholar] [CrossRef]
- Bednarz, B. Theranostics and Patient-Specific Dosimetry. Semin. Radiat. Oncol. 2023, 33, 317–326. [Google Scholar] [CrossRef]
- Lodge, M.A.; Wahl, R.L. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.; Pruim, J.; Price, P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- Kitajima, K.; Watabe, T.; Nakajo, M.; Ishibashi, M.; Daisaki, H.; Soeda, F.; Tanemura, A.; Kanekura, T.; Yamazaki, N.; Ito, K. Tumor response evaluation in patients with malignant melanoma undergoing immune checkpoint inhibitor therapy and prognosis prediction using 18F-FDG PET/CT: Multicenter study for comparison of EORTC, PERCIST, and imPERCIST. Jpn. J. Radiol. 2021, 40, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Vogsen, M.; Bülow, J.L.; Ljungstrøm, L.; Oltmann, H.R.; Alamdari, T.A.; Naghavi-Behzad, M.; Braad, P.-E.; Gerke, O.; Hildebrandt, M.G. FDG-PET/CT for Response Monitoring in Metastatic Breast Cancer: The Feasibility and Benefits of Applying PERCIST. Diagnostics 2021, 11, 723. [Google Scholar] [CrossRef]
- Min, S.J.; Jang, H.J.; Kim, J.H. Comparison of the RECIST and PERCIST criteria in solid tumors: A pooled analysis and review. Oncotarget 2016, 7, 27848–27854. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L. PERCIST in Perspective. Nucl. Med. Mol. Imaging 2017, 52, 1–4. [Google Scholar] [CrossRef]
- Weiss, G.J.; Korn, R.L. Interpretation of PET Scans: Do Not Take SUVs at Face Value. J. Thorac. Oncol. 2012, 7, 1744–1746. [Google Scholar] [CrossRef]
- Bouchet, L.G.; Bolch, W.E.; Weber, D.A.; Atkins, H.L.; Poston, J.W. MIRD Pamphlet No. 15: Radionuclide S values in a revised dosimetric model of the adult head and brain. Med. Intern. Radiat. Dose. 1999, 40, 62S–101S. [Google Scholar]
- Kesner, A.L.; Carter, L.M.; Ramos, J.C.O.; Lafontaine, D.; Olguin, E.A.; Brown, J.L.; President, B.; Jokisch, D.W.; Fisher, D.R.; Bolch, W.E. MIRD Pamphlet No. 28, Part 1: MIRDcalc—A Software Tool for Medical Internal Radiation Dosimetry. J. Nucl. Med. 2023, 64, 1117–1124. [Google Scholar] [CrossRef]
- Ringheim, A.; Campos Neto, G.C.; Anazodo, U.; Cui, L.; da Cunha, M.L.; Vitor, T.; Martins, K.M.; Miranda, A.C.C.; de Barboza, M.F.; Fuscaldi, L.L.; et al. Kinetic modeling of 68Ga-PSMA-11 and validation of simplified methods for quantification in primary prostate cancer patients. EJNMMI Res. 2020, 10, 12. [Google Scholar] [CrossRef]
- Strauss, D.S.; Sachpekidis, C.; Kopka, K.; Pan, L.; Haberkorn, U.; Dimitrakopoulou-Strauss, A. Pharmacokinetic studies of [68 Ga]Ga-PSMA-11 in patients with biochemical recurrence of prostate cancer: Detection, differences in temporal distribution and kinetic modelling by tissue type. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4472–4482. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.J.; Green, M.A.; Bahler, C.D.; Tann, M.; Territo, W.; Smith, A.M.; Hutchins, G.D. Comparison of tracer kinetic models for 68Ga-PSMA-11 PET in intermediate-risk primary prostate cancer patients. EJNMMI Res. 2024, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Pryma, D.A.; Chin, B.B.; Noto, R.B.; Dillon, J.S.; Perkins, S.; Solnes, L.; Kostakoglu, L.; Serafini, A.N.; Pampaloni, M.H.; Jensen, J.; et al. Efficacy and Safety of High-Specific-Activity 131I-MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma. J. Nucl. Med. 2018, 60, 623–630. [Google Scholar] [CrossRef]
- Kryza, D.; Vinceneux, A.; Bidaux, A.-S.; Garin, G.; Tatu, D.; Cropet, C.; Badel, J.-N.; Perol, D.; Giraudet, A.-L. A multicentric, single arm, open-label, phase I/II study evaluating PSMA targeted radionuclide therapy in adult patients with metastatic clear cell renal cancer (PRadR). BMC Cancer 2024, 24, 163. [Google Scholar] [CrossRef]
- Huynh, E.; Hosny, A.; Guthier, C.; Bitterman, D.S.; Petit, S.F.; Haas-Kogan, D.A.; Kann, B.; Aerts, H.J.W.L.; Mak, R.H. Artificial intelligence in radiation oncology. Nat. Rev. Clin. Oncol. 2020, 17, 771–781. [Google Scholar] [CrossRef]
- Fu, Y.; Lei, Y.; Wang, T.; Curran, W.J.; Liu, T.; Yang, X. Deep learning in medical image registration: A review. Phys. Med. Biol. 2020, 65, 20TR01. [Google Scholar] [CrossRef]
- Rusanov, B.; Hassan, G.M.; Reynolds, M.; Sabet, M.; Kendrick, J.; Rowshanfarzad, P.; Ebert, M. Deep learning methods for enhancing cone-beam CT image quality toward adaptive radiation therapy: A systematic review. Med. Phys. 2022, 49, 6019–6054. [Google Scholar] [CrossRef]
- Castiglioni, I.; Rundo, L.; Codari, M.; Di Leo, G.; Salvatore, C.; Interlenghi, M.; Gallivanone, F.; Cozzi, A.; D’AMico, N.C.; Sardanelli, F. AI applications to medical images: From machine learning to deep learning. Phys. Medica 2021, 83, 9–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H. Convolutional Neural Network Based Metal Artifact Reduction in X-Ray Computed Tomography. IEEE Trans. Med. Imaging 2018, 37, 1370–1381. [Google Scholar] [CrossRef]
- Tamada, D.; Kromrey, M.-L.; Ichikawa, S.; Onishi, H.; Motosugi, U. Motion Artifact Reduction Using a Convolutional Neural Network for Dynamic Contrast Enhanced MR Imaging of the Liver. Magn. Reson. Med. Sci. 2020, 19, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xing, Y.; Angelini, E.D.; Landman, B.A. CT artifact reduction via U-net CNN. In Proceedings of the Medical Imaging 2018: Image Processing, Houston, TX, USA, 5 March 2018; Volume 10574, p. 105741R. [Google Scholar] [CrossRef]
- Fantini, I.; Yasuda, C.; Bento, M.; Rittner, L.; Cendes, F.; Lotufo, R. Automatic MR image quality evaluation using a Deep CNN: A reference-free method to rate motion artifacts in neuroimaging. Comput. Med. Imaging Graph. 2021, 90, 101897. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liang, X.; Shen, C.; Jiang, S.; Wang, J. Synthetic CT generation from CBCT images via deep learning. Med. Phys. 2019, 47, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, D.; Lenkowicz, J.; Votta, C.; Boldrini, L.; Placidi, L.; Catucci, F.; Dinapoli, N.; Antonelli, M.V.; Romano, A.; De Luca, V.; et al. A deep learning approach to generate synthetic CT in low field MR-guided adaptive radiotherapy for abdominal and pelvic cases. Radiother. Oncol. 2020, 153, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Podgorsak, A.R.; Venkatesulu, B.P.; Abuhamad, M.; Harkenrider, M.M.; Solanki, A.A.; Roeske, J.C.; Kang, H. Dosimetric and workflow impact of synthetic-MRI use in prostate high-dose-rate brachytherapy. Brachytherapy 2023, 22, 686–696. [Google Scholar] [CrossRef]
