Effects of Massage Therapy in Breast Cancer Survivors with Mastectomy: Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Eligibility Criteria
- Type of participant: Female participants aged 45–64 years who had undergone mastectomy.
- Type of intervention: Interventions involving massage therapy as an adjuvant treatment.
- Type of study: Randomized controlled trials (RCTs), uncontrolled trials, secondary analyses of clinical trials, quasi-experimental studies, and retrospective analyses.
- Studies published within the last 16 years.
- Publications in English or Spanish.
- Research published in the last 16 years to assess the most recent advancements in massage therapy as an adjuvant treatment and to update the available scientific evidence on this topic.
- Outcome measures: Any outcome measure assessed with a standardized or validated assessment tool.Exclusion criteria:
- Study protocols, systematic reviews, and meta-analyses.
- Studies with fewer than 4 participants.
- Interventions not classified as massage therapy techniques.
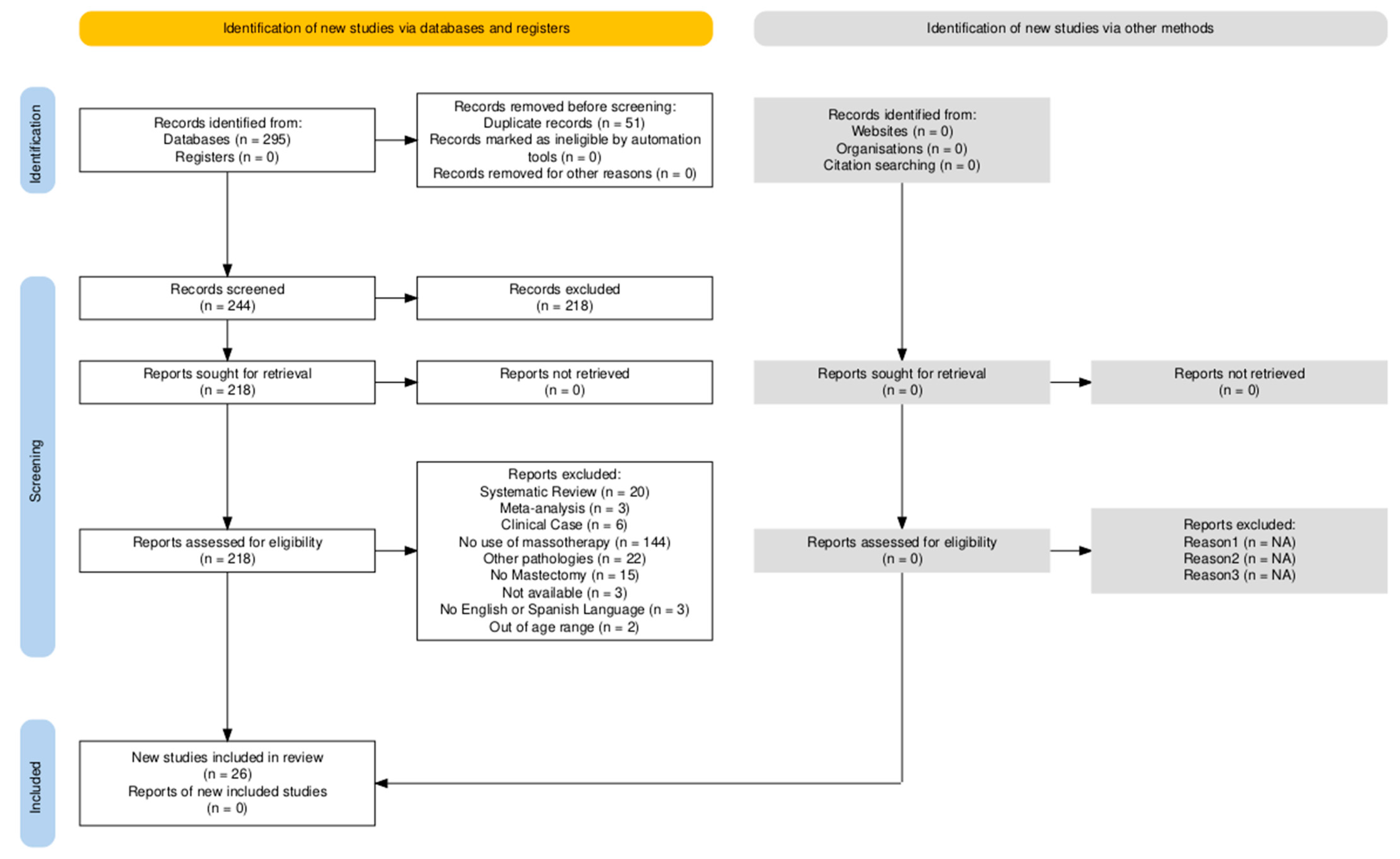
2.4. Study Selection
2.5. Methodological Quality Analysis
2.6. Risk of Bias Analysis
3. Results
| Authors | Sample, Mean Age | Study Design and Intervention | Treatment and Follow-up Period | Type of Massage Therapy | Assessment Tools | Main Findings |
|---|---|---|---|---|---|---|
| Arinaga Y, Piller N, Sato F et al. [33] | N = 43 (CG: 21; EG: 22), Mean age ≥ 20 | Randomized controlled pilot study. CG received standard hospital care; EG participated in a self-care program for breast cancer-related lymphedema (BCRL). | EG performed a 10-min daily holistic self-care routine (radio Taiso: 3 min; arm exercises: 1 min; central lymphatic drainage: 1 min; hydration: 3 min). CG received standard care. Follow-up at baseline, week 1, and months 1, 3, and 6. | Central MLD at subclavian drainage points during bathing; skin care with milk lotion using MLD techniques toward thoracic duct. | L-Dex, Relative Edema Volume (REV), Relative Volume Change (RVC), Transepidermal Water Loss (TEWL), skin induration, BCRL symptoms, SF-8, QOL. | No significant differences in extracellular fluid or skin induration. Significant differences observed in hand measurements (REV and RVC), but not in the arm. TEWL differed significantly in breast (EG: p = 0.043), hand, and arm. EG showed greater improvement in BCRL symptoms and quality of life. |
| Baklaci M, Eyigör S, Tanlgör G et al. [34] | N = 74 Mean age: 56 ± 10.74 | Descriptive observational clinical trial. All participants enrolled in complex decongestive therapy (CDT). | Program included patient education, skin care, 20 min exercises, 30 min walking, 45 min MLD, and compression bandaging, at least 5 days/week in two phases. Weekly evaluations. | Patients were taught to perform slow, rhythmic MLD on the affected limb. | Handgrip dynamometry (JAMAR), volumetric measurements. | Volume decreased by 46%, resulting in significant inter-arm difference (p < 0.01), which persisted. No significant improvement in handgrip strength. |
| Xiong Q, Luo F, Zhan J et al. [11] | N = 104 (EG1: 52, EG2: 52), Mean age: 51.9 ± 8.0 | Randomized controlled trial (RCT). EG1: regular functional exercise; EG2: MLD with rehabilitation and functional exercise. | EG1 began active exercise day 1 post-op, 4×/day, 20 min each. EG2 received 1–2 daily MLD sessions (20–25 min), acupressure, and compression bandaging. Evaluations at pre-op, day 5, months 1–3. | MLD applied from central to adjacent drainage, anastomotic, and edematous zones. | Goniometry for shoulder ROM; arm circumference measured via tape. | EG2 showed significant ROM improvement over EG1 (p < 0.05); no significant differences in arm circumference. BCRL risk increased over time. |
| Uzkeser H, Karatay S, Erdemce B et al. [19] | N = 31 (G1: 15; G2: 16), Mean age: 37–75 | RCT. G1: CDT (MLD, compression bandages and garments, exercises). G2: CDT with intermittent pneumatic compression pump. | All groups treated 5 days/week for 3 weeks. Evaluations: baseline, end of treatment (week 3), and 1 month post-treatment. | MLD in G1 only. | Measuring tape, water immersion method, ultrasonography for dermis thickness, VAS scale. | Significant volume reduction in both groups during treatment. At 7 months, only G2 maintained improvements. Circumference and dermal thickness improved only in G1. No significant intergroup difference in VAS, though pre–post improvements were seen. |
