Predictive Factors Aiding in the Estimation of Intraoperative Resources in Gastric Cancer Oncologic Surgery
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Patients diagnosed with malignant gastric adenocarcinomas (ICD-10 code C16), confirmed with histopathological diagnosis, who underwent a gastrectomy during the study period in the study center;
- As per institutional policy, only patients with documented general consent for secondary use of data (for research and education) were included.
- Patients suffering from benign gastric tumors;
- Patients suffering from gastric inflammations (e.g., gastritis);
- Patients suffering from gastric infections (e.g., gastric actinomycosis);
- Patients with a major contraindication for surgery due to the general anesthesia;
- Patients who have explicitly declined the use of their medical data for research and/or medical education purposes, as documented by their non-consent in the institutional records.
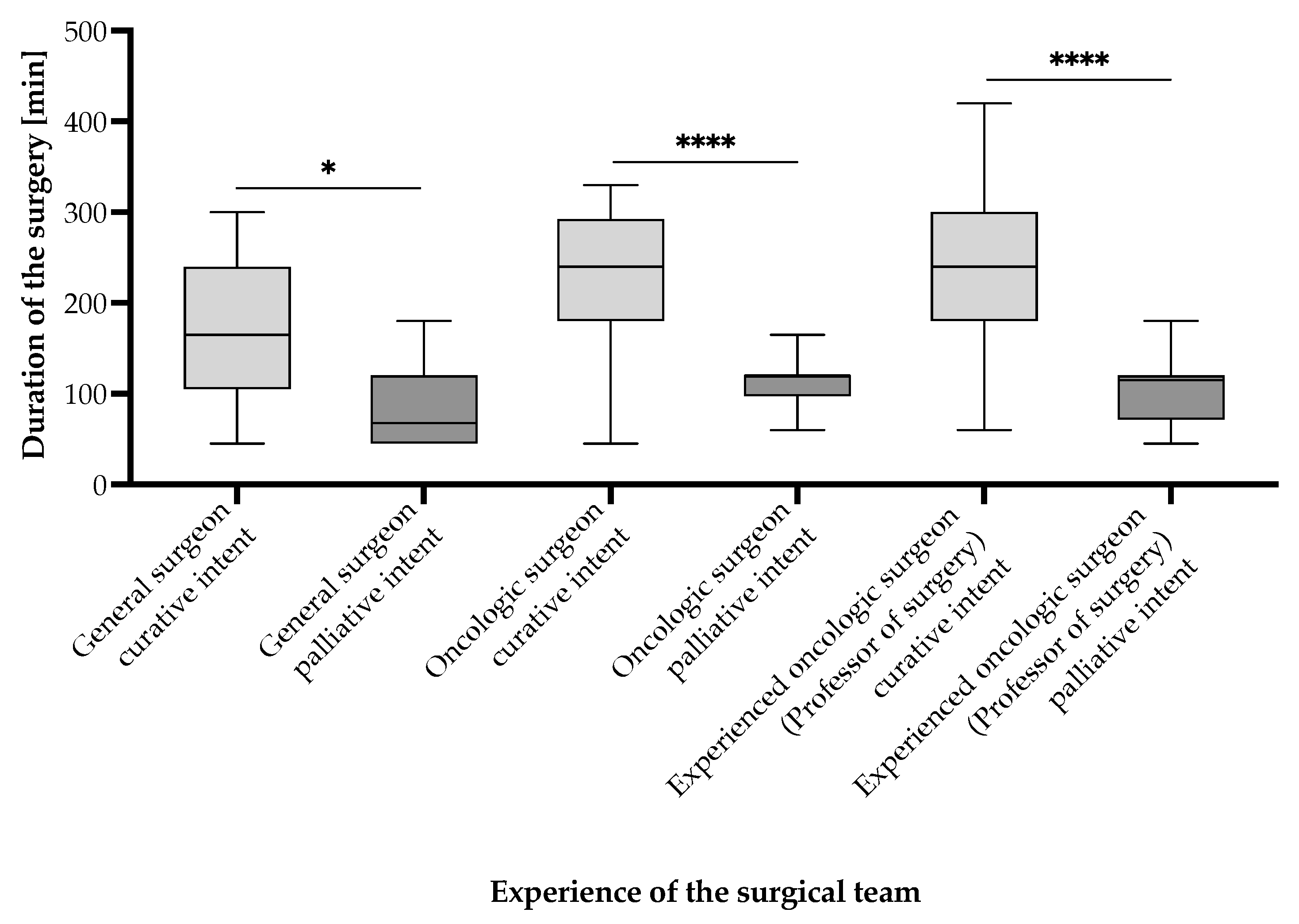
- Group 1: Patients undergoing surgery with curative intent;
- Group 2: Patients undergoing surgery with palliative intent.