- Liao, R.; Miao, S.; De Tournemire, P.; Grbic, S.; Kamen, A.; Mansi, T.; Comaniciu, D. An Artificial Agent for Robust Image Registration. In Proceedings of the AAAI Conference on Artificial Intelligence, San Francisco, CA, USA, 4–9 February 2017; Volume 31. [Google Scholar] [CrossRef]
- de Vos, B.D.; Berendsen, F.F.; Viergever, M.A.; Sokooti, H.; Staring, M.; Išgum, I. A deep learning framework for unsupervised affine and deformable image registration. Med. Image Anal. 2019, 52, 128–143. [Google Scholar] [CrossRef]
- Wong, J.; Fong, A.; McVicar, N.; Smith, S.; Wells, D.; Kolbeck, C.; Giambattista, J.; Gondara, L.; Alexander, A. Comparing deep learning-based auto-segmentation of organs at risk and clinical target volumes to expert inter-observer variability in radiotherapy planning. Radiother. Oncol. 2020, 144, 152–158. [Google Scholar] [CrossRef]
- Radici, L.; Ferrario, S.; Borca, V.C.; Cante, D.; Paolini, M.; Piva, C.; Baratto, L.; Franco, P.; La Porta, M.R. Implementation of a Commercial Deep Learning-Based Auto Segmentation Software in Radiotherapy: Evaluation of Effectiveness and Impact on Workflow. Life 2022, 12, 2088. [Google Scholar] [CrossRef]
- Harrison, K.; Pullen, H.; Welsh, C.; Oktay, O.; Alvarez-Valle, J.; Jena, R. Machine Learning for Auto-Segmentation in Radiotherapy Planning. Clin. Oncol. 2022, 34, 74–88. [Google Scholar] [CrossRef]
- Ge, Y.; Wu, Q.J. Knowledge-based planning for intensity-modulated radiation therapy: A review of data-driven approaches. Med. Phys. 2019, 46, 2760–2775. [Google Scholar] [CrossRef]
- Simon, L.; Robert, C.; Meyer, P. Artificial intelligence for quality assurance in radiotherapy. Cancer Radiother. 2021, 25, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.F.; Witztum, A.; Valdes, G. Integration of AI and Machine Learning in Radiotherapy QA. Front. Artif. Intell. 2020, 3, 577620. [Google Scholar] [CrossRef]
- Fh, T.; Cyw, C.; Eyw, C. Radiomics AI prediction for head and neck squamous cell carcinoma (HNSCC) prognosis and recurrence with target volume approach. BJR Open 2021, 3, 20200073. [Google Scholar] [CrossRef] [PubMed]
- Niraula, D.; Sun, W.; Jin, J.; Dinov, I.D.; Cuneo, K.; Jamaluddin, J.; Matuszak, M.M.; Luo, Y.; Lawrence, T.S.; Jolly, S.; et al. A clinical decision support system for AI-assisted decision-making in response-adaptive radiotherapy (ARCliDS). Sci. Rep. 2023, 13, 5279. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yang, J.; Fong, S.; Zhao, Q. Artificial intelligence in cancer diagnosis and prognosis: Opportunities and challenges. Cancer Lett. 2020, 471, 61–71. [Google Scholar] [CrossRef]
- Bourbonne, V.; Da-Ano, R.; Jaouen, V.; Lucia, F.; Dissaux, G.; Bert, J.; Pradier, O.; Visvikis, D.; Hatt, M.; Schick, U. Radiomics analysis of 3D dose distributions to predict toxicity of radiotherapy for lung cancer. Radiother. Oncol. 2021, 155, 144–150. [Google Scholar] [CrossRef]
- Lou, B.; Doken, S.; Zhuang, T.; Wingerter, D.; Gidwani, M.; Mistry, N.; Ladic, L.; Kamen, A.; Abazeed, M.E. An image-based deep learning framework for individualising radiotherapy dose: A retrospective analysis of outcome prediction. Lancet Digit. Health 2019, 1, e136–e147. [Google Scholar] [CrossRef]
- Pastor-Serrano, O.; Perkó, Z. Millisecond speed deep learning based proton dose calculation with Monte Carlo accuracy. Phys. Med. Biol. 2022, 67, 105006. [Google Scholar] [CrossRef]
- Oh, Y.; Park, S.; Byun, H.K.; Cho, Y.; Lee, I.J.; Kim, J.S.; Ye, J.C. LLM-driven multimodal target volume contouring in radiation oncology. Nat. Commun. 2024, 15, 9186. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, P.; Li, Y.; Holmes, J.; Shu, P.; Zhang, L.; Liu, C.; Liu, N.; Zhu, D.; Li, X.; et al. RadOnc-GPT: A Large Language Model for Radiation Oncology. arXiv 2023, arXiv:2309.10160. [Google Scholar]
- Lipkova, J.; Chen, R.J.; Chen, B.; Lu, M.Y.; Barbieri, M.; Shao, D.; Vaidya, A.J.; Chen, C.; Zhuang, L.; Williamson, D.F.; et al. Artificial intelligence for multimodal data integration in oncology. Cancer Cell 2022, 40, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, X.; Zeng, J.; Xiao, J.; Nie, D.; Zu, C.; Wu, X.; Zhou, J.; Wang, Y. Explainable attention guided adversarial deep network for 3D radiotherapy dose distribution prediction. Knowledge-Based Syst. 2022, 241, 108324. [Google Scholar] [CrossRef]
- Chen, R.J.; Wang, J.J.; Williamson, D.F.K.; Chen, T.Y.; Lipkova, J.; Lu, M.Y.; Sahai, S.; Mahmood, F. Algorithmic fairness in artificial intelligence for medicine and healthcare. Nat. Biomed. Eng. 2023, 7, 719–742. [Google Scholar] [CrossRef]
- Lamy, J.-B.; Sekar, B.; Guezennec, G.; Bouaud, J.; Séroussi, B. Explainable artificial intelligence for breast cancer: A visual case-based reasoning approach. Artif. Intell. Med. 2019, 94, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.P.; Jacobs, S.; Senadeera, M.; Le, V.; Coghlan, S. The three ghosts of medical AI: Can the black-box present deliver? Artif. Intell. Med. 2022, 124, 102158. [Google Scholar] [CrossRef]
- Sadre, R.; Sundaram, B.; Majumdar, S.; Ushizima, D. Validating deep learning inference during chest X-ray classification for COVID-19 screening. Sci. Rep. 2021, 11, 16075. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regulatory Considerations on Artificial Intelligence for Health; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- International Atomic Energy Agency; Dosimetry and Medical Radiation Physics Section. Artificial Intelligence in Medical Physics Roles, Responsibilities, Education and Training of Clinically Qualified Medical Physicists Endorsed by the American Association of Physicists in Medicine. December 2024. Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/TCS83web.pdf (accessed on 10 June 2025).
- Vokinger, K.N.; Gasser, U. Regulating AI in medicine in the United States and Europe. Nat. Mach. Intell. 2021, 3, 738–739. [Google Scholar] [CrossRef] [PubMed]
- Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD); U.S. Food & Drug Administration: Silver Spring, MD, USA, 2019.
- Larkin, B.; Ilyukha, V.; Sorokin, M.; Buzdin, A.; Vannier, E.; Poltorak, A. Cutting Edge: Activation of STING in T Cells Induces Type I IFN Responses and Cell Death. J. Immunol. 2017, 199, 397–402. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Farhood, B.; Musa, A.E.; Taeb, S.; Rezaeyan, A.; Najafi, M. Abscopal effect in radioimmunotherapy. Int. Immunopharmacol. 2020, 85, 106663. [Google Scholar] [CrossRef]
