A Pragmatic Grouping Model for Bone-Only De Novo Metastatic Breast Cancer (MetS Protocol MF22-03) †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
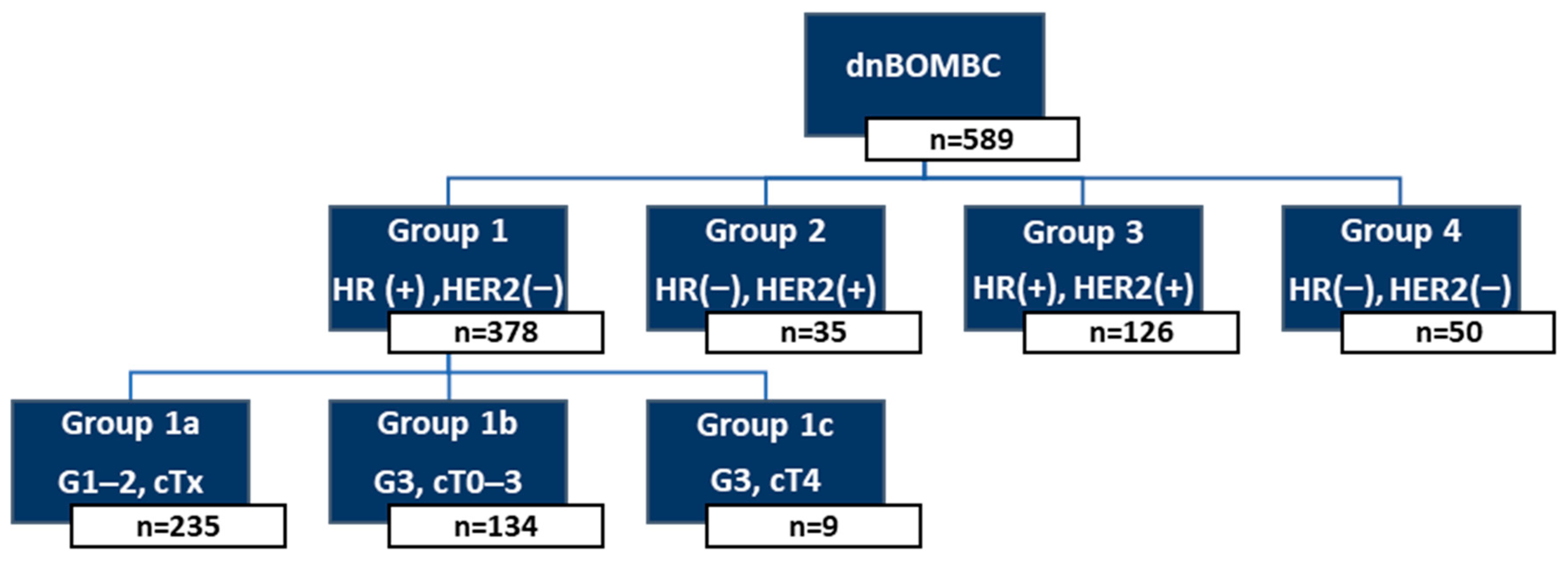
2.1. Patient Classification and Risk Grouping
- Group 1a: HR(+)/HER2(−), Grade 1–2, any cT stage.
- Group 1b: HR(+)/HER2(−), Grade 3, cT0–3.
- Group 1c: HR(+)/HER2(−), Grade 3, cT4.
- Group 2: HR(−)/HER2(+).
- Group 3: HR(+)/HER2(+).
- Group 4: HR(−)/HER2(−) (TN).