| Guerrero R, das Neves L, Guirro R et al. [28] | N = 16 (G1: 8; G2: 8), Mean age: 64 ± 11.44 | RCT. G1: MLD. G2: MLD with 30° upper limb elevation. | G2 performed therapy with arm elevated to 30°. Evaluations: before, immediately after, and 30 min post-treatment. | MLD using Leduc method in supine position with or without arm elevation. | Portable continuous-wave Doppler ultrasound, measuring tape, truncated cone volume estimation method. | Significant increase in blood flow velocity with elevation. Values returned to baseline after 30 min. |
| Oliveira M, Gurgel M, Amorim B et al. [24] | N = 106 (G1: 52; G2: 53), Mean age: 55 | Non-randomized controlled clinical trial. G1: MLD. G2: active exercise. | Postop education on day 1. Starting 48 h after surgery: 40 min MLD or active exercise sessions, 2/week for 30 days. Evaluations: pre-op, and 2 and 3 months post-op. | MLD techniques applied. | Goniometer, measuring tape, lymphoscintigraphy (node uptake and visualization speed). | ROM recovery was partial and similar in both groups. Significant correlation (p = 0.003) between initial lymphoscintigraphy scores and lymphedema development. |
| Martín M, Hernández M, Avendaño C et al. [13] | N = 58 (CG: 29; EG: 29) | RCT. CG: standard treatment (skin care, exercises, compression garments/bandaging). EG: standard care plus MLD. | Outpatient treatment for 1 month. CG: 4 weeks of multilayer bandaging, then specific exercises. EG: 4 weeks MLD + bandaging, + specific exercises. Follow-up T0, 1, 3, 6 months. | MLD starting at neck and trunk, 45–60 min/day for 2–4 weeks. | Circumference and volume via truncated cone formula, QoL questionnaires: EORTC QLQ-C30 and QLQ-BR23. | No results reported. |
| Dayes I, Whelan T, Julian J et al. [25] | N = 103 (CG: 46, Mean age: 59; EG: 57, Mean age: 61) | RCT. CG: compression garments. EG: MLD, compression bandaging, and garments. | CG wore elastic sleeves/gloves for 12 h/day. EG received 1 h MLD (5 days/week, 4 weeks), compression bandaging (23 h/day), and lifestyle counseling. Evaluations: baseline, weeks 3, 6, 12, 24, and 52. | MLD performed by Vodder/Földi-certified therapists. | SF-36, DASH, National Cancer Institute Common Toxicity Criteria. | EG showed greater mean and absolute lymphedema volume reduction, especially in long-standing cases. No DASH score differences between groups. |
| Cho Y, Do J, Jung S et al. [20] | N = 48 (G1: 24, Mean age: 50.7 ± 9.6; G2: 24, Mean age: 46.6 ± 6.8) | RCT. G1: physical therapy. G2: physical therapy + MLD. | Both groups received physical therapy 3×/week for 4 weeks: warm-up, stretching, strengthening, tissue mobilization, and MLD (30 min). Evaluated at baseline and after 4 weeks. | MLD using stationary circles, pumping, and rotary techniques. | Measuring tape, Power Track II dynamometer, digital inclinometer, EORTC QLQ-C30, QLQ-BR23, DASH, NRS. | Significant improvement in physical, emotional, and social function, fatigue, pain, arm/breast symptoms, strength, and DASH scores (p < 0.05). NRS and arm volume lower in G2. Lymphedema occurred only in G1. |
| Serra-Añó, Inglés M, Bou-Catalá et al. [7] | N = 24 (CG: 11; EG: 13), Mean age: 30–60 | RCT. CG: MLD. EG: MFR. | Patients treated supine, arms extended, affected limb elevated 30°. Evaluations: pre-treatment, post-treatment, and 1-month follow-up. | Pilat’s MFR technique (10 min per technique, no cream). | VAS, manual goniometer, DASH, PHQ-9, FACT-B+4. | Pain improved significantly in EG (p < 0.05), but not in CG. ROM and function improved in both, sustained only in EG. Depression decreased only in CG. Physical well-being and FACT improved in both. |
| Dion L, Engen D, Lemaine V et al. [10] | N = 40 (G1: 20; G2: 20), Mean age: 47.7 ± 8.4 | Randomized pilot study. G1: massage. G2: massage + guided meditation. | Therapy in private hospital room; 20 min massage for 3 days; G2 began with 15 min meditation. Evaluated pre-op, days 1–3, and week 3. | Swedish massage, acupressure, foot reflexology using oils. | VAS, EVA, PSS-14. | Significant improvement in VAS in both groups. G1 improved PSS-14 at week 3. No major differences between groups. |
