Ultrasound as a New Method for the Release and Identification of Novel microRNAs and Proteins as Candidate Biomarkers in Pancreatic Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. US Treatment
2.3. RNA Extraction and miRNA Expression Analysis
2.4. Protein Extraction and Analysis
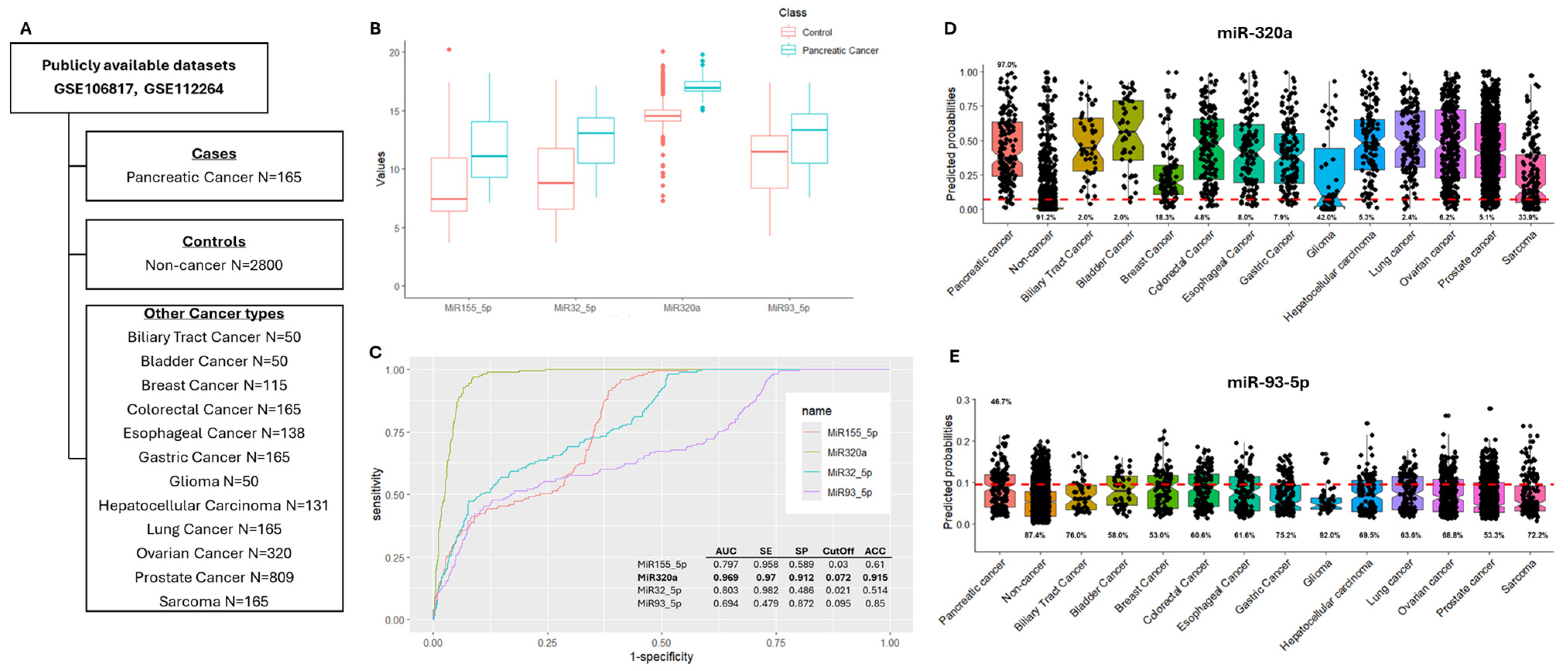
2.5. Publicly Available Datasets for Results Validation
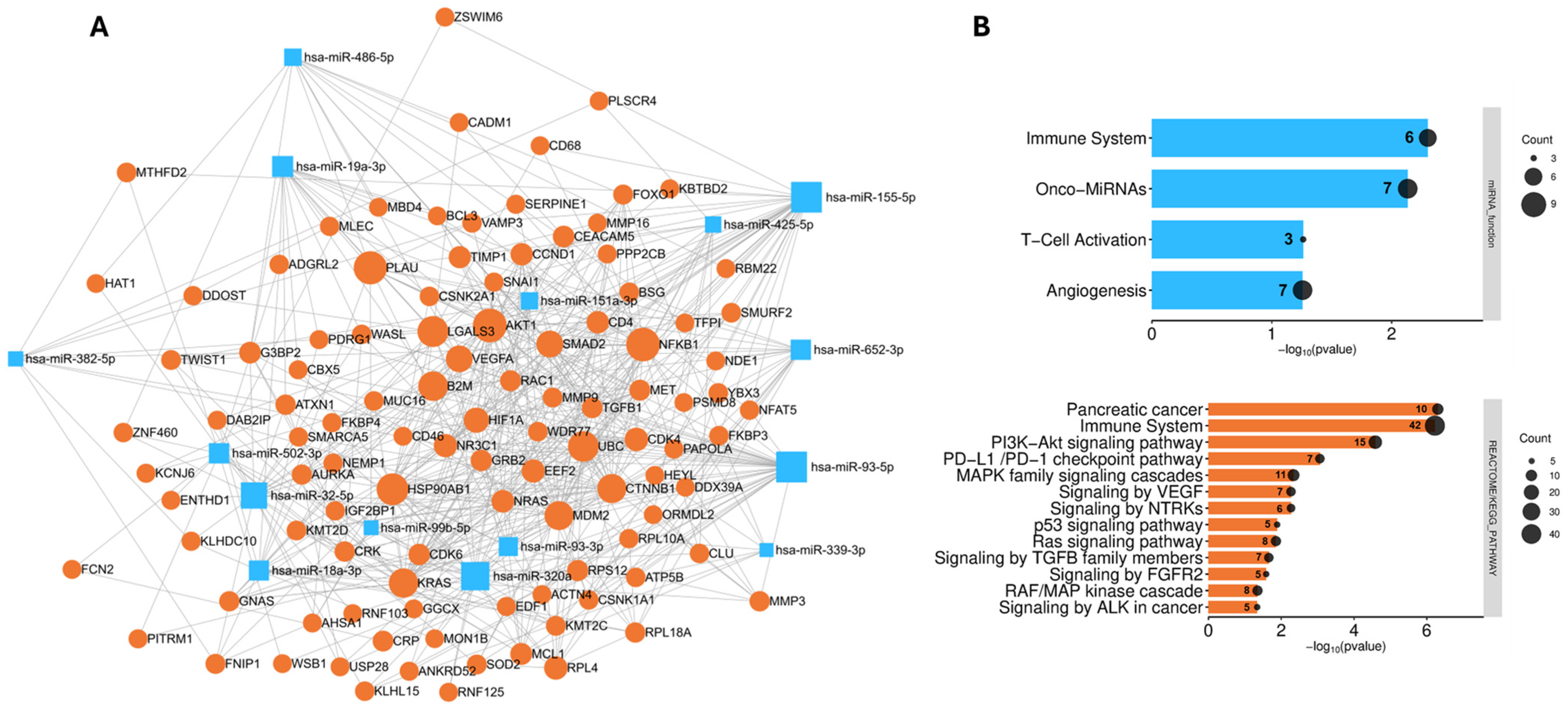
2.6. Network and Pathway-Based Analysis
3. Results
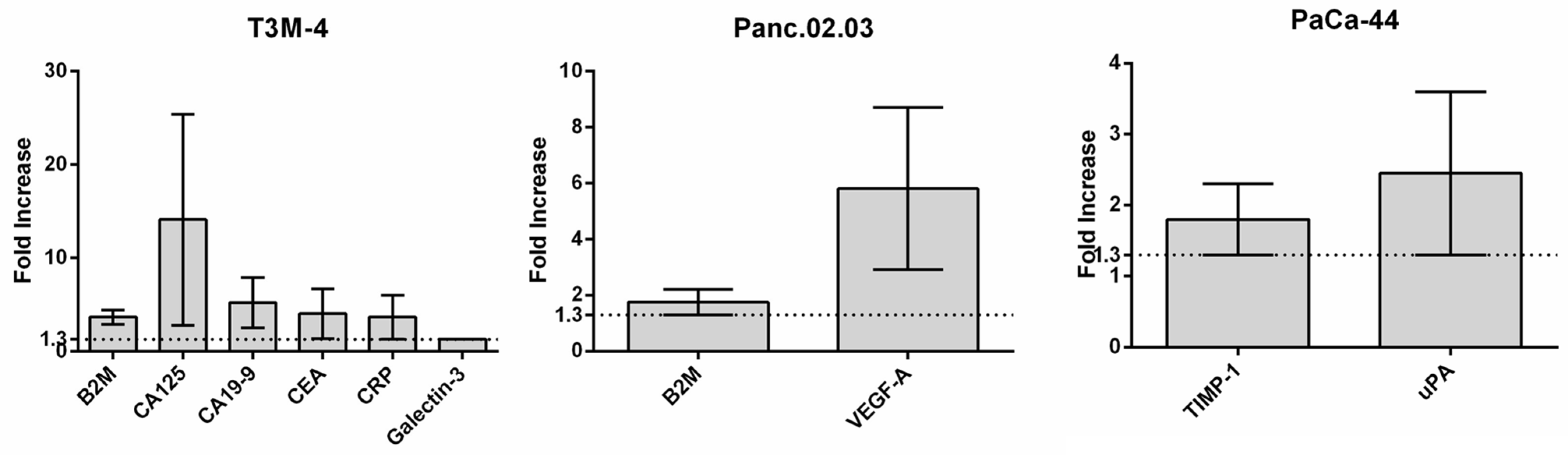
3.1. Assessment of Effective Molecule Release After US Treatment
3.2. MiRNA Profiling in US Supernatants
3.3. In Silico Validation of Differentially Expressed miRNAs
3.4. US Protein Secretome Analysis
3.5. Analysis of the Biological Role and Molecular Interactions of the Identified Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Accuracy |
| AP | Acoustic Pressure (negative peak) |
| AUC | Area Under the Curve |
| B2M | Beta-2 microglobulin |
| dbDEMC | Database of Differentially Expressed miRNAs in Human Cancers |
| DC | Duty Cycle |
| FC | Fold Change |
| FUS | Focused Ultrasound |
| Isppa | Intensity of Spatial Peak Pressure Average |
| Ispta | Intensity of Spatial Peak Time Average |
| miRNA | microRNA |
| PC | Pancreatic Cancer |
| ROC | Receiver Operating Characteristic |
| RQ | Relative Quantification |
| SE | Sensitivity |
| SP | Specificity |
| TBD | Tone Burst Duration |
| US | Ultrasound |
References
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic Cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Puckett, Y.; Garfield, K. Pancreatic Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Scialpi, M.; Reginelli, A.; D’Andrea, A.; Gravante, S.; Falcone, G.; Baccari, P.; Manganaro, L.; Palumbo, B.; Cappabianca, S. Pancreatic Tumors Imaging: An Update. Int. J. Surg. 2016, 28 (Suppl. 1), S142–S155. [Google Scholar] [CrossRef]
- Poruk, K.E.; Gay, D.Z.; Brown, K.; Mulvihill, J.D.; Boucher, K.M.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. The Clinical Utility of CA 19-9 in Pancreatic Adenocarcinoma: Diagnostic and Prognostic Updates. Curr. Mol. Med. 2013, 13, 340–351. [Google Scholar] [CrossRef]
- Zofia Rogowska, A. Ultrasound-Guided Percutaneous Core-Needle Biopsy of Focal Pancreatic Lesions—Practical Aspectss. J. Ultrason. 2022, 22, 117–120. [Google Scholar] [CrossRef]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid Biopsy Enters the Clinic—Implementation Issues and Future Challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Juźwik, C.A.; Drake, S.S.; Zhang, Y.; Paradis-Isler, N.; Sylvester, A.; Amar-Zifkin, A.; Douglas, C.; Morquette, B.; Moore, C.S.; Fournier, A.E. microRNA Dysregulation in Neurodegenerative Diseases: A Systematic Review. Prog. Neurobiol. 2019, 182, 101664. [Google Scholar] [CrossRef]