2.2. Statistical Analysis
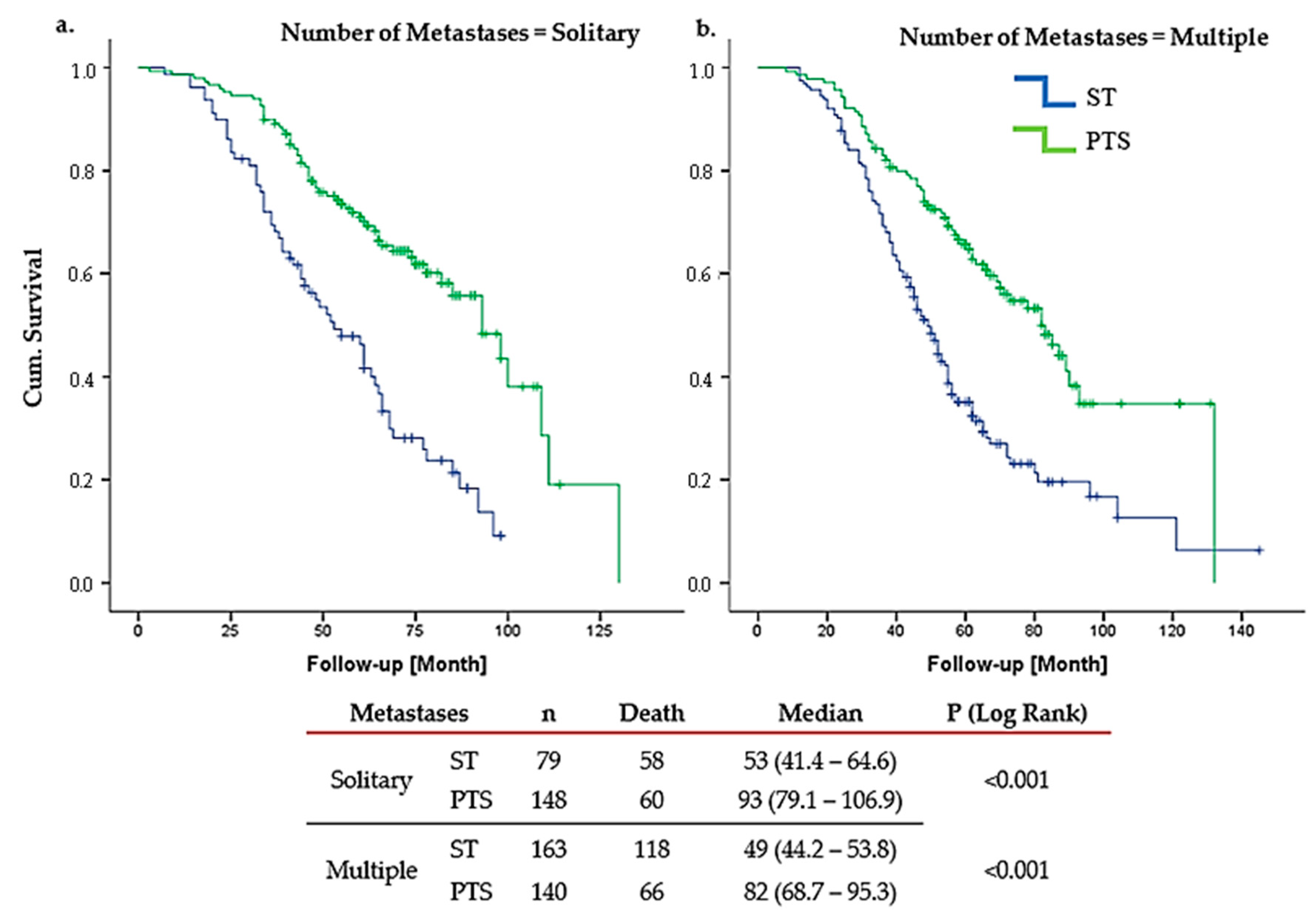
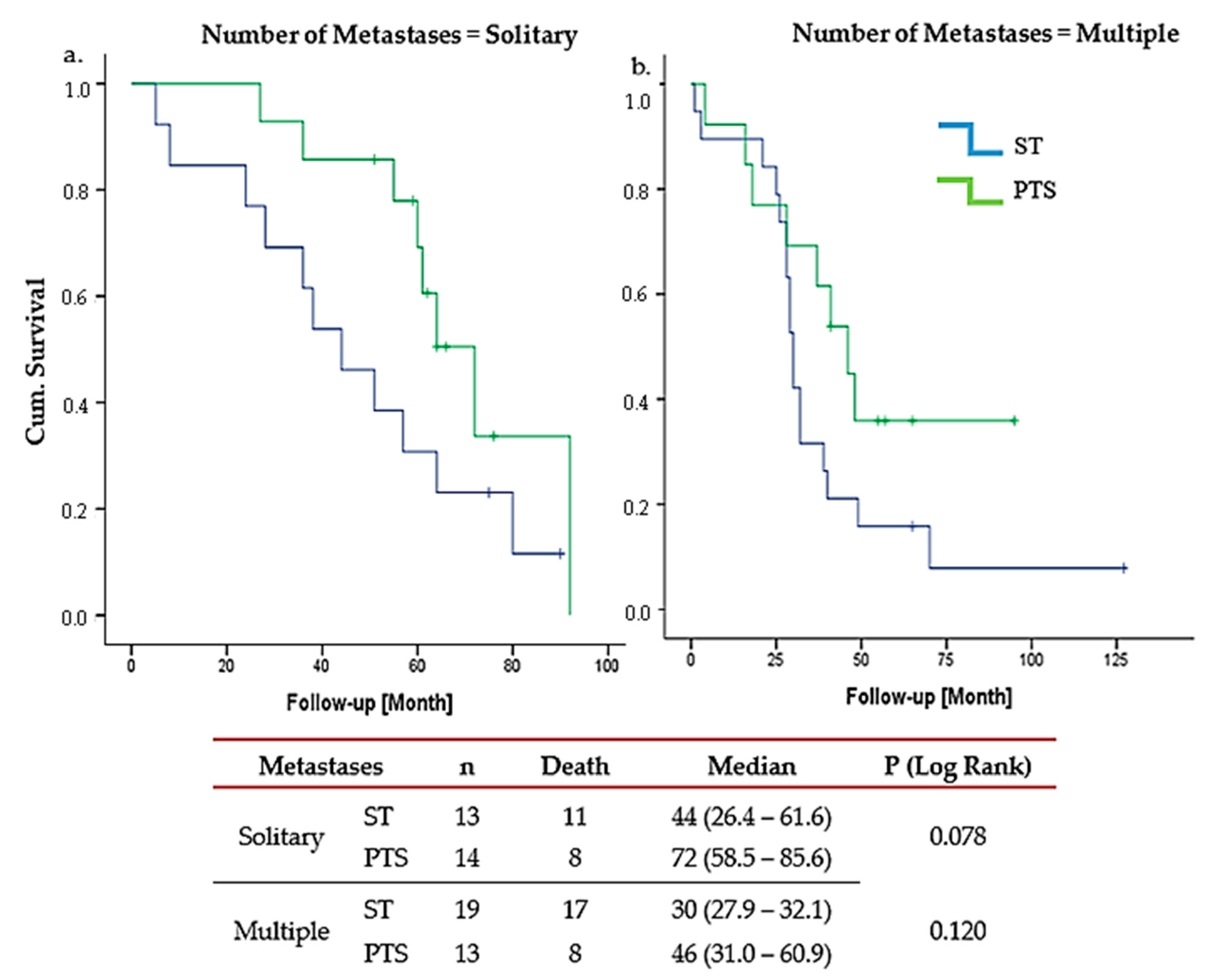
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OS | Overall survival |
| LRT | Locoregional therapy |
| dnMBC | De novo metastatic breast cancer |
| cT | clinical T |
| HER2 | Human epidermal human Epidermal growth factor Receptor 2 |
| mFT | Median follow-up time |
| GrA | Group A |
| HR | Hormone receptor |
| GrB | Group B |
| HoD | The hazard of death |
| PTS | Primary tumor surgery |
| ST | Systemic therapy |
| BC | Breast cancer |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| MBC | Metastatic breast cancer |
| RT | Radiotherapy |
| AJCC | American Joint Committee on Cancer |
| NCDB | National Cancer Database |
| SEER | Surveillance, Epidemiology, and End Results |
| IHC | Immunohistochemistry |
| CI | Confidence intervals |
| BOM | Bone-only metastatic |
| LPFS | Locoregional progression-free survival |
| PFS | Progression-free survival |
| TN | Triple-negative |
References
- Taskindoust, M.; Thomas, S.M.; Sammons, S.L.; Fayanju, O.M.; DiLalla, G.; Hwang, E.S.; Plichta, J.K. Survival Outcomes Among Patients with Metastatic Breast Cancer: Review of 47,000 Patients. Ann. Surg. Oncol. 2021, 28, 7441–7449. [Google Scholar] [CrossRef] [PubMed]
- Barcenas, C.H.; Song, J.; Murthy, R.K.; Raghavendra, A.S.; Li, Y.; Hsu, L.; Carlson, R.W.; Tripathy, D.; Hortobagyi, G.N. Prognostic Model for De Novo and Recurrent Metastatic Breast Cancer. JCO Clin. Cancer Inform. 2021, 5, 789–804. [Google Scholar] [CrossRef]
- Leone, B.A.; Vallejo, C.T.; Romero, A.O.; Machiavelli, M.R.; Pérez, J.E.; Leone, J.; Leone, J.P. Prognostic impact of metastatic pattern in stage IV breast cancer at initial diagnosis. Breast Cancer Res. Treat. 2017, 161, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Press, D.J.; Miller, M.E.; Liederbach, E.; Yao, K.; Huo, D. De novo metastasis in breast cancer: Occurrence and overall survival stratified by molecular subtype. Clin. Exp. Metastasis 2017, 34, 457–465. [Google Scholar] [CrossRef]
- Soran, A.; Ozmen, V.; Ozbas, S.; Karanlik, H.; Muslumanoglu, M.; Igci, A.; Canturk, N.Z.; Utkan, Z.; Evrensel, T.; Sezgin, E. Primary Surgery with Systemic Therapy in Patients with de Novo Stage IV Breast Cancer: 10-year Follow-up; Protocol MF07-01 Randomized Clinical Trial. J. Am. Coll. Surg. 2021, 233, 742–751e5. [Google Scholar] [CrossRef]