| Kim Y, Park E, Lee H [12] | N = 30 (GA: 15, Mean age: 48 ± 8.3; GB: 15, Mean age: 47.8 ± 5.2) | Crossover RCT. GA: MFR + CDT, then placebo MFR + CDT; GB: reverse sequence. | Each group received 30 min MFR + 30 min CDT (MLD, compression, education, stretching). Evaluations: weeks 0, 4, 8 (washout), 12. | MFR applied using hand/finger to extend fascia opposite muscle direction. MLD also applied. | Measuring tape, NRS, goniometer, chest circumference, DASH, FACT-B. | Significant differences in limb volume, pain, ROM, and DASH scores (p < 0.05) post-intervention. Significant chest circumference and FACT-B improvement after MFR only. |
| Cruz-Ramos J, Cedeño-Meza A, Bernal-Gallardo J et al. [29] | N = 32, Mean age: 57.16 ± 11.98 | Descriptive observational clinical trial. CDT with MLD. | Daily MLD sessions (40–60 min) for 5 days, followed by 24 h multilayer bandaging. Evaluations: baseline, each session, and after 5 sessions. | MLD using Vodder-Földi technique, distal to proximal. | Measuring tape, Kuhnke method, EORTC QLQ-C30. | Significant volume reduction (p < 0.0005), improved quality of life. Reduced fatigue, pain, dyspnea, and diarrhea. Circumference decreased only 4%. |
| Demirci P, Tasci S, Öztunç G [3] | N = 30 (CG: 15; EG: 15), Age ≥ 18 | Mixed-method RCT. CG: standard care. EG: foot massage. | EG received 30 min foot massages twice weekly for 3 weeks. Evaluations: pre–post massage and weekly for 7 wks. | Foot massage using effleurage, petrissage, superficial friction. | VAS, EVA, Personal Information Form, EORTC QLQ-C30. | VAS showed significant pre–post improvement. EG had significant time-based EVA changes; CG did not. Health status improved in both, but more in EG (p < 0.05). |
| Rao M, Pattanshetty R [8] | N = 22, Age: 18–70 | Pre–post study. MFR, stretching, and strengthening. | Four sessions/week for 3 weeks (12 total). Each included 15 min MFR + stretching (45 s) + strengthening (2 × 10 reps). Evaluations: baseline and after 12 sessions. | MFR applied to pectoralis major/minor, SCM, scalenes. | MB-Ruler v5.1 (photogrammetry), digital inclinometer, MMT, hand dynamometer, SPADI, FACT-B. | Significant improvements in posture (p = 0.001), spinal curvature, ROM, strength, SPADI, and FACT-B scores (p = 0.001). |
| Kasseroller R, Brenner E [39] | N = 62 (GA: 31; GB: 30), Mean age: 57.4 ± 8.926 | Retrospective analysis. GA: conventional bandage + MLD; GB: alginate bandage + MLD. | CDT: MLD twice/day, 5 days/week (90–120 min). Bandaging (conventional or alginate), education, and exercise included. Evaluations: days 1, 5, 8, 12, 15, 19, 22. | MLD using Vodder technique. | Kuhnke method for arm volume. | Weekday volume decreased (−155.23 mL week 1, −101.02 mL week 2, −61.69 mL week 3). Slight weekend increases. Overall downward trend. |
| Castro-Sánchez A, Moreno-Lorenzo C, Matarán-Peñarrocha G et al. [35] | N = 48 (CG: 24; EG: 24), Mean age: 30–60 | RCT. CG: postural measures. EG: elastic containment orthosis + MLD. | Eight-month intervention. MLD 5 days/week followed by containment orthosis. CG received hygiene and postural advice. Evaluated pre- and post-intervention. | MLD using Leduc method. | Measuring tape, EVA, bioimpedance system, Dermatemp infrared thermography, EORTC QLQ-C30. | EG showed significant improvement (p < 0.05) in quality of life, extracellular water, function, and mastectomy-side limb volume. CG showed similar gains except in social function. |
| Listing M, Reibhauer A, Krohn M et al. [4] | N = 115 (CG: 57, Mean age: 61.4; EG: 58, Mean age: 57.6) | RCT. CG: usual care. EG: classical massage (back, head, neck). | Private and quiet setting. EG: 30 min massage for 6 weeks. Evaluated pre-treatment, week 5, and 6 weeks post-treatment. | Swedish massage using rose and calendula oils (stroking, kneading, friction, pressure). | SF-8, EORTC QLQ-BR23, GBB, BSF. | Significant reduction in fatigue and physical discomfort (body pain, extremities, breast symptoms) in EG. Reduced apathy and anger, but effects not long-lasting. |
| Meer T, Noor R, Bashir M et al. [30] | N = 36 (GA: 19; GB: 17), Age: 18–60 | RCT. GA: MLD. GB: soft tissue mobilization, therapeutic exercise, stretching, strengthening, ROM exercises. | Both groups received 5 sessions/week for 4 weeks. GA: 25 min MLD seated/lying. GB: 20 min limb mobilization. Evaluated pre- and post-intervention. | MLD on shoulder and arm (5–7 movements per section). | EORTC QLQ-C30, QLQ-BR23, DASH, NPRS, PSFS, dynamometer, goniometer. | Both treatments improved QoL, NPRS, MMT, and ROM (p > 0.05). MLD more beneficial in DASH and PSFS (p < 0.05). |
