The Impact of Social Determinants on Pancreatic Cancer Care in the United States
Simple Summary
Abstract
1. Introduction
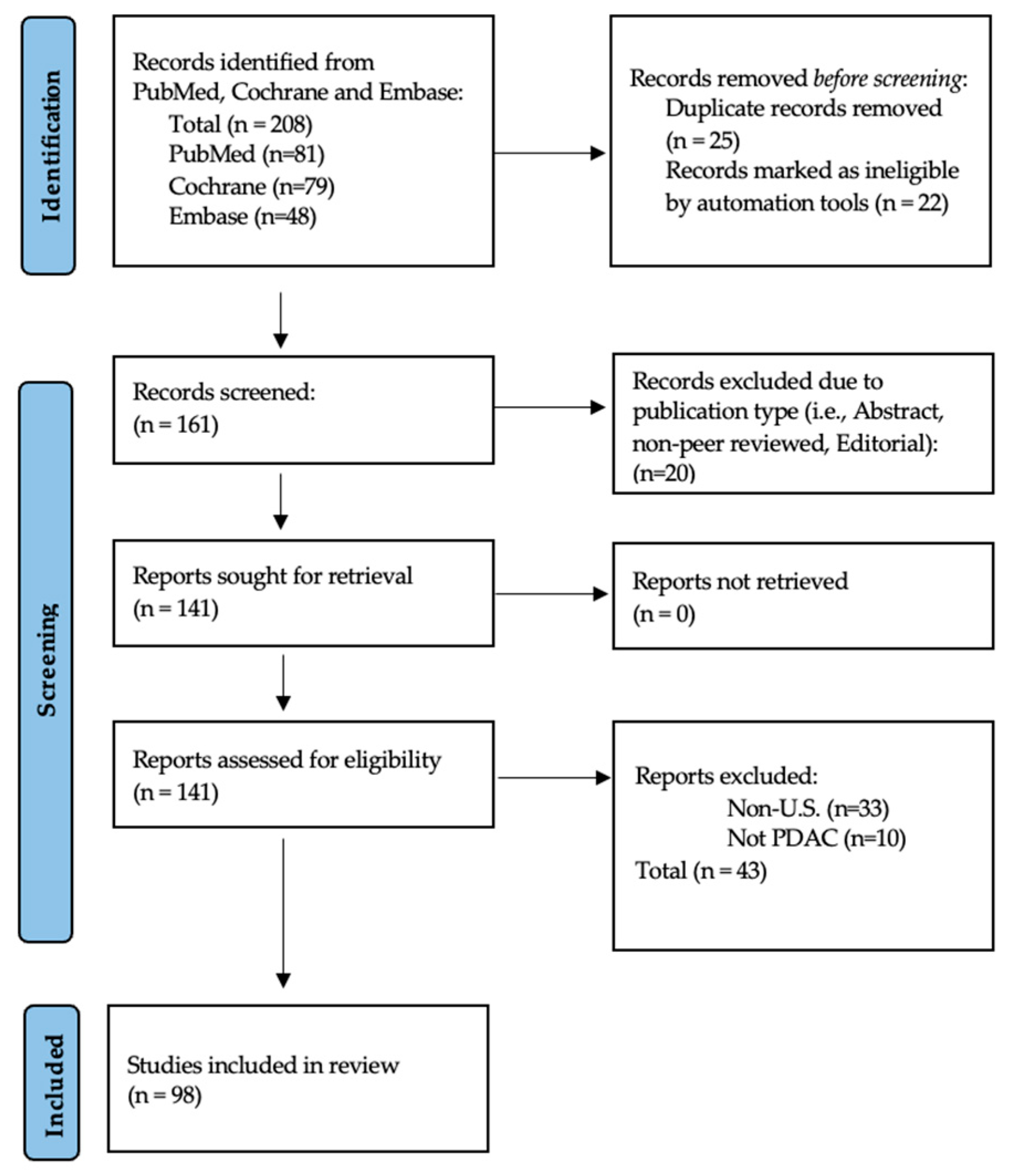
2. Materials and Methods
3. Results
3.1. Socioeconomic Status
3.2. Insurance Status
3.3. Educational Background
3.4. Race
4. Discussion and Future Directions
4.1. Existing Theoretical Framework and Areas for Future Study
4.2. Potential Interventions to Address Disparities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDAC | Pancreatic ductal adenocarcinoma |
| SES | Socioeconomic status |
| MDC | Multidisciplinary center |
| CHW | Community health worker |
| LOS | Length of stay |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Habib, J.R.; Kinny-Köster, B.; van Oosten, F.; Javed, A.A.; Cameron, J.L.; Lafaro, K.J.; Burkhart, R.A.; Burns, W.R.; He, J.; Thompson, E.D.; et al. Periadventitial dissection of the superior mesenteric artery for locally advanced pancreatic cancer: Surgical planning with the “halo sign” and “string sign”. Surgery 2021, 169, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Habib, J.R.; Kinny-Köster, B.; Bou-Samra, P.; Alsaad, R.; Sereni, E.; Javed, A.A.; Ding, D.; Cameron, J.L.; Lafaro, K.J.; Burns, W.R.; et al. Surgical Decision-Making in Pancreatic Ductal Adenocarcinoma: Modeling Prognosis Following Pancreatectomy in the Era of Induction and Neoadjuvant Chemotherapy. Ann. Surg. 2023, 277, 151–158. Available online: https://journals.lww.com/annalsofsurgery/fulltext/2023/01000/surgical_decision_making_in_pancreatic_ductal.24.aspx (accessed on 15 January 2025). [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Conroy, T.; Bouché, O.; Bécouarn, Y.; Gourgou-Bourgade, S.; Bennouna, J.; Khemissa-Akouz, F.; Assenat, E.; Montoto-Grillot, C.; Chem, M.; Ducreux, M. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Noel, M.; Fiscella, K. Disparities in Pancreatic Cancer Treatment and Outcomes. Health Equity 2019, 3, 532–540. [Google Scholar] [CrossRef]
- Alcaraz, K.I.; Wiedt, T.L.; Daniels, E.C.; Yabroff, K.R.; Guerra, C.E.; Wender, R.C. Understanding and addressing social determinants to advance cancer health equity in the United States: A blueprint for practice, research, and policy. CA Cancer J. Clin. 2020, 70, 31–46. [Google Scholar] [CrossRef]
- Abdel-Rahman, O. Impact of socioeconomic status on presentation, treatment and outcomes of patients with pancreatic cancer. J. Comp. Eff. Res. 2020, 9, 1233–1241. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Garritty, C.; Hamel, C.; Trivella, M.; Gartlehner, G.; Nussbaumer-Streit, B.; Devane, D.; Kamel, C.; Griebler, U.; King, V.J. Updated recommendations for the Cochrane rapid review methods guidance for rapid reviews of effectiveness. BMJ 2024, 384, e076335. [Google Scholar] [CrossRef]
