Impact of Duodenal Stump Reinforcement in Preventing Duodenal Stump Fistula/Leakage After Distal or Total Gastrectomy for Malignant Disease: A Meta-Analysis of Comparative Studies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
- PubMed/MEDLINE
- Scopus
- Web of Science
- Embase
- Cochrane Library
2.2. Inclusion Criteria
2.3. Outcomes
2.4. Data Extraction
- Demographic data (author’s surname and year of publication, study type, study centers, study country, study period, population size, size of population with duodenal stump reinforcement, DSF rate, gender and age, body mass index (BMI), American Society of Anesthesiologists (ASA) score, neoadjuvant chemotherapy ± radiotherapy);
- Surgical data (surgical approach, type of gastrectomy and lymph node dissection);
- Pathological data (pT, pN, stage of disease);
- Duodenal stump reinforcement data (stapler type, cartridge length and closure height, reinforcement method, suture thread type).
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Search Results
3.2. Quality of Studies
3.3. Study and Population Characteristics
3.4. Duodenal Stump Reinforcement Methods
3.5. Meta-Analyses Results
3.5.1. Duodenal Stump Fistula/Leakage
3.5.2. Operative Time
3.5.3. Estimated Blood Loss
3.5.4. Overall Postoperative Complications
3.5.5. Major Postoperative Complications (Clavien–Dindo or CD ≥ III)
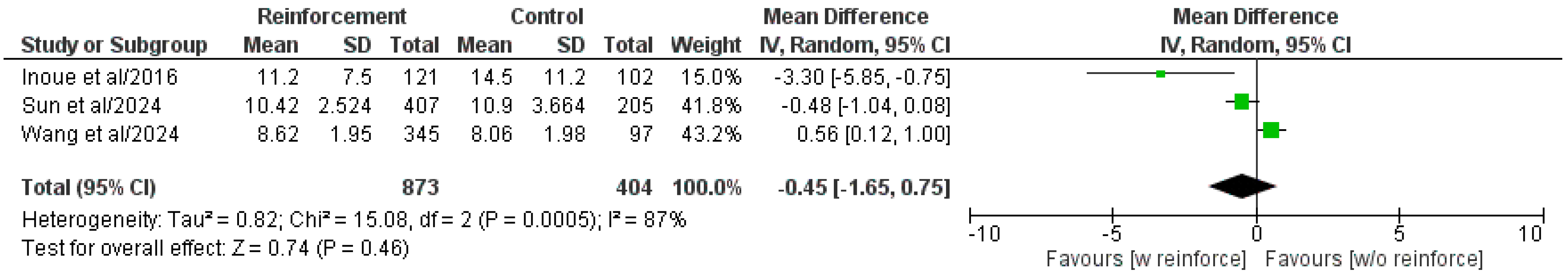
3.5.6. Length of Hospital Stay
3.5.7. Sensitivity and Subgroup Analyses
3.5.8. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IARC | International Agency for Research on Cancer |
| GC | Gastric cancer |
| DSF | Duodenal stump fistula |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | Randomized clinical trial |
| EBL | Estimated blood loss |
| CD | Clavien–Dindo |
| BMI | Body mass index |
| ASA | American Society of Anesthesiologists |
| ROB | Risk of Bias |
| ROBIN | Risk Of Bias In Non-randomized Studies |
| OR | Odds ratio |
| CI | Confidence interval |
| MH | Mantel–Haenszel |
| WMD | Weighted mean difference |
| IV | Inverse variance |
| SD | Standard deviation |
| SVM | Support vector machine |
| BPA | Bioabsorbable polyglycolic acid |
| ICG | Indocyanine green |
| DUCA | Dutch Upper Gastrointestinal Cancer Audit |
| ECOG | Eastern Cooperative Oncology Group |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C.; ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.H.; Zhang, X.T.; Tang, L.; Wu, Q.; Cai, M.Y.; Li, Y.F.; Qu, X.J.; Qiu, H.; Zhang, Y.J.; Ying, J.E.; et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2023. Cancer Commun. 2024, 44, 127–172. [Google Scholar] [CrossRef]
- Kim, I.H.; Kang, S.J.; Choi, W.; Seo, A.N.; Eom, B.W.; Kang, B.; Kim, B.J.; Min, B.H.; Tae, C.H.; Choi, C.I.; et al. Korean Practice Guidelines for Gastric Cancer 2024: An Evidence-based, Multidisciplinary Approach (Update of 2022 Guideline). J. Gastric Cancer 2025, 25, 5–114. [Google Scholar] [CrossRef]
- Schneider, M.A.; Kim, J.; Berlth, F.; Sugita, Y.; Grimminger, P.P.; Sano, T.; Rosati, R.; Baiocchi, G.L.; Bencivenga, M.; De Manzoni, G.; et al. Defining benchmarks for total and distal gastrectomy: Global multicentre analysis. Br. J. Surg. 2024, 111, znad379. [Google Scholar] [CrossRef]
- Baiocchi, G.L.; Giacopuzzi, S.; Marrelli, D.; Reim, D.; Piessen, G.; Matos da Costa, P.; Reynolds, J.V.; Meyer, H.J.; Morgagni, P.; Gockel, I.; et al. International consensus on a complications list after gastrectomy for cancer. Gastric Cancer 2019, 22, 172–189. [Google Scholar] [CrossRef]
- Zizzo, M.; Ugoletti, L.; Manzini, L.; Castro Ruiz, C.; Nita, G.E.; Zanelli, M.; De Marco, L.; Besutti, G.; Scalzone, R.; Sassatelli, R.; et al. Management of duodenal stump fistula after gastrectomy for malignant disease: A systematic review of the literature. BMC Surg. 2019, 19, 55. [Google Scholar]
- Cozzaglio, L.; Coladonato, M.; Biffi, R.; Coniglio, A.; Corso, V.; Dionigi, P.; Gianotti, L.; Mazzaferro, V.; Morgagni, P.; Rosa, F.; et al. Duodenal fistula after elective gastrectomy for malignant disease: An italian retrospective multicenter study. J. Gastrointest. Surg. 2010, 14, 805–811. [Google Scholar] [CrossRef]