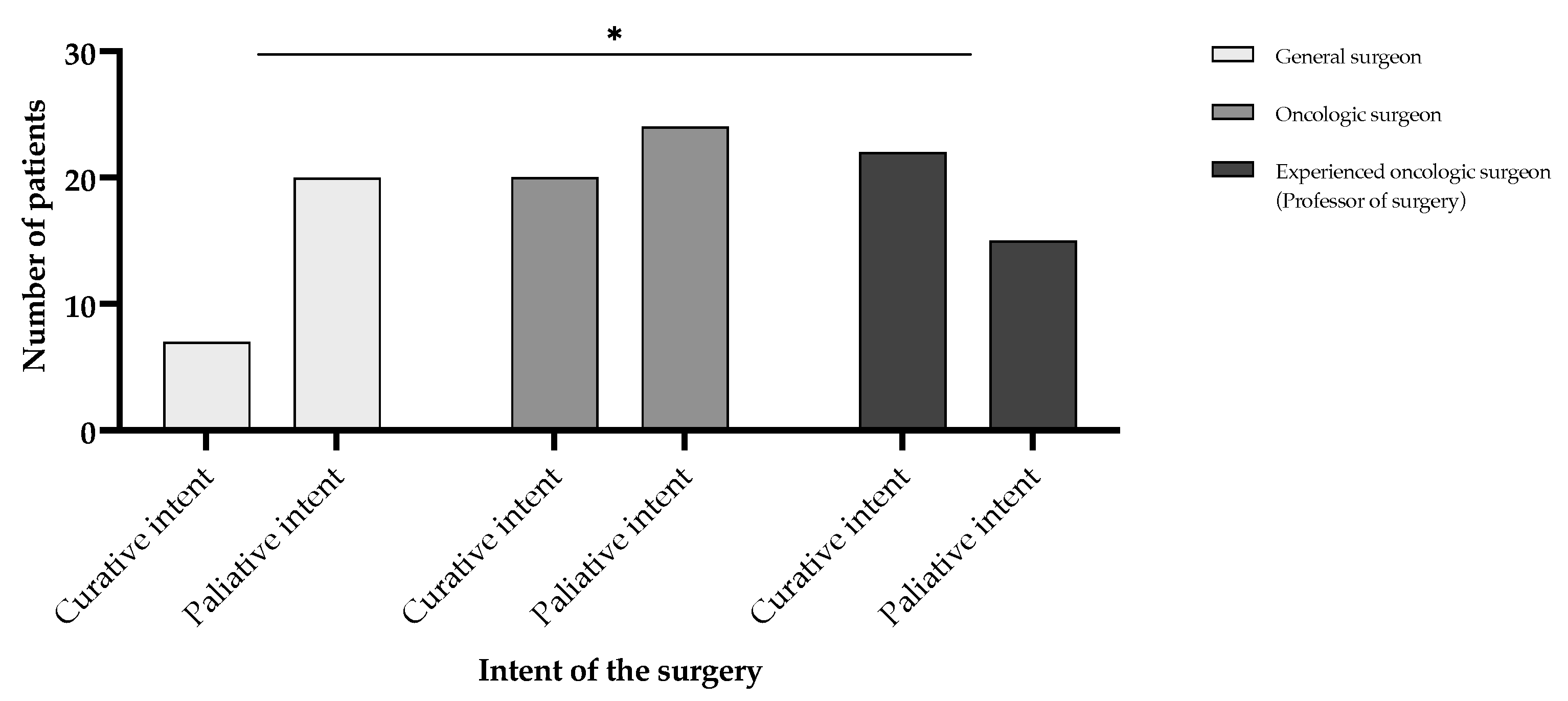
3. Results
- Group 1: Patients undergoing surgery with curative intent—63 (58.33%) patients;
- Group 2: Patients undergoing surgery with palliative intent—45 (32.41%) patients.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gür, Ş.; Pınarbaşı, M.; Alakaş, H.M.; Eren, T. Operating room scheduling with surgical team: A new approach with constraint programming and goal programming. Cent. Eur. J. Oper. Res. 2022, 31, 1061–1085. [Google Scholar] [CrossRef]
- Bellini, V.; Guzzon, M.; Bigliardi, B.; Mordonini, M.; Filippelli, S.; Bignami, E. Artificial Intelligence: A New Tool in Operating Room Management. Role of Machine Learning Models in Operating Room Optimization. J. Med. Syst. 2019, 44, 20. [Google Scholar] [CrossRef] [PubMed]
- Schofield, W.N.; Rubin, G.L.; Piza, M.; Lai, Y.Y.; Sindhusake, D.; Fearnside, M.R.; Klineberg, P.L. Cancellation of operations on the day of intended surgery at a major Australian referral hospital. Med. J. Aust. 2005, 182, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.P.; Brown, H.N. Turnover of licensed nurses in skilled nursing facilities. Nurs. Econ. 2002, 20, 66. [Google Scholar]
- Strachota, E.; Normandin, P.; O’brien, N.; Clary, M.; Krukow, B. Reasons Registered Nurses Leave or Change Employment Status. Jona J. Nurs. Adm. 2003, 33, 111–117. [Google Scholar] [CrossRef]
- Armoeyan, M.; Aarabi, A.; Akbari, L. The Effects of Surgery Cancellation on Patients, Families, and Staff: A Prospective Cross-Sectional Study. J. PeriAnesth. Nurs. 2021, 36, 695–701.e2. [Google Scholar] [CrossRef]
- Han, L. Prevalence, risk factors and prognostic role of anxiety and depression in surgical gastric cancer patients. Transl. Cancer Res. 2020, 9, 1371–1383. [Google Scholar] [CrossRef]
- Dinkel, M.; Kamp, H.D.; Schweiger, H. Somatosensory evoked potentials in carotid surgery. Anaesthesist 1991, 40, 72–78. [Google Scholar]
- Baqué, P.; Iannelli, A.; Delotte, J.; de Peretti, F.; Bourgeon, A. Division of the right posterior attachments of the head of the pancreas with a linear stapler during pancreaticoduodenectomy: Vascular and oncological considerations based on an anatomical cadaver-based study. Surg. Radiol. Anat. 2008, 31, 13–17. [Google Scholar] [CrossRef]
- Ishiyama, Y.; Maeda, C.; Shimada, S.; Kudo, S.-E. Propensity-score-matched analysis of short- and long-term outcomes in patients with an ileocolic artery crossing anterior vs. posterior to the superior mesenteric vein during curative resection for right-sided colon cancer. Surg. Endosc. 2020, 34, 5384–5392. [Google Scholar] [CrossRef]