- Hidalgo Salinas, C.; Wolfgang, C.L.; Habib, J.R. Clinical and radiological predictive features for high-grade and invasive carcinoma in intraductal papillary mucinous neoplasms: A systematic review. J. Hepatobiliary Pancreat. Sci. 2025. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.H. Socioeconomic Status, Definition. In The Wiley Blackwell Encyclopedia of Health, Illness, Behavior, and Society; John Wiley and Sons: Hoboken, NJ, USA, 2014; pp. 2210–2214. [Google Scholar] [CrossRef]
- Hao, S.; Mitsakos, A.; Irish, W.; Tuttle-Newhall, J.E.; Parikh, A.A.; Snyder, R.A. Differences in receipt of multimodality therapy by race, insurance status, and socioeconomic disadvantage in patients with resected pancreatic cancer. J. Surg. Oncol. 2022, 126, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Eskander, M.F.; Hamad, A.; Li, Y.; Fisher, J.L.; Oppong, B.; Obeng-Gyasi, S.; Tsung, A. From street address to survival: Neighborhood socioeconomic status and pancreatic cancer outcomes. Surgery 2022, 171, 770–776. [Google Scholar] [CrossRef]
- Muaddi, H.; Glasgow, A.; Abou Chaar, M.K.; Habermann, E.; Starlinger, P.; Warner, S.; Smoot, R.; Kendrick, M.; Truty, M.; Thiels, C.A. The Influence of Area Deprivation Index on Surgical Outcomes in Pancreas Cancer. J. Surg. Oncol. 2025, 131, 827–833. [Google Scholar] [CrossRef]
- Rashid, Z.; Munir, M.M.; Woldesenbet, S.; Khan, M.M.M.; Khalil, M.; Endo, Y.; Tsilimigras, D.I.; Dillhoff, M.; Ejaz, A.; Pawlik, T.M. Association between social determinants of health and delayed postoperative adjuvant therapy among patients undergoing resection of pancreatic cancer. J. Surg. Oncol. 2024, 129, 850–859. [Google Scholar] [CrossRef]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zülke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant Chemotherapy with Gemcitabine and Long-term Outcomes Among Patients With Resected Pancreatic Cancer: The CONKO-001 Randomized Trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef]
- Azap, R.A.; Paredes, A.Z.; Diaz, A.; Hyer, J.M.; Pawlik, T.M. The association of neighborhood social vulnerability with surgical textbook outcomes among patients undergoing hepatopancreatic surgery. Surgery 2020, 168, 868–875. [Google Scholar] [CrossRef]
- Kutlu, O.C.; Vega, E.A.; Salehi, O.; Lathan, C.; Kim, S.; Krishnan, S.; Stallwood, C.; Kozyreva, O.; Conrad, C. Laparoscopic pancreatectomy for cancer in high volume centers is associated with an increased use and fewer delays of adjuvant chemotherapy. HPB 2021, 23, 625–632. [Google Scholar] [CrossRef]
- Lidsky, M.E.; Sun, Z.; Nussbaum, D.P.; Adam, M.A.; Speicher, P.J.; Blazer, D.G.I. Going the Extra Mile: Improved Survival for Pancreatic Cancer Patients Traveling to High-volume Centers. Ann. Surg. 2017, 266, 333–338. Available online: https://journals.lww.com/annalsofsurgery/fulltext/2017/08000/going_the_extra_mile__improved_survival_for.21.aspx (accessed on 15 February 2025). [CrossRef]