- Cozzaglio, L.; Giovenzana, M.; Biffi, R.; Cobianchi, L.; Coniglio, A.; Framarini, M.; Gerard, L.; Gianotti, L.; Marchet, A.; Mazzaferro, V.; et al. Surgical management of duodenal stump fistula after elective gastrectomy for malignancy: An Italian retrospective multicenter study. Gastric Cancer 2016, 19, 273–279. [Google Scholar] [CrossRef]
- Orsenigo, E.; Bissolati, M.; Socci, C.; Chiari, D.; Muffatti, F.; Nifosi, J.; Staudacher, C. Duodenal stump fistula after gastric surgery for malignancies: A retrospective analysis of risk factors in a single centre experience. Gastric Cancer 2014, 17, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Paik, H.J.; Lee, S.H.; Choi, C.I.; Kim, D.H.; Jeon, T.Y.; Kim, D.H.; Jeon, U.B.; Choi, C.W.; Hwang, S.H. Duodenal stump fistula after gastrectomy for gastric cancer: Risk factors, prevention, and management. Ann. Surg. Treat. Res. 2016, 90, 157–163. [Google Scholar] [CrossRef]
- Ali, B.I.; Park, C.H.; Song, K.Y. Outcomes of Non-Operative Treatment for Duodenal Stump Leakage after Gastrectomy in Patients with Gastric Cancer. J. Gastric Cancer 2016, 16, 28–33. [Google Scholar] [CrossRef]
- Ramos, M.F.K.P.; Pereira, M.A.; Barchi, L.C.; Yagi, O.K.; Dias, A.R.; Szor, D.J.; Zilberstein, B.; Ribeiro-Júnior, U.; Cecconello, I. Duodenal fistula: The most lethal surgical complication in a case series of radical gastrectomy. Int. J. Surg. 2018, 53, 366–370. [Google Scholar] [CrossRef]
- Po Chu Patricia, Y.; Ka Fai Kevin, W.; Fong Yee, L.; Kiu Jing, F.; Kylie, S.; Siu Kee, L. Duodenal stump leakage. Lessons to learn from a large-scale 15-year cohort study. Am. J. Surg. 2020, 220, 976–981. [Google Scholar] [CrossRef]
- Inoue, K.; Michiura, T.; Fukui, J.; Mukaide, H.; Ozaki, T.; Miki, H.; Kobayashi, T.; Oishi, M.; Inada, R.; Matsumoto, T.; et al. Staple-Line Reinforcement of the Duodenal Stump with Intracorporeal Lembert’s Sutures in Laparoscopic Distal Gastrectomy with Roux-en-Y Reconstruction for Gastric Cancer. Surg. Laparosc. Endosc. Percutaneous Tech. 2016, 26, 338–342. [Google Scholar] [CrossRef]
- Kim, S.Y.; Nam, S.H.; Min, J.S.; Kim, M.C. Laparoscopic reinforcement suture on staple-line of duodenal stump using barbed suture during laparoscopic gastrectomy for gastric cancer. Ann. Surg. Treat. Res. 2017, 93, 305–309. [Google Scholar] [CrossRef]
- Kim, M.C.; Kim, S.Y.; Kim, K.W. Laparoscopic Reinforcement Suture (LARS) on Staple Line of Duodenal Stump Using Barbed Suture in Laparoscopic Gastrectomy for Gastric Cancer: A Prospective Single Arm Phase II Study. J. Gastric Cancer 2017, 17, 354–362. [Google Scholar] [CrossRef]
- Ri, M.; Hiki, N.; Ishizuka, N.; Ida, S.; Kumagai, K.; Nunobe, S.; Ohashi, M.; Sano, T. Duodenal stump reinforcement might reduce both incidence and severity of duodenal stump leakage after laparoscopic gastrectomy with Roux-en-Y reconstruction for gastric cancer. Gastric Cancer 2019, 22, 1053–1059. [Google Scholar] [CrossRef]
- He, H.; Li, H.; Ye, B.; Liu, F. Single Purse-String Suture for Reinforcement of Duodenal Stump During Laparoscopic Radical Gastrectomy for Gastric Cancer. Front. Oncol. 2019, 9, 1020. [Google Scholar] [CrossRef]
- Sun, L.; Wang, W.; Zhou, J.; Ji, L.; Zhao, S.; Fu, Y.; Li, R.; Wang, J.; Qian, C.; Sun, Q.; et al. Modified Q-type purse-string suture duodenal stump embedding method for laparoscopic gastrectomy for gastric cancer. BMC Surg. 2024, 24, 123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Z.; Jin, S.; Ju, Y.; Sun, P.; Wei, Y.; Zhu, G.; Wang, K. Double Half Purse-String Sutures Plus “8” Pattern of Stitching for Prevention of Duodenal Stump Fistula after Laparoscopic Gastrectomy. J. Laparoendosc. Adv. Surg. Tech. A 2024, 34, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Cochrane Review Manager (RevMan). Available online: https://training.cochrane.org/online-learning/core-software/revman (accessed on 19 March 2025).