- Songun, I.; Putter, H.; Kranenbarg, E.M.-K.; Sasako, M.; van de Velde, C.J. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Freytag, D.; Pape, J.; Dhanawat, J.; Günther, V.; Maass, N.; Gitas, G.; Laganà, A.S.; Allahqoli, L.; Meinhold-Heerlein, I.; Moawad, G.N.; et al. Challenges Posed by Embryonic and Anatomical Factors in Systematic Lymphadenectomy for Endometrial Cancer. J. Clin. Med. 2020, 9, 4107. [Google Scholar] [CrossRef] [PubMed]
- Natsume, T.; Shuto, K.; Yanagawa, N.; Akai, T.; Kawahira, H.; Hayashi, H.; Matsubara, H. The classification of anatomic variations in the perigastric vessels by dual-phase CT to reduce intraoperative bleeding during laparoscopic gastrectomy. Surg. Endosc. 2010, 25, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chen, B.P.; Soleas, I.M.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged operative duration increases risk of surgical site infections: A systematic re-view. Surg. Infect. 2017, 18, 722–735. [Google Scholar] [CrossRef]
- Chen, K.-W.; Yang, H.-L.; Lu, J.; Wang, G.-L.; Ji, Y.-M.; Bao, Z.-H.; Wu, G.-Z.; Gu, Y.; Sun, Z.-Y.; Zhu, R.-F. Risk Factors for Postoperative Wound Infections of Sacral Chordoma After Surgical Excision. J. Spinal Disord. Tech. 2011, 24, 230–234. [Google Scholar] [CrossRef]
- Nandi, P.L.; Soundara Rajan, S.; Mak, K.C.; Chan, S.C.; So, Y.P. Surgical wound infection. Hong Kong Med. J. 1999, 5, 82–86. [Google Scholar] [PubMed]
- Schricker, T.; Lattermann, R. Perioperative catabolism. Can. J. Anaesth. 2015, 62, 182–193. [Google Scholar] [CrossRef]
- Gu, A.; Wei, C.; Chen, A.Z.; Malahias, M.-A.; Fassihi, S.C.; Ast, M.P.; Liu, J.; Cross, M.B.; Sculco, P.K. Operative time greater than 120 minutes is associated with increased pulmonary and thromboembolic complications following revision total hip arthroplasty. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 1393–1400. [Google Scholar] [CrossRef]
- Lucas, C.E.; Benishek, D.J.; Ledgerwood, A.M. Reduced Oncotic Pressure After Shock. Arch. Surg. 1982, 117, 675–679. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Y.; Qian, H.; Li, H.; Zhu, X. Longer Operating Time During Gastrectomy Has Adverse Effects on Short-Term Surgical Outcomes. J. Surg. Res. 2019, 243, 151–159. [Google Scholar] [CrossRef]