- Thomas, A.S.; Sharma, R.K.; Kwon, W.; Sugahara, K.N.; Chabot, J.A.; Schrope, B.A.; Kluger, M.D. Socioeconomic Predictors of Access to Care for Patients with Operatively Managed Pancreatic Cancer in New York State. J. Gastrointest. Surg. 2022, 26, 1647–1662. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T.L.; Beneville, B.; Johnson, A.J.; Mayo, S.C.; Gilbert, E.W.; Lopez, C.D.; Grossberg, A.J.; Rocha, F.G.; Sheppard, B.C. Socioeconomic and Geographic Disparities in the Referral and Treatment of Pancreatic Cancer at High-Volume Centers. JAMA Surg. 2023, 158, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Murimwa, G.Z.; Meier, J.; Nehrubabu, M.; Zeh, H.J.; Yopp, A.C.; Polanco, P.M. Implications of the interaction between travel burden and area deprivation for patients with pancreatic cancer. Am. J. Surg. 2023, 226, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Hegland, T.A.; Owens, P.L.; Selden, T.M. New evidence on geographic disparities in United States hospital capacity. Health Serv. Res. 2022, 57, 1006–1019. [Google Scholar] [CrossRef]
- Segel, J.E.; Hollenbeak, C.S.; Gusani, N.J. Rural-Urban Disparities in Pancreatic Cancer Stage of Diagnosis: Understanding the Interaction with Medically Underserved Areas. J. Rural. Health 2020, 36, 476–483. [Google Scholar] [CrossRef]
- Siegel, J.; Engelhardt, K.E.; Hornor, M.A.; Morgan, K.A.; Lancaster, W.P. Travel distance and its interaction with patient and hospital factors in pancreas cancer care. Am. J. Surg. 2021, 221, 819–825. [Google Scholar] [CrossRef]
- Chu, Q.D.; Hsieh, M.-C.; Gibbs, J.F.; Wu, X.-C. Social determinants of health associated with poor outcome for rural patients following resected pancreatic cancer. J. Gastrointest. Oncol. 2021, 12, 2567–2578. [Google Scholar] [CrossRef]
- Drewnowski, A. Food insecurity has economic root causes. Nat. Food 2022, 3, 555–556. [Google Scholar] [CrossRef]
- Chinaemelum, A.; Munir, M.M.; Azap, L.; Woldesenbet, S.; Dillhoff, M.; Cloyd, J.; Ejaz, A.; Pawlik, T.M. Impact of Food Insecurity on Outcomes Following Resection of Hepatopancreaticobiliary Cancer. Ann. Surg. Oncol. 2023, 30, 5365–5373. [Google Scholar] [CrossRef]
- Swords, D.S.; Mulvihill, S.J.; Brooke, B.S.; Skarda, D.E.; Firpo, M.A.; Scaife, C.L. Disparities in utilization of treatment for clinical stage I-II pancreatic adenocarcinoma by area socioeconomic status and race/ethnicity. Surgery 2019, 165, 751–759. [Google Scholar] [CrossRef]
- Madnick, D.; Handorf, E.; Ortiz, A.; Sorice, K.; Nagappan, L.; Moccia, M.; Cheema, K.; Vijayvergia, N.; Dotan, E.; Lynch, S.M. Investigating disparities: The effect of social environment on pancreatic cancer survival in metastatic patients. J. Gastrointest. Oncol. 2020, 11, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Yabroff, K.R.; Reeder-Hayes, K.; Zhao, J.; Halpern, M.T.; Lopez, A.M.; Bernal-Mizrachi, L.; Collier, A.B.; Neuner, J.; Phillips, J.; Blackstock, W.; et al. Health Insurance Coverage Disruptions and Cancer Care and Outcomes: Systematic Review of Published Research. JNCI J. Natl. Cancer Inst. 2020, 112, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Han, X.; Nogueira, L.; Fedewa, S.A.; Jemal, A.; Halpern, M.T.; Yabroff, K.R. Health insurance status and cancer stage at diagnosis and survival in the United States. CA Cancer J. Clin. 2022, 72, 542–560. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, J.M.; Taylor, A.; Vardell, V.; Silberstein, P.T. Socioeconomic Factors Associated With a Late-Stage Pancreatic Cancer Diagnosis: An Analysis of the National Cancer Database. Cureus 2023, 15, e35857. [Google Scholar] [CrossRef]
- Poulson, M.R.; Papageorge, M.V.; LaRaja, A.S.; Kenzik, K.M.; Sachs, T.E. Socioeconomic Mediation of Racial Segregation in Pancreatic Cancer Treatment and Outcome Disparities. Ann. Surg. 2023, 278, 246–252. [Google Scholar] [CrossRef]
- Fabregas, J.C.; Riley, K.E.; Brant, J.M.; George, T.J.; Orav, E.J.; Lam, M.B. Association of social determinants of health with late diagnosis and survival of patients with pancreatic cancer. J. Gastrointest. Oncol. 2022, 13, 1204–1214. [Google Scholar] [CrossRef]
- Hoehn, R.S.; Rieser, C.J.; Phelos, H.; Sabik, L.M.; Nassour, I.; Khan, S.; Kaltenmeier, C.; Paniccia, A.; Zureikat, A.H.; Tohme, S.T. Medicaid expansion and the management of pancreatic cancer. J. Surg. Oncol. 2021, 124, 324–333. [Google Scholar] [CrossRef]
- Fonseca, A.L.; Cherla, D.; Kothari, A.N.; Tzeng, C.-W.D.; Heslin, M.J.; Mehari, K.R.; Johnston, F.M.; Tran-Cao, H.S. Association of Medicaid Expansion with Pancreatic Cancer Treatment and Outcomes: Evidence from the National Cancer Database. Ann. Surg. Oncol. 2022, 29, 342–351. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, H.; Ashamalla, H. Racial and Socioeconomic Disparities in the Treatments and Outcomes of Pancreatic Cancer Among Different Treatment Facility Types. Pancreas 2020, 49, 1355–1363. [Google Scholar] [CrossRef]
- Thomas, A.S.; Tehranifar, P.; Kwon, W.; Shridhar, N.; Sugahara, K.N.; Schrope, B.A.; Chabot, J.A.; Manji, G.A.; Genkinger, J.M.; Kluger, M.D. Trends in the Care of Locally Advanced Pancreatic Cancer in the Modern Era of Chemotherapy. J. Surg. Oncol. 2024, 130, 1589–1604. [Google Scholar] [CrossRef]
- Hackert, T.; Sachsenmaier, M.; Hinz, U.; Schneider, L.; Michalski, C.W.; Springfeld, C.; Strobel, O.; Jäger, D.; Ulrich, A.; Büchler, M.W. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy with Folfirinox Results in Resectability in 60% of the Patients. Ann. Surg. 2016, 264, 457–463. Available online: https://journals.lww.com/annalsofsurgery/fulltext/2016/09000/locally_advanced_pancreatic_cancer__neoadjuvant.8.aspx (accessed on 10 January 2025). [CrossRef] [PubMed]
- Shavers, V.L.; Harlan, L.C.; Jackson, M.; Robinson, J. Racial/Ethnic Patterns of Care for Pancreatic Cancer. J. Palliat. Med. 2009, 12, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Flick, K.F.; Sublette, C.M.; Yip-Schneider, M.T.; Maatman, T.K.; Colgate, C.L.; Soufi, M.; Kelley, K.E.; Schmidt, C.M. Insurance Type and Marital Status Impact Hospital Length of Stay After Pancreatoduodenectomy. J. Surg. Res. 2021, 257, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Seldomridge, A.N.; Rasic, G.; Papageorge, M.V.; Ng, S.C.; de Geus, S.W.; Woods, A.P.; McAneny, D.; Tseng, J.F.; Sachs, T.E. Trends in access to minimally invasive pancreaticoduodenectomy for pancreatic cancers. HPB 2024, 26, 333–343. [Google Scholar] [CrossRef]