- Zhou, S.-S.; Jin, J.-P.; Wang, J.-Q.; Zhang, Z.-G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in Cardiovascular Diseases: Potential Biomarkers, Therapeutic Targets and Challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 Complexes Carry a Population of Circulating microRNAs Independent of Vesicles in Human Plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs Are Transported in Plasma and Delivered to Recipient Cells by High-Density Lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, K.; Yang, S.; Liu, J.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Yuan, W. Effect of Exosomal miRNA on Cancer Biology and Clinical Applications. Mol. Cancer 2018, 17, 147. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.L.; Tseng, J.R.; Pauly, K.B.; Guccione, S.; Rosenberg, J.; Gambhir, S.S.; Glazer, G.M. A Strategy for Blood Biomarker Amplification and Localization Using Ultrasound. Proc. Natl. Acad. Sci. USA 2009, 106, 17152–17157. [Google Scholar] [CrossRef]
- D’Souza, A.L.; Chevillet, J.R.; Ghanouni, P.; Yan, X.; Tewari, M.; Gambhir, S.S. Tumor Characterization by Ultrasound-Release of Multiple Protein and microRNA Biomarkers, Preclinical and Clinical Evidence. PLoS ONE 2018, 13, e0194268. [Google Scholar] [CrossRef]
- Zhu, L.; Nazeri, A.; Pacia, C.P.; Yue, Y.; Chen, H. Focused Ultrasound for Safe and Effective Release of Brain Tumor Biomarkers into the Peripheral Circulation. PLoS ONE 2020, 15, e0234182. [Google Scholar] [CrossRef] [PubMed]
- Cornice, J.; Capece, D.; Di Vito Nolfi, M.; Di Padova, M.; Compagnoni, C.; Verzella, D.; Di Francesco, B.; Vecchiotti, D.; Flati, I.; Tessitore, A.; et al. Ultrasound-Based Method for the Identification of Novel MicroRNA Biomarkers in Prostate Cancer. Genes 2021, 12, 1726. [Google Scholar] [CrossRef]
- Krasovitski, B.; Frenkel, V.; Shoham, S.; Kimmel, E. Intramembrane Cavitation as a Unifying Mechanism for Ultrasound-Induced Bioeffects. Proc. Natl. Acad. Sci. USA 2011, 108, 3258–3263. [Google Scholar] [CrossRef] [PubMed]
- Azhari, H. Basics of Biomedical Ultrasound for Engineers; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 313–314. [Google Scholar] [CrossRef]
- Beekers, I.; Vegter, M.; Lattwein, K.R.; Mastik, F.; Beurskens, R.; van der Steen, A.F.W.; de Jong, N.; Verweij, M.D.; Kooiman, K. Opening of Endothelial Cell-Cell Contacts Due to Sonoporation. J. Control Release 2020, 322, 426–438. [Google Scholar] [CrossRef]