- Wennerberg, E.; Vanpouille-Box, C.; Bornstein, S.; Yamazaki, T.; Demaria, S.; Galluzzi, L. Immune recognition of irradiated cancer cells. Immunol. Rev. 2017, 280, 220–230. [Google Scholar] [CrossRef]
- Shevtsov, M.; Sato, H.; Multhoff, G.; Shibata, A. Novel Approaches to Improve the Efficacy of Immuno-Radiotherapy. Front. Oncol. 2019, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, M.E.; Rodriguez, I.; Leaman, O.; López-Campos, F.; Montero, A.; Conde, A.J.; Aristu, J.; Lara, P.; Calvo, F.M.; Melero, I. Immune mechanisms mediating abscopal effects in radioimmunotherapy. Pharmacol. Ther. 2019, 196, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Chen, D.; Yu, J. Radiotherapy combined with immunotherapy: The dawn of cancer treatment. Signal Transduct. Target. Ther. 2022, 7, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Mondini, M.; Levy, A.; Meziani, L.; Milliat, F.; Deutsch, E. Radiotherapy–immunotherapy combinations—Perspectives and challenges. Mol. Oncol. 2020, 14, 1529–1537. [Google Scholar] [CrossRef]
- Welsh, J.W.; Tang, C.; de Groot, P.; Naing, A.; Hess, K.R.; Heymach, J.V.; Papadimitrakopoulou, V.A.; Cushman, T.R.; Subbiah, V.; Chang, J.Y.; et al. Phase II Trial of Ipilimumab with Stereotactic Radiation Therapy for Metastatic Disease: Outcomes, Toxicities, and Low-Dose Radiation–Related Abscopal Responses. Cancer Immunol. Res. 2019, 7, 1903–1909. [Google Scholar] [CrossRef]
- Goto, H.; Shiraishi, Y.; Okada, S. Recent preclinical and clinical advances in radioimmunotherapy for non-Hodgkin’s lymphoma. Explor. Target. Anti-Tumor Ther. 2024, 5, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dahal, P.K.; Mosharaf, P.; Shahjalal; Alam Mahumud, R. Assessing the Clinical Effectiveness of Radioimmunotherapy with Combined Radionuclide/Monoclonal Antibody Conjugates in Cancer Treatment: Insights from Randomised Clinical Trials. Cancers 2025, 17, 1413. [Google Scholar] [CrossRef]
- Deutsch, E.; Chargari, C.; Galluzzi, L.; Kroemer, G. Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol. 2019, 20, e452–e463. [Google Scholar] [CrossRef]
- Barsoumian, H.B.; Hsu, J.; Nanez, S.; Hu, Y.; Hsu, E.Y.; Riad, T.S.; Puebla-Osorio, N.; Cortez, M.A.; Welsh, J.W. The RadScopal Technique as an Immune Adjuvant to Treat Cancer. Immuno 2023, 3, 74–85. [Google Scholar] [CrossRef]
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.-K.V.; Press, O.W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer 2015, 15, 347–360. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, B.; Wang, W.; Tian, T.; Qian, H.; Li, X.; Yu, Y. Multifunctional Au@AgBiS2 Nanoparticles as High-Efficiency Radiosensitizers to Induce Pyroptosis for Cancer Radioimmunotherapy. Adv. Sci. 2023, 10, e2302141. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Chen, C.; Wang, T.; Li, X.; Liu, Y.; Wang, H.; Zhao, S.; Zuo, C.; Sun, G.; Bu, W. Radionuclide-labeled gold nanoparticles for nuclei-targeting internal radio-immunity therapy. Mater. Horizons 2019, 7, 1115–1125. [Google Scholar] [CrossRef]
- Pei, P.; Shen, W.; Zhou, H.; Sun, Y.; Zhong, J.; Liu, T.; Yang, K. Radionuclide labeled gold nanoclusters boost effective anti-tumor immunity for augmented radio-immunotherapy of cancer. Nano Today 2021, 38, 101144. [Google Scholar] [CrossRef]
- Cheal, S.M.; Chung, S.K.; Vaughn, B.A.; Cheung, N.-K.V.; Larson, S.M. Pretargeting: A Path Forward for Radioimmunotherapy. J. Nucl. Med. 2022, 63, 1302–1315. [Google Scholar] [CrossRef]
- Zaheer, J.; Kim, H.; Lee, Y.-J.; Kim, J.S.; Lim, S.M. Combination Radioimmunotherapy Strategies for Solid Tumors. Int. J. Mol. Sci. 2019, 20, 5579. [Google Scholar] [CrossRef]
- Zhou, Z.; Guan, B.; Xia, H.; Zheng, R.; Xu, B. Particle radiotherapy in the era of radioimmunotherapy. Cancer Lett. 2023, 567, 216268. [Google Scholar] [CrossRef]
- Liberson, F. The Value of a Multi-perforated Screen in Deep X-ray Therapy. Radiology 1933, 20, 186–195. [Google Scholar] [CrossRef]
- Prezado, Y. Divide and conquer: Spatially fractionated radiation therapy. Expert Rev. Mol. Med. 2022, 24, e3. [Google Scholar] [CrossRef]
- Billena, C.; Khan, A.J. A Current Review of Spatial Fractionation: Back to the Future? Int. J. Radiat. Oncol. 2019, 104, 177–187. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Stevens, J.H.; Reiff, J.E.; Huq, M.S.; Suntharalingam, N. Spatially Fractionated (GRID) Radiation for Palliative Treatment of Advanced Cancer. Radiat. Oncol. Investig. 1996, 4, 41–47. [Google Scholar] [CrossRef]
- Huhn, J.L.; Regine, W.F.; Valentino, J.P.; Meigooni, A.S.; Kudrimoti, M.; Mohiuddin, M. Spatially Fractionated GRID Radiation Treatment of Advanced Neck Disease Associated with Head and Neck Cancer. Technol. Cancer Res. Treat. 2006, 5, 607–612. [Google Scholar] [CrossRef]
- Edwards, J.; Shah, P.; Huhn, J.; Clair, W.S.; Regine, W.; Mohiuddin, M.; Kudrimoti, M. Definitive GRID and Fractionated Radiation in Bulky Head and Neck Cancer Associated with Low Rates of Distant Metastasis. Int. J. Radiat. Oncol. 2015, 93, E334. [Google Scholar] [CrossRef]
- Neuner, G.; Mohiuddin, M.M.; Walde, N.V.; Goloubeva, O.; Ha, J.; Yu, C.X.; Regine, W.F. High-Dose Spatially Fractionated GRID Radiation Therapy (SFGRT): A Comparison of Treatment Outcomes with Cerrobend vs. MLC SFGRT. Int. J. Radiat. Oncol. 2012, 82, 1642–1649. [Google Scholar] [CrossRef]
- Wu, X.; Perez, N.C.; Zheng, Y.; Li, X.; Jiang, L.; Amendola, B.E.; Xu, B.; Mayr, N.A.; Lu, J.J.; Hatoum, G.F.; et al. The Technical and Clinical Implementation of LATTICE Radiation Therapy (LRT). Radiat. Res. 2020, 194, 737–746. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Zhang, X.; Chang, S.X.; Megooni, A.; Donnelly, E.D.; Ahmed, M.M.; Griffin, R.J.; Welsh, J.S.; Simone, C.B.; et al. Photon GRID Radiation Therapy: A Physics and Dosimetry White Paper from the Radiosurgery Society (RSS) GRID/LATTICE, Microbeam and FLASH Radiotherapy Working Group. Radiat. Res. 2020, 194, 665–677. [Google Scholar] [CrossRef]
- Iori, F.; Cappelli, A.; D’Angelo, E.; Cozzi, S.; Ghersi, S.F.; De Felice, F.; Ciammella, P.; Bruni, A.; Iotti, C. Lattice Radiation Therapy in clinical practice: A systematic review. Clin. Transl. Radiat. Oncol. 2022, 39, 100569. [Google Scholar] [CrossRef]