| De Baets L, De Groef A, Hagen M et al. [9] | N = 48 (CG: 24, Mean age: 52.6; EG: 24, Mean age: 54.8) | Secondary analysis of an RCT. CG: placebo therapy. EG: MFR. | Twelve-week program: weeks 1–8 (2 individual sessions/week), weeks 9–12 (1 session/week). Session: 30 min mobilizations, stretches, massage, exercise. EG received MFR. | MFR techniques on trigger points and fascial adhesions. | Infrared cameras (100 Hz sampling), height index bar. | Significant reduction in scapular protraction (p = 0.043) and anterior tilt (p = 0.049) in EG. No changes in humerothoracic, trunk, or elbow motion. |
| De Oliveira M, De Rezende L, Do Amaral M et al. [36] | N = 89 (G1: 43, Mean age: 55.6 ± 11.9; G2: 46, Mean age: 56.7 ± 15.1) | Non-randomized controlled clinical trial. G1: MLD. G2: active exercise. | All received postoperative care education; 48 h after surgery, G1 had 40 min MLD sessions and G2 had active group exercise, both 2×/week for 30 days. Evaluated pre-op and 60 days post-op. | MLD with lymph node evacuation and absorption maneuvers. | Flexible measuring tape, goniometer, palpation. | No significant pre–post differences between groups in ROM or circumference. |
| Freire de Oliveira M, Costa Gurgel M, Pace do Amaral M et al. [26] | N = 105 (G1: 52, Mean age: 56.9 ± 11.9; G2: 53, Mean age: 57.3 ± 15.1) | Non-randomized clinical trial. G1: MLD. G2: active exercise. | Postoperative care education; 48 h post-op: G1 received individual MLD, G2 group active exercise, both 40 min, 2×/week, 30 days. Evaluated pre-op and 60 days post-op. | MLD using lymph node evacuation and absorption techniques. | Flexible tape, goniometer, lymphoscintigraphy. | No significant group differences in ROM or circumference; 48% in G1 worsened tracer uptake speed; 13% improved uptake intensity. |
| Drackley N, Degnim A, Jakub J et al. [37] | N = 64, Mean age: 57.6 | Pilot study. Manual massage. | Performed in private, relaxing setting with music. Evaluated pre- and post-massage. | Manual massage in area chosen by patient using unscented organic lotion. | VAS, anonymous questionnaire, Likert scale. | Significant post-massage reductions in pain, anxiety, and tension; increased relaxation and general well-being. |
| Marshall-Mckenna R, Paul L, McFadyen A et al. [31] | N = 24 (G1: 14, Mean age: 63.5; G2: 10, Mean age: 51.4) | Pilot study. G1: MFR. G2: usual care. | G1: 4 MFR (1 before radiotherapy, 3 weekly). G2: no physiotherapy. Evaluated at baseline, week 4, and 3 months post-treatment. | MFR techniques. | BMI, digital inclinometer, McGill Pain Questionnaire, DASH, HADS. | G1 showed significant improvement (p = 0.001) in all motions. Pain decreased in both groups. DASH improved in G1, no significant difference vs. G2. G2 had higher anxiety and depression. |
| Mogahed H, Mohamed N, Wahed M [32] | N = 40 (CG: 20, Mean age: 43.3 ± 9.28; EG: 20, Mean age: 44.25 ± 9.21) | RCT. CG: traditional shoulder exercises. EG: Cyriax, scapular proprioceptive neuromuscular facilitation + traditional exercises. | Massage for 15 min/session, 3×/week. CG: traditional shoulder exercises 15 min/session, 3×/week (24 sessions total). Evaluated pre- and post-intervention. | Cyriax technique on bicipital groove and serratus anterior. | Goniometer. | EG had significant shoulder ROM improvement and pain reduction (p < 0.001) in post-mastectomy adhesive capsulitis compared to CG. |
| Haghighat S, Lofti-Tokaldany M, Maboudi A et al. [27] | N = 137, Mean age: 53.5 ± 10 | Descriptive observational clinical trial. CDT. | Daily CDT: 5 days/week (10–15 sessions), including skin care, 45 min light therapy, MLD, exercise, and compression bandaging. Evaluated at first and last session. | MLD using Vodder technique. | Water displacement method, 4-point scale questionnaire. | Significant reductions in edema volume (p = 0.03) and lymphedema duration (p = 0.002) after phase I CDT. |
3.1. Type of Techniques
3.2. Efficacy of Massage Therapy
3.3. Methodological Quality
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sánchez Portela, J.R.; Verga Tirado, B. Cáncer de mama: ¿Es posible prevenirlo? Rev. Cienc. Médicas Pinar Río 2011, 15, 14–28. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1561-31942011000100003&lng=es (accessed on 1 May 2024).