- Kobayashi, T.; Watanabe, Y.; Aizawa, J.; Suzuki, K.S. Factors affecting the early post-operative prognosis in morbidly obese surgical patients after laparoscopic sleeve gastrectomy—A retrospective cohort study. JA Clin. Rep. 2017, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Borta, S.M.; Dumitra, S.; Miklos, I.; Popetiu, R.; Pilat, L.; Pușchiță, M.; Marian, C. Clinical Relevance of Plasma Concentrations of MBL in Accordance with IgE Levels in Children Diagnosed with Bronchial Asthma. Medicina 2020, 56, 594. [Google Scholar] [CrossRef] [PubMed]
- Muntean, C.; Blidari, A.R.; Faur, A.M.; Curca, R.O.; Feier, C.V.I. Evaluating the Outcomes in Patients with Colorectal Cancer Using the Malnutrition Universal Screening Tool: A Systematic Review. J. Multidiscip. Healthc. 2024, 17, 4277–4289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, D.; Yoo, K.Y. Fluid management in perioperative and critically ill patients. Acute Crit. Care 2019, 34, 235–245. [Google Scholar] [CrossRef]
- Lim, H.-S.; Lee, B.; Cho, I.; Cho, G.S. Nutritional and Clinical Factors Affecting Weight and Fat-Free Mass Loss after Gastrectomy in Patients with Gastric Cancer. Nutrients 2020, 12, 1905. [Google Scholar] [CrossRef]
- Knight, S.R.; Qureshi, A.U.; Drake, T.M.; Lapitan, M.C.M.; Maimbo, M.; Yenli, E.; Tabiri, S.; Ghosh, D.; Kingsley, P.A.; Sundar, S.; et al. The impact of preoperative oral nutrition supplementation on outcomes in patients undergoing gastrointestinal surgery for cancer in low- and middle-income countries: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 12456. [Google Scholar] [CrossRef]
- Park, S.-H.; Shin, Y.-R.; Hur, H.; Lee, C.M.; Min, J.S.; Ryu, S.W.; Chae, H.D.; Jeong, O.; Choi, C.-I.; Song, K.-Y.; et al. Exploring ideal operative time for best outcomes in gastric cancer surgery: A multi-institutional study based on KLASS-07 database. Chin. J. Cancer Res. 2023, 35, 660–674. [Google Scholar] [CrossRef]
- Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/7-stomach-fact-sheet.pdf (accessed on 27 January 2025).
- Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/642-romania-fact-sheet.pdf (accessed on 27 January 2025).
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef]
- Nelen, S.D.; Verhoeven, R.H.A.; Lemmens, V.E.P.P.; de Wilt, J.H.W.; Bosscha, K. Increasing survival gap between young and elderly gastric cancer patients. Gastric Cancer 2017, 20, 919–928. [Google Scholar] [CrossRef]
- Available online: https://gco.iarc.who.int/tomorrow/en/dataviz/bars?multiple_populations=1&mode=cancer&multiple_cancers=1&cancers=7&types=0&populations=8_40_56_70_100_112_191_196_203_208_233_246_250_276_300_348_352_372_380_428_440_442_470_498_499_528_578_616_620_642_643_688_703_705_724_752_756_804_807_826&group_populations=1&years=2050 (accessed on 27 January 2025).