- Larrea, L.; Gonzalez, V.; Antonini, P.; Lopez, E.; Banos, M. Lattice Radiotherapy (LRT)-Spatially Fractionated Radiotherapy (SFRT): Advanced Non-Small Cell Lung Cancer (NSCLC): Early Experience. Int. J. Radiat. Oncol. 2021, 111, e443. [Google Scholar] [CrossRef]
- Grams, M.P.; Deufel, C.L.; Kavanaugh, J.A.; Corbin, K.S.; Ahmed, S.K.; Haddock, M.G.; Lester, S.C.; Ma, D.J.; Petersen, I.A.; Finley, R.R.; et al. Clinical aspects of spatially fractionated radiation therapy treatments. Phys. Medica 2023, 111, 102616. [Google Scholar] [CrossRef]
- Amendola, B.E.; Perez, N.C.; Mayr, N.A.; Wu, X.; Amendola, M. Spatially Fractionated Radiation Therapy Using Lattice Radiation in Far-advanced Bulky Cervical Cancer: A Clinical and Molecular Imaging and Outcome Study. Radiat. Res. 2020, 194, 724–736. [Google Scholar] [CrossRef]
- Rivera, J.N.; Kierski, T.M.; Kasoji, S.K.; Abrantes, A.S.; Dayton, P.A.; Chang, S.X.; Zheng, D. Conventional dose rate spatially-fractionated radiation therapy (SFRT) treatment response and its association with dosimetric parameters—A preclinical study in a Fischer 344 rat model. PLoS ONE 2020, 15, e0229053. [Google Scholar] [CrossRef]
- Butterworth, K.T.; Ghita, M.; McMahon, S.J.; Mcgarry, C.K.; Griffin, R.J.; Hounsell, A.R.; Prise, K.M. Modelling responses to spatially fractionated radiation fields using preclinical image-guided radiotherapy. Br. J. Radiol. 2017, 90, 20160485. [Google Scholar] [CrossRef]
- Trappetti, V.; Fazzari, J.M.; Fernandez-Palomo, C.; Scheidegger, M.; Volarevic, V.; Martin, O.A.; Djonov, V.G. Microbeam Radiotherapy—A Novel Therapeutic Approach to Overcome Radioresistance and Enhance Anti-Tumour Response in Melanoma. Int. J. Mol. Sci. 2021, 22, 7755. [Google Scholar] [CrossRef]
- Fernandez-Palomo, C.; Chang, S.; Prezado, Y. Should Peak Dose Be Used to Prescribe Spatially Fractionated Radiation Therapy?—A Review of Preclinical Studies. Cancers 2022, 14, 3625. [Google Scholar] [CrossRef]
- Johnstone, C.D.; Bazalova-Carter, M. A review of small animal dosimetry techniques: Image-guided and spatially fractionated therapy. J. Phys. Conf. Ser. 2023, 2630, 012013. [Google Scholar] [CrossRef]
- Asur, R.; Butterworth, K.T.; Penagaricano, J.A.; Prise, K.M.; Griffin, R.J. High dose bystander effects in spatially fractionated radiation therapy. Cancer Lett. 2015, 356, 52–57. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, T.; Liu, W.; Zuo, L. Molecular mechanism of bystander effects and related abscopal/cohort effects in cancer therapy. Oncotarget 2018, 9, 18637–18647. [Google Scholar] [CrossRef]
- Daguenet, E.; Louati, S.; Wozny, A.-S.; Vial, N.; Gras, M.; Guy, J.-B.; Vallard, A.; Rodriguez-Lafrasse, C.; Magné, N. Radiation-induced bystander and abscopal effects: Important lessons from preclinical models. Br. J. Cancer 2020, 123, 339–348. [Google Scholar] [CrossRef]
- Toossi, M.T.B.; Khademi, S.; Azimian, H.; Mohebbi, S.; Soleymanifard, S. Assessment of The Dose-Response Relationship of Radiation-Induced Bystander Effect in Two Cell Lines Exposed to High Doses of Ionizing Radiation (6 and 8 Gy). Cell J. 2017, 19, 434–442. [Google Scholar] [CrossRef]
- Park, H.J.; Griffin, R.J.; Hui, S.; Levitt, S.H.; Song, C.W. Radiation-induced vascular damage in tumors: Implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat. Res. 2012, 177, 311–327. [Google Scholar] [CrossRef]
- Venkatesulu, B.P.; Mahadevan, L.S.; Aliru, M.L.; Yang, X.; Bodd, M.H.; Singh, P.K.; Yusuf, S.W.; Abe, J.-I.; Krishnan, S. Radiation-Induced Endothelial Vascular Injury. JACC Basic Transl. Sci. 2018, 3, 563–572. [Google Scholar] [CrossRef]
- Castle, K.D.; Kirsch, D.G. Establishing the Impact of Vascular Damage on Tumor Response to High-Dose Radiation Therapy. Cancer Res. 2019, 79, 5685–5692. [Google Scholar] [CrossRef]
- Yamazaki, T.; Young, K.H. Effects of radiation on tumor vasculature. Mol. Carcinog. 2021, 61, 165–172. [Google Scholar] [CrossRef]
- Frey, B.; Rückert, M.; Deloch, L.; Rühle, P.F.; Derer, A.; Fietkau, R.; Gaipl, U.S. Immunomodulation by ionizing radiation—Impact for design of radio-immunotherapies and for treatment of inflammatory diseases. Immunol. Rev. 2017, 280, 231–248. [Google Scholar] [CrossRef]
- Savage, T.; Pandey, S.; Guha, C. Postablation Modulation after Single High-Dose Radiation Therapy Improves Tumor Control via Enhanced Immunomodulation. Clin. Cancer Res. 2020, 26, 910–921. [Google Scholar] [CrossRef]
- Kumari, S.; Mukherjee, S.; Sinha, D.; Abdisalaam, S.; Krishnan, S.; Asaithamby, A. Immunomodulatory Effects of Radiotherapy. Int. J. Mol. Sci. 2020, 21, 8151. [Google Scholar] [CrossRef]
- Stoecklein, V.M.; Osuka, A.; Ishikawa, S.; Lederer, M.R.; Wanke-Jellinek, L.; Lederer, J.A. Radiation Exposure Induces Inflammasome Pathway Activation in Immune Cells. J. Immunol. 2015, 194, 1178–1189. [Google Scholar] [CrossRef]
- Ji, H.; Zhou, Z. A ‘Hybrid’ Radiotherapy Regimen Designed for Immunomodulation: Combining High-Dose Radiotherapy with Low-Dose Radiotherapy. Cancers 2022, 14, 3505. [Google Scholar] [CrossRef]
- Amendola, B.E.; Mahadevan, A.; Suarez, J.M.B.; Griffin, R.J.; Wu, X.; Perez, N.C.; Hippe, D.S.; Simone, C.B.; Mohiuddin, M.; Mohiuddin, M.; et al. An International Consensus on the Design of Prospective Clinical–Translational Trials in Spatially Fractionated Radiation Therapy for Advanced Gynecologic Cancer. Cancers 2022, 14, 4267. [Google Scholar] [CrossRef]
- Mayr, N.A.; Snider, J.W.; Regine, W.F.; Mohiuddin, M.; Hippe, D.S.; Peñagarícano, J.; Mohiuddin, M.; Kudrimoti, M.R.; Zhang, H.; Limoli, C.L.; et al. An International Consensus on the Design of Prospective Clinical–Translational Trials in Spatially Fractionated Radiation Therapy. Adv. Radiat. Oncol. 2021, 7, 100866. [Google Scholar] [CrossRef]
- Lu, Q.; Yan, W.; Zhu, A.; Tubin, S.; Mourad, W.F.; Yang, J. Combining spatially fractionated radiation therapy (SFRT) and immunotherapy opens new rays of hope for enhancing therapeutic ratio. Clin. Transl. Radiat. Oncol. 2023, 44, 100691. [Google Scholar] [CrossRef]
- Vozenin, M.-C.; De Fornel, P.; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.-F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin. Cancer Res. 2019, 25, 35–42. [Google Scholar] [CrossRef]
- Di Martino, F.; Barca, P.; Barone, S.; Bortoli, E.; Borgheresi, R.; De Stefano, S.; Di Francesco, M.; Faillace, L.; Giuliano, L.; Grasso, L.; et al. FLASH Radiotherapy with Electrons: Issues Related to the Production, Monitoring, and Dosimetric Characterization of the Beam. Front. Phys. 2020, 8, 570697. [Google Scholar] [CrossRef]