- Martín, M.; Herrero, A.; Echavarría, I. El cáncer de mama. Arbor 2015, 191, 234. [Google Scholar] [CrossRef][Green Version]
- Demirci, P.Y.; Taşcı, S.; Öztunç, G. Effect of foot massage on upper extremity pain level and quality of life in women who had a mastectomy operation: A mixed-method study. Eur. J. Integr. Med. 2022, 54, 102160. [Google Scholar] [CrossRef]
- Listing, M.; Reisshauer, A.; Krohn, M.; Voigt, B.; Tjahono, G.; Becker, J.; Rauchfuß, M. Massage therapy reduces physical discomfort and improves mood disturbances in women with breast cancer. Psychooncology 2009, 18, 1290–1299. [Google Scholar] [CrossRef]
- Kinkead, B.; Schettler, P.J.; Larson, E.R.; Carroll, D.; Sharenko, M.; Nettles, J.; Rapaport, M.H. Massage therapy decreases cancer-related fatigue: Results from a randomized early phase trial: Massage Decreases Cancer-Related Fatigue in Breast Cancer Survivors. Cancer 2018, 124, 546–554. [Google Scholar] [CrossRef]
- Rios, M.C.V.; Pedraza, R.S. Anxiety and depression disorders in relation to the quality of life of breast cancer patients with locally advanced or disseminated stage. Rev. Colomb. Psiquiatr. 2018, 47, 211–220. [Google Scholar] [CrossRef]
- Serra-Añó, P.; Inglés, M.; Bou-Catalá, C.; Iraola-Lliso, A.; Espí-López, G.V. Effectiveness of myofascial release after breast cancer surgery in women undergoing conservative surgery and radiotherapy: A randomized controlled trial. Support. Care Cancer 2019, 27, 2633–2641. [Google Scholar] [CrossRef]
- Rao, M.S.; Pattanshetty, R.B. Effect of myofascial release, stretching, and strengthening on upper torso posture, spinal curvatures, range of motion, strength, shoulder pain and disability, and quality of life in breast cancer survivors. Physiother. Res. Int. 2022, 27, e1939. [Google Scholar] [CrossRef]
- Baets, D.; Groef, D.; Hagen, A.; Neven, M.; Dams, P.; Geraerts, L. The effect of myofascial and physical therapy on trunk, shoulder, and elbow movement patterns in women with pain and myofascial dysfunctions after breast cancer surgery: Secondary analyses of a randomized controlled trial. PM R 2023, 15, 1382–1391. [Google Scholar] [CrossRef]
- Dion, L.J.; Engen, D.J.; Lemaine, V.; Lawson, D.K.; Brock, C.G.; Thomley, B.S.; Cha, S.; Wahner-Roedler, D. Massage therapy alone and in combination with meditation for breast cancer patients undergoing autologous tissue reconstruction: A randomized pilot study. Complement. Ther. Clin. Pract. 2016, 23, 82–87. [Google Scholar] [CrossRef]
- Xiong, Q.; Luo, F.; Zhan, J.; Qiao, J.; Duan, Y.; Huang, J.; Jin, P. Effect of manual lymphatic drainage combined with targeted rehabilitation therapies on the recovery of upper limb function in patients with modified radical mastectomy: A randomized controlled trial. Turk. J. Phys. Med. Rehabil. 2023, 69, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, E.Y.; Lee, H. The effect of myofascial release in patients with breast cancer-related lymphedema: A cross-over randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2023, 59, 85–93. [Google Scholar] [CrossRef]
- Martín, M.L.; Hernández, M.A.; Avendaño, C.; Rodríguez, F.; Martínez, H. Manual lymphatic drainage therapy in patients with breast cancer related lymphoedema. BMC Cancer 2011, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Devoogdt, N.; Van Kampen, M.; Geraerts, I.; Coremans, T.; Christiaens, M.R. Different physical treatment modalities for lymphoedema developing after axillary lymph node dissection for breast cancer: A review. Eur. J. Obs. Gynecol. Reprod. Biol. 2010, 149, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.K. Rehabilitation in women with breast cancer. Phys. Med. Rehabil. Clin. N. Am. 2007, 18, 521–537. [Google Scholar] [CrossRef]
- Fialka-Moser, V.; Korpan, M.; Varela, E.; Ward, A.; Gutenbrunner, C.; Casillas, J.M.; Delarque, A.; Berteanu, M.; Christodoulou, N. The role of physical and rehabilitation medicine specialist in lymphoedema. Ann. Phys. Rehabil. Med. 2013, 56, 396–410. [Google Scholar] [CrossRef]
- Masis Tenorio, E.; Molina Vargas, V.M. Physical Consequences of Surgery for Breast Cancer in the Affected Upper Limb and Proposal of Preventive Physiotherapeutic Treatment. Ph.D. Thesis, Universidad de Costa Rica, Facultad de Medicina, Escuela de Tecnologias de Salud (Costa Rica), San José, Costa Rica, 2008. [Google Scholar]
- Cole, J.S.; Olson, A.D.; Dupont-Versteegden, E.E. The Effects of Massage Therapy in Decreasing Pain and Anxiety in Post-Surgical Patients with Breast Cancer: A Systematic Review and Meta-Analysis. Glob. Adv. Integr. Med. Health 2024, 13, 27536130241245099. [Google Scholar] [CrossRef]
- Uzkeser, H.; Karatay, S.; Erdemci, B.; Koc, M.; Senel, K. Efficacy of manual lymphatic drainage and intermittent pneumatic compression pump use in the treatment of lymphedema after mastectomy: A randomized controlled trial. Breast Cancer 2015, 22, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Do, J.; Jung, S.; Kwon, O.; Jeon, J.Y. Effects of a physical therapy program combined with manual lymphatic drainage on shoulder function, quality of life, lymphedema incidence, and pain in breast cancer patients with axillary web syndrome following axillary dissection. Support. Care Cancer 2016, 24, 2047–2057. [Google Scholar] [CrossRef]
- Ajimsha, M.S.; Al-Mudahka, N.R.; Al-Madzhar, J.A. Effectiveness of myofascial release: Systematic review of randomized controlled trials. J. Bodyw. Mov. Ther. 2015, 19, 102–112. [Google Scholar] [CrossRef]
- Hutton, B.; Catalá-López, F.; Moher, D. La extensión de la declaración PRISMA para revisiones sistemáticas que incorporan metaanálisis en red: PRISMA-NMA. Med. Clin. 2016, 147, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Escala PEDro [Internet]. PEDro-Physiotherapy Evidence Database. PEDro. 2016. Available online: https://pedro.org.au/spanish/resources/pedro-scale/ (accessed on 24 May 2024).