- Mocan, L. Surgical Management of Gastric Cancer: A Systematic Review. J. Clin. Med. 2021, 10, 2557. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Robb, W.B.; Messager, M.; Gronnier, C.; Tessier, W.; Hec, F.; Piessen, G.; Mariette, C.; FREGAT (French EsoGastric Tumor) Working Group. High-grade toxicity to neoadjuvant treatment for upper gastrointestinal carcinomas: What is the impact on perioperative and oncologic outcomes? Ann. Surg. Oncol. 2015, 22, 3632–3639. [Google Scholar] [CrossRef] [PubMed]
- Charalampakis, N.; Xiao, L.; Lin, Q.; Elimova, E.; Shimodaira, Y.; Harada, K.; Rogers, J.E.; Mares, J.; Amlashi, F.G.; Minsky, B.D.; et al. Co-morbidities rather than age impact outcomes in patients receiving preoperative therapy for gastroesophageal adenocarcinoma. Ann. Surg. Oncol. 2017, 24, 2291–2301. [Google Scholar] [CrossRef] [PubMed]
- Institutul National de Statistica. Recensamantul Populatiei si Locuintelor. Romania. 2021. Available online: https://www.recensamantromania.ro/rezultate-rpl-2021/rezultate-definitive-caracteristici-demografice/ (accessed on 27 January 2025).
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Marano, L.; Verre, L.; Carbone, L.; Poto, G.E.; Fusario, D.; Venezia, D.F.; Calomino, N.; Kaźmierczak-Siedlecka, K.; Polom, K.; Marrelli, D.; et al. Current Trends in Volume and Surgical Outcomes in Gastric Cancer. J. Clin. Med. 2023, 12, 2708. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef]
- Degiuli, M.; De Manzoni, G.; Di Leo, A.; D’ugo, D.; Galasso, E.; Marrelli, D.; Petrioli, R.; Polom, K.; Roviello, F.; Santullo, F.; et al. Gastric cancer: Current status of lymph node dissection. World J. Gastroenterol. 2016, 22, 2875–2893. [Google Scholar] [CrossRef]
- Marano, L.; Marrelli, D.; Roviello, F. Focus on research: Nodal dissection for gastric cancer—A dilemma worthy of King Solomon! Eur. J. Surg. Oncol. (EJSO) 2016, 42, 1623–1624. [Google Scholar] [CrossRef]
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, J.; Maciejewski, R.; Polkowski, W. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018, 10, 239–248. [Google Scholar] [CrossRef]
- Jensen, L.; Nielsen, H.; Mortensen, P.; Pilegaard, H.; Johnsen, S. Enforcing centralization for gastric cancer in Denmark. Eur. J. Surg. Oncol. (EJSO) 2010, 36, S50–S54. [Google Scholar] [CrossRef]
