KAT/3BP: A Metabolism-Targeting Agent with Single and Combination Activity in Aggressive B-Cell Lymphomas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Compounds
2.3. In Vitro Cytotoxic Activity
2.4. Cell Cycle and Apoptosis Assessment
2.5. In Vivo Syngeneic Mouse Models
2.6. Statistical Analysis
3. Results
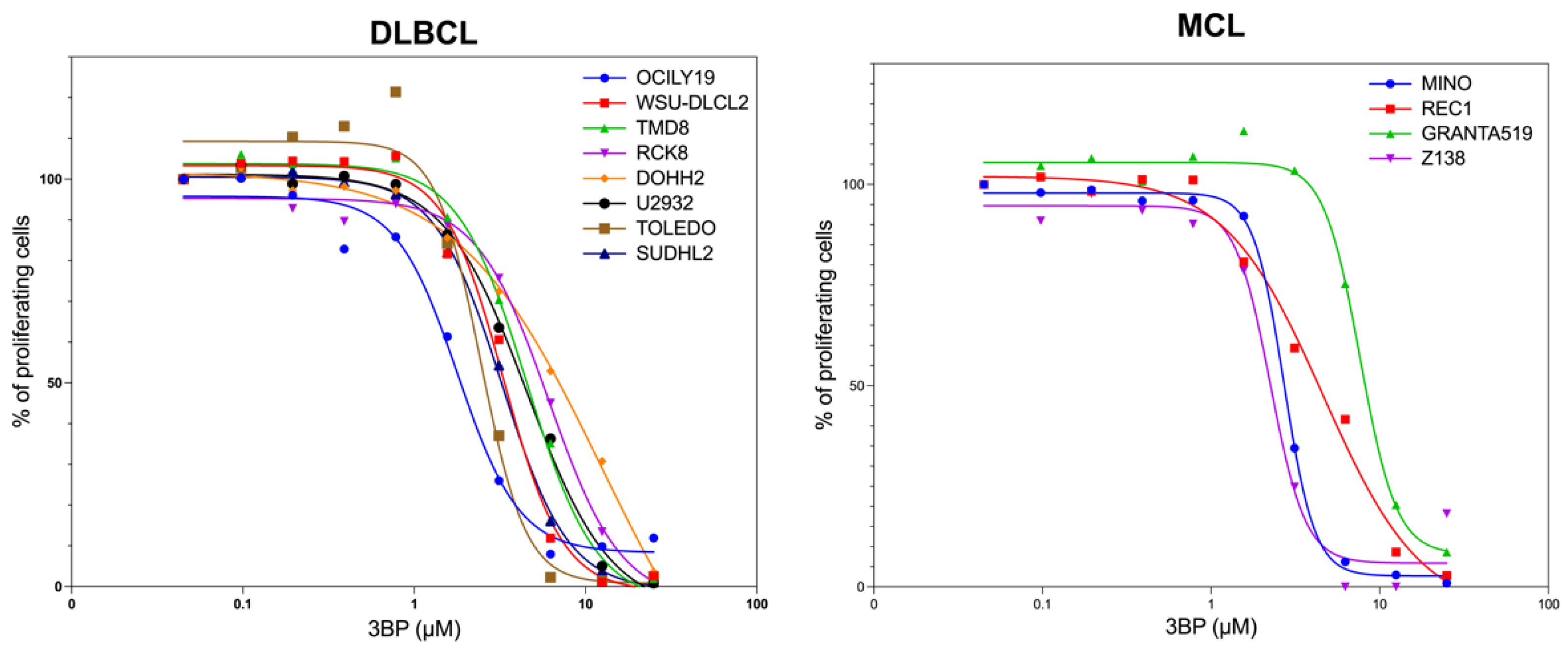
3.1. KAT/3BP as a Single Agent Is Cytotoxic in Lymphoma Cell Lines
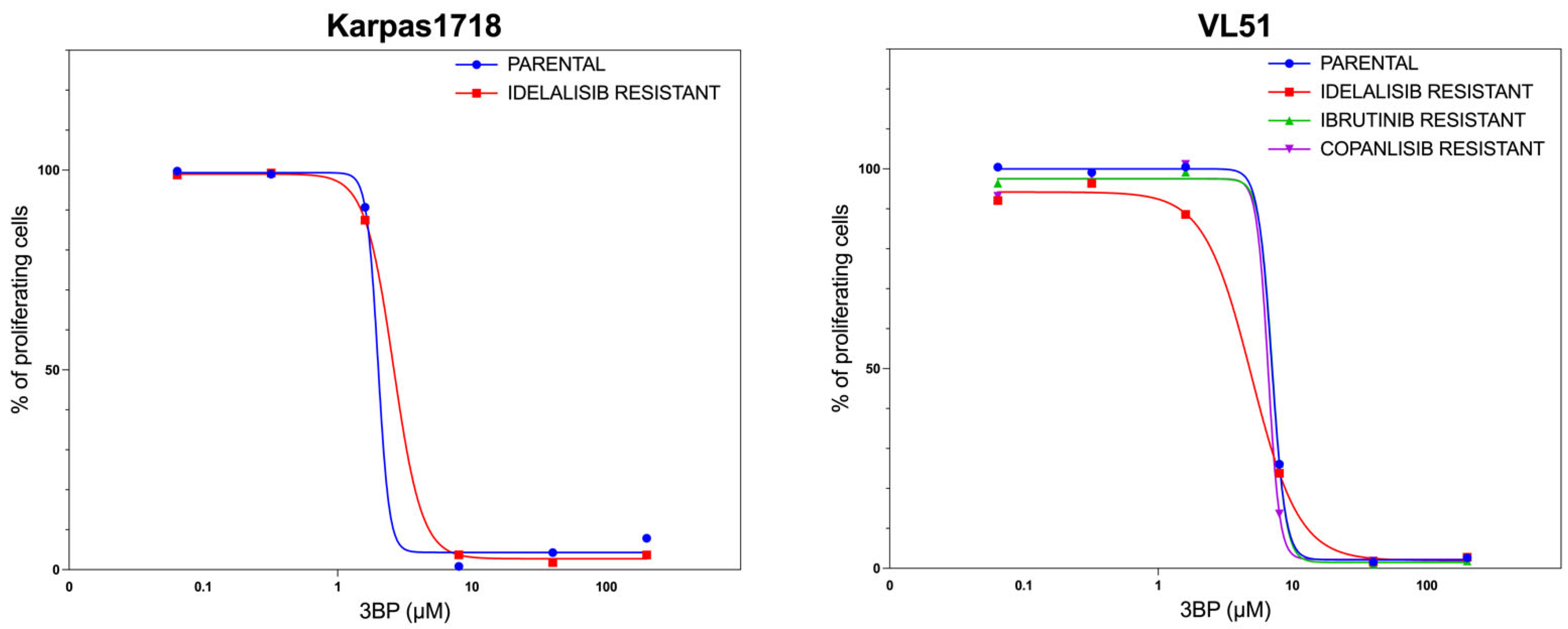
3.2. KAT/3BP as a Single Agent Exerts Anti-Lymphoma Activity in Models of Secondary Resistance to FDA-Approved Agents
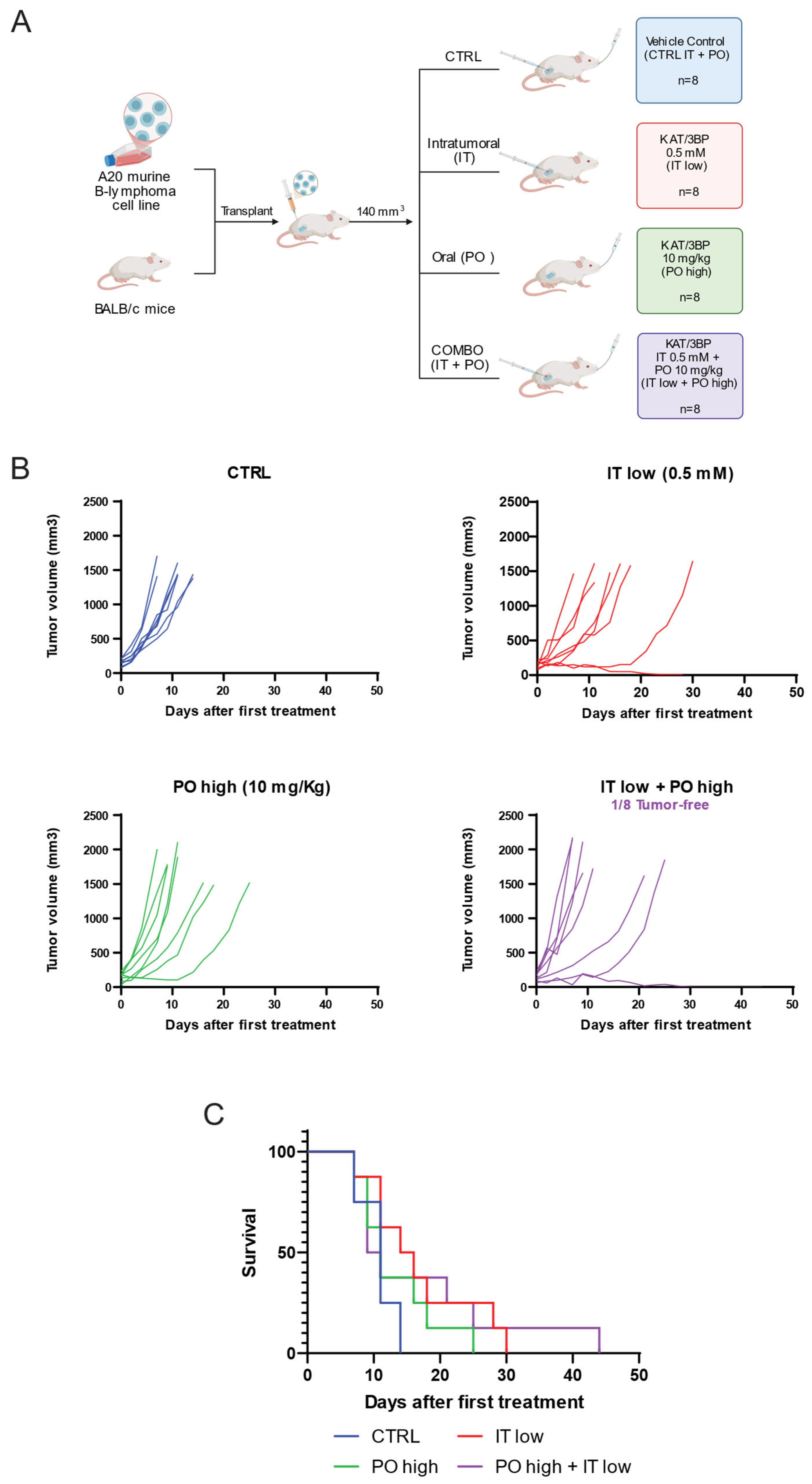
3.3. KAT/3BP Has In Vivo Antitumor Activity in a Syngeneic Mouse Model
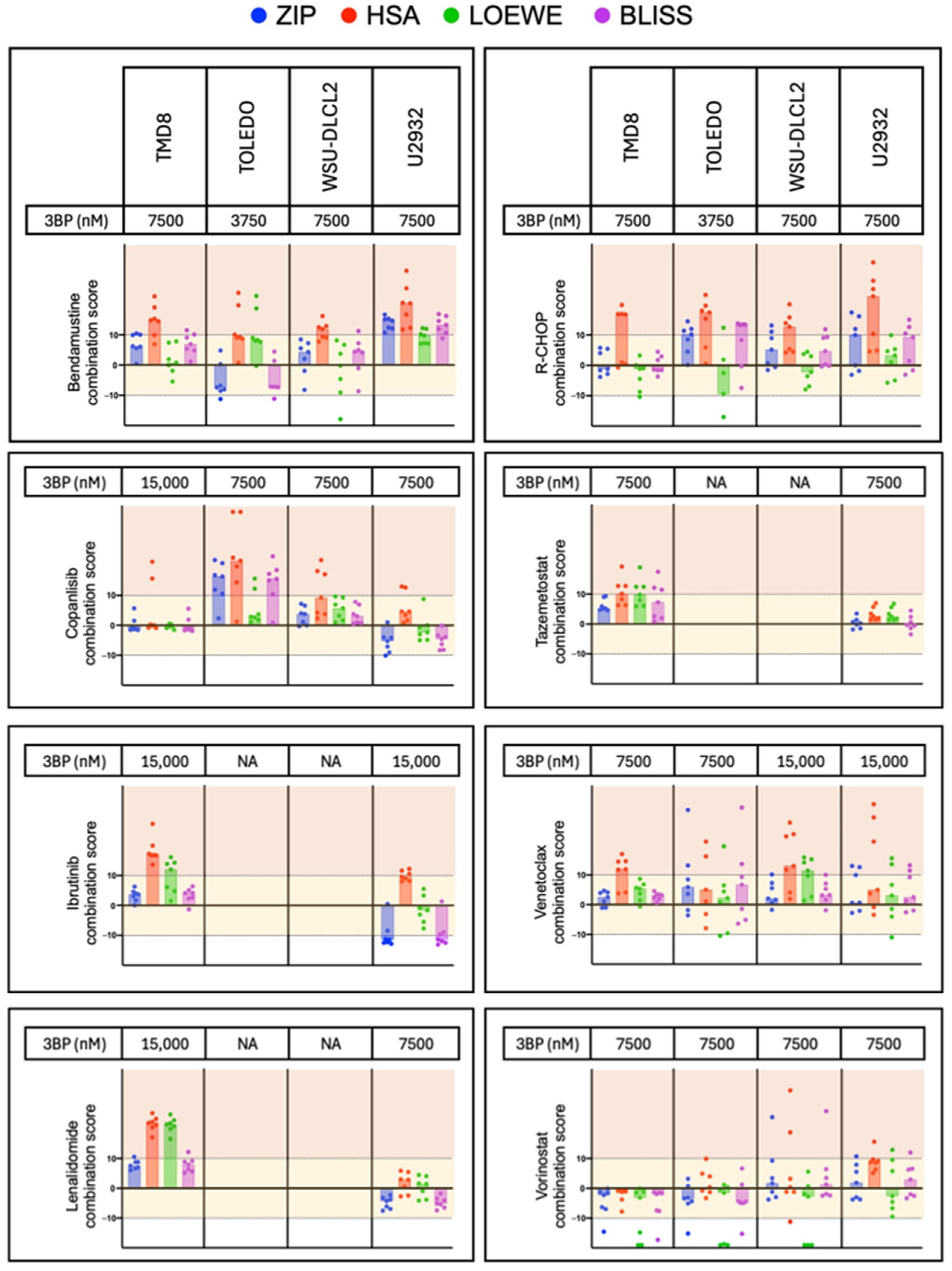
3.4. KAT/3BP-Based Combinations Are Active in Lymphoma Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teicher, B.A.; Linehan, W.M.; Helman, L.J. Targeting Cancer Metabolism. Clin. Cancer Res. 2012, 18, 5537–5545. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.L. 3-bromopyruvate (3BP) a fast acting, promising, powerful, specific, and effective “small molecule” anti-cancer agent taken from labside to bedside: Introduction to a special issue. J. Bioenerg. Biomembr. 2012, 44, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cardaci, S.; Desideri, E.; Ciriolo, M.R. Targeting aerobic glycolysis: 3-bromopyruvate as a promising anticancer drug. J. Bioenerg. Biomembr. 2012, 44, 17–29. [Google Scholar] [CrossRef]
- Rodrigues-Ferreira, C.; da Silva, A.P.P.; Galina, A. Effect of the antitumoral alkylating agent 3-bromopyruvate on mitochondrial respiration: Role of mitochondrially bound hexokinase. J. Bioenerg. Biomembr. 2012, 44, 39–49. [Google Scholar] [CrossRef]