- Iwase, T.; Shrimanker, T.V.; Rodriguez-Bautista, R.; Sahin, O.; James, A.; Wu, J.; Shen, Y.; Ueno, N.T. Changes in Overall Survival over Time for Patients with de novo Metastatic Breast Cancer. Cancers 2021, 13, 2650. [Google Scholar] [CrossRef]
- Weiser, M.R. AJCC 8th Edition: Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 1454–1455. [Google Scholar] [CrossRef]
- Lababede, O.; Meziane, M.A. The Eighth Edition of TNM Staging of Lung Cancer: Reference Chart and Diagrams. Oncologist 2018, 23, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 61–85. [Google Scholar] [CrossRef]
- Lamartina, L.; Grani, G.; Arvat, E.; Nervo, A.; Zatelli, M.C.; Rossi, R.; Puxeddu, E.; Morelli, S.; Torlontano, M.; Massa, M.; et al. 8th edition of the AJCC/TNM staging system of thyroid cancer: What to expect (ITCO#2). Endocr. Relat. Cancer 2018, 25, L7–L11. [Google Scholar]
- Tamirisa, N.P.; Ren, Y.; Campbell, B.M.; Thomas, S.M.; Fayanju, O.M.; Plichta, J.K.; Rosenberger, L.H.; Force, J.; Hyslop, T.; Hwang, E.S.; et al. Treatment Patterns and Outcomes of Women with Breast Cancer and Supraclavicular Nodal Metastases. Ann. Surg. Oncol. 2021, 28, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Plichta, J.K.; Ren, Y.; Thomas, S.M.; Greenup, R.A.; Fayanju, O.M.; Rosenberger, L.H.; Hyslop, T.; Hwang, E.S. Implications for Breast Cancer Restaging Based on the 8th Edition AJCC Staging Manual. Ann. Surg. 2020, 271, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, B.; Chen, J.; Wu, Z.; Gu, H.; Wang, Y.; Dai, X. A Novel Nomogram Model to Identify Candidates and Predict the Possibility of Benefit From Primary Tumor Resection Among Female Patients With Metastatic Infiltrating Duct Carcinoma of the Breast: A Large Cohort Study. Front. Oncol. 2022, 12, 798016. [Google Scholar] [CrossRef]
- Kommalapati, A.; Tella, S.H.; Goyal, G.; Ganti, A.K.; Krishnamurthy, J.; Tandra, P.K. A prognostic scoring model for survival after locoregional therapy in de novo stage IV breast cancer. Breast Cancer Res. Treat. 2018, 170, 677–685. [Google Scholar] [CrossRef]