- Bourhis, J.; Montay-Gruel, P.; Jorge, P.G.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; et al. Clinical translation of FLASH radiotherapy: Why and how? Radiother. Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef]
- Harrington, K.J. Ultrahigh Dose-rate Radiotherapy: Next Steps for FLASH-RT. Clin. Cancer Res. 2019, 25, 3–5. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Corde, S.; Laissue, J.A.; Bazalova-Carter, M. FLASH radiotherapy with photon beams. Med. Phys. 2021, 49, 2055–2067. [Google Scholar] [CrossRef]
- Diffenderfer, E.S.; Sørensen, B.S.; Mazal, A.; Carlson, D.J. The current status of preclinical proton FLASH radiation and future directions. Med. Phys. 2021, 49, 2039–2054. [Google Scholar] [CrossRef]
- Weber, U.A.; Scifoni, E.; Durante, M. FLASH radiotherapy with carbon ion beams. Med. Phys. 2021, 49, 1974–1992. [Google Scholar] [CrossRef]
- Dewey, D.L.; Boag, J.W. Modification of the Oxygen Effect when Bacteria are given Large Pulses of Radiation. Nature 1959, 183, 1450–1451. [Google Scholar] [CrossRef]
- Town, C.D. Effect of High Dose Rates on Survival of Mammalian Cells. Nature 1967, 215, 847–848. [Google Scholar] [CrossRef]
- Berry, R.J.; Hall, E.J.; Forster, D.W.; Storr, T.H.; Goodman, M.J. Survival of mammalian cells exposed to X rays at ultra-high dose-rates. Br. J. Radiol. 1969, 42, 102–107. [Google Scholar] [CrossRef]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Petersson, K.; Jaccard, M.; Boivin, G.; Germond, J.-F.; Petit, B.; Doenlen, R.; Favaudon, V.; Bochud, F.; Bailat, C.; et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother. Oncol. 2017, 124, 365–369. [Google Scholar] [CrossRef]
- Loo, B.W.; Schuler, E.; Lartey, F.M.; Rafat, M.; King, G.J.; Trovati, S.; Koong, A.C.; Maxim, P.G. (P003) Delivery of Ultra-Rapid Flash Radiation Therapy and Demonstration of Normal Tissue Sparing After Abdominal Irradiation of Mice. Int. J. Radiat. Oncol. 2017, 98, E16. [Google Scholar] [CrossRef]
- Field, S.; Bewley, D. Effects of Dose-rate on the Radiation Response of Rat Skin. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1974, 26, 259–267. [Google Scholar] [CrossRef]
- Cunningham, S.; McCauley, S.; Vairamani, K.; Speth, J.; Girdhani, S.; Abel, E.; Sharma, R.A.; Perentesis, J.P.; Wells, S.I.; Mascia, A.; et al. FLASH Proton Pencil Beam Scanning Irradiation Minimizes Radiation-Induced Leg Contracture and Skin Toxicity in Mice. Cancers 2021, 13, 1012. [Google Scholar] [CrossRef]
- Hornsey, S.; Bewley, D. Hypoxia in Mouse Intestine Induced by Electron Irradiation at High Dose-rates. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1971, 19, 479–483. [Google Scholar] [CrossRef]
- Berry, R.J.; Stedeford, J.B.H. Reproductive survival of mammalian cells after irradiation at ultra-high dose-rates: Further observations and their importance for radiotherapy. Br. J. Radiol. 1972, 45, 171–177. [Google Scholar] [CrossRef]
- Weiss, H.; Epp, E.; Heslin, J.; Ling, C.; Santomasso, A. Oxygen Depletion in Cells Irradiated at Ultra-high Dose-rates and at Conventional Dose-rates. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1974, 26, 17–29. [Google Scholar] [CrossRef]
- Michaels, H.B.; Epp, E.R.; Ling, C.C.; Peterson, E.C. Oxygen Sensitization of CHO Cells at Ultrahigh Dose Rates: Prelude to Oxygen Diffusion Studies. Radiat. Res. 1978, 76, 510–521. [Google Scholar] [CrossRef]
- Del Debbio, F.; Bertilacchi, M.S.; Gonnelli, A.; Da Pozzo, E.; Tozzini, V.; Martini, C.; Capaccioli, S.; Costa, B. An insight into hypothesized biological mechanisms contributing to the Flash effect. Front. Phys. 2023, 11, 1201708. [Google Scholar] [CrossRef]
- Cooper, C.R.; Jones, D.; Jones, G.D.; Petersson, K. FLASH irradiation induces lower levels of DNA damage ex vivo, an effect modulated by oxygen tension, dose, and dose rate. Br. J. Radiol. 2022, 95, 20211150. [Google Scholar] [CrossRef]
- Chow, J.C.L.; Ruda, H.E. Mechanisms of Action in FLASH Radiotherapy: A Comprehensive Review of Physicochemical and Biological Processes on Cancerous and Normal Cells. Cells 2024, 13, 835. [Google Scholar] [CrossRef]
- Bogaerts, E.; Macaeva, E.; Isebaert, S.; Haustermans, K. Potential Molecular Mechanisms behind the Ultra-High Dose Rate “FLASH” Effect. Int. J. Mol. Sci. 2022, 23, 12109. [Google Scholar] [CrossRef]
- Iturri, L.; Bertho, A.; Lamirault, C.; Brisebard, E.; Juchaux, M.; Gilbert, C.; Espenon, J.; Sébrié, C.; Jourdain, L.; de Marzi, L.; et al. Oxygen supplementation in anesthesia can block FLASH effect and anti-tumor immunity in conventional proton therapy. Commun. Med. 2023, 3, 183. [Google Scholar] [CrossRef]
- Iturri, L.; Bertho, A.; Lamirault, C.; Juchaux, M.; Gilbert, C.; Espenon, J.; Sebrie, C.; Jourdain, L.; Pouzoulet, F.; Verrelle, P.; et al. Proton FLASH Radiation Therapy and Immune Infiltration: Evaluation in an Orthotopic Glioma Rat Model. Int. J. Radiat. Oncol. 2022, 116, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Konradsson, E.; Arendt, M.L.; Jensen, K.B.; Børresen, B.; Hansen, A.E.; Bäck, S.; Kristensen, A.T.; Rosenschöld, P.M.A.; Ceberg, C.; Petersson, K. Establishment and Initial Experience of Clinical FLASH Radiotherapy in Canine Cancer Patients. Front. Oncol. 2021, 11, 658004. [Google Scholar] [CrossRef]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.-F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef]
- Mascia, A.E.; Daugherty, E.C.; Zhang, Y.; Lee, E.; Xiao, Z.; Sertorio, M.; Woo, J.; Backus, L.R.; McDonald, J.M.; McCann, C.; et al. Proton FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases. JAMA Oncol. 2023, 9, 62–69. [Google Scholar] [CrossRef]
- Daugherty, E.C.; Mascia, A.; Zhang, Y.; Lee, E.; Xiao, Z.; Sertorio, M.; Woo, J.; McCann, C.; Russell, K.; Levine, L.; et al. FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases (FAST-01): Protocol for the First Prospective Feasibility Study. JMIR Res. Protoc. 2023, 12, e41812. [Google Scholar] [CrossRef]
- Locher, G.L. Biological Effects and Therapeutic Possibilities of Neutrons. Am. J. Roentgenol. Radium Ther. 1936, 36, 1–13. [Google Scholar]
- Chadwick, J.; Goldhaber, M. The nuclear photoelectric effect. Proc. R. Soc. Lond. Ser. A. Math. Phys. Sci. 1935, 151, 479–493. [Google Scholar] [CrossRef]