- Shoshan, M.C. 3-bromopyruvate: Targets and outcomes. J. Bioenerg. Biomembr. 2012, 44, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Azevedo-Silva, J.; Queirós, O.; Baltazar, F.; Ułaszewski, S.; Goffeau, A.; Ko, Y.H.; Pedersen, P.L.; Preto, A.; Casal, M. The anticancer agent 3-bromopyruvate: A simple but powerful molecule taken from the lab to the bedside. J. Bioenerg. Biomembr. 2016, 48, 349–362. [Google Scholar] [CrossRef]
- Majkowska-Skrobek, G.; Augustyniak, D.; Lis, P.; Bartkowiak, A.; Gonchar, M.; Ko, Y.H.; Pedersen, P.L.; Goffeau, A.; Ułaszewski, S. Killing multiple myeloma cells with the small molecule 3-bromopyruvate: Implications for therapy. Anticancer Drugs. 2014, 25, 673–682. [Google Scholar] [CrossRef]
- Chapiro, J.; Sur, S.; Savic, L.J.; Ganapathy-Kanniappan, S.; Reyes, J.; Duran, R.; Thiruganasambandam, S.C.; Moats, C.R.; Lin, M.; Luo, W.; et al. Systemic delivery of microencapsulated 3-bromopyruvate for the therapy of pancreatic cancer. Clin. Cancer Res. 2014, 20, 6406–6417. [Google Scholar] [CrossRef]
- Gan, L.; Xiu, R.; Ren, P.; Yue, M.; Su, H.; Guo, G.; Xiao, D.; Yu, J.; Jiang, H.; Liu, H.; et al. Metabolic targeting of oncogene MYC by selective activation of the proton-coupled monocarboxylate family of transporters. Oncogene 2016, 35, 3037–3048. [Google Scholar] [CrossRef]
- Pichla, M.; Sroka, J.; Pienkowska, N.; Piwowarczyk, K.; Madeja, Z.; Bartosz, G.; Sadowska-Bartosz, I. Metastatic prostate cancer cells are highly sensitive to 3-bromopyruvic acid. Life Sci. 2019, 227, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Skaripa-Koukelli, I.; Hauton, D.; Walsby-Tickle, J.; Thomas, E.; Owen, J.; Lakshminarayanan, A.; Able, S.; McCullagh, J.; Carlisle, R.C.; Vallis, K.A. 3-Bromopyruvate-mediated MCT1-dependent metabolic perturbation sensitizes triple negative breast cancer cells to ionizing radiation. Cancer Metab. 