- Oliveira, M.M.F.D.; Gurgel, M.S.C.; Amorim, B.J.; Ramos, C.D.; Derchain, S.; Furlan-Santos, N.; Sarian, L.O. Long term effects of manual lymphatic drainage and active exercises on physical morbidities, lymphoscintigraphy parameters and lymphedema formation in patients operated due to breast cancer: A clinical trial. PLoS ONE 2018, 13, e0189176. [Google Scholar] [CrossRef]
- Dayes, I.S.; Whelan, T.J.; Julian, J.A.; Parpia, S.; Pritchard, K.I.; D’Souza, D.P.; Levine, M.N. Randomized trial of decongestive lymphatic therapy for the treatment of lymphedema in women with breast cancer. J. Clin. Oncol. 2013, 31, 3758–3763. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.M.F.; Gurgel, M.S.C.; do Amaral, M.T.P.; Amorim, B.J.; Ramos, C.D.; Almeida Filho, J.G.; Sarian, L.O.Z. Manual lymphatic drainage and active exercise effects on lymphatic function do not translate into morbidities in women who underwent breast cancer surgery. Arch. Phys. Med. Rehabil. 2017, 98, 256–263. [Google Scholar] [CrossRef]
- Las Lesiones de la Mano en Fisioterapeutas: Factores de Riesgo y Estrategias de Prevención. Fisiocampus. Available online: https://www.fisiocampus.com/las-lesiones-de-la-mano-en-fisioterapeutas-factores-de-riesgo-y-estrategias-de-prevencion (accessed on 21 May 2024).
- Guerero, R.M.; das Neves, L.M.S.; Guirro, R.R.D.J.; Guirro, E.C.D.O. Manual lymphatic drainage in blood circulation of upper limb with lymphedema after breast cancer surgery. J. Manip. Physiol. Ther. 2017, 40, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Ramos, J.A.; Cedeño-Meza, A.; Bernal-Gallardo, J.A.; De La Mora-Jiménez, E.; Cervantes-Cardona, G.A.; Rivas-Rivera, F. Efecto de terapia descongestiva compleja en linfedema secundario al tratamiento quirúrgico y calidad de vida en mujeres con cáncer de mama. Salud Soc. 2018, 9, 88–96. [Google Scholar] [CrossRef]
- Meer, T.A.; Noor, R.; Bashir, M.S.; Ikram, M. Comparative effects of lymphatic drainage and soft tissue mobilization on pain threshold, shoulder mobility and quality of life in patients with axillary web syndrome after mastectomy. BMC Women’s Health 2023, 23, 588. [Google Scholar] [CrossRef]
- Marshall-McKenna, R.; Paul, L.; McFadyen, A.K.; Gilmartin, A.; Armstrong, A.; Rice, A.M.; McIlroy, P. Myofascial release for women undergoing radiotherapy for breast cancer: A pilot study. Eur. J. Physiother. 2014, 16, 58–64. [Google Scholar] [CrossRef]
- Mogahed, H.G.H.; Mohamed, N.A.; Wahed, M.H.M.A. Impact of 8-weeks combined cyriax soft tissue release and proprioceptive neuromuscular facilitation on glenohumeral rhythm in post mastectomy adhesive capsulitis. Res. J. Pharm. Technol. 2020, 13, 4903–4908. [Google Scholar] [CrossRef]
- Arinaga, Y.; Piller, N.; Sato, F.; Ishida, T.; Ohtake, T.; Kikuchi, K. The 10-min holistic self-care for patients with breast cancer- related lymphedema: Pilot randomized controlled study. Tohoku J. Exp. Med. 2019, 247, 139–147. [Google Scholar] [CrossRef]
- Baklaci, M.; Eyigör, S.; Tanıgör, G.; Özgür İnbat, M.; Çalışkan Kabayel, S. Assessment of muscle strength and volume changes in patients with breast cancer-related lymphedema. Oncol. Res. Treat. 2020, 43, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sánchez, A.M.; Moreno-Lorenzo, C.; Matarán-Peñarrocha, G.A.; Aguilar-Ferrándiz, M.E.; Almagro-Céspedes, I.; Anaya-Ojeda, J. Prevención del linfedema tras cirugía de cáncer de mama mediante ortesis elástica de contención y drenaje linfático manual: Ensayo clínico aleatorizado. Med. Clin. 2011, 137, 204–207. [Google Scholar] [CrossRef]
- De Oliveira, M.M.F.; De Rezende, L.F.; Do Amaral, M.T.P.; Pinto e Silva, M.P.; Morais, S.S.; Costa Gurgel, M.S. Manual lymphatic drainage versus exercise in the early postoperative period for breast cancer. Physiother. Theory Pract. 2014, 30, 384–389. [Google Scholar] [CrossRef]
- Drackley, N.L.; Degnim, A.C.; Jakub, J.W.; Cutshall, S.M.; Thomley, B.S.; Brodt, J.K.; Boughey, J.C. Effect of massage therapy for postsurgical mastectomy recipients. Clin. J. Oncol. Nurs. 2012, 16, 121–124. [Google Scholar] [CrossRef]
- Haghighat, S.; Lotfi-Tokaldany, M.; Maboudi, A.A.K.; Karami, M.; Bahadori, A.; Weiss, J. Predictive factors of response to phase i complete decongestive therapy in upper extremity lymphedema following breast carcinoma in Iran. Lymphology 2013, 46, 97–104. [Google Scholar]
- Kasseroller, R.G.; Brenner, E. Effectiveness of manual lymphatic drainage in intensive phase I therapy of breast cancer-related lymphedema-a retrospective analysis. Support. Care Cancer 2024, 32, 5. [Google Scholar] [CrossRef]
- de Fisioterapia, G. Efectos del Ejercicio de Linfático Manual en Mujeres Secundario a Cáncer. Unavarra.es. Available online: https://academica-e.unavarra.es/xmlui/bitstream/handle/2454/41234/Perez%20Miranda%20Vanessa%20_TFG%20def.pdf?sequence=1&isAllowed=y (accessed on 21 May 2024).