- Farr, L.E.; Sweet, W.H.; Locksley, H.B.; Robertson, J.S. Neutron capture therapy of gliomas using boron. Trans. Am. Neurol. Assoc. 1954, 13, 110–113. [Google Scholar] [PubMed]
- Godwin, J.T.; Farr, L.E.; Sweet, W.H.; Robertson, J.S. Pathological study of eight patients with glioblastoma multiforme treated by neutron-capture therapy using boron 10. Cancer 1955, 8, 601–615. [Google Scholar] [CrossRef]
- Hatanaka, H.; Sano, K.; Yasukochi, H. Clinical Results of Boron Neutron Capture Therapy. In Progress in Neutron Capture Therapy for Cancer; Springer: Boston, MA, USA, 1992; pp. 561–568. [Google Scholar] [CrossRef]
- Mishima, Y.; Ichihashi, M.; Hatta, S.; Honda, C.; Yamamura, K.; Nakagawa, T.; Obara, H.; Shirakawa, J.; Hiratsuka, J.; Taniyama, K. First human clinical trial of melanoma neutron capture. Diagnosis and therapy. Strahlenther. Onkol. 1989, 165, 251–254. [Google Scholar] [PubMed]
- Kumada, H.; Matsumura, A.; Sakurai, H.; Sakae, T.; Yoshioka, M.; Kobayashi, H.; Matsumoto, H.; Kiyanagi, Y.; Shibata, T.; Nakashima, H. Project for the development of the linac based NCT facility in University of Tsukuba. Appl. Radiat. Isot. 2014, 88, 211–215. [Google Scholar] [CrossRef]
- Yoshioka, M. Review of Accelerator-Based Boron Neutron Capture Therapy Machines. In Proceedings of the 7th International Particle Accelerator Conference, Busan, Republic of Korea, 8–13 May 2016. [Google Scholar]
- He, H.; Li, J.; Jiang, P.; Tian, S.; Wang, H.; Fan, R.; Liu, J.; Yang, Y.; Liu, Z.; Wang, J. The basis and advances in clinical application of boron neutron capture therapy. Radiat. Oncol. 2021, 16, 216. [Google Scholar] [CrossRef]
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.H.; Blue, T.E. Boron Neutron Capture Therapy of Cancer: Current Status and Future Prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef]
- Barth, R.F.; Albert, H.; Fairchild, R.G. Boron Neutron Capture Therapy of Cancer. Cancer Res. 1990, 50, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Sauerwein, W.A.G.; Sancey, L.; Hey-Hawkins, E.; Kellert, M.; Panza, L.; Imperio, D.; Balcerzyk, M.; Rizzo, G.; Scalco, E.; Herrmann, K.; et al. Theranostics in Boron Neutron Capture Therapy. Life 2021, 11, 330. [Google Scholar] [CrossRef]
- Kreiner, A.J.; Bergueiro, J.; Cartelli, D.; Baldo, M.; Castell, W.; Asoia, J.G.; Padulo, J.; Sandín, J.C.S.; Igarzabal, M.; Erhardt, J.; et al. Present status of Accelerator-Based BNCT. Rep. Pract. Oncol. Radiother. 2016, 21, 95–101. [Google Scholar] [CrossRef]
- Mitsumoto, T.; Yajima, S.; Tsutsui, H.; Ogasawara, T.; Fujita, K.; Tanaka, H.; Sakurai, Y.; Maruhashi, A. Cyclotron-based neutron source for BNCT. AIP Conf. Proc. 2013, 1525, 319–322. [Google Scholar] [CrossRef]
- Snyder, H.R.; Reedy, A.J.; Lennarz, W.J. Synthesis of Aromatic Boronic Acids. Aldehydo Boronic Acids and a Boronic Acid Analog of Tyrosine1. J. Am. Chem. Soc. 1958, 80, 835–838. [Google Scholar] [CrossRef]
- Soloway, A.H.; Hatanaka, H.; Davis, M.A. Penetration of Brain and Brain Tumor. VII. Tumor-Binding Sulfhydryl Boron Compounds. J. Med. Chem. 1967, 10, 714–717. [Google Scholar] [CrossRef]
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun. 2020, 40, 406–421. [Google Scholar] [CrossRef]
- Chen, W.; Mehta, S.C.; Lu, D. Selective boron drug delivery to brain tumors for boron neutron capture therapy. Adv. Drug Deliv. Rev. 1997, 26, 231–247. [Google Scholar] [CrossRef]
- Torresan, V.; Guadagnini, A.; Badocco, D.; Pastore, P.; Medina, G.A.M.; van Raap, M.B.F.; Postuma, I.; Bortolussi, S.; Bekić, M.; Čolić, M.; et al. Biocompatible Iron–Boron Nanoparticles Designed for Neutron Capture Therapy Guided by Magnetic Resonance Imaging. Adv. Healthc. Mater. 2020, 10, e2001632. [Google Scholar] [CrossRef] [PubMed]
- Geninatti-Crich, S.; Alberti, D.; Szabo, I.; Deagostino, A.; Toppino, A.; Barge, A.; Ballarini, F.; Bortolussi, S.; Bruschi, P.; Protti, N.; et al. MRI-Guided Neutron Capture Therapy by Use of a Dual Gadolinium/Boron Agent Targeted at Tumour Cells through Upregulated Low-Density Lipoprotein Transporters. Chem.—Eur. J. 2011, 17, 8479–8486. [Google Scholar] [CrossRef] [PubMed]
- Aihara, T.; Hiratsuka, J.; Morita, N.; Uno, M.; Sakurai, Y.; Maruhashi, A.; Ono, K.; Harada, T. First clinical case of boron neutron capture therapy for head and neck malignancies using 18F-BPA PET. Head Neck 2006, 28, 850–855. [Google Scholar] [CrossRef]
- Hanaoka, K.; Watabe, T.; Naka, S.; Kanai, Y.; Ikeda, H.; Horitsugi, G.; Kato, H.; Isohashi, K.; Shimosegawa, E.; Hatazawa, J. FBPA PET in boron neutron capture therapy for cancer: Prediction of 10B concentration in the tumor and normal tissue in a rat xenograft model. EJNMMI Res. 2014, 4, 70. [Google Scholar] [CrossRef]
- Silberstein, E.B. Radioiodine: The Classic Theranostic Agent. Semin. Nucl. Med. 2012, 42, 164–170. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Suzuki, O.; Sakurai, Y.; Tanaka, H.; Kondo, N.; Kinashi, Y.; Masunaga, S.-I.; Maruhashi, A.; Ono, K. Reirradiation for locally recurrent lung cancer in the chest wall with boron neutron capture therapy (BNCT). Int. Cancer Conf. J. 2012, 1, 235–238. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.H.; Chou, F.; Jiang, S. Clinical trials for treating recurrent head and neck cancer with boron neutron capture therapy using the Tsing-Hua Open Pool Reactor. Cancer Commun. 2018, 38, 37. [Google Scholar] [CrossRef]
- Farías, R.O.; Bortolussi, S.; Menéndez, P.R.; González, S.J. Exploring Boron Neutron Capture Therapy for non-small cell lung cancer. Phys. Medica 2014, 30, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Nakai, K.; Matsumura, A. Boron neutron capture therapy for glioblastoma. Cancer Lett. 2008, 262, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T. The current status and novel advances of boron neutron capture therapy clinical trials. Am. J. Cancer Res. 2024, 14, 429–447. [Google Scholar] [CrossRef]
- Kankaanranta, L.; Seppälä, T.; Koivunoro, H.; Saarilahti, K.; Atula, T.; Collan, J.; Salli, E.; Kortesniemi, M.; Uusi-Simola, J.; Välimäki, P.; et al. Boron Neutron Capture Therapy in the Treatment of Locally Recurred Head-and-Neck Cancer: Final Analysis of a Phase I/II Trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e67–e75. [Google Scholar] [CrossRef]