- Zajacova, A.; Lawrence, E.M. The Relationship Between Education and Health: Reducing Disparities Through a Contextual Approach. Annu. Rev. Public Health 2024, 39, 273–289. [Google Scholar] [CrossRef]
- Ben, Q.-W.; Liu, J.; Sun, Y.-W.; Wang, L.-F.; Zou, D.-W.; Yuan, Y.-Z. Cigarette Smoking and Mortality in Patients with Pancreatic Cancer: A Systematic Review and Meta-analysis. Pancreas 2019, 48, 985–995. Available online: https://journals.lww.com/pancreasjournal/fulltext/2019/09000/cigarette_smoking_and_mortality_in_patients_with.4.aspx (accessed on 10 January 2025). [CrossRef]
- Gram, I.T.; Park, S.-Y.; Wilkens, L.R.; Le Marchand, L.; Setiawan, V.W. Smoking and pancreatic cancer: A sex-specific analysis in the Multiethnic Cohort study. Cancer Causes Control 2023, 34, 89–100. [Google Scholar] [CrossRef]
- Weissman, S.; Takakura, K.; Eibl, G.; Pandol, S.J.; Saruta, M. The Diverse Involvement of Cigarette Smoking in Pancreatic Cancer Development and Prognosis. Pancreas 2020, 49, 612–620. [Google Scholar] [CrossRef]
- Zanini, S.; Renzi, S.; Limongi, A.R.; Bellavite, P.; Giovinazzo, F.; Bermano, G. A review of lifestyle and environment risk factors for pancreatic cancer. Eur. J. Cancer 2021, 145, 53–70. [Google Scholar] [CrossRef]
- Cao, P.; Jeon, J.; Tam, J.; Fleischer, N.L.; Levy, D.T.; Holford, T.R.; Meza, R. Smoking Disparities by Level of Educational Attainment and Birth Cohort in the U.S. Am. J. Prev. Med. 2023, 64, S22–S31. [Google Scholar] [CrossRef]
- Hamad, A.; DePuccio, M.; Reames, B.N.; Dave, A.; Kurien, N.; Cloyd, J.M.; Shen, C.; Pawlik, T.M.; Tsung, A.; McAlearney, A.S.; et al. Disparities in Stage-Specific Guideline-Concordant Cancer-Directed Treatment for Patients with Pancreatic Adenocarcinoma. J. Gastrointest. Surg. 2021, 25, 2889–2901. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.; Rademakers, J.; Waverijn, G.; Verheij, R.; Osborne, R.; Heijmans, M. The role of health literacy in explaining the association between educational attainment and the use of out-of-hours primary care services in chronically ill people: A survey study. BMC Health Serv. Res. 2018, 18, 394. [Google Scholar] [CrossRef] [PubMed]
- Humphrys, E.; Burt, J.; Rubin, G.; Emery, J.D.; Walter, F.M. The influence of health literacy on the timely diagnosis of symptomatic cancer: A systematic review. Eur. J. Cancer Care 2019, 28, e12920. [Google Scholar] [CrossRef] [PubMed]
- England, B.; Habib, J.R.; Sharma, A.R.; Hewitt, D.B.; Bridges, J.F.P.; Javed, A.A.; Wolfgang, C.L.; Braithwaite, R.S.; Sacks, G.D. Decisional Control Preferences in Managing Intraductal Papillary Mucinous Neoplasms of the Pancreas. Pancreas 2025, 54, e391–e396. Available online: https://journals.lww.com/pancreasjournal/fulltext/9900/decisional_control_preferences_in_managing.243.aspx (accessed on 10 January 2025). [CrossRef]