2021, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Sołek, P.; Mytych, J.; Łannik, E.; Majchrowicz, L.; Koszła, O.; Koziorowska, A.; Koziorowski, M. Cancer on-target: Selective enhancement of 3-bromopyruvate action by an electromagnetic field in vitro. Free Radic. Biol. Med. 2022, 180, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Zhang, Q.; Zhao, C.; Li, X.; Chen, Z.; Sun, X.; Yu, J. 3-Bromopyruvate overcomes cetuximab resistance in human colorectal cancer cells by inducing autophagy-dependent ferroptosis. Cancer Gene Ther. 2023, 30, 1414–1425. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Lu, W.; Huang, P. Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim. Biophys. Acta 2009, 1787, 553–560. [Google Scholar] [CrossRef]
- Schaefer, N.G.; Geschwind, J.F.; Engles, J.; Buchanan, J.W.; Wahl, R.L. Systemic administration of 3-bromopyruvate in treating disseminated aggressive lymphoma. Transl. Res. 2012, 159, 51–57. [Google Scholar] [CrossRef]
- Yadav, S.; Pandey, S.K.; Goel, Y.; Kujur, P.K.; Maurya, B.N.; Verma, A.; Kumar, A.; Singh, R.P.; Singh, S.M. Protective and recuperative effects of 3-bromopyruvate on immunological, hepatic and renal homeostasis in a murine host bearing ascitic lymphoma: Implication of niche dependent differential roles of macrophages. Biomed. Pharmacother. 2018, 99, 970–985. [Google Scholar] [CrossRef]