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Deeks, J.J. Chapter 7: Selecting studies and collecting data. In Cochrane Handbook for Systematic Reviews of Interventions; [Version 5.1.0]; Available online: https://handbook-5-1.cochrane.org/chapter_7/table_7_7_a_formulae_for_combining_groups.htm (accessed on 19 March 2025).
- Gu, L.; Zhang, K.; Shen, Z.; Wang, X.; Zhu, H.; Pan, J.; Zhong, X.; Khadaroo, P.A.; Chen, P. Risk Factors for Duodenal Stump Leakage after Laparoscopic Gastrectomy for Gastric Cancer. J. Gastric Cancer 2020, 20, 81–94. [Google Scholar] [CrossRef]
- Sano, A.; Imai, Y.; Yamaguchi, T.; Bamba, T.; Shinno, N.; Kawashima, Y.; Tokunaga, M.; Enokida, Y.; Tsukada, T.; Hatakeyama, S.; et al. Importance of duodenal stump reinforcement to prevent stump leakage after gastrectomy: A large-scale multicenter retrospective study (KSCC DELICATE study). Gastric Cancer 2024, 27, 1320–1330. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M.; Moher, D.; on behalf of the Cochrane Bias Methods Group. Chapter 10: Addressing reporting biases. In Cochrane Handbook for Systematic Reviews of Interventions; [Version 5.1.0]; Available online: https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm (accessed on 19 March 2025).
- Kim, K.H.; Kim, M.C.; Jung, G.J. Risk factors for duodenal stump leakage after gastrectomy for gastric cancer and management technique of stump leakage. Hepatogastroenterology 2014, 61, 1446–1453. [Google Scholar]
- Aurello, P.; Sirimarco, D.; Magistri, P.; Petrucciani, N.; Berardi, G.; Amato, S.; Gasparrini, M.; D’Angelo, F.; Nigri, G.; Ramacciato, G. Management of duodenal stump fistula after gastrectomy for gastric cancer: Systematic review. World J. Gastroenterol. 2015, 21, 7571–7576. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Shao, S.; Wang, T.; Liu, X.; Qin, J. Machine learning-based prediction of duodenal stump leakage following laparoscopic gastrectomy for gastric cancer. Surgery 2025, 180, 108999. [Google Scholar] [CrossRef]
- Du, J.; Xue, H.; Zhao, L.; Zhang, Z.; Hu, J. Handover method: Simple, classic and harmonized intracorporeal closure of stapled duodenal stump during laparoscopic gastrectomy. J. Surg. Oncol. 2021, 124, 41–48. [Google Scholar] [CrossRef]
- Liu, X.; Kong, W.; Ying, R.; Shan, Y.; Yin, G. Reinforcement methods of duodenal stump after laparoscopic gastrectomy for gastric cancer: A review. Heliyon 2023, 9, e17272. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Zhou, Z.; Chen, Y. Reinforcement of the stapled duodenal stump during laparoscopic gastrectomy. Br. J. Surg. 2024, 111, znad305. [Google Scholar] [CrossRef]
- Misawa, K.; Yoshikawa, T.; Ito, S.; Cho, H.; Ito, Y.; Ogata, T. Safety and Feasibility of Linear Stapling Device with Bioabsorbable Polyglycolic Acid Sheet for Duodenal Closure in Gastric Cancer Surgery: A Multi-institutional Phase II Study. World J. Surg. 2019, 43, 192–198. [Google Scholar] [CrossRef]
- Cai, Z.H.; Zang, L.; Yang, H.K.; Kitano, S.; Zheng, M.H. Survey on laparoscopic total gastrectomy at the 11th China-Korea-Japan Laparoscopic Gastrectomy Joint Seminar. Asian J. Endosc. Surg. 2017, 10, 259–267. [Google Scholar] [CrossRef]