- Keating, N.L.; Beth Landrum, M.; Arora, N.K.; Malin, J.L.; Ganz, P.A.; Van Ryn, M.; Weeks, J.C. Cancer Patients’ Roles in Treatment Decisions: Do Characteristics of the Decision Influence Roles? J. Clin. Oncol. 2010, 28, 4364–4370. [Google Scholar] [CrossRef]
- Khawja, S.N.; Mohammed, S.; Silberfein, E.J.; Musher, B.L.; Fisher, W.E.; Van Buren, G. Pancreatic Cancer Disparities in African Americans. Pancreas 2015, 44, 522–527. [Google Scholar] [CrossRef]
- Wray, C.J.; Castro-Echeverry, E.; Silberfein, E.J.; Ko, T.C.; Kao, L.S. A Multi-institutional Study of Pancreatic Cancer in Harris County, Texas: Race Predicts Treatment and Survival. Ann. Surg. Oncol. 2012, 19, 2776–2781. [Google Scholar] [CrossRef]
- Singal, V.; Singal, A.K.; Kuo, Y.-F. Racial disparities in treatment for pancreatic cancer and impact on survival: A population-based analysis. J. Cancer Res. Clin. Oncol. 2012, 138, 715–722. [Google Scholar] [CrossRef]
- Chang, K.J.; Parasher, G.; Christie, C.; Largent, J.; Anton-Culver, H. Risk of pancreatic adenocarcinoma: Disparity between African Americans and other race/ethnic groups. Cancer 2005, 103, 349–357. [Google Scholar] [CrossRef]
- Shah, A.; Chao, K.S.C.; Østbye, T.; Castleberry, A.W.; Pietrobon, R.; Gloor, B.; Clary, B.M.; White, R.R.; Worni, M. Trends in Racial Disparities in Pancreatic Cancer Surgery. J. Gastrointest. Surg. 2013, 17, 1897–1906. [Google Scholar] [CrossRef]
- Eloubeidi, M.A.; Desmond, R.A.; Wilcox, C.M.; Wilson, R.J.; Manchikalapati, P.; Fouad, M.M.; Eltoum, I.; Vickers, S.M. Prognostic factors for survival in pancreatic cancer: A population-based study. Am. J. Surg. 2006, 192, 322–329. [Google Scholar] [CrossRef]
- Tohme, S.; Kaltenmeier, C.; Bou-Samra, P.; Varley, P.R.; Tsung, A. Race and Health Disparities in Patient Refusal of Surgery for Early-Stage Pancreatic Cancer: An NCDB Cohort Study. Ann. Surg. Oncol. 2018, 25, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Simons, J.P.; Hill, J.S.; McDade, T.P.; Chau Ng, S.; Whalen, G.F.; Shah, S.A.; Harrison, L.H.; Tseng, J.F. Pancreatic resection: A key component to reducing racial disparities in pancreatic adenocarcinoma. Cancer 2009, 115, 3979–3990. [Google Scholar] [CrossRef]
- Riner, A.N.; Girma, S.; Vudatha, V.; Mukhopadhyay, N.; Skoro, N.; Gal, T.S.; Freudenberger, D.C.; Herremans, K.M.; George, T.J.; Trevino, J.G. Eligibility Criteria Perpetuate Disparities in Enrollment and Participation of Black Patients in Pancreatic Cancer Clinical Trials. J. Clin. Oncol. 2022, 40, 2193–2202. [Google Scholar] [CrossRef]