- Plichta, J.K.; Thomas, S.M.; Sergesketter, A.R.; Greenup, R.A.; Rosenberger, L.H.; Fayanju, O.M.; Kimmick, G.; Force, J.; Hyslop, T.; Hwang, E.S. A Novel Staging System for De Novo Metastatic Breast Cancer Refines Prognostic Estimates. Ann. Surg. 2022, 275, 784–792. [Google Scholar] [CrossRef]
- Soran, A.; Dogan, L.; Isik, A.; Ozbas, S.; Trabulus, D.C.; Demirci, U.; Karanlik, H.; Soyder, A.; Dag, A.; Bilici, A.; et al. The Effect of Primary Surgery in Patients with De Novo Stage IV Breast Cancer with Bone Metastasis Only (Protocol BOMET MF 14-01): A Multi-Center, Prospective Registry Study. Ann. Surg. Oncol. 2021, 28, 5048–5057. [Google Scholar] [CrossRef]
- Goktepe, B.; Demirors, B.; Senol, K.; Ozbas, S.; Cabioglu, N.; Sezgin, E.; Soran, A. Primary tumor surgery in patients with de novo bone only stage IVA/B breast cancer; Mets: Protocol MF22-03, combination data analysis of prospective studies. In Proceedings of the American Society of Breast Surgeons 24th Annual Meeting, Boston, MA, USA, 26–30 April 2023. [Google Scholar]
- Soran, A.; Ozmen, V.; Ozbas, S.; Karanlik, H.; Muslumanoglu, M.; Igci, A.; Canturk, Z.; Utkan, Z.; Ozaslan, C.; Evrensel, T.; et al. Randomized Trial Comparing Resection of Primary Tumor with No Surgery in Stage IV Breast Cancer at Presentation: Protocol MF07-01. Ann. Surg. Oncol. 2018, 25, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Pons-Tostivint, E.; Alouani, E.; Kirova, Y.; Dalenc, F.; Vaysse, C. Is there a role for locoregional treatment of the primary tumor in de novo metastatic breast cancer in the era of tailored therapies? Crit. Rev. Oncol. Hematol. 2021, 157, 103146. [Google Scholar] [CrossRef]
- Daily, K.; Douglas, E.; Romitti, P.A.; Thomas, A. Epidemiology of De Novo Metastatic Breast Cancer. Clin. Breast Cancer 2021, 21, 302–308. [Google Scholar] [CrossRef]
- Weichselbaum, R.R.; Hellman, S. Oligometastases revisited. Nat. Rev. Clin. Oncol. 2011, 8, 378–382. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef] [PubMed]