- Yadav, S.; Pandey, S.K.; Kumar, A.; Kujur, P.K.; Singh, R.P.; Singh, S.M. Antitumor and chemosensitizing action of 3-bromopyruvate: Implication of deregulated metabolism. Chem.Biol. Interact. 2017, 270, 73–89. [Google Scholar] [CrossRef]
- El Sayed, S.M. Enhancing anticancer effects, decreasing risks and solving practical problems facing 3-bromopyruvate in clinical oncology: 10 years of research experience. Int. J. Nanomed. 2018, 13, 4699–4709. [Google Scholar] [CrossRef]
- Feldwisch-Drentrup, H. Candidate cancer drug suspected after death of three patients at an alternative medicine clinic. Science 2016. [Google Scholar] [CrossRef]
- Ko, Y.H.; Verhoeven, H.A.; Lee, M.J.; Corbin, D.J.; Vogl, T.J.; Pedersen, P.L. A translational study “case report” on the small molecule “energy blocker” 3-bromopyruvate (3BP) as a potent anticancer agent: From bench side to bedside. J. Bioenerg. Biomembr. 2012, 44, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, K.; Jaber, S.; Nahid, M.N.; Underwood, K.; Beheshti, A.; Landon, A.; Bhandary, B.; Bastian, P.; Evens, A.M.; Haley, J.; et al. Role of hypoxia in Diffuse Large B-cell Lymphoma: Metabolic repression and selective translation of HK2 facilitates development of DLBCL. Sci. Rep. 2018, 8, 744. [Google Scholar]
- Noble, R.A.; Bell, N.; Blair, H.; Sikka, A.; Thomas, H.; Phillips, N.; Nakjang, S.; Miwa, S.; Crossland, R.; Rand, V.; et al. Inhibition of monocarboxyate transporter 1 by AZD3965 as a novel therapeutic approach for diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica 2017, 102, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Wennerberg, K.; Aittokallio, T.; Tang, J. Searching for Drug Synergy in Complex Dose-Response Landscapes Using an Interaction Potency Model. Comput. Struct. Biotechnol. J. 2015, 13, 504–513. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, W.; Aldahdooh, J.; Malyutina, A.; Shadbahr, T.; Tanoli, Z.; Pessia, A.; Tang, J. SynergyFinder Plus: Toward Better Interpretation and Annotation of Drug Combination Screening Datasets. Genom. Proteom. Bioinform. 2022, 20, 587–596. [Google Scholar] [CrossRef]
- Golay, J.; Semenzato, G.; Rambaldi, A.; Foà, R.; Gaidano, G.; Gamba, E.; Pane, F.; Pinto, A.; Specchia, G.; Zaja, F.; et al. Lessons for the clinic from rituximab pharmacokinetics and pharmacodynamics. MAbs 2013, 5, 826–837. [Google Scholar] [CrossRef]
- Habermann, T.M.; Weller, E.A.; Morrison, V.A.; Gascoyne, R.D.; Cassileth, P.A.; Cohn, J.B.; Dakhil, S.R.; Woda, B.; Fisher, R.I.; Peterson, B.A.; et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J. Clin. Oncol. 2006, 24, 3121–3127. [Google Scholar] [CrossRef]
- de Jong, M.R.W.; Langendonk, M.; Reitsma, B.; Nijland, M.; Berg, A.v.D.; Ammatuna, E.; Visser, L.; van Meerten, T. Heterogeneous Pattern of Dependence on Anti-Apoptotic BCL-2 Family Proteins upon CHOP Treatment in Diffuse Large B-Cell Lymphoma. Int. J. Mol. Sci. 2019, 20, 6036. [Google Scholar] [CrossRef]
- Arribas, A.J.; Napoli, S.; Cascione, L.; Sartori, G.; Barnabei, L.; Gaudio, E.; Tarantelli, C.; Mensah, A.A.; Spriano, F.; Zucchetto, A.; et al. Resistance to PI3Kdelta inhibitors in marginal zone lymphoma can be reverted by targeting the IL-6/PDGFRA axis. Haematologica 2022, 107, 2685–2697. [Google Scholar] [CrossRef]