- Dulucq, J.; Wintringer, P.; Stabilini, C.; Solinas, L.; Perissat, J.; Mahajna, A. Laparoscopic and open gastric resections for malignant lesions: A prospective comparative study. Surg. Endosc. 2005, 19, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zou, J.; Jia, M.; Li, P.; Zhang, R.; Han, J.; Huang, K.; Qiao, Y.; Xu, T.; Peng, R.; et al. Palliative Gastrectomy and Survival in Patients With Metastatic Gastric Cancer: A Propensity Score–Matched Analysis of a Large Population-Based Study. Clin. Transl. Gastroenterol. 2019, 10, e00048. [Google Scholar] [CrossRef] [PubMed]
- Gertsen, E.C.; Brenkman, H.J.; Goense, L.; Mohammad, N.H.; Weusten, B.L.; van Hillegersberg, R.; Ruurda, J.P. Non-curative gastrectomy for advanced gastric cancer does not result in additional risk of postoperative morbidity compared to curative gastrectomy. Surg. Oncol. 2020, 35, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kopecky, K.; Monton, O.; Rosman, L.; Johnston, F. Palliative interventions for patients with advanced gastric cancer: A systematic review. Chin. Clin. Oncol. 2022, 11, 47. [Google Scholar] [CrossRef]





| Topography of the Tumor | Number of Patients | Percentage of Patients [%] | Average Surgical Duration (Incl. Anesthesiology) [min] |
|---|---|---|---|
| Gastro-esophageal junction | 2 | 1.85 | 60.00 [CI not available due to low sample size] |
| Cardia | 4 | 3.70 | 142.50 [95% CI (6.78–278.22)] |
| Fundus | 2 | 1.85 | 180.00 [CI not available due to low sample size] |
| Corpus | 31 | 28.70 | 172.26 [95% CI (136.58–207.94)] |
| Antrum | 55 | 50.93 | 182.82 [95% CI (157.55–208.09)] |
| Pyloric antrum | 14 | 12.96 | 176.79 [95% CI (128.60–224.97)] |
| TNM Classification for Gastric Cancer (According to AJCC 8th Edition) [38] | Number of Patients | Percentage of Patients [%] | Average Surgical Duration (Incl. Anesthesiology) [min] |
|---|---|---|---|
| Size and extent of the main tumor | |||
| pTis | 1 | 0.93 | 45.00 [CI not available due to low sample size] |
| pT1 | 2 | 1.85 | 112.50 [CI not available due to low sample size] |
| pT2 | 23 | 21.30 | 156.1 [95% CI (114.4–197.8)] |
| pT3 | 20 | 18.52 | 192.8 [95% CI (146.1–239.4)] |
| pT4a | 33 | 30.56 | 201.4 [95% CI (174.0–228.7)] |