- Kostakis, I.D.; Alexandrou, A.; Armeni, E.; Damaskos, C.; Kouraklis, G.; Diamantis, T.; Tsigris, C. Comparison Between Minimally Invasive and Open Gastrectomy for Gastric Cancer in Europe: A Systematic Review and Meta-analysis. Scand. J. Surg. 2017, 106, 3–20. [Google Scholar] [CrossRef]
- Voeten, D.M.; Busweiler, L.A.D.; van der Werf, L.R.; Wijnhoven, B.P.L.; Verhoeven, R.H.A.; van Sandick, J.W.; van Hillegersberg, R.; van Berge Henegouwen, M.I.; Dutch Upper Gastrointestinal Cancer Audit (DUCA) Group. Outcomes of Esophagogastric Cancer Surgery During Eight Years of Surgical Auditing by the Dutch Upper Gastrointestinal Cancer Audit (DUCA). Ann. Surg. 2021, 274, 866–873. [Google Scholar] [CrossRef]
- Salehi, O.; Vega, E.A.; Kutlu, O.C.; James, D.; Alarcon, S.V.; Herrick, B.; Kozyreva, O.; Conrad, C. Western population-based study of oncologic surgical quality and outcomes of laparoscopic versus open gastrectomy for gastric adenocarcinoma. Surg. Endosc. 2021, 35, 4786–4793. [Google Scholar] [CrossRef]
- Baiocchi, G.L.; Giacopuzzi, S.; Reim, D.; Piessen, G.; Costa, P.M.D.; Reynolds, J.V.; Meyer, H.J.; Morgagni, P.; Gockel, I.; Santos, L.L.; et al. Incidence and Grading of Complications After Gastrectomy for Cancer Using the GASTRODATA Registry: A European Retrospective Observational Study. Ann. Surg. 2020, 272, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K. Multicenter Studies: Relevance, Design and Implementation. Indian. Pediatr. 2022, 59, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Chaparro, M. Tips and tricks for successfully conducting a multicenter study. Gastroenterol. Hepatol. 2024, 47, 649–660. [Google Scholar] [CrossRef] [PubMed]
- van den Boorn, H.G.; Engelhardt, E.G.; van Kleef, J.; Sprangers, M.A.G.; van Oijen, M.G.H.; Abu-Hanna, A.; Zwinderman, A.H.; Coupé, V.M.H.; van Laarhoven, H.W.M. Prediction models for patients with esophageal or gastric cancer: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0192310. [Google Scholar] [CrossRef]
- Watanabe, M.; Miyata, H.; Gotoh, M.; Baba, H.; Kimura, W.; Tomita, N.; Nakagoe, T.; Shimada, M.; Kitagawa, Y.; Sugihara, K.; et al. Total gastrectomy risk model: Data from 20,011 Japanese patients in a nationwide internet-based database. Ann. Surg. 2014, 260, 1034–1039. [Google Scholar] [CrossRef]
- Kurita, N.; Miyata, H.; Gotoh, M.; Shimada, M.; Imura, S.; Kimura, W.; Tomita, N.; Baba, H.; Kitagawa, Y.; Sugihara, K.; et al. Risk Model for Distal Gastrectomy When Treating Gastric Cancer on the Basis of Data From 33,917 Japanese Patients Collected Using a Nationwide Web-based Data Entry System. Ann. Surg. 2015, 262, 295–303. [Google Scholar] [CrossRef]
- Pera, M.; Gibert, J.; Gimeno, M.; Garsot, E.; Eizaguirre, E.; Miró, M.; Castro, S.; Miranda, C.; Reka, L.; Leturio, S.; et al. Machine Learning Risk Prediction Model of 90-day Mortality After Gastrectomy for Cancer. Ann. Surg. 2022, 276, 776–783. [Google Scholar] [CrossRef]
- Hong, Q.Q.; Yan, S.; Zhao, Y.L.; Fan, L.; Yang, L.; Zhang, W.B.; Liu, H.; Lin, H.X.; Zhang, J.; Ye, Z.J.; et al. Machine learning identifies the risk of complications after laparoscopic radical gastrectomy for gastric cancer. World J. Gastroenterol. 2024, 30, 79–90. [Google Scholar] [CrossRef]