- Lievens, Y.; Guckenberger, M.; Gomez, D.; Hoyer, M.; Iyengar, P.; Kindts, I.; Méndez Romero, A.; Nevens, D.; Palma, D.; Park, C.; et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother. Oncol. 2020, 148, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.T.; Thukral, A.D.; Hwang, W.T.; Solin, L.J.; Vapiwala, N. Incidence and Patterns of Distant Metastases for Patients With Early-Stage Breast Cancer After Breast Conservation Treatment. Clin. Breast Cancer 2013, 13, 88–94. [Google Scholar] [CrossRef]
- Thomas, A.; Khan, S.A.; Chrischilles, E.A.; Schroeder, M.C. Initial Surgery and Survival in Stage IV Breast Cancer in the United States, 1988–2011. JAMA Surg. 2016, 151, 424. [Google Scholar] [CrossRef]
- Lane, W.O.; Thomas, S.M.; Blitzblau, R.C.; Plichta, J.K.; Rosenberger, L.H.; Fayanju, O.M.; Hyslop, T.; Hwang, E.S.; Greenup, R.A. Surgical Resection of the Primary Tumor in Women With De Novo Stage IV Breast Cancer: Contemporary Practice Patterns and Survival Analysis. Ann. Surg. 2019, 269, 537–544. [Google Scholar] [CrossRef]
- Pons-Tostivint, E.; Kirova, Y.; Lusque, A.; Campone, M.; Geffrelot, J.; Mazouni, C.; Mailliez, A.; Pasquier, D.; Madranges, N.; Firmin, N.; et al. Survival Impact of Locoregional Treatment of the Primary Tumor in De Novo Metastatic Breast Cancers in a Large Multicentric Cohort Study: A Propensity Score-Matched Analysis. Ann. Surg. Oncol. 2019, 26, 356–365. [Google Scholar] [CrossRef]
- Lopez-Tarruella, S.; Escudero, M.J.; Pollan, M.; Martín, M.; Jara, C.; Bermejo, B.; Guerrero-Zotano, A.; García-Saenz, J.; Santaballa, A.; Alba, E.; et al. Survival impact of primary tumor resection in de novo metastatic breast cancer patients (GEICAM/El Alamo Registry). Sci. Rep. 2019, 9, 20081. [Google Scholar] [CrossRef] [PubMed]
- Cady, B.; Nathan, N.R.; Michaelson, J.S.; Golshan, M.; Smith, B.L. Matched Pair Analyses of Stage IV Breast Cancer with or Without Resection of Primary Breast Site. Ann. Surg. Oncol. 2008, 15, 3384–3395. [Google Scholar] [CrossRef]
- Shien, T.; Kinoshita, T.; Shimizu, C.; Hojo, T.; Taira, N.; Doihara, H.; Akashi-Tanaka, S. Primary Tumor Resection Improves the Survival of Younger Patients with Metastatic Breast Cancer. Oncol. Rep. 2009, 21, 827–832. [Google Scholar] [CrossRef]
- Xiong, Z.; Deng, G.; Wang, J.; Li, X.; Xie, X.; Shuang, Z.; Wang, X. Could local surgery improve survival in de novo stage IV breast cancer? BMC Cancer 2018, 18, 885. [Google Scholar] [CrossRef]