- Hu, Y.; Wei, J.; Shen, Y.; Chen, S.; Chen, X. Barrier-Breaking Effects of Ultrasonic Cavitation for Drug Delivery and Biomarker Release. Ultrason. Sonochem. 2023, 94, 106346. [Google Scholar] [CrossRef]
- Forbrich, A.; Paproski, R.; Hitt, M.; Zemp, R. Comparing Efficiency of Micro-RNA and mRNA Biomarker Liberation with Microbubble-Enhanced Ultrasound Exposure. Ultrasound. Med. Biol. 2014, 40, 2207–2216. [Google Scholar] [CrossRef]
- Martin, E.; Aubry, J.-F.; Schafer, M.; Verhagen, L.; Treeby, B.; Pauly, K.B. ITRUSST Consensus on Standardised Reporting for Transcranial Ultrasound Stimulation. Brain Stimul. 2024, 17, 607–615. [Google Scholar] [CrossRef]
- Yokoi, A.; Matsuzaki, J.; Yamamoto, Y.; Yoneoka, Y.; Takahashi, K.; Shimizu, H.; Uehara, T.; Ishikawa, M.; Ikeda, S.-I.; Sonoda, T.; et al. Integrated Extracellular microRNA Profiling for Ovarian Cancer Screening. Nat. Commun. 2018, 9, 4319. [Google Scholar] [CrossRef] [PubMed]
- Urabe, F.; Matsuzaki, J.; Yamamoto, Y.; Kimura, T.; Hara, T.; Ichikawa, M.; Takizawa, S.; Aoki, Y.; Niida, S.; Sakamoto, H.; et al. Large-Scale Circulating microRNA Profiling for the Liquid Biopsy of Prostate Cancer. Clin. Cancer Res. 2019, 25, 3016–3025. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kondo, S.; Matsuzaki, J.; Esaki, M.; Okusaka, T.; Shimada, K.; Murakami, Y.; Enomoto, M.; Tamori, A.; Kato, K.; et al. Highly Sensitive Circulating MicroRNA Panel for Accurate Detection of Hepatocellular Carcinoma in Patients with Liver Disease. Hepatol. Commun. 2019, 4, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Usuba, W.; Urabe, F.; Yamamoto, Y.; Matsuzaki, J.; Sasaki, H.; Ichikawa, M.; Takizawa, S.; Aoki, Y.; Niida, S.; Kato, K.; et al. Circulating miRNA panels for specific and early detection in bladder cancer. Cancer Sci. 2019, 110, 408–419. [Google Scholar] [CrossRef]
- Mazzara, S.; Rossi, R.L.; Grifantini, R.; Donizetti, S.; Abrignani, S.; Bombaci, M. CombiROC: An Interactive Web Tool for Selecting Accurate Marker Combinations of Omics Data. Sci. Rep. 2017, 7, 45477. [Google Scholar] [CrossRef]
- Tu, J.; Yu, A.C.H. Ultrasound-Mediated Drug Delivery: Sonoporation Mechanisms, Biophysics, and Critical Factors. BME Front. 2022, 2022, 9807347. [Google Scholar] [CrossRef]