- Bracale, U.; Corcione, F.; Pignata, G.; Andreuccetti, J.; Dolce, P.; Boni, L.; Cassinotti, E.; Olmi, S.; Uccelli, M.; Gualtierotti, M.; et al. Impact of neoadjuvant therapy followed by laparoscopic radical gastrectomy with D2 lymph node dissection in Western population: A multi-institutional propensity score-matched study. J. Surg. Oncol. 2021, 124, 1338–1346. [Google Scholar] [CrossRef]
- Nelen, S.D.; Bosscha, K.; Lemmens, V.E.P.P.; Hartgrink, H.H.; Verhoeven, R.H.A.; de Wilt, J.H.W.; Dutch Upper Gastrointestinal Cancer Audit group. Morbidity and mortality according to age following gastrectomy for gastric cancer. Br. J. Surg. 2018, 105, 1163–1170. [Google Scholar]
- Ueno, T.; Iida, M.; Yoshino, S.; Takeda, S.; Kubota, H.; Higashida, M.; Oka, Y.; Tsuruta, A.; Matsumoto, H.; Nagano, H. East Versus West: Differences in Surgical Management in Asia Compared with Europe and North America. Surg. Clin. N. Am. 2017, 97, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.D.; Yamashita, H.; Seto, Y. Gastric cancer surgery: Historical background and perspective in Western countries versus Japan. Ann. Transl. Med. 2019, 7, 493. [Google Scholar] [CrossRef] [PubMed]
- van der Wielen, N.; Straatman, J.; Cuesta, M.A.; Daams, F.; van der Peet, D.L. Short-term outcomes in minimally invasive versus open gastrectomy: The differences between East and West. A systematic review of the literature. Gastric Cancer 2018, 21, 19–30. [Google Scholar] [CrossRef]
- Lin, J.X.; Lin, J.P.; Desiderio, J.; Xie, J.W.; Gemini, A.; Wang, J.B.; Lu, J.; Chen, Q.Y.; Cao, L.L.; Lin, M.; et al. Difference in the short-term outcomes of laparoscopic gastrectomy for gastric carcinoma between the east and west: A retrospective study from the IMIGASTRIC trial. J. Cancer 2019, 10, 4106–4113. [Google Scholar] [CrossRef]
- Stillman, M.D.; Yoon, S.S. Open and minimally invasive gastrectomy in Eastern and Western patient populations: A review of the literature and reasons for differences in outcomes. J. Surg. Oncol. 2022, 126, 279–291. [Google Scholar] [CrossRef]
- Ji, J.; Shi, L.; Ying, X.; Lu, X.; Shan, F. Associations of Annual Hospital and Surgeon Volume with Patient Outcomes After Gastrectomy: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2022, 29, 8276–8297. [Google Scholar] [CrossRef]
- Ning, F.L.; Gu, W.J.; Zhao, Z.M.; Du, W.Y.; Sun, M.; Cao, S.Y.; Zeng, Y.J.; Abe, M.; Zhang, C.D. Association between hospital surgical case volume and postoperative mortality in patients undergoing gastrectomy for gastric cancer: A systematic review and meta-analysis. Int. J. Surg. 2023, 109, 936–945. [Google Scholar] [CrossRef]
- Chan, K.S.; Oo, A.M. Establishing the Learning Curve of Laparoscopic and Robotic Distal Gastrectomy: A Systematic Review and Meta-Regression Analysis. J. Gastrointest. Surg. 2023, 27, 2946–2982. [Google Scholar] [CrossRef]
- Chan, K.S.; Oo, A.M. Learning curve of laparoscopic and robotic total gastrectomy: A systematic review and meta-analysis. Surg. Today. 2024, 54, 509–522. [Google Scholar] [CrossRef]
- Zizzo, M.; Zanelli, M.; Sanguedolce, F.; Torricelli, F.; Morini, A.; Tumiati, D.; Mereu, F.; Zuliani, A.L.; Palicelli, A.; Ascani, S.; et al. Robotic versus Laparoscopic Gastrectomy for Gastric Cancer: An Updated Systematic Review. Medicina 2022, 58, 834. [Google Scholar] [CrossRef]