| pT4b | 29 | 26.85 | 150.5 [95% CI (116.6–184.4)] |
| Number of nearby lymph nodes invaded by the tumor | |||
| pN0 | 17 | 15.74 | 199.4 [95% CI (147.3–251.6)] |
| pN1 | 36 | 33.33 | 171.7 [95% CI (139.5–203.9)] |
| pN2 | 25 | 23.25 | 174.6 [95% CI (133.9–215.3)] |
| pN3a | 13 | 12.04 | 174.6 [95% CI (125.5–223.7)] |
| pN3b | 11 | 10.19 | 151.4 [95% CI (106.9–195.9)] |
| pNx | 6 | 5.56 | 142.5 [95% CI (66.2–218.8)] |
| Presence of metastases | |||
| M0 | 64 | 59.26 | 192.42 [95% CI (168.49–216.36)] |
| M1 | 44 | 40.74 | 145.68 [95% CI (122.43–168.93)] |
| Pathological TNM staging (pTNM) | |||
| 0 | 1 | 0.93 | 45 [CI not available due to low sample size] |
| IA | 1 | 0.93 | 330 [CI not available due to low sample size] |
| IB | 7 | 6.48 | 182.14 [95% CI (99.13–265.16)] |
| IIA | 9 | 8.33 | 218.33 [95% CI (119.13–317.54)] |
| IIB | 5 | 4.63 | 120 [95% CI (48.09–197.91)] |
| IIIA | 26 | 24.07 | 222.69 [95% CI (189.52–255.86)] |
| IIIB | 10 | 9.26 | 180 [95% CI (102.13–257.87)] |
| IIIC | 6 | 5.56 | 167.50 [95% CI (85.76–249.25)] |
| IV | 43 | 39.81 | 142.09 [95% CI (119.46–164.72)] |
| Classification | Number of Patients | Percentage of Patients [%] | Average Surgical Duration (Incl. Anesthesiology) [min] |
|---|---|---|---|
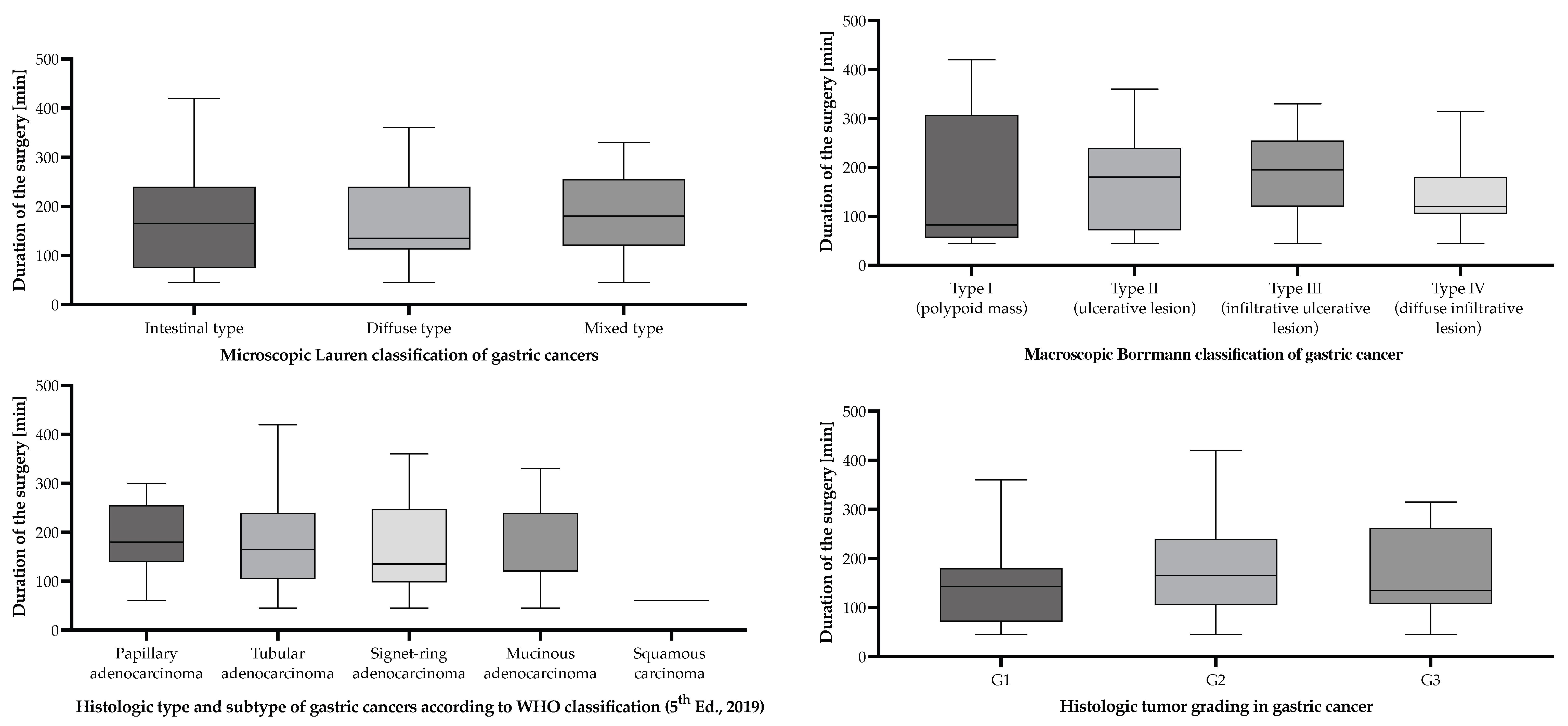
| Macroscopic Borrmann classification of gastric cancers | |||
| Borrmann I | 10 | 9.30 | 154.5 [95% CI (54.2–254.8)] |
| Borrmann II | 22 | 20.40 | 173.9 [95% CI (133.2–214.5)] |
| Borrmann III | 47 | 43.50 | 190.6 [95% CI (165.3–216.0)] |
| Borrmann IV | 29 | 26.9 | 151.6 [95% CI (122.9–180.2)] |
| Microscopic Lauren classification of gastric cancers | |||
| Intestinal | 62 | 57.40 | 168.7 [95% CI (144.2–193.2)] |
| Diffuse | 21 | 19.40 | 163.6 [95% CI (124.2–202.9)] |
| Mixed | 25 | 23.10 | 193.2 [95% CI (159.7–226.7)] |
| Histologic type and subtype of gastric cancers according to WHO classification (5th Ed., 2019) | |||
| Adenocarcinoma | |||
| Tubular pattern | 73 | 67.60 | 176.2 [95% CI (154.4–198.0)] |
| Signet-ring cell pattern | 13 | 12.00 | 173.1 [95% CI (111.5–234.6)] |
| Papillary pattern | 6 | 5.60 | 187.5 [95% CI (103.2–271.8)] |
| Mucinous adenocarcinoma | 15 | 13.90 | 162.0 [95% CI (118.7–205.3) |
| Squamous carcinoma | |||