- Chen, D.; Wu, X.; Xia, M.; Wu, F.; Ding, J.; Jiao, Y.; Zhan, Q.; An, F. Upregulated Exosomic miR-23b-3p Plays Regulatory Roles in the Progression of Pancreatic Cancer. Oncol. Rep. 2017, 38, 2182–2188. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Zhang, G.; Wu, X.; Zhou, L.; Lin, Y.; Ding, J.; An, F.; Zhan, Q. MicroRNA-23b-3p Promotes Pancreatic Cancer Cell Tumorigenesis and Metastasis via the JAK/PI3K and Akt/NF-κB Signaling Pathways. Oncol. Lett. 2020, 20, 160. [Google Scholar] [CrossRef]
- López-Beas, J.; Capilla-González, V.; Aguilera, Y.; Mellado, N.; Lachaud, C.C.; Martín, F.; Smani, T.; Soria, B.; Hmadcha, A. miR-7 Modulates hESC Differentiation into Insulin-Producing Beta-like Cells and Contributes to Cell Maturation. Mol. Ther. Nucleic. Acids 2018, 12, 463–477. [Google Scholar] [CrossRef]
- Przystupski, D.; Ussowicz, M. Landscape of Cellular Bioeffects Triggered by Ultrasound-Induced Sonoporation. Int. J. Mol. Sci. 2022, 23, 11222. [Google Scholar] [CrossRef]
- Chevillet, J.R.; Khokhlova, T.D.; Giraldez, M.D.; Schade, G.R.; Starr, F.; Wang, Y.-N.; Gallichotte, E.N.; Wang, K.; Hwang, J.H.; Tewari, M. Release of Cell-Free MicroRNA Tumor Biomarkers into the Blood Circulation with Pulsed Focused Ultrasound: A Noninvasive, Anatomically Localized, Molecular Liquid Biopsy. Radiology 2017, 283, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Madadjim, R.; An, T.; Cui, J. MicroRNAs in Pancreatic Cancer: Advances in Biomarker Discovery and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 3914. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, D.; Dehlendorff, C.; Boisen, M.K.; Hasselby, J.P.; Schultz, N.A.; Werner, J.; Immervoll, H.; Molven, A.; Hansen, C.P.; Johansen, J.S. Tissue MicroRNA Profiles as Diagnostic and Prognostic Biomarkers in Patients with Resectable Pancreatic Ductal Adenocarcinoma and Periampullary Cancers. Biomark. Res. 2017, 5, 8. [Google Scholar] [CrossRef]
- Wu, Y.; Hong, Q.; Lu, F.; Zhang, Z.; Li, J.; Nie, Z.; He, B. The Diagnostic and Prognostic Value of miR-155 in Cancers: An Updated Meta-Analysis. Mol. Diagn. Ther. 2023, 27, 283–301. [Google Scholar] [CrossRef]
- Nakamura, S.; Sadakari, Y.; Ohtsuka, T.; Okayama, T.; Nakashima, Y.; Gotoh, Y.; Saeki, K.; Mori, Y.; Nakata, K.; Miyasaka, Y.; et al. Pancreatic Juice Exosomal MicroRNAs as Biomarkers for Detection of Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2019, 26, 2104–2111. [Google Scholar] [CrossRef]
- Wnuk, J.; Strzelczyk, J.K.; Gisterek, I. Clinical Value of Circulating miRNA in Diagnosis, Prognosis, Screening and Monitoring Therapy of Pancreatic Ductal Adenocarcinoma-A Review of the Literature. Int. J. Mol. Sci. 2023, 24, 5113. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.S.; Park, S.B.; Kim, C.; Kim, K.; Jung, D.E.; Song, S.Y. Identification of Circulating Serum miRNAs as Novel Biomarkers in Pancreatic Cancer Using a Penalized Algorithm. Int. J. Mol. Sci. 2021, 22, 1007. [Google Scholar] [CrossRef]