- Kossenas, K.; Moutzouri, O.; Georgopoulos, F. Robotic vs laparoscopic distal gastrectomy with Billroth I and II reconstruction: A systematic review and meta-analysis. J. Robot. Surg. 2024, 19, 30. [Google Scholar] [CrossRef]
- Du, R.; Wan, Y.; Shang, Y.; Lu, G. Robotic Versus Laparoscopic Gastrectomy for Gastric Cancer: The Largest Systematic Reviews of 68,755 Patients and Meta-analysis. Ann. Surg. Oncol. 2025, 32, 351–373. [Google Scholar] [CrossRef]
- Huang, W.; Tang, G.; Sun, H. Robotic vs. laparoscopic gastrectomy for patients with locally advanced gastric cancer: A meta-analysis of randomized controlled trials and propensity-score-matched studies. Int. J. Surg. 2025, 111, 2240–2256. [Google Scholar] [CrossRef]
- Kossenas, K.; Moutzouri, O.; Georgopoulos, F. Evaluating the safety of robotic total gastrectomy with D2 lymphadenectomy for gastric cancer against the conventional laparoscopic approach: A systematic review and meta-analysis. J. Robot. Surg. 2025, 19, 59. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, B.; Huang, Y.; Lu, H.; Luo, R.; Huang, B. Feasibility of laparoscopy gastrectomy for gastric cancer in the patients with high body mass index: A systematic review and meta-analysis. Asian J. Surg. 2020, 43, 69–77. [Google Scholar] [CrossRef]
- Tsekrekos, A.; Lovece, A.; Chrysikos, D.; Ndegwa, N.; Schizas, D.; Kumagai, K.; Rouvelas, I. Impact of obesity on the outcomes after gastrectomy for gastric cancer: A meta-analysis. Asian J. Surg. 2022, 45, 15–26. [Google Scholar] [CrossRef]
- Lou, S.; Yin, X.; Wang, Y.; Zhang, Y.; Xue, Y. Laparoscopic versus open gastrectomy for gastric cancer: A systematic review and meta-analysis of randomized controlled trials. Int. J. Surg. 2022, 102, 106678. [Google Scholar] [CrossRef]
- Aiolfi, A.; Lombardo, F.; Matsushima, K.; Sozzi, A.; Cavalli, M.; Panizzo, V.; Bonitta, G.; Bona, D. Systematic review and updated network meta-analysis of randomized controlled trials comparing open, laparoscopic-assisted, and robotic distal gastrectomy for early and locally advanced gastric cancer. Surgery 2021, 170, 942–951. [Google Scholar] [CrossRef]
- Davey, M.G.; Temperley, H.C.; O’Sullivan, N.J.; Marcelino, V.; Ryan, O.K.; Ryan, É.J.; Donlon, N.E.; Johnston, S.M.; Robb, W.B. Minimally Invasive and Open Gastrectomy for Gastric Cancer: A Systematic Review and Network Meta-Analysis of Randomized Clinical Trials. Ann. Surg. Oncol. 2023, 30, 5544–5557. [Google Scholar] [CrossRef]
- Bittar, V.; Boneli, M.F.; Reis, P.C.A.; Felix, N.; Braga, M.A.P.; Rocha, K.M.; Fogaroli, L.O.; Costa, G.B.; Comini, A.C.; Amaral, G.; et al. Laparoscopic Versus Open Gastrectomy for Advanced Gastric Cancer: A Meta-Analysis of Randomized Controlled Trials. J. Gastrointest. Cancer 2024, 55, 652–661. [Google Scholar] [CrossRef]
- Li, Z.; Bai, B.; Xie, F.; Zhao, Q. Distal versus total gastrectomy for middle and lower-third gastric cancer: A systematic review and meta-analysis. Int. J. Surg. 2018, 53, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, F.; Ma, J.; Zhang, N.; Zhang, C.; Li, G.; Li, Z. Surgical and oncological outcomes of distal gastrectomy compared to total gastrectomy for middle-third gastric cancer: A systematic review and meta-analysis. Oncol. Lett. 2022, 24, 291. [Google Scholar] [CrossRef]

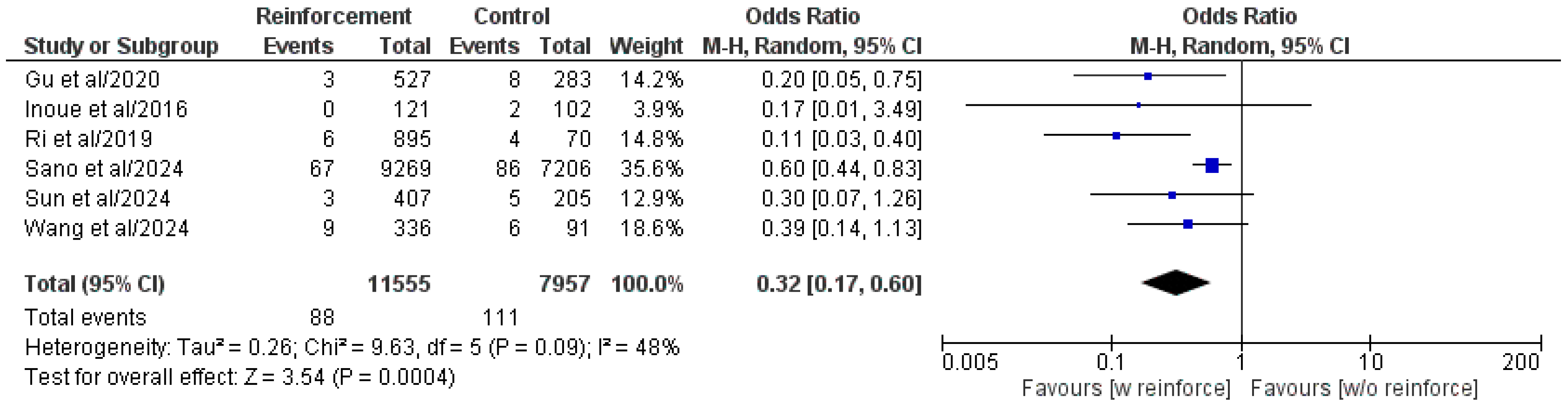