- Arribas, A.J.; Napoli, S.; Cascione, L.; Barnabei, L.; Sartori, G.; Cannas, E.; Gaudio, E.; Tarantelli, C.; Mensah, A.A.; Spriano, F.; et al. ERBB4-Mediated Signaling Is a Mediator of Resistance to PI3K and BTK Inhibitors in B-cell Lymphoid Neoplasms. Mol. Cancer Ther. 2024, 23, 368–380. [Google Scholar] [CrossRef]
- Arribas, A.J.; Guidetti, F.; Cannas, E.; Cascione, L.; Napoli, S.; Sartori, G.; Fuzio, F.; Pesenti, E.; Tarantelli, C.; Spriano, F.; et al. IL-16 production is a mechanism of resistance to BTK inhibitors and R-CHOP in lymphomas. bioRxiv 2025. bioRxiv:2025.05.07.652612. [Google Scholar]
- Arribas, A.; Napoli, S.; Cascione, L.; Gaudio, E.; Bordone-Pittau, R.; Barreca, M.; Sartori, G.; Chiara, T.; Spriano, F.; Rinaldi, A.; et al. Secondary resistance to the PI3K inhibitor copanlisib in marginal zone lymphoma. Eur. J. Cancer 2020, 138, S40. [Google Scholar] [CrossRef]
- Silkenstedt, E.; Salles, G.; Campo, E.; Dreyling, M. B-cell non-Hodgkin lymphomas. Lancet 2024, 403, 1791–1807. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef]
- Armitage, J.O.; Longo, D.L. Mantle-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 2495–2506. [Google Scholar] [CrossRef]
- Tarantelli, C.; Wald, D.; Munz, N.; Spriano, F.; Bruscaggin, A.; Cannas, E.; Cascione, L.; Gaudio, E.; Arribas, A.J.; Manjappa, S.; et al. Targeting CD19-positive lymphomas with the antibodydrug conjugate loncastuximab tesirine: Preclinical evidence of activity as a single agent and in combination therapy. Haematologica 2024, 109, 3314–3326. [Google Scholar] [CrossRef]
- Kawasaki, N.; Nishito, Y.; Yoshimura, Y.; Yoshiura, S. The molecular rationale for the combination of polatuzumab vedotin plus rituximab in diffuse large B-cell lymphoma. Br. J. Haematol. 2022, 199, 245–255. [Google Scholar] [CrossRef]