- Xue, J.; Jia, E.; Ren, N.; Lindsay, A.; Yu, H. Circulating microRNAs as Promising Diagnostic Biomarkers for Pancreatic Cancer: A Systematic Review. Onco Targets Ther. 2019, 12, 6665–6684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-X.; Yang, W.; Wu, J.-Z.; Zhou, C.; Liu, S.; Shi, H.-B.; Zhou, W.-Z. MicroRNA-32-5p Inhibits Epithelial-Mesenchymal Transition and Metastasis in Lung Adenocarcinoma by Targeting SMAD Family 3. J. Cancer 2021, 12, 2258–2267. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Zhou, H.-G.; Chen, L.-H.; Qu, D.-C.; Wang, C.-J.; Xia, Z.-Y.; Zheng, J.-H. MiR-32-5p Regulates the Proliferation and Metastasis of Cervical Cancer Cells by Targeting HOXB8. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 87–95. [Google Scholar] [CrossRef]
- Yuan, P.; Tang, C.; Chen, B.; Lei, P.; Song, J.; Xin, G.; Wang, Z.; Hui, Y.; Yao, W.; Wang, G.; et al. miR-32-5p Suppresses the Proliferation and Migration of Pancreatic Adenocarcinoma Cells by Targeting TLDC1. Mol. Med. Rep. 2021, 24, 752. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, W.; Yang, Y.; Zhang, Z. miRNA-93-5p Promotes Gemcitabine Resistance in Pancreatic Cancer Cells by Targeting the PTEN-Mediated PI3K/Akt Signaling Pathway. Ann. Clin. Lab. Sci. 2021, 51, 310–320. [Google Scholar] [PubMed]
- Mądro, A.; Kazimierak, W.; Grenda, A.; Krawczyk, P. Circulating microRNAs as a Potential Diagnostic Marker in Chronic Pancreatitis, Pancreatic Cancer and Colorectal Cancer. Oncol. Clin. Pract. 2023, 19, 34–42. [Google Scholar] [CrossRef]
- Despotović, J.; Bogdanović, A.; Dragičević, S.; Galun, D.; Krivokapić, Z.; Nikolić, A. Prognostic Potential of Circulating miR-93-5p in Patients with Colorectal Cancer Liver Metastases. Neoplasma 2022, 69, 430–442. [Google Scholar] [CrossRef]
- Costa, C.; Indovina, P.; Mattioli, E.; Forte, I.M.; Iannuzzi, C.A.; Luzzi, L.; Bellan, C.; De Summa, S.; Bucci, E.; Di Marzo, D.; et al. Correction: P53-Regulated miR-320a Targets PDL1 and Is Downregulated in Malignant Mesothelioma. Cell Death Dis. 2020, 11, 867. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Huang, Y.; Li, J.-P.; Zhang, X.; Wang, L.; Meng, Y.-L.; Yan, B.; Bian, Y.-Q.; Zhao, J.; Wang, W.-Z.; et al. MicroRNA-320a Suppresses Human Colon Cancer Cell Proliferation by Directly Targeting β-Catenin. Biochem. Biophys. Res. Commun. 2012, 420, 787–792. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Z.; Wang, H.; Cao, Z.; Zhao, Y.; Gong, C.; Ma, L.; Wang, X.; Hu, X.; Chen, S. MicroRNA-320a Inhibits Proliferation and Invasion of Breast Cancer Cells by Targeting RAB11A. Am. J. Cancer Res. 2015, 5, 2719–2729. [Google Scholar] [CrossRef]