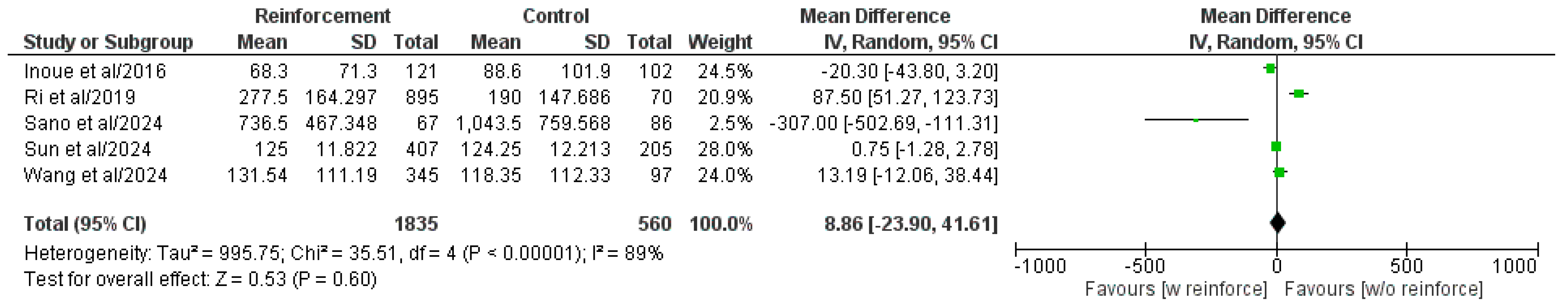
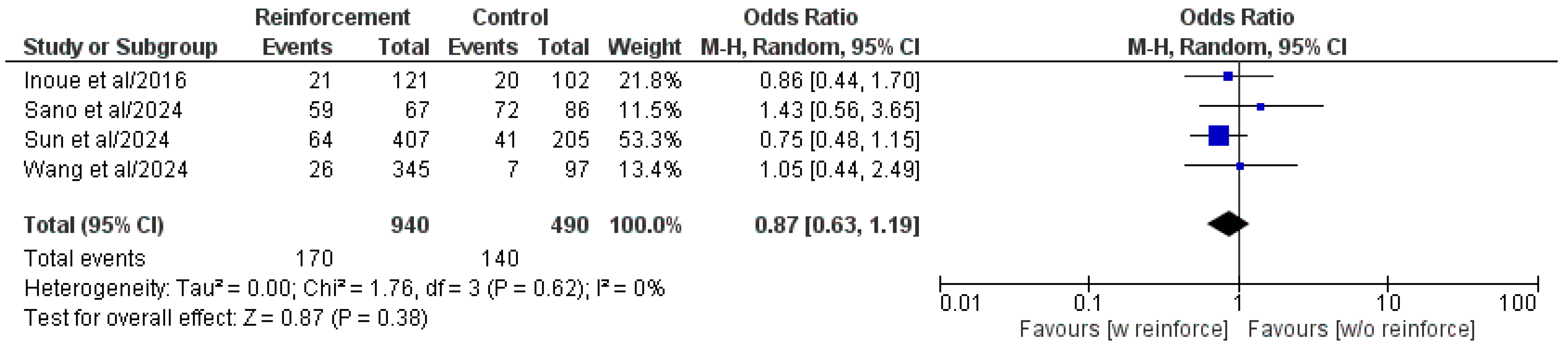
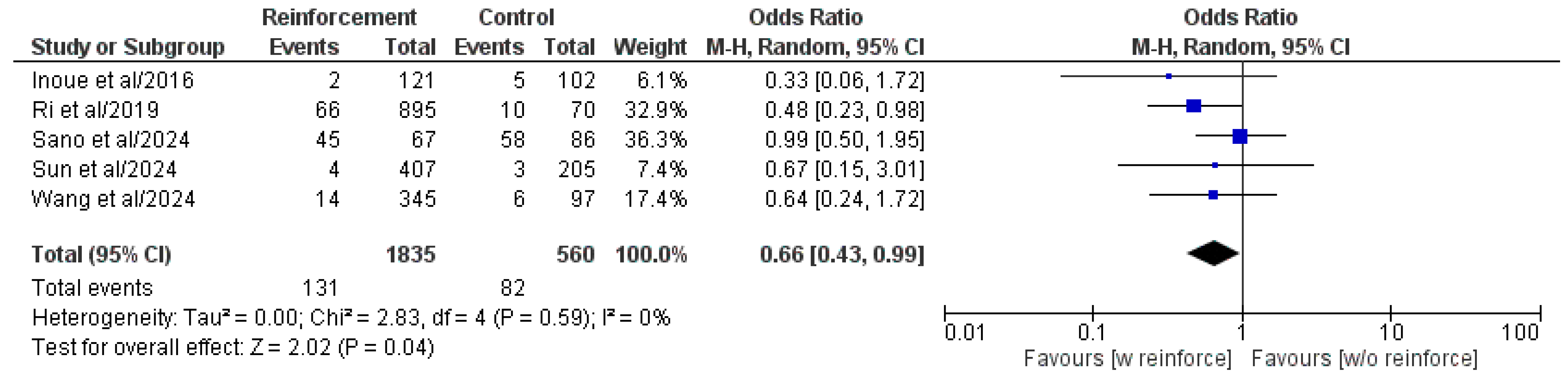
| Authors/Year | Study Type | Study Centers, n | Study Country | Study Period | Patient Population, n | Duodenal Reinforcement, n | DSF, n (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | ||||||||
| Sano et al./2024 [31] | Retrospective | Multicenter | 57 | Japan | 2012–2021 | 16,475 | 9269 | 7206 | 153 (0.93) |
| Wang et al./2024 [22] | Retrospective | Single-center | 1 | China | 2022–2023 | 442 | 345 | 97 | 15 (3.39) |
| Sun et al./2024 [21] | Retrospective | Multicenter | 2 | China | 2019–2023 | 612 | 407 | 205 | 8 (1.31) |
| Gu et al./2020 [30] | Retrospective | Multicenter | 2 | China | 2013–2018 | 810 | 527 | 283 | 11 (1.36) |
| Ri et al./2019 [19] | Retrospective | Single-center | 1 | Japan | 2005–2016 | 965 | 895 | 70 | 10 (1.04) |
| Inoue et al./2016 [16] | Retrospective | Single-center | 1 | Japan | 2009–2014 | 223 | 102 | 121 | 2 (0.89) |
| Authors/Year | Duodenal Reinforcement | Patient Population, n | DSF, n (%) | Gender, n | Age (Years), Mean ± SD | BMI (kg/m2), Mean ± SD | ASA Score, n | Neoadjuvant CT ± RT, n | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | I–II | III–IV | Yes | No | ||||||
| Sano et al./2024 [31] | No | 7206 | 86 (1.19) | 11,729 | 4746 | n/a | n/a | n/a | n/a | 1538 | 14,937 |
| Yes | 9269 | 67 (0.72) | n/a | n/a | n/a | n/a | |||||
| Wang et al./2024 [22] | No | 97 | 6 (6.19) | 71 | 26 | 60.85 ± 8.22 | 22.93 ± 3.07 | 70 | 27 | n/a | |
| Yes | 345 | 9 (2.61) | 243 | 102 | 60.05 ± 10.0089 | 22.746 ± 3.7624 | 261 | 84 | n/a | ||
| Sun et al./2024 [21] | No | 205 | 5 (2.44) | 128 | 253 | 67.90 ± 10.2 | 24.325 ± 0.674 | 174 | 31 | n/a | |
| Yes | 407 | 3 (0.74) | 77 | 154 | 69.22 ± 9.268 | 24.525 ± 0.659 | 322 | 85 | n/a | ||
| Gu et al./2020 [30] | No | 283 | 8 (2.83) | 596 | 214 | 62.5 ± 12.9 | n/a | 761 | 49 | 19 | 791 |
| Yes | 527 | 3 (0.57) | n/a | ||||||||
| Ri et al./2019 [19] | No | 70 | 4 (5.71) | 60 | 10 | 63 ± 9.283 | 24.375 ± 3.101 | 895 | 0 | n/a | |
| Yes | 895 | 6 (0.67) | 626 | 269 | 61.5 ± 10.014 | 24.85 ± 3.849 | 70 | 0 | n/a | ||
| Inoue et al./2016 [16] | No | 102 | 2 (1.96) | 75 | 27 | n/a | 22.9 ± 3.2 | 99 | 3 | n/a | |
| Yes | 121 | 0 | 79 | 42 | n/a | 23.4 ± 3.7 | 109 | 12 | n/a | ||
| Authors/Year | Duodenal Reinforcement | Patient Population, n | Surgical Approach, n | Gastrectomy, n | Lymph Node Dissection, n | Drain | pT, n | pN, n | Stage, n | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Open | Lap | Rob | Distal | Total | D1+ | D2 | Y/N | n | 1 | 2 | 3 | 4 | 0 | ≥1 | I | II | III | IV | |||
| Sano et al./2024 [31] | No | 7206 | 9046 | 6556 | 873 | 7884 | 8591 | 7518 | 8957 | n/a | 6247 | 1759 | 4213 | 4256 | 8362 | 8113 | n/a | ||||
| Yes | 9269 | n/a | |||||||||||||||||||
| Wang et al./2024 [22] | No | 97 | 0 | 97 | 0 | 89 | 8 | n/a | n/a | 21 | 19 | 19 | 38 | 48 | 49 | n/a | |||||
| Yes | 345 | 0 | 345 | 0 | 294 | 51 | n/a | 103 | 56 | 89 | 97 | 160 | 185 | n/a | |||||||
| Sun et al./2024 [21] | No | 205 | 0 | 205 | 0 | 75 | 122 | n/a | n/a | n/a | n/a | 18 | 55 | 108 | 24 | ||||||
| Yes | 407 | 0 | 407 | 0 | 130 | 285 | n/a | n/a | n/a | 45 | 91 | 236 | 35 | ||||||||