- Eskander, M.F.; Gil, L.; Beal, E.W.; Li, Y.; Hamad, A.; Oppong, B.; Obeng-Gyasi, S.; Tsung, A. Access Denied: Inequities in Clinical Trial Enrollment for Pancreatic Cancer. Ann. Surg. Oncol. 2022, 29, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Halder, R.; Veeravelli, S.; Cheng, C.; Estrada-Mendizabal, R.J.; Recio-Boiles, A. Health Disparities in Presentation, Treatment, Genomic Testing, and Outcomes of Pancreatic Cancer in Hispanic and Non-Hispanic Patients. J. Racial Ethn. Health Disparities 2023, 10, 3131–3139. [Google Scholar] [CrossRef] [PubMed]
- Makar, M.; Worple, E.; Dove, J.; Hunsinger, M.; Arora, T.; Oxenberg, J.; Blansfield, J.A. Disparities in Care: Impact of Socioeconomic Factors on Pancreatic Surgery: Exploring the National Cancer Database. Am. Surg. 2019, 85, 327–334. [Google Scholar] [CrossRef]
- Nieuwenhuis, J. Neighborhood and community effects in East and Southeast Asia: A systematic review and meta-analytical exploration of publication bias. Asian J. Soc. Sci. 2022, 50, 237–249. [Google Scholar] [CrossRef]
- Yom, S.; Lor, M. Advancing Health Disparities Research: The Need to Include Asian American Subgroup Populations. J. Racial Ethn. Health Disparities 2022, 9, 2248–2282. [Google Scholar] [CrossRef]
- Johnson, S.B.; Park, H.S.; Gross, C.P.; Yu, J.B. Use of Alternative Medicine for Cancer and Its Impact on Survival. JNCI J. Natl. Cancer Inst. 2018, 110, 121–124. [Google Scholar] [CrossRef]
- Johnson, S.B.; Park, H.S.; Gross, C.P.; Yu, J.B. Complementary Medicine, Refusal of Conventional Cancer Therapy, and Survival Among Patients with Curable Cancers. JAMA Oncol. 2018, 4, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- So, T.-H.; Chan, S.-K.; Lee, V.H.-F.; Chen, B.-Z.; Kong, F.-M.; Lao, L.-X. Chinese Medicine in Cancer Treatment—How is it Practised in the East and the West? Clin. Oncol. 2019, 31, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; McLeod, M.C.; Song, Z.; Chen, H.; Rose, J.B.; Bhatia, S.; Gillis, A. Exploring Disparities in Pancreatic Ductal Adenocarcinoma Outcomes among Asian and Pacific Islander Subgroups. Cancer Res. Commun. 2024, 4, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Melkonian, S.C.; Jim, M.A.; Pete, D.; Poel, A.; Dominguez, A.E.; Echo-Hawk, A.; Zhang, S.; Wilson, R.J.; Haverkamp, D.; Petras, L.; et al. Cancer disparities among non-Hispanic urban American Indian and Alaska Native populations in the United States 1999–2017. Cancer 2022, 128, 1626–1636. [Google Scholar] [CrossRef]
- Commonly Asked Questions and Answers: Regarding Limited English Proficient (LEP) Individuals; Federal Coordination and Compliance Section: Washington, DC, USA, 2011. Available online: https://www.lep.gov/sites/lep/files/media/document/2020-03/042511_QA_LEP_General_0.pdf (accessed on 10 January 2025).
- Gonzalez-Barrera, A.; Hamel, L.; Artiga, S.; Presaido, M. Language Barriers in Health Care: Findings from the KFF Survey on Racism, Discrimination, and Health; KFF: San Francisco, CA, USA, 2024; Available online: https://www.kff.org/racial-equity-and-health-policy/poll-finding/language-barriers-in-health-care-findings-from-the-kff-survey-on-racism-discrimination-and-health/ (accessed on 10 January 2025).