- Kwong, A.; Co, M. Long term survival study of de-novo metastatic breast cancers with or without primary tumour resection. Cancer Treat Res. Commun. 2019, 20, 100148. [Google Scholar] [CrossRef] [PubMed]
- Abo-Touk, N.A. The Benefit of Locoregional Surgical Intervention in Metastatic Breast Cancer at Initial Presentation. Cancer Res. J. 2016, 4, 32. [Google Scholar] [CrossRef]
- Badwe, R.; Hawaldar, R.; Nair, N.; Kaushik, R.; Parmar, V.; Siddique, S.; Budrukkar, A.; Mittra, I.; Gupta, S. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: An open-label randomised controlled trial. Lancet Oncol. 2015, 16, 1380–1388. [Google Scholar] [CrossRef]
- Khan, S.A.; Zhao, F.; Goldstein, L.J.; Cella, D.; Basik, M.; Golshan, M.; Julian, T.B.; Pockaj, B.A.; Lee, C.A.; Razaq, W.; et al. Early Local Therapy for the Primary Site in De Novo Stage IV Breast Cancer: Results of a Randomized Clinical Trial (E2108). J. Clin. Oncol. 2022, 40, 978–987. [Google Scholar] [CrossRef]
- Shien, T.; Nakamura, K.; Shibata, T.; Kinoshita, T.; Aogi, K.; Fujisawa, T.; Masuda, N.; Inoue, K.; Fukuda, H.; Iwata, H. A Randomized Controlled Trial Comparing Primary Tumour Resection Plus Systemic Therapy With Systemic Therapy Alone in Metastatic Breast Cancer (PRIM-BC): Japan Clinical Oncology Group Study JCOG1017. Jpn. J. Clin. Oncol. 2012, 42, 970–973. [Google Scholar] [CrossRef]
- Ren, C.; Sun, J.; Kong, L.; Wang, H. Breast surgery for patients with de novo metastatic breast cancer: A meta-analysis of randomized controlled trials. Eur. J. Surg. Oncol. 2024, 50, 107308. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hong, H.; Wang, Y.; Fu, T.; Chen, Y.; Zhao, J.; Chen, P.; Cai, R.; Tan, Y.; He, Z.; et al. Clinical Evidence for Locoregional Surgery of the Primary Tumor in Patients with De Novo Stage IV Breast Cancer. Ann. Surg. Oncol. 2021, 28, 5059–5070. [Google Scholar] [CrossRef]
- Xiao, W.; Zou, Y.; Zheng, S.; Hu, X.; Liu, P.; Xie, X.; Yu, P.; Tang, H.; Xie, X. Primary tumor resection in stage IV breast cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2018, 44, 1504–1512. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.B.; Im, Y.H.; Im, S.A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Slamon, D.J.; Diéras, V.; Rugo, H.S.; Harbeck, N.; Im, S.A.; Gelmon, K.A.; Lipatov, O.N.; Walshe, J.M.; Martin, M.; Chavez-MacGregor, M.; et al. Overall Survival With Palbociclib Plus Letrozole in Advanced Breast Cancer. J. Clin. Oncol. 2024, 42, 994–1000. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Jerusalem, G.; De Laurentiis, M.; Im, S.; Petrakova, K.; Valeria Bianchi, G.; Martín, M.; Nusch, A.; et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: Updated overall survival. Ann. Oncol. 2021, 32, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, D.J.A.; Van Kampen, R.J.W.; Voogd, A.C.; Dercksen, M.W.; Van Den Berkmortel, F.; Smilde, T.J.; Van De Wouw, A.J.; Peters, F.P.J.; Van Riel, J.M.G.H.; Peters, N.A.J.B.; et al. Prognosis of metastatic breast cancer subtypes: The hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res. Treat. 2013, 141, 507–514. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Liu, X.; Liao, X.; He, J.; Niu, L. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer 2019, 19, 1091. [Google Scholar] [CrossRef]
- Lin, C.; Wu, J.; Ding, S.; Goh, C.; Andriani, L.; Lu, S.; Shen, K.; Zhu, L. Subdivision of M1 Stage for De Novo Metastatic Breast Cancer to Better Predict Prognosis and Response to Primary Tumor Surgery. J. Natl. Compr. Canc. Netw. 2019, 17, 1521–1528. [Google Scholar] [CrossRef]
- Yoo, T.K.; Chae, B.J.; Kim, S.J.; Lee, J.; Yoon, T.I.; Lee, S.J.; Park, H.Y.; Park, H.K.; Eom, Y.H.; Kim, H.S.; et al. Identifying long-term survivors among metastatic breast cancer patients undergoing primary tumor surgery. Breast Cancer Res. Treat. 2017, 165, 109–118. [Google Scholar] [CrossRef]
- Plichta, J.K.; Thomas, S.M.; Hayes, D.F.; Chavez-MacGregor, M.; Allison, K.; De Los Santos, J.; Fowler, A.M.; Giuliano, A.E.; Sharma, P.; Smith, B.D.; et al. Novel Prognostic Staging System for Patients With De Novo Metastatic Breast Cancer. J. Clin. Oncol. 2023, 41, 2546–2560. [Google Scholar] [CrossRef]