- Yang, H.; Yu, J.; Wang, L.; Ding, D.I.; Zhang, L.; Chu, C.; Chen, Q.; Xu, Z.; Zou, Q.; Liu, X. miR-320a Is an Independent Prognostic Biomarker for Invasive Breast Cancer. Oncol. Lett. 2014, 8, 1043–1050. [Google Scholar] [CrossRef]
- Wang, J.; Shi, C.; Wang, J.; Cao, L.; Zhong, L.; Wang, D. MicroRNA-320a Is Downregulated in Non-Small Cell Lung Cancer and Suppresses Tumor Cell Growth and Invasion by Directly Targeting Insulin-like Growth Factor 1 Receptor. Oncol. Lett. 2017, 13, 3247–3252. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, Q.; Yu, Z.; Mao, S.; Jin, Y.; Li, J.; Jiang, Z.; Zhang, Y.; Chen, M.; Chen, P.; et al. MiR-320a-3p/ELF3 Axis Regulates Cell Metastasis and Invasion in Non-Small Cell Lung Cancer via PI3K/Akt Pathway. Gene 2018, 670, 31–37. [Google Scholar] [CrossRef]
- Ge, X.; Cui, H.; Zhou, Y.; Yin, D.; Feng, Y.; Xin, Q.; Xu, X.; Liu, W.; Liu, S.; Zhang, Q. miR-320a Modulates Cell Growth and Chemosensitivity via Regulating ADAM10 in Gastric Cancer. Mol. Med. Rep. 2017, 16, 9664–9670. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Liang, L.-H.; Zhang, Y.; Ding, J.; Tian, Q.; Li, J.-J.; He, X.-H. GNAI1 Suppresses Tumor Cell Migration and Invasion and Is Post-Transcriptionally Regulated by Mir-320a/c/d in Hepatocellular Carcinoma. Cancer Biol. Med. 2012, 9, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, L.; Wei, X.; Wang, L.; Liu, S.; Yang, Y.; Wang, F.; Sun, G.; Zhang, J.; Ma, Y.; et al. MicroRNA-320a Promotes 5-FU Resistance in Human Pancreatic Cancer Cells. Sci. Rep. 2016, 6, 27641. [Google Scholar] [CrossRef]
- Vila-Navarro, E.; Duran-Sanchon, S.; Vila-Casadesús, M.; Moreira, L.; Ginès, À.; Cuatrecasas, M.; Lozano, J.J.; Bujanda, L.; Castells, A.; Gironella, M. Novel Circulating miRNA Signatures for Early Detection of Pancreatic Neoplasia. Clin. Transl. Gastroenterol. 2019, 10, e00029. [Google Scholar] [CrossRef]
- Zelli, V.; Compagnoni, C.; Capelli, R.; Cannita, K.; Sidoni, T.; Ficorella, C.; Capalbo, C.; Zazzeroni, F.; Tessitore, A.; Alesse, E. Circulating MicroRNAs as Prognostic and Therapeutic Biomarkers in Breast Cancer Molecular Subtypes. J. Pers. Med. 2020, 10, 98. [Google Scholar] [CrossRef]