- Muñoz-Sanz, J.J.; Jiménez-Palomares, M.; Garrido-Ardila, E.M.; Rodríguez-Mansilla, J. Non-Participation in Breast Cancer Screening in Spain and Potential Application in the Present and Future: A Cross Sectional Study. Cancers 2021, 13, 4331. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Yang, L.-N.; Kong, Y.-H.; Huang, X.; Li, Y.; Bai, D.-Q. Effect of manual lymphatic drainage on breast cancer-related postmastectomy lymphedema: A meta-analysis of randomized controlled trials: A meta-analysis of randomized controlled trials. Cancer Nurs. 2023, 46, 159–166. [Google Scholar] [CrossRef]
- Claver, I.C. Masaje Y Drenaje Linfático Manual, en Pacientes Bajo Tratamiento Para el Cancer de Mama. Masoterapiachile.cl. Available online: https://www.masoterapiachile.cl/intranet-files/DLM%20CANCER%20MAMAS.pdf (accessed on 24 May 2024).
- Dilaveri, C.A.; Croghan, I.T.; Mallory, M.J.; Dion, L.J.; Fischer, K.M.; Schroeder, D.R.; Wahner-Roedler, D.L. Massage compared with massage plus acupuncture for breast cancer patients undergoing reconstructive surgery. J. Altern. Complement. Med. 2020, 26, 602–609. [Google Scholar] [CrossRef]
- Krohn, M.; Listing, M.; Tjahjono, G.; Reisshauer, A.; Peters, E.; Klapp, B.F.; Rauchfuss, M. Depression, mood, stress, and Th1/Th2 immune balance in primary breast cancer patients undergoing classical massage therapy. Support. Care Cancer 2011, 19, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.I.; Scherer, R.W.; Snyder, C.; Geigle, P.M.; Berlanstein, D.R.; Topaloglu, O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst. Rev. 2012, 8, CD008465. [Google Scholar] [CrossRef] [PubMed]
| Database | Syntax |
|---|---|
| PubMed | ‘Mastectomy AND breast neoplasms AND massage’; ‘Mastectomy AND breast neoplasms AND manual lymphatic drainage’; ‘myofascial release therapy AND breast neoplasms’ |
| Cochrane | ‘Mastectomy AND breast neoplasms AND massage’; ‘Mastectomy AND breast neoplasms AND manual lymphatic drainage’; ‘mastectomy AND myofascial release’ |
| PEDRO | ‘Mastectomy AND massage’; ‘Mastectomy AND manual lymphatic drainage’; ‘mastectomy AND myofascial release’ |
| Dialnet | ‘Mastectomy AND breast neoplasms AND massage’; ‘Mastectomy AND manual lymphatic drainage’; ‘mastectomy AND myofascial release’ |
| ScienceDirect | ‘Mastectomy AND massage’; ‘Mastectomy AND manual lymphatic drainage’; ‘mastectomy AND myofascial release’ |
| Scopus | ‘Mastectomy AND breast neoplasms AND massage’; ‘Mastectomy AND breast neoplasms AND manual lymphatic drainage’; ‘mastectomy AND myofascial release’ |
| Authors | Intervention Types | Session Frequency | Duration |
|---|---|---|---|
| Arinaga Y, Piller N, Sato F et al. [33] | MLD | Daily | 6 weeks |
| Baklaci M, Eyigör S, Tanlgör G et al. [34] | MLD | 5 days/week | Not specified |
| Xiong Q, Luo F, Zhan J et al. [11] | MLD | Daily | 3 months |
| Uzkeser H, Karatay S, Erdemce B et al. [19] | MLD | 5 days/week | 3 weeks |
| Guerrero R, das Neves L, Guirro R et al. [28] | MLD | Not specified | Not specified |
| Oliveira M, Gurgel M, Amorim B et al. [24] | MLD | 2 days/week | 30 days |
| Martín M, Hernández M, Avendaño C et al. [13] | MLD | Not specified | 4 weeks |
| Dayes I, Whelan T, Julian J et al. [25] | MLD | 5 days/week | 4 weeks |
| Cho Y, Do J, Jung S et al. [20] | MLD | 3 days/week | 4 weeks |
| Cruz-Ramos J, Cedeño-Meza A, Bernal-Gallardo J et al. [29] | MLD | Daily | 5 days |
| Kasseroller R, Brenner E [39] | MLD | 2 days/week | 5 days |
| Castro-Sánchez A, Moreno-Lorenzo C, Matarán-Peñarrocha G et al. [35] | MLD | 5 days/week | 8 months |
| Meer T, Noor R, Bashir M et al. [30] | MLD | 5 days/week. | 4 weeks |
| De Oliveira M, De Rezende L, Do Amaral M et al. [36] | MLD | 2 days/week | Not specified |
| Freire de Oliveira M, Costa Gurgel M, Pace do Amaral M et al. [26] | MLD | 2 days/week | 30 days |
| Haghighat S, Lofti-Tokaldany M, Maboudi A et al. [27] | MLD | 5 days/week | 10–15 sessions |
| Serra-Añó, Inglés M, Bou-Catalá et al. [7] | MFR | Not specified | Not specified |
| Kim Y, Park E, Lee H [12] | MFR | Not specified | Not specified |
| Rao M, Pattanshetty R [8] | MFR | 4 sessions/week | 3 weeks |
| De Baets L, De Groef A, Hagen M et al. [9] | MFR | 1 or 2 sessions/week | 12 weeks |