| Gu et al./2020 [30] | No | 283 | 0 | 283 | 0 | 480 | 330 | n/a | Y | 1 or 2 | 220 | 98 | 151 | 341 | 353 | 457 | 261 | 172 | 377 | 0 | |
| Yes | 527 | 0 | 527 | 0 | n/a | ||||||||||||||||
| Ri et al./2019 [19] | No | 70 | 0 | 70 | 0 | 32 | 38 | 62 | 8 | n/a | n/a | n/a | n/a | ||||||||
| Yes | 895 | 0 | 895 | 0 | 725 | 170 | 731 | 164 | n/a | n/a | n/a | ||||||||||
| Inoue et al./2016 [16] | No | 102 | 0 | 102 | 0 | 102 | 0 | 70 | 32 | Y | 1 | n/a | n/a | 96 | 6 | 0 | 0 | ||||
| Yes | 121 | 0 | 121 | 0 | 121 | 0 | 82 | 39 | n/a | n/a | 106 | 10 | 5 | 0 | |||||||
| Authors/Year | Duodenal Transection | Reinforcement Method | Suture Thread | |||
|---|---|---|---|---|---|---|
| Stapler | Cartridge Length, mm | Cartridge Closure Height, mm | Absorbable/Non-Absorbable | USP | ||
| Sano et al./2024 [31] | Linear Stapler | n/a | n/a | Unspecified suture | n/a | n/a |
| Reinforced stapler | none | none | ||||
| Wang et al./2024 [22] | Linear Stapler | n/a | n/a | Continuous suture | n/a | n/a |
| Double half purse-string suture plus “8” pattern of stitching | ||||||
| Sun et al./2024 [21] | Linear Stapler | 60 | 3.5 | Interrupted suture | Absorbable | 3-0 |
| Purse-string suture | ||||||
| Gu et al./2020 [30] | Linear Stapler | n/a | n/a | Interrupted suture | n/a | n/a |
| Continuous suture | ||||||
| Semi-pouch suture | ||||||
| Complete-pouch suture | ||||||
| Ri et al./2019 [19] | Linear Stapler | n/a | n/a | Interrupted suture | n/a | n/a |
| Inoue et al./2016 [16] | Linear Stapler | 60 | 2.5 | Interrupted suture | Absorbable | 3-0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zizzo, M.; Morini, A.; Zanelli, M.; Broggi, G.; Sanguedolce, F.; Koufopoulos, N.I.; Palicelli, A.; Mangone, L.; Fabozzi, M.; Giuffrida, M.; et al. Impact of Duodenal Stump Reinforcement in Preventing Duodenal Stump Fistula/Leakage After Distal or Total Gastrectomy for Malignant Disease: A Meta-Analysis of Comparative Studies. Cancers 2025, 17, 1735. https://doi.org/10.3390/cancers17111735
Zizzo M, Morini A, Zanelli M, Broggi G, Sanguedolce F, Koufopoulos NI, Palicelli A, Mangone L, Fabozzi M, Giuffrida M, et al. Impact of Duodenal Stump Reinforcement in Preventing Duodenal Stump Fistula/Leakage After Distal or Total Gastrectomy for Malignant Disease: A Meta-Analysis of Comparative Studies. Cancers. 2025; 17(11):1735. https://doi.org/10.3390/cancers17111735
Chicago/Turabian StyleZizzo, Maurizio, Andrea Morini, Magda Zanelli, Giuseppe Broggi, Francesca Sanguedolce, Nektarios I. Koufopoulos, Andrea Palicelli, Lucia Mangone, Massimiliano Fabozzi, Mario Giuffrida, and et al. 2025. "Impact of Duodenal Stump Reinforcement in Preventing Duodenal Stump Fistula/Leakage After Distal or Total Gastrectomy for Malignant Disease: A Meta-Analysis of Comparative Studies" Cancers 17, no. 11: 1735. https://doi.org/10.3390/cancers17111735
APA StyleZizzo, M., Morini, A., Zanelli, M., Broggi, G., Sanguedolce, F., Koufopoulos, N. I., Palicelli, A., Mangone, L., Fabozzi, M., Giuffrida, M., Bonelli, C., & Marchesi, F. (2025). Impact of Duodenal Stump Reinforcement in Preventing Duodenal Stump Fistula/Leakage After Distal or Total Gastrectomy for Malignant Disease: A Meta-Analysis of Comparative Studies. Cancers, 17(11), 1735. https://doi.org/10.3390/cancers17111735