- Cheng, J.; Holland-Letz, T.; Wallwiener, M.; Surowy, H.; Cuk, K.; Schott, S.; Trumpp, A.; Pantel, K.; Sohn, C.; Schneeweiss, A.; et al. Circulating free DNA integrity and concentration as independent prognostic markers in metastatic breast cancer. Breast Cancer Res. Treat. 2018, 169, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Pairawan, S.; Hess, K.R.; Janku, F.; Sanchez, N.S.; Mills Shaw, K.R.; Eng, C.; Damodaran, S.; Javle, M.; Kaseb, A.O.; Hong, D.S.; et al. Cell-free Circulating Tumor DNA Variant Allele Frequency Associates with Survival in Metastatic Cancer. Clin. Cancer Res. 2020, 26, 1924–1931. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Liu, J.; Shen, Y. Prognostic factors and survival according to tumour subtype in women presenting with breast cancer bone metastases at initial diagnosis: A SEER-based study. BMC Cancer 2020, 20, 1102. [Google Scholar] [CrossRef]
- Ai, X.; Liao, X.; Li, J.; Tang, P.; Jiang, J. Clinical Outcomes of N3 Breast Cancer: A Real-World Study of a Single Institution and the US Surveillance, Epidemiology, and End Results (SEER) Database. Cancer Manag. Res. 2020, 12, 5331–5343. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chen, H.; Dai, Y.; Bao, B.; Tian, L.; Chen, Y. Nontherapeutic Risk Factors of Different Grouped Stage IIIC Breast Cancer Patients’ Mortality: A Study of the US Surveillance, Epidemiology, and End Results Database. Breast J. 2022, 2022, 6705052. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goktepe, B.; Demirors, B.; Senol, K.; Ozbas, S.; Sezgin, E.; Lucci, A.; Soran, A. A Pragmatic Grouping Model for Bone-Only De Novo Metastatic Breast Cancer (MetS Protocol MF22-03). Cancers 2025, 17, 2033. https://doi.org/10.3390/cancers17122033
Goktepe B, Demirors B, Senol K, Ozbas S, Sezgin E, Lucci A, Soran A. A Pragmatic Grouping Model for Bone-Only De Novo Metastatic Breast Cancer (MetS Protocol MF22-03). Cancers. 2025; 17(12):2033. https://doi.org/10.3390/cancers17122033
Chicago/Turabian StyleGoktepe, Berk, Berkay Demirors, Kazim Senol, Serdar Ozbas, Efe Sezgin, Anthony Lucci, and Atilla Soran. 2025. "A Pragmatic Grouping Model for Bone-Only De Novo Metastatic Breast Cancer (MetS Protocol MF22-03)" Cancers 17, no. 12: 2033. https://doi.org/10.3390/cancers17122033
APA StyleGoktepe, B., Demirors, B., Senol, K., Ozbas, S., Sezgin, E., Lucci, A., & Soran, A. (2025). A Pragmatic Grouping Model for Bone-Only De Novo Metastatic Breast Cancer (MetS Protocol MF22-03). Cancers, 17(12), 2033. https://doi.org/10.3390/cancers17122033