- Simone, B.A.; Champ, C.E.; Rosenberg, A.L.; Berger, A.C.; A Monti, D.; P Dicker, A.; Simone, N.L. Selectively starving cancer cells through dietary manipulation: Methods and clinical implications. Future Oncol. 2013, 9, 959–976. [Google Scholar] [CrossRef]
- O’Flanagan, C.H.; Smith, L.A.; McDonell, S.B.; Hursting, S.D. When less may be more: Calorie restriction and response to cancer therapy. BMC Med. 2017, 15, 106. [Google Scholar] [CrossRef]
- Antunes, F.; Erustes, A.G.; Costa, A.J.; Nascimento, A.C.; Bincoletto, C.; Ureshino, R.P.; Pereira, G.J.S.; Smaili, S.S. Autophagy and intermittent fasting: The connection for cancer therapy? Clinics 2018, 73 (Suppl. S1), e814s. [Google Scholar] [CrossRef]
- Qin, J.Z.; Xin, H.; Nickoloff, B.J. 3-Bromopyruvate induces necrotic cell death in sensitive melanoma cell lines. Biochem. Biophys. Res. Commun. 2010, 396, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Calviño, E.; Estañ, M.C.; Sánchez-Martín, C.; Brea, R.; de Blas, E.; Boyano-Adánez, M.d.C.; Rial, E.; Aller, P. Regulation of death induction and chemosensitizing action of 3-bromopyruvate in myeloid leukemia cells: Energy depletion, oxidative stress, and protein kinase activity modulation. J. Pharmacol. Exp. Ther. 2014, 348, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Valenti, D.; Vacca, R.A.; de Bari, L. 3-Bromopyruvate induces rapid human prostate cancer cell death by affecting cell energy metabolism, GSH pool and the glyoxalase system. J. Bioenerg. Biomembr. 2015, 47, 493–506. [Google Scholar] [CrossRef] [PubMed]
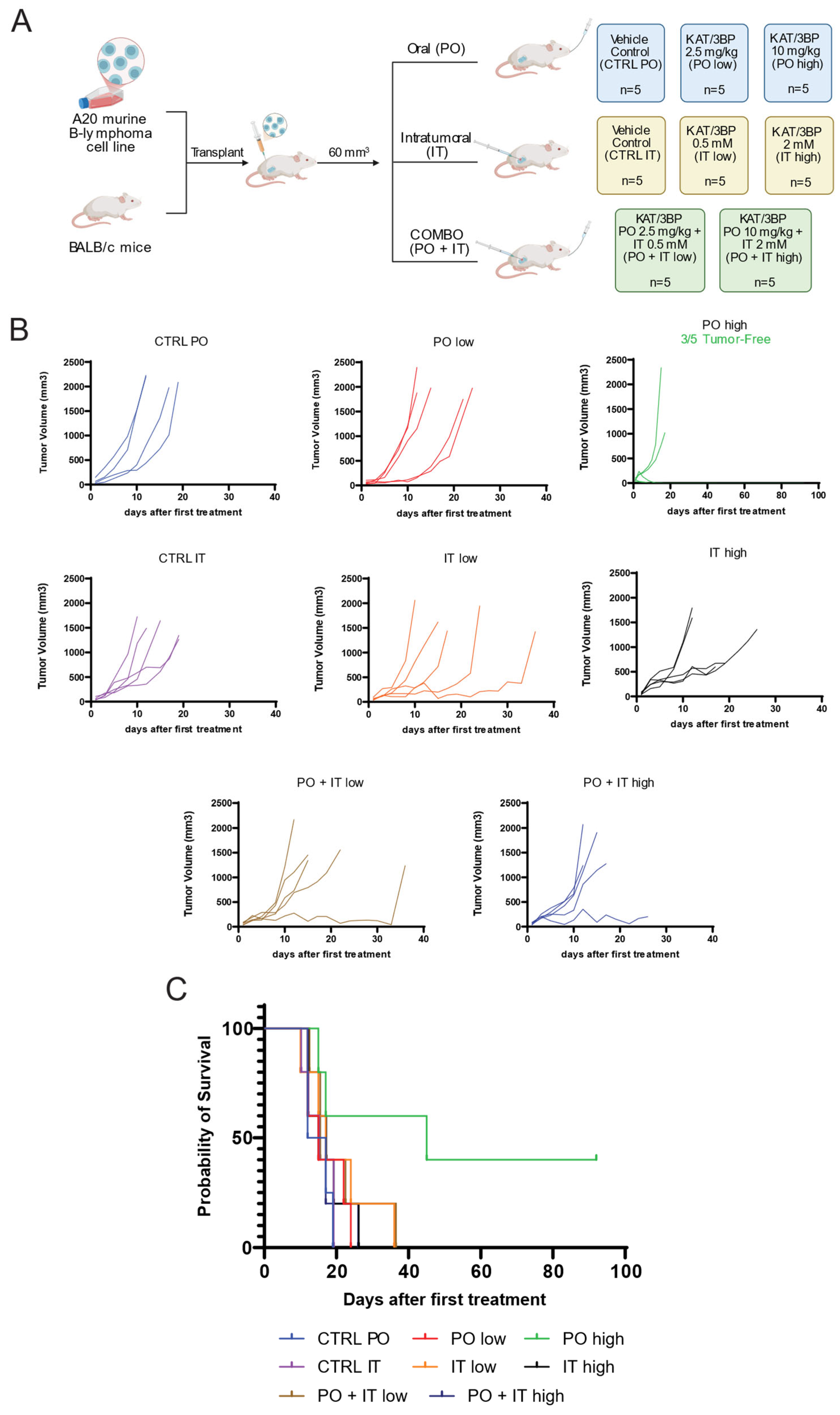
| CTRL PO | PO Low | PO High | CTRL IT | IT Low | IT High | PO + IT Low | PO + IT High | |