- Sauerwein, W.; Moss, R.; Hideghéty, K.; Stecher-Rasmussen, F.; De Vries, M.; Reulen, H.-J.; Götz, C.; Paquis, P.; Grochulla, F.; Haselsberge, K.; et al. Status Report on the European Clinical Trial of BNCT at Petten (EORTC Protocol 11961). In Frontiers in Neutron Capture Therapy; Springer: New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Kyrgias, G.; Hajiioannou, J.; Tolia, M.; Kouloulias, V.; Lachanas, V.; Skoulakis, C.; Skarlatos, I.; Rapidis, A.; Bizakis, I. Intraoperative radiation therapy (IORT) in head and neck cancer. Medicine 2016, 95, e5035. [Google Scholar] [CrossRef]
- Sarria, G.R.; Petrova, V.; Wenz, F.; Abo-Madyan, Y.; Sperk, E.; Giordano, F.A. Intraoperative radiotherapy with low energy x-rays for primary and recurrent soft-tissue sarcomas. Radiat. Oncol. 2020, 15, 110. [Google Scholar] [CrossRef]
- Calvo, F.A.; Krengli, M.; Asencio, J.M.; Serrano, J.; Poortmans, P.; Roeder, F.; Krempien, R.; Hensley, F.W. ESTRO IORT Task Force/ACROP recommendations for intraoperative radiation therapy in unresected pancreatic cancer. Radiother. Oncol. 2020, 148, 57–64. [Google Scholar] [CrossRef]
- Giordano, F.A.; Brehmer, S.; Mürle, B.; Welzel, G.; Sperk, E.; Keller, A.; Abo-Madyan, Y.; Scherzinger, E.; Clausen, S.; Schneider, F.; et al. Intraoperative Radiotherapy in Newly Diagnosed Glioblastoma (INTRAGO): An Open-Label, Dose-Escalation Phase I/II Trial. Neurosurgery 2018, 84, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.; Simiele, E.; Lozko, Y.; Romero, I.; Skinner, L.; Binkley, M.S.; Hoppe, R.; Kovalchuk, N.; Hiniker, S.M. Volumetric modulated arc therapy total body irradiation improves toxicity outcomes compared to 2D total body irradiation. Front. Oncol. 2024, 14, 1459287. [Google Scholar] [CrossRef] [PubMed]
- Zhang-Velten, E.R.; Parsons, D.; Lee, P.; Chambers, E.; Abdulrahman, R.; Desai, N.B.; Dan, T.; Wardak, Z.; Timmerman, R.; Vusirikala, M.; et al. Volumetric Modulated Arc Therapy Enabled Total Body Irradiation (VMAT-TBI): Six-year Clinical Experience and Treatment Outcomes. Biol. Blood Marrow Transplant. 2022, 28, 113.e1–113.e8. [Google Scholar] [CrossRef] [PubMed]
- Kerbauy, M.N.; Arcuri, L.J.; Favareto, S.L.; de Rezende, A.C.P.; Hamerschlak, N. Total marrow irradiation in hematopoietic stem cell transplantation for hematologic malignancies. Front. Med. 2023, 10, 1155954. [Google Scholar] [CrossRef]
- Spohn, S.K.; Draulans, C.; Kishan, A.U.; Spratt, D.; Ross, A.; Maurer, T.; Tilki, D.; Berlin, A.; Blanchard, P.; Collins, S.; et al. Genomic Classifiers in Personalized Prostate Cancer Radiation Therapy Approaches: A Systematic Review and Future Perspectives Based on International Consensus. Int. J. Radiat. Oncol. 2022, 116, 503–520. [Google Scholar] [CrossRef]
- Rooney, M.K.; Rosenberg, D.M.; Braunstein, S.; Cunha, A.; Damato, A.L.; Ehler, E.; Pawlicki, T.; Robar, J.; Tatebe, K.; Golden, D.W. Three-dimensional printing in radiation oncology: A systematic review of the literature. J. Appl. Clin. Med. Phys. 2020, 21, 15–26. [Google Scholar] [CrossRef]
- Shamsabadi, R. 3D-Printing Advances in Radiotherapy. In Advances in 3D Printing; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Parker, C.; Lewington, V.; Shore, N.; Kratochwil, C.; Levy, M.; Lindén, O.; Noordzij, W.; Park, J.; Saad, F. Targeted Alpha Therapy, an Emerging Class of Cancer Agents. JAMA Oncol. 2018, 4, 1765–1772. [Google Scholar] [CrossRef]
- Perrin, J.; Capitao, M.; Allard, M.; Chouin, N.; Gouard, S.; Marionneau-Lambot, S.; Louvet, C.; Donnadieu, E.; Bruchertseifer, F.; Morgenstern, A.; et al. Targeted Alpha Particle Therapy Remodels the Tumor Microenvironment and Improves Efficacy of Immunotherapy. Int. J. Radiat. Oncol. 2022, 112, 790–801. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Pirovano, G.; Wilson, T.C.; Reiner, T. Auger: The future of precision medicine. Nucl. Med. Biol. 2021, 96, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liao, R.; Sheng, X.; Luo, X.; Zhang, X.; Wen, X.; Zhou, J.; Peng, K. Hydrogen Gas in Cancer Treatment. Front. Oncol. 2019, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Noor, M.N.Z.M.; Alauddin, A.S.; Wong, Y.H.; Looi, C.Y.; Wong, E.H.; Madhavan, P.; Yeong, C.H. A Systematic Review of Molecular Hydrogen Therapy in Cancer Management. Asian Pac. J. Cancer Prev. 2023, 24, 37–47. [Google Scholar] [CrossRef]
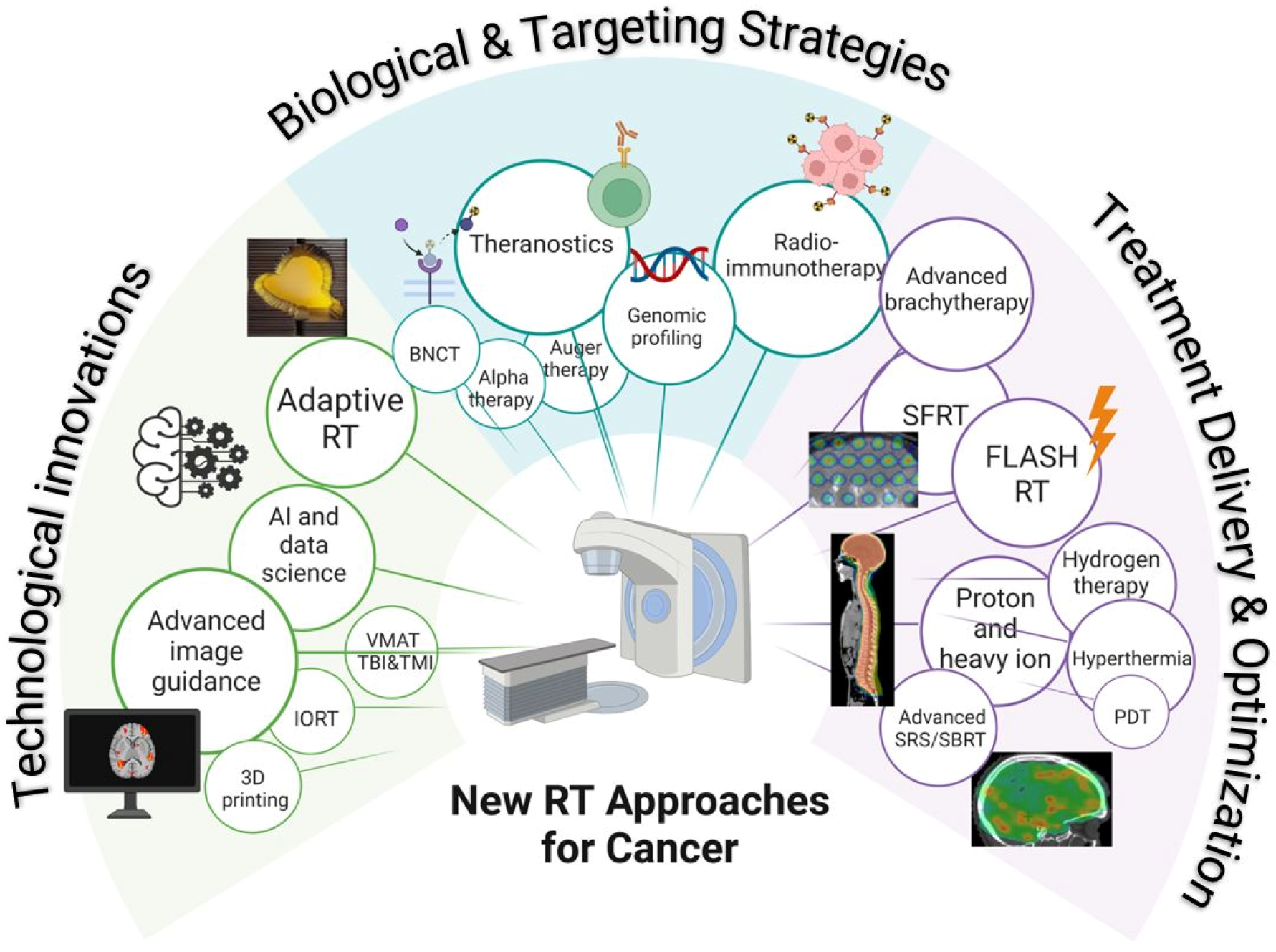
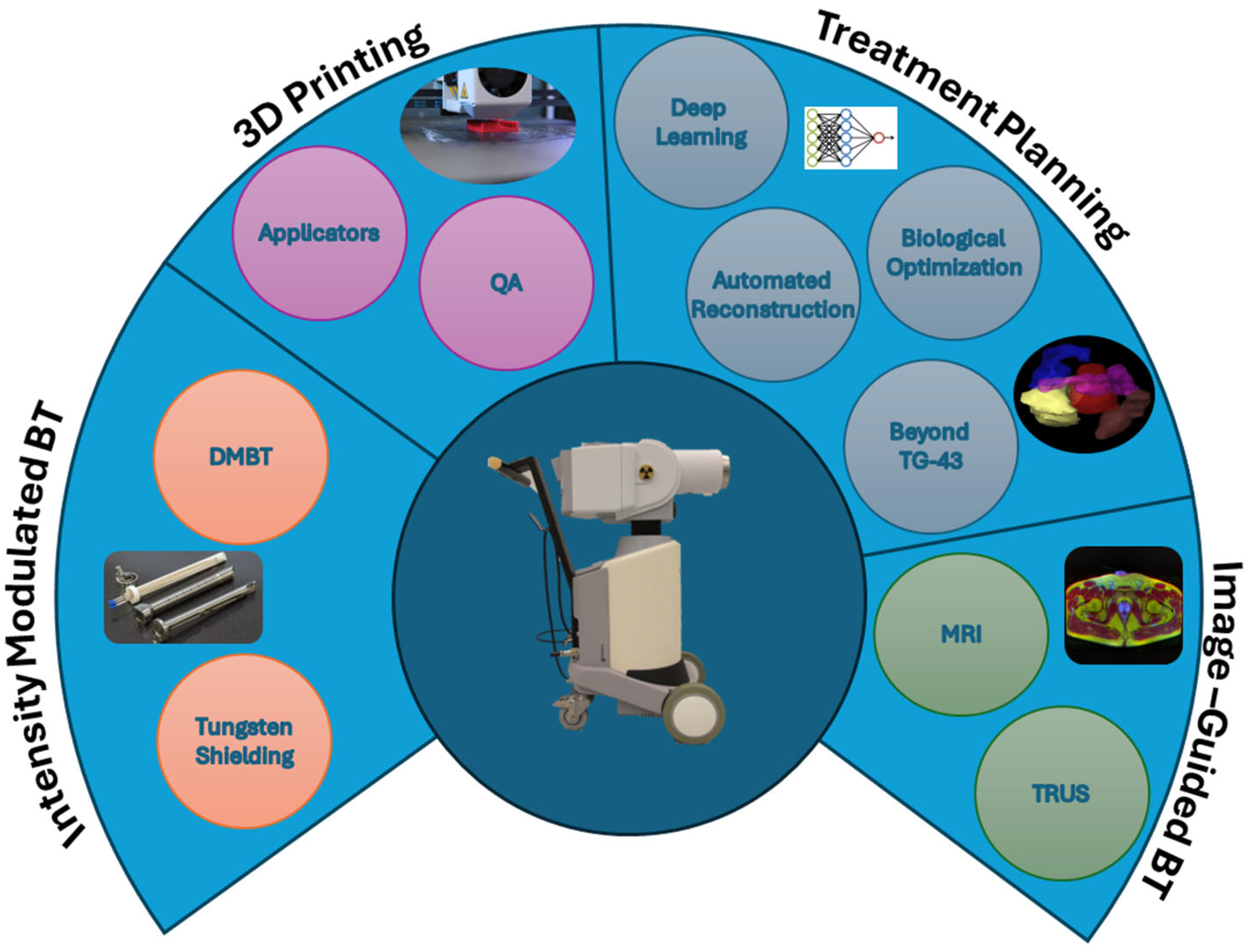
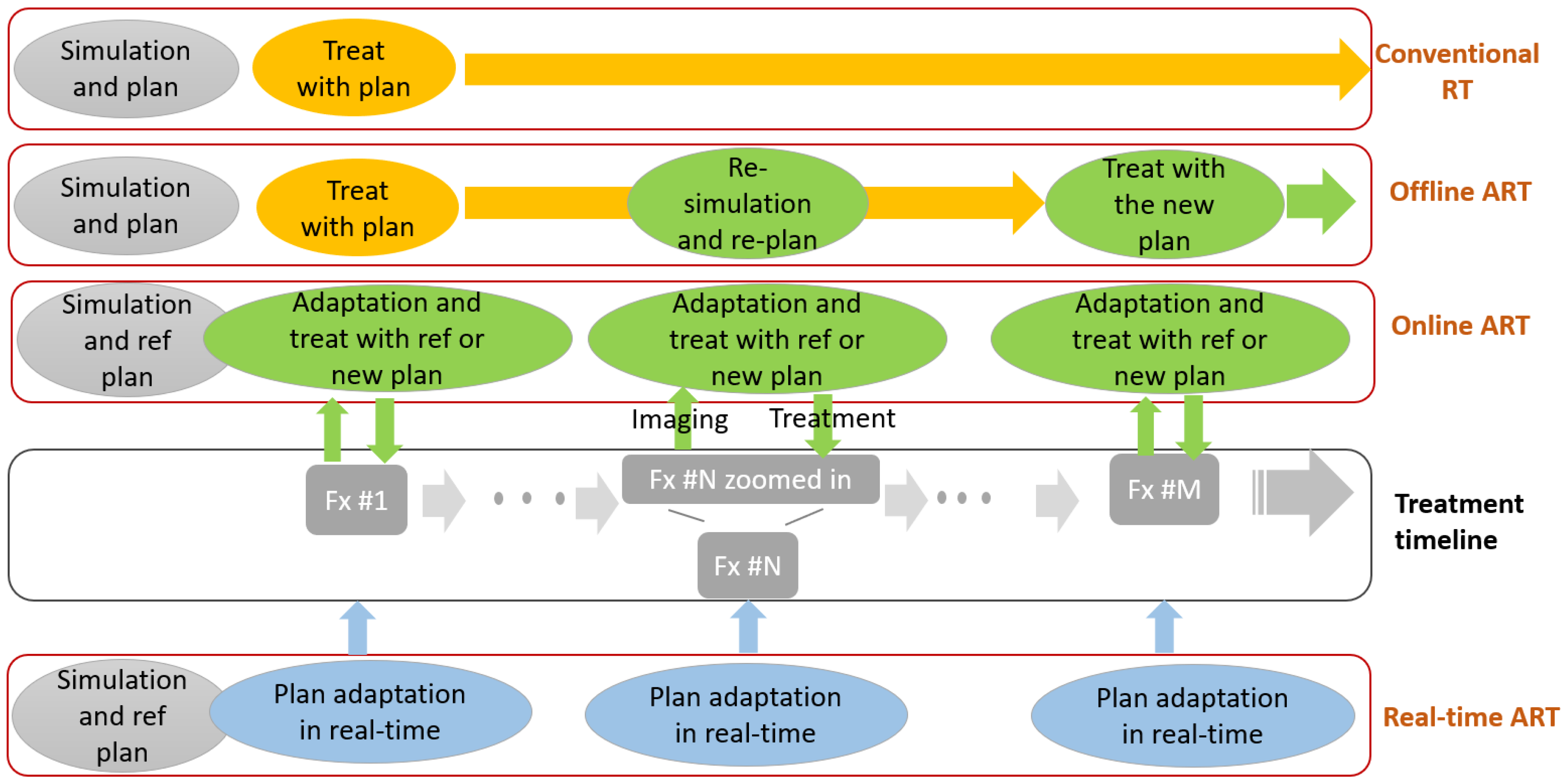
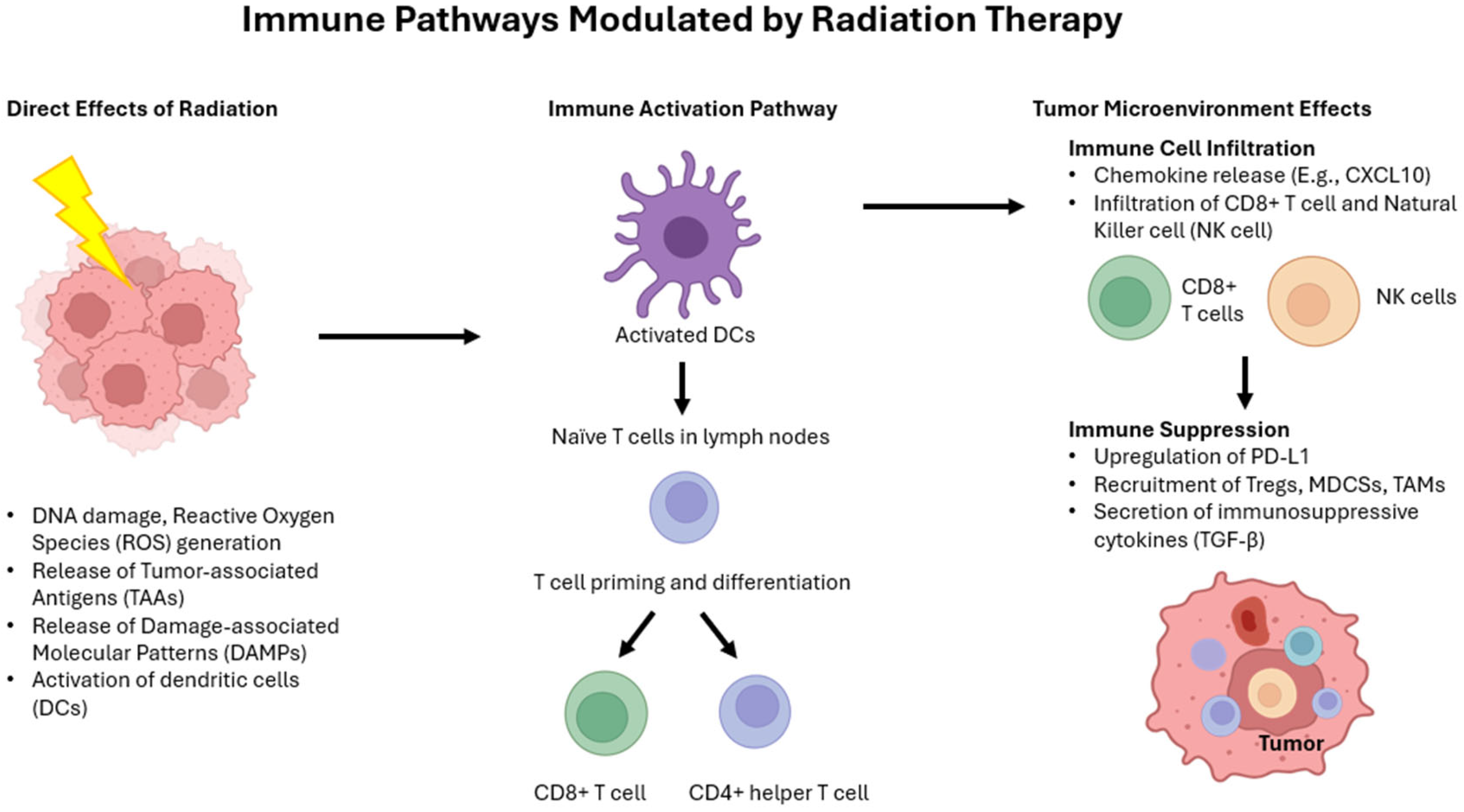
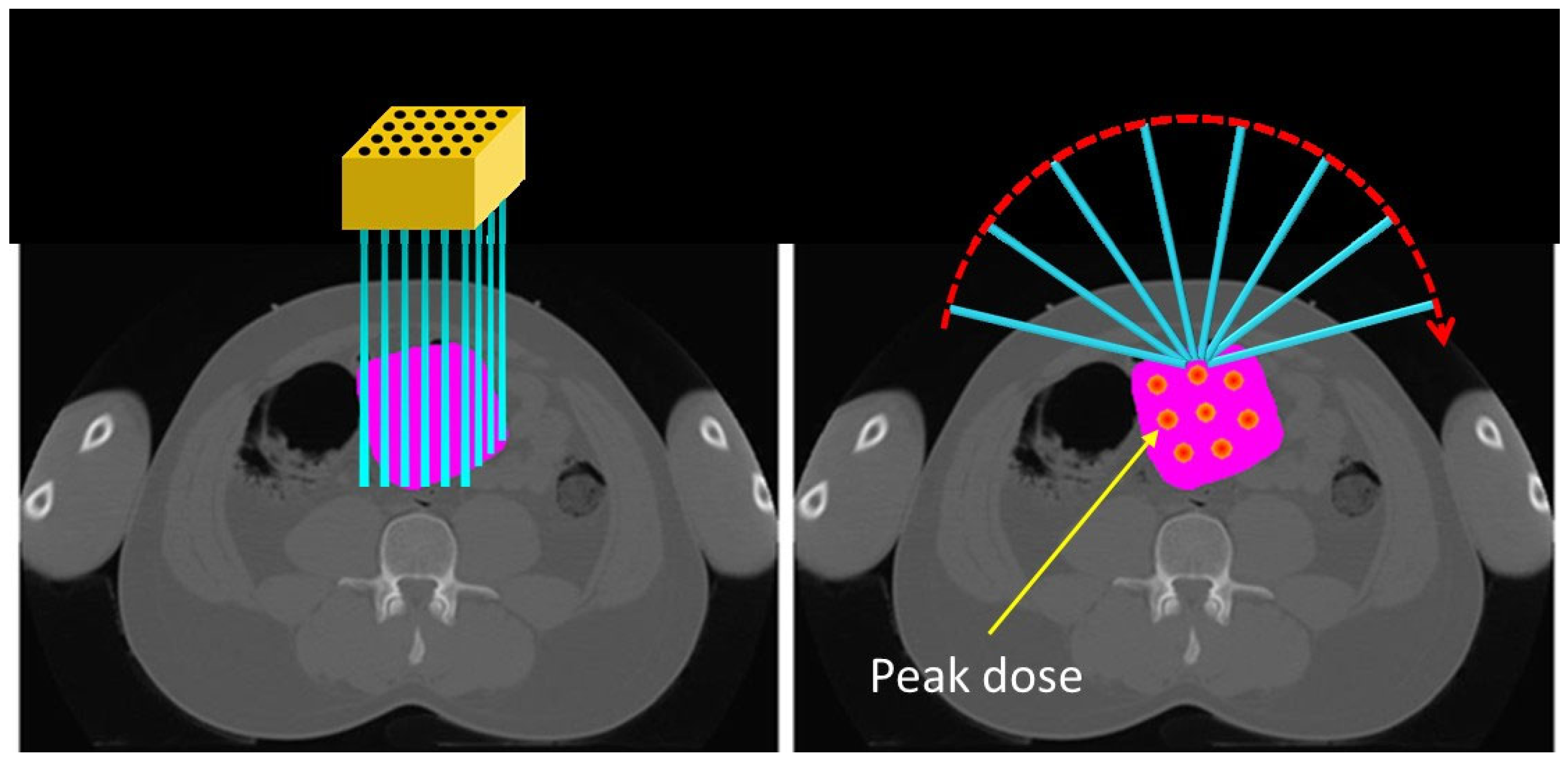
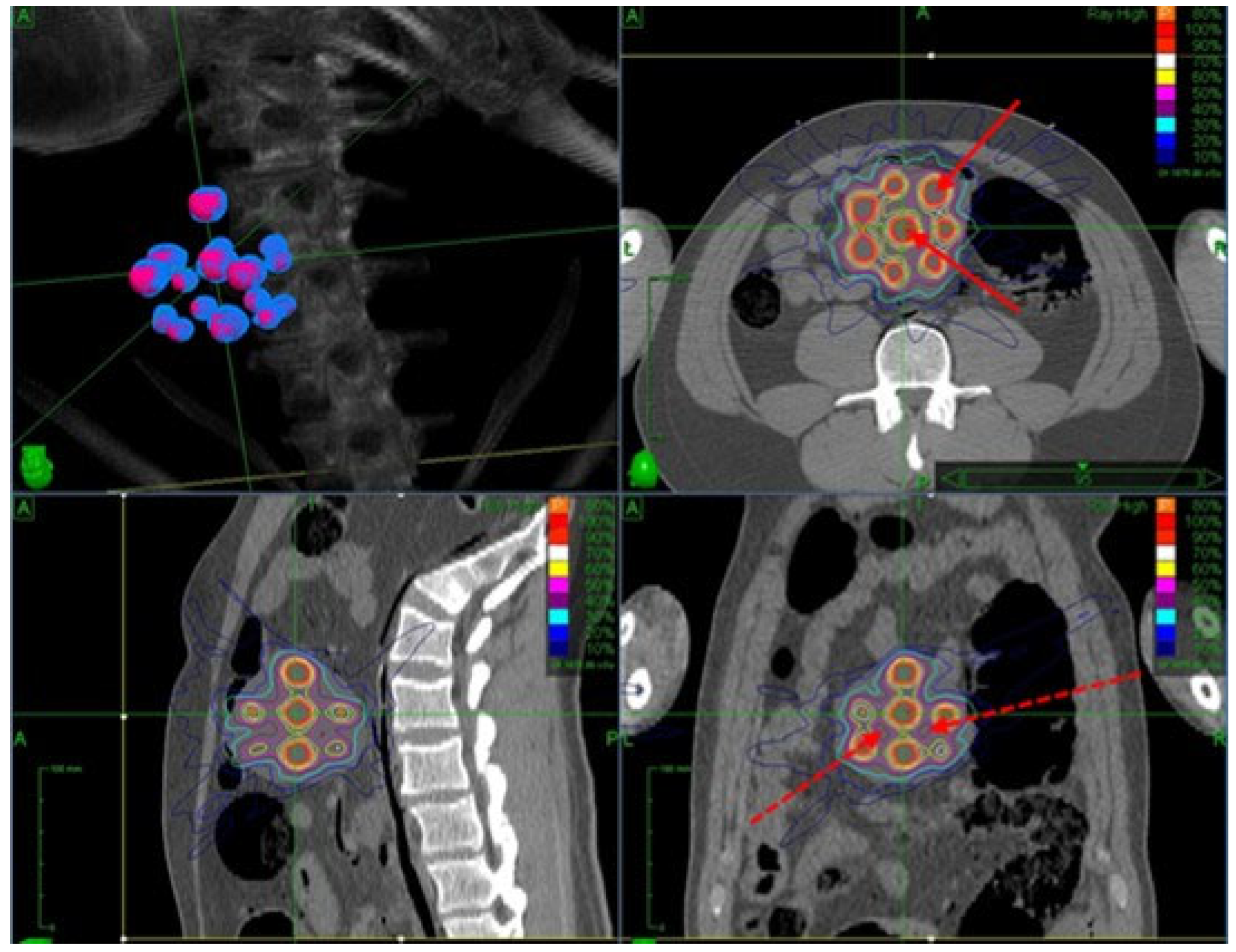
| Agents (Trade Name) | Therapy (Imaging) | Trial | Dosage | Indications |
|---|---|---|---|---|
| PSMA [210] (Locametz) | 177Lu (68Ga) | VISION | 7.4 GBq every 6 weeks up to 6 doses. | PSMA metastatic castration-resistant prostate cancer |
| MIBG [211] (Azedra) | 131I (123I) | IB12B | 18.5 GBq or 296 MBq/kg, based on weight | Pheochromocytoma or paraganglioma |
| DOTATATE [212] (Luthathera) | 177Lu (177Lu) | NETTER-1 | 7.4 GBq over 30 min every 8 weeks (4 doses total) | Somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumor |
| NaI | 131I (123I) | 3.7–5.55 GBq | Malignant and benign thyroid cancer |
| Agent Therapy (Imaging) | Institute | Study Objective | Trial Identifiers | Target | Indication |
|---|---|---|---|---|---|
| 177Lu (68Ga) 177Lu (68Ga) SABR + 177Lu (68Ga) 177Lu (68Ga) 177Lu-ITG-PSMA-1 (68Ga-PSMA-11) 68Ga-DOTA-5G (177Lu-DOTA-ABM-5G) 177Lu-TLX591 (68Ga-PSMA-11) 212Pb-VMT01 (203Pb-VMT01 or 68Ga-VMT02) 212Pb-VMT--NET (203Pb-VMT--NET) | Centre Leon Berard, France CIUSS de l’Estrie-CHUS hospital, Canada Peter MacCallum Cancer Centre, Australia St. Olavs hospital, Norway University of Laussanne Hospitals (Switzerland) University of California, Davis, USA Telix Pharmaceuticals Pty Limited Perspective therapeutics University of Iowa | Safety and efficacy Eligibility for endoradiotherapy SABR alone vs. SABR + 177Lu-PSMA Improve diagnostic and therapeutic glioma management Feasibility study Safety and efficacy Safety, Biodistribution, and dosimetry Safety and efficacy Safety and efficacy | NCT06059014 (Phase I/II) NCT05214820 (Phase II) NCT05560659 POPSTAR II (Phase II) NCT05644080 NCT05420727 NCT06389123 NCT04786847 (ProstACTSelect) NCT05655312 NCT06148636 | PSMA PSMA PSMA PSMA Vasculature of soft tissue sarcoma integrin PSMA Melanocortin subtype 1 receptor Somatostatin receptor type 2 | Metastatic clear cell renal cancer Upper Gastrointestinal cancer Oligometastatic prostate cancer Recurrent Grade 3 and 4 Glioma Soft tissue sarcoma Metastatic carcinomas, Pancreatic Cancer, non-small lung cancer Metastatic castration-resistant prostate cancer Unresectable and metastatic melanoma Neuroendocrine tumors |