| Marshall-Mckenna R, Paul L, McFadyen A et al. [31] | MFR | 3 sessions/week | 4 weeks |
| Dion L, Engen D, Lemaine V et al. [10] | Swedish massage | 3 days | Not specified |
| Listing M, Reibhauer A, Krohn M et al. [4] | Swedish massage | Not specified | 6 weeks |
| Demirci P, Tasci S, Öztunç G [3] | Other massage techniques | 2 sessions/week | 3 weeks |
| Drackley N, Degnim A, Jakub J et al. [37] | Other massage techniques | 3 sessions/week | Not specified |
| Mogahed H, Mohamed N, Wahed M [32] | Other massage techniques | 3 sessions/week. | 4 weeks |
| Study | Criteria | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Score | Results | |
| Arinaga Y et al. [33] | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 7 | Good |
| Baklaci M et al. [34] | Y | N | N | Y | N | N | N | Y | Y | Y | N | 5 | Fair |
| Xiong Q et al. [11] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 9 | Excellent |
| Uzkeser H et al. [19] | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | 10 | Excellent |
| Guerero R et al. [28] | Y | Y | N | Y | N | N | N | Y | Y | N | N | 5 | Fair |
| De Oliveira M et al. [24] | Y | N | N | Y | N | N | N | N | Y | Y | Y | 5 | Fair |
| Martín M et al. [13] | Y | Y | N | Y | Y | N | N | Y | Y | N | N | 6 | Good |
| Dayes I et al. [25] | Y | Y | N | Y | Y | Y | N | Y | Y | Y | Y | 9 | Excellent |
| Cho Y et al. [20] | Y | Y | N | Y | Y | N | Y | N | Y | Y | Y | 8 | Good |
| Serra-Añó et al. [7] | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | 10 | Excellent |
| Dion L et al. [10] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 9 | Excellent |
| Kim Y, Park E, Lee H [12] | Y | Y | Y | Y | Y | N | N | N | Y | Y | Y | 8 | Good |
| Cruz-Ramos J et al. [29] | Y | N | N | N | N | N | N | Y | Y | Y | Y | 5 | Fair |
| Demirci P, Tasci S, Öztunç G [3] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 9 | Excellent |
| Rao M, Pattanshetty R [8] | Y | N | N | N | N | N | N | Y | Y | Y | Y | 5 | Fair |
| Kasseroller R, Brenner E [39] | Y | Y | Y | Y | N | N | N | Y | Y | Y | N | 7 | Good |
| Castro-Sánchez A et al. [35] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 9 | Excellent |
| Listing M et al. [4] | Y | Y | Y | N | Y | N | N | N | Y | Y | Y | 7 | Good |
| Meer T et al. [30] | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | 10 | Excellent |
| De Baets L et al. [9] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | 10 | Excellent |
| De Oliveira M et al. [36] | Y | N | N | Y | N | N | N | Y | Y | Y | Y | 6 | Good |
| Freire de Oliveira M et al. [26] | Y | N | N | Y | N | N | N | Y | Y | Y | N | 5 | Fair |
| Drackley N et al. [37] | Y | N | N | Y | N | N | N | Y | N | N | N | 3 | Poor |
| Marshall-Mckenna R et al. [31] | Y | Y | N | N | Y | N | Y | Y | Y | Y | Y | 8 | Good |
| Mogahed H, Mohamed N, Wahed M [32] | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | 9 | Excellent |
| Haghighat S et al. [38] | Y | N | N | Y | N | N | N | Y | Y | Y | N | 5 | Fair |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansilla, J.R.; Díaz, A.S.; Sánchez, B.G.; Ramírez-Durán, M.d.V.; Ardila, E.M.G.; Sánchez, M.d.C.C.; Palomares, M.J. Effects of Massage Therapy in Breast Cancer Survivors with Mastectomy: Systematic Review. Cancers 2025, 17, 2023. https://doi.org/10.3390/cancers17122023
Mansilla JR, Díaz AS, Sánchez BG, Ramírez-Durán MdV, Ardila EMG, Sánchez MdCC, Palomares MJ. Effects of Massage Therapy in Breast Cancer Survivors with Mastectomy: Systematic Review. Cancers. 2025; 17(12):2023. https://doi.org/10.3390/cancers17122023
Chicago/Turabian StyleMansilla, Juan Rodríguez, Ana Sánchez Díaz, Blanca González Sánchez, María del Valle Ramírez-Durán, Elisa María Garrido Ardila, María del Carmen Cilleros Sánchez, and María Jiménez Palomares. 2025. "Effects of Massage Therapy in Breast Cancer Survivors with Mastectomy: Systematic Review" Cancers 17, no. 12: 2023. https://doi.org/10.3390/cancers17122023
APA StyleMansilla, J. R., Díaz, A. S., Sánchez, B. G., Ramírez-Durán, M. d. V., Ardila, E. M. G., Sánchez, M. d. C. C., & Palomares, M. J. (2025). Effects of Massage Therapy in Breast Cancer Survivors with Mastectomy: Systematic Review. Cancers, 17(12), 2023. https://doi.org/10.3390/cancers17122023