| Squamous cell carcinoma | 1 | 0.93 | 330 [CI not available due to low sample size] |
| Microscopic tumor grading of gastric cancers according to the WHO classification (5th Ed., 2019) | |||
| G1 | 8 | 7.40 | 151.9 [95% CI (69.4–234.4)] |
| G2 | 87 | 80.60 | 175.3 [95% CI (156.0–194.7)] |
| G3 | 13 | 12.00 | 173.5 [95% CI (116.6–230.3)] |
| Classification | Number of Patients | Percentage of Patients [%] | Average Surgical Duration (Incl. Anesthesiology) [min] |
|---|---|---|---|
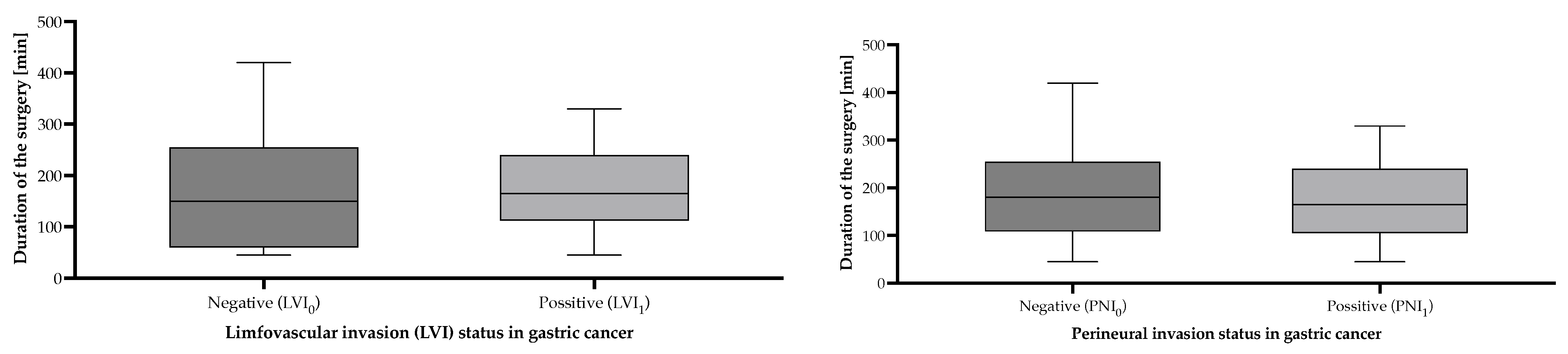
| Lymphovascular invasion status | |||
| LVI0 | 35 | 32.40 | 170.3 [95% CI (133.7–206.9)] |
| LVI1 | 73 | 67.60 | 174.9 [95% CI (155.4–194.3)] |
| Perineural invasion status | |||
| PNI0 | 46 | 42.60 | 184.3 [95% CI (155.5–213.2)] |
| PNI1 | 62 | 57.40 | 165.2 [95% CI (143.3–187.1)] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blidișel, A.; Roșu, M.-C.; Neamțu, A.-A.; Totolici, B.D.; Pop-Moldovan, R.-O.; Ardelean, A.; Iovin, V.-C.; Faur, I.F.; Dehelean, C.A.; Dema, S.A.; et al. Predictive Factors Aiding in the Estimation of Intraoperative Resources in Gastric Cancer Oncologic Surgery. Cancers 2025, 17, 2038. https://doi.org/10.3390/cancers17122038
Blidișel A, Roșu M-C, Neamțu A-A, Totolici BD, Pop-Moldovan R-O, Ardelean A, Iovin V-C, Faur IF, Dehelean CA, Dema SA, et al. Predictive Factors Aiding in the Estimation of Intraoperative Resources in Gastric Cancer Oncologic Surgery. Cancers. 2025; 17(12):2038. https://doi.org/10.3390/cancers17122038
Chicago/Turabian StyleBlidișel, Alexandru, Mihai-Cătălin Roșu, Andreea-Adriana Neamțu, Bogdan Dan Totolici, Răzvan-Ovidiu Pop-Moldovan, Andrei Ardelean, Valentin-Cristian Iovin, Ionuț Flaviu Faur, Cristina Adriana Dehelean, Sorin Adalbert Dema, and et al. 2025. "Predictive Factors Aiding in the Estimation of Intraoperative Resources in Gastric Cancer Oncologic Surgery" Cancers 17, no. 12: 2038. https://doi.org/10.3390/cancers17122038
APA StyleBlidișel, A., Roșu, M.-C., Neamțu, A.-A., Totolici, B. D., Pop-Moldovan, R.-O., Ardelean, A., Iovin, V.-C., Faur, I. F., Dehelean, C. A., Dema, S. A., & Neamțu, C. (2025). Predictive Factors Aiding in the Estimation of Intraoperative Resources in Gastric Cancer Oncologic Surgery. Cancers, 17(12), 2038. https://doi.org/10.3390/cancers17122038