| Types | Immune Drugs | RT Indications | Cancers |
|---|---|---|---|
| PD-1/PD-L1 inhibitors | Pembrolizumab, Nivolumab, Atezolizumab, Avelumab, Durvalumab | Boost immune responses | NSCLC, GBM, bladder |
| CTLA-4 inhibitors | Ipilimumab | Enhance systemic anti-tumor responses | Melanoma, NSCLC |
| TGF-β inhibitors | Fresolimumab, LY2109761, LY364947 | Modulate the tumor microenvironment and enhance immune responses/radio-resistance | NSCLC, metastatic breast, GBM |
| Cytokines | GM-CSF | Abscopal effect | Laryngeal |
| Peak Dose | Beam Size | Beam Space | Peak Valley Dose Ratio | |
|---|---|---|---|---|
| GRID Therapy | 10–20 Gy | 1–2 cm | 1–4 cm | 3–7 |
| LATTICE Therapy | 8–20 Gy | 1–2 cm | 1–4 cm | 3–7 |
| MBRT | 50–100 Gy | 0.3–1 mm | 1–4 mm | 10–20 |
| MRT | 300–600 Gy | 25–100 µm | 200–400 µm | >50 |
| GRID | LATTICE | MBRT | MRT | |
|---|---|---|---|---|
| Clinical Readiness | Limited | Limited and early phased | Preclinical | Preclinical |
| Beam scale | cm scale 2D | cm scale 3D | mm scale | μm scale |
| Dose delivery | Static | VMAT/IMRT | Preclinical systems | Preclinical systems |
| Need Grid/Slit Collimator? | Yes | No | Yes | Yes |
| Need SFRT structure contour? | No | Yes | No | No |
| Clinical Trials | |||||
|---|---|---|---|---|---|
| Authors | Number of Patients | Primary Tumor Site | SFRT Rx | Control Rate | |
| GRID therapy | Mohiuddin et al., 1996 [272] | 87 | Diverse (including SCC, sarcoma) | 10–25 Gy single fx SFRT only, 10–15 Gy single fx SFRT + EBRT | Response rate: 91% |
| Huhn et al., 2006 [273] | 27 | SCC of H&N | 15–20 Gy single fx SFRT + EBRT | Neck control: 92–93% | |
| LATTICE therapy | Larrea et al., 2022 [279] | 11 | NSCLC | 15 Gy single fx SFRT + EBRT | Complete response 18.2%, response > 50% is 54.4% |
| Amendola et al., 2020 [281] | 10 | Cervix Cancer | 24 Gy in three fx SFRT + EBRT | Complete metabolic response: 88.9% | |
| Technique | Advantages | Disadvantages | Clinical Readiness | Key Challenges |
|---|---|---|---|---|
| Stereotactic RT | High precision, few fractions, established efficacy | Requires advanced imaging, not suitable for all tumors | Routine | Motion management, expanding to new indications |
| Brachytherapy | High local dose, short treatment time | Invasive, declining usage in some regions, procedural, resource-intensive | Routine/ evolving | Training, accessibility |
| Advanced Image Guidance | Enhances precision, real-time targeting, biological and functional imaging | Cost, increased treatment time | Routine | Workflow integration |
| Proton/Heavy Ion RT | Superior dosimetry | High cost, limited availability | Niche | Cost-effectiveness, clinical evidence, still evolving |
| Adaptive RT | Personalized treatment, daily replanning | Resource-intensive, complex workflow | Expanding | Automation, clinician training |
| AI/Data Science | Faster planning, predictive analytics | Data quality, validation, regulatory hurdles | Early Adoption | Transparency, data privacy, generalizability |
| Hyperthermia | Radiosensitization, immune stimulation | Limited availability, specialized equipment, resource-intensive | Niche | Infrastructure, standardization |
| Theranostics | Dual imaging + therapy, systemic/local combo | Radiopharmaceutical logistics | Expanding | Integration with RT workflows |
| Radioimmunotherapy | Systemic effect, synergy with RT | Targeting specificity, toxicity, limited approvals | Niche | Drug delivery, trial validation |
| Spatially Fractionated RT | Possible immune boost, bulk tumor targeting | Planning complexity, experimental | Early Adoption | Lack of guidelines, validation |
| FLASH RT | Reduced toxicity, ultrafast delivery | Technological challenges | Experimental | Dose control, machine availability |
| Boron Neutron Capture Therapy | Tumor-selective targeting | Requires neutron sources, complex dosimetry | Experimental | Infrastructure, boron delivery agents |
| Technique | Advantages | Disadvantages | Clinical Readiness | Key Challenges |
|---|---|---|---|---|
| VMAT for TBI/TMI | Conformal dose to target, organ sparing | Complex planning, time-intensive | Expanding | Automation, access in resource-limited settings |
| Intraoperative RT (IORT) | One-time RT during surgery, reduces recurrence | Specialized equipment, limited tumor sites, procedural | Niche | Logistics, standardization |
| Genomic Profiling | Personalized therapy, predicts RT response | Interpretation complexity, limited RT-specific use | Early Adoption | Validation, integration with RT decisions |
| 3D Printing | Personalized bolus/applicators, QA tools | Material cost, regulatory approval for devices | Expanding | Standardization, clinical outcome validation |
| Photodynamic Therapy | Non-ionizing, minimal systemic toxicity | Depth limitation, photosensitivity | Niche | Agent development, tumor accessibility |
| Alpha-Particle Therapy | High LET, short range, ideal for micrometastases | Limited isotopes, toxicity control | Expanding | Delivery, safety, cost |
| Auger Therapy | High LET at DNA-level, minimal bystander damage | Inefficient targeting, low efficacy so far | Preclinical | Compound design, delivery to DNA |
| Hydrogen Therapy | Radioprotective, antioxidant potential | Unproven in clinical oncology | Preclinical | Mechanistic validation, delivery systems |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Webster, M.; Podgorsak, A.; Li, F.; Zhou, Y.; Jung, H.; Yoon, J.; Dona Lemus, O.; Zheng, D. New Approaches in Radiotherapy. Cancers 2025, 17, 1980. https://doi.org/10.3390/cancers17121980
Webster M, Podgorsak A, Li F, Zhou Y, Jung H, Yoon J, Dona Lemus O, Zheng D. New Approaches in Radiotherapy. Cancers. 2025; 17(12):1980. https://doi.org/10.3390/cancers17121980
Chicago/Turabian StyleWebster, Matthew, Alexander Podgorsak, Fiona Li, Yuwei Zhou, Hyunuk Jung, Jihyung Yoon, Olga Dona Lemus, and Dandan Zheng. 2025. "New Approaches in Radiotherapy" Cancers 17, no. 12: 1980. https://doi.org/10.3390/cancers17121980
APA StyleWebster, M., Podgorsak, A., Li, F., Zhou, Y., Jung, H., Yoon, J., Dona Lemus, O., & Zheng, D. (2025). New Approaches in Radiotherapy. Cancers, 17(12), 1980. https://doi.org/10.3390/cancers17121980