- Martini, R.; Amankwah, M.; Sahler, J.; Stonaker, B.; Sadullozoda, M.; Radzio, P.; August, A.; Newman, L.; Davis, M. Abstract C009: Race- and TNBC-Specific Circulating Biomarker Profiles among Breast Cancer Patients. Cancer Epidemiol. Biomark. Prev. 2023, 32, C009. [Google Scholar] [CrossRef]
- Collisson, E.A.; Bailey, P.; Chang, D.K.; Biankin, A.V. Molecular Subtypes of Pancreatic Cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.-Y.; Byrd, C.J. Diagnostic Bioliquid Markers for Pancreatic Cancer: What We Have vs. What We Need. Cancers 2023, 15, 2446. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kang, K.N.; Shin, Y.S.; Byun, Y.; Han, Y.; Kwon, W.; Kim, C.W.; Jang, J.-Y. Biomarker Panel for the Diagnosis of Pancreatic Ductal Adenocarcinoma. Cancers 2020, 12, 1443. [Google Scholar] [CrossRef]
- Nurmi, A.M.; Mustonen, H.K.; Stenman, U.-H.; Seppänen, H.E.; Haglund, C.H. Combining CRP and CA19-9 in a Novel Prognostic Score in Pancreatic Ductal Adenocarcinoma. Sci. Rep. 2021, 11, 781. [Google Scholar] [CrossRef]
- Slater, E.P.; Fendrich, V.; Strauch, K.; Rospleszcz, S.; Ramaswamy, A.; Mätthai, E.; Chaloupka, B.; Gress, T.M.; Langer, P.; Bartsch, D.K. LCN2 and TIMP1 as Potential Serum Markers for the Early Detection of Familial Pancreatic Cancer. Transl. Oncol. 2013, 6, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Yi, N.; Zhao, X.; Ji, J.; Xu, M.; Jiao, Y.; Qian, T.; Zhu, S.; Jiang, F.; Chen, J.; Xiao, M. Serum Galectin-3 as a Biomarker for Screening, Early Diagnosis, Prognosis and Therapeutic Effect Evaluation of Pancreatic Cancer. J. Cell. Mol. Med. 2020, 24, 11583–11591. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.A.; Buckley, B.J.; Ranson, M. The Urokinase Plasminogen Activation System in Pancreatic Cancer: Prospective Diagnostic and Therapeutic Targets. Biomolecules 2022, 12, 152. [Google Scholar] [CrossRef]
- Pistol-Tanase, C.; Raducan, E.; Dima, S.O.; Albulescu, L.; Alina, I.; Marius, P.; Cruceru, L.M.; Codorean, E.; Neagu, T.M.; Popescu, I. Assessment of Soluble Angiogenic Markers in Pancreatic Cancer. Biomark. Med. 2008, 2, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic Cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor Biomarkers for Diagnosis, Prognosis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef]
- Felekkis, K.; Papaneophytou, C. The Circulating Biomarkers League: Combining miRNAs with Cell-Free DNAs and Proteins. Int. J. Mol. Sci. 2024, 25, 3403. [Google Scholar] [CrossRef]

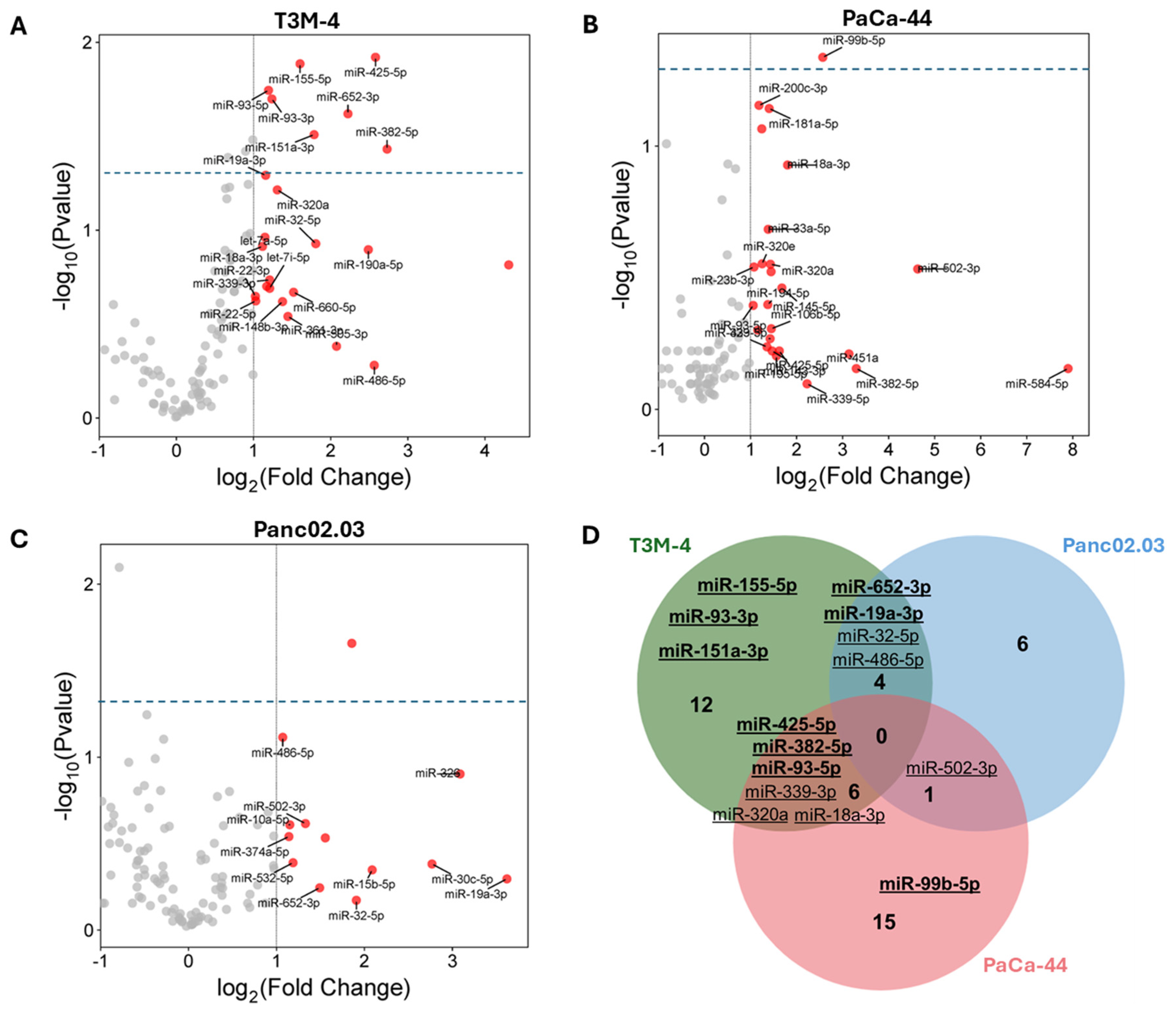