|---|---|---|---|---|---|---|---|---|
| Best-fit values Slope | 101.8 | 67.84 | −2.610 | 68.24 | 18.71 | 39.26 | 13.95 | 22.39 |
| CTRL PO | PO High | IT Low | PO High + IT Low | |
|---|---|---|---|---|
| Best-fit values Slope | 104.2 | 19.80 | 54.30 | −0.3001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarantelli, C.; Spriano, F.; Civanelli, E.; Aresu, L.; Risi, G.; Cannas, E.; Kayali, O.; Cascione, L.; Arribas, A.J.; Stathis, A.; et al. KAT/3BP: A Metabolism-Targeting Agent with Single and Combination Activity in Aggressive B-Cell Lymphomas. Cancers 2025, 17, 2034. https://doi.org/10.3390/cancers17122034
Tarantelli C, Spriano F, Civanelli E, Aresu L, Risi G, Cannas E, Kayali O, Cascione L, Arribas AJ, Stathis A, et al. KAT/3BP: A Metabolism-Targeting Agent with Single and Combination Activity in Aggressive B-Cell Lymphomas. Cancers. 2025; 17(12):2034. https://doi.org/10.3390/cancers17122034
Chicago/Turabian StyleTarantelli, Chiara, Filippo Spriano, Elisa Civanelli, Luca Aresu, Giorgia Risi, Eleonora Cannas, Omar Kayali, Luciano Cascione, Alberto J. Arribas, Anastasios Stathis, and et al. 2025. "KAT/3BP: A Metabolism-Targeting Agent with Single and Combination Activity in Aggressive B-Cell Lymphomas" Cancers 17, no. 12: 2034. https://doi.org/10.3390/cancers17122034
APA StyleTarantelli, C., Spriano, F., Civanelli, E., Aresu, L., Risi, G., Cannas, E., Kayali, O., Cascione, L., Arribas, A. J., Stathis, A., Ko, Y. H., & Bertoni, F. (2025). KAT/3BP: A Metabolism-Targeting Agent with Single and Combination Activity in Aggressive B-Cell Lymphomas. Cancers, 17(12), 2034. https://doi.org/10.3390/cancers17122034