| EXP00529 (GSE106817) | EXP00620 (GSE112264) | EXP00609 (GSE113740) | EXP00538 (GSE113486) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| miRNA | T3M-4 | Pnc02.03 | PaCa-44 | log2FC | adj p-Value | log2FC | adj p-Value | log2FC | adj p-Value | log2FC | adj p-Value |
| hsa-miR-151a-3p | 0.031 | 1.466 | 0.005 | ||||||||
| hsa-miR-155-5p | 0.013 | 1.502 | 1.15 × 10−13 | 1.337 | 0.0006 | ||||||
| hsa-miR-18a-3p | |||||||||||
| hsa-miR-19a-3p | 0.050 | ||||||||||
| hsa-miR-320a | 1.907 | 5.62 × 10−129 | 1.795 | 5.808 × 10−23 | 1.572 | 2.93 × 10−15 | 1.643 | 4.848 × 10−21 | |||
| hsa-miR-32-5p | 1.719 | 2.932 × 10−14 | 2.420 | 9.082 × 10−7 | 1.358 | 0.003 | |||||
| hsa-miR-339-3p | 1.014 | 6.419 × 10−8 | |||||||||
| hsa-miR-382-5p | 0.037 | ||||||||||
| hsa-miR-425-5p | 0.012 | ||||||||||
| hsa-miR-486-5p | |||||||||||
| hsa-miR-652-3p | 0.024 | 0.959 | 1.773 × 10−7 | ||||||||
| hsa-miR-93-3p | 0.020 | 0.977 | 1.12 × 10−5 | ||||||||
| hsa-miR-93-5p | 0.018 | 0.721 | 0.003 | 1.527 | 0.001 | ||||||
| hsa-miR-502-3p | 0.583 | 0.002 | |||||||||
| hsa-miR-99b-5p | 0.046 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelli, V.; Corrente, A.; Compagnoni, C.; Colaianni, F.; Miscione, M.S.; Di Padova, M.; Capece, D.; Barbato, G.; Alesse, E.; Zazzeroni, F.; et al. Ultrasound as a New Method for the Release and Identification of Novel microRNAs and Proteins as Candidate Biomarkers in Pancreatic Cancer. Cancers 2025, 17, 1979. https://doi.org/10.3390/cancers17121979
Zelli V, Corrente A, Compagnoni C, Colaianni F, Miscione MS, Di Padova M, Capece D, Barbato G, Alesse E, Zazzeroni F, et al. Ultrasound as a New Method for the Release and Identification of Novel microRNAs and Proteins as Candidate Biomarkers in Pancreatic Cancer. Cancers. 2025; 17(12):1979. https://doi.org/10.3390/cancers17121979
Chicago/Turabian StyleZelli, Veronica, Alessandra Corrente, Chiara Compagnoni, Francesco Colaianni, Martina Sara Miscione, Monica Di Padova, Daria Capece, Gaetano Barbato, Edoardo Alesse, Francesca Zazzeroni, and et al. 2025. "Ultrasound as a New Method for the Release and Identification of Novel microRNAs and Proteins as Candidate Biomarkers in Pancreatic Cancer" Cancers 17, no. 12: 1979. https://doi.org/10.3390/cancers17121979
APA StyleZelli, V., Corrente, A., Compagnoni, C., Colaianni, F., Miscione, M. S., Di Padova, M., Capece, D., Barbato, G., Alesse, E., Zazzeroni, F., & Tessitore, A. (2025). Ultrasound as a New Method for the Release and Identification of Novel microRNAs and Proteins as Candidate Biomarkers in Pancreatic Cancer. Cancers, 17(12), 1979. https://doi.org/10.3390/cancers17121979