- Calvillo-Ortiz, R.; Polanco-Santana, J.C.; Castillo-Angeles, M.; Allar, B.G.; Anguiano-Landa, L.; Ghaffarpasand, E.; Barrows, C.; Callery, M.P.; Kent, T.S. Language Proficiency and Survival in Pancreatic Cancer: A Propensity Score-Matched Analysis. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2022, 26, 94–103. [Google Scholar] [CrossRef]
- Williams, C.; Chong, G.; Tacey, M.; Barnett, F.; Mooi, J.; Jalali, A.; Hodgson, R.; Yong, T.; Lee, B. Addressing disparities in health outcomes for patients with advanced pancreatic cancer and limited English proficiency. Asia Pac. J. Clin. Oncol. 2024, 20, 531–536. [Google Scholar] [CrossRef]
- Sridharan, A.; Dotan, E.; Dorta, M.; Vemula, N.; Handorf, E.; Deng, M.; Renning, A.; Sorice, K.; Laderman, L.; Whittington, K.; et al. Racial/Ethnic Differences and Effects of Clinical/Socioeconomic Factors on Time from Diagnosis to Treatment in Pancreatic Cancer. J. Gastrointest. Cancer 2025, 56, 67. [Google Scholar] [CrossRef]
- Kim, J.; Ji, E.; Jung, K.; Jung, I.H.; Park, J.; Lee, J.-C.; Kim, J.W.; Hwang, J.-H.; Kim, J. Gender Differences in Patients with Metastatic Pancreatic Cancer Who Received FOLFIRINOX. J. Pers. Med. 2021, 11, 83. [Google Scholar] [CrossRef]
- Pijnappel, E.N.; Schuurman, M.; Wagner, A.D.; de Vos-Geelen, J.; van der Geest, L.G.M.; de Groot, J.-W.B.; Koerkamp, B.G.; de Hingh, I.H.J.T.; Homs, M.Y.V.; Creemers, G.-J.; et al. Sex, Gender and Age Differences in Treatment Allocation and Survival of Patients with Metastatic Pancreatic Cancer: A Nationwide Study. Front. Oncol. 2022, 12, 839779. [Google Scholar] [CrossRef]
- Gehrels, A.M.; Wagner, A.D.; Besselink, M.G.; Verhoeven, R.H.A.; van Eijck, C.H.J.; van Laarhoven, H.W.M.; Wilmink, J.W.; van der Geest, L.G.; Dutch Pancreatic Cancer Group. Gender differences in tumor characteristics, treatment allocation and survival in stage I-III pancreatic cancer: A nationwide study. Eur. J. Cancer 2024, 206, 114117. [Google Scholar] [CrossRef]
- Bauer, M.R.; Bright, E.E.; MacDonald, J.J.; Cleary, E.H.; Hines, O.J.; Stanton, A.L. Quality of Life in Patients with Pancreatic Cancer and Their Caregivers: A Systematic Review. Pancreas 2018, 47, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.; Crowe, L.; Mentor, K.; Pandanaboyana, S.; Sharp, L. Systematic review of caregiver burden, unmet needs and quality-of-life among informal caregivers of patients with pancreatic cancer. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2022, 31, 74. [Google Scholar] [CrossRef] [PubMed]
- Fong, Z.V.; Teinor, J.; Engineer, L.; Yeo, T.P.; Rinaldi, D.; Greer, J.B.; Lavu, H.; Qadan, M.; Johnston, F.M.; Ferrone, C.R.; et al. Caregiver-reported quality of communication in pancreatic and periampullary cancer. Cancer 2024, 130, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Willis, K.D.; Jacobs, J.M. Closing the Caregiving Gap: Considerations for Pancreatic and Periampullary Cancer Caregivers. JCO Oncol. Pract. 2023, 19, 523–525. [Google Scholar] [CrossRef]
- Engebretson, A.; Matrisian, L.; Thompson, C. Pancreatic cancer: Patient and caregiver perceptions on diagnosis, psychological impact, and importance of support. Pancreatology 2015, 15, 701–707. [Google Scholar] [CrossRef]
- Sridhar, P.; Misir, P.; Kwak, H.; deGeus, S.W.; Drake, F.T.; Cassidy, M.R.; McAneny, D.A.; Tseng, J.F.; Sachs, T.E. Impact of Race, Insurance Status, and Primary Language on Presentation, Treatment, and Outcomes of Patients with Pancreatic Adenocarcinoma at a Safety-Net Hospital. J. Am. Coll. Surg. 2019, 229, 389–396. [Google Scholar] [CrossRef]
- Prevention Institute. What? Why? How? Answers to Frequently Asked Questions About the Adverse Community Experiences and Resilience Framework; Prevention Institute: Oakland, CA, USA, 2017. [Google Scholar]
- Feliciano, E.J.G.; Ho, F.D.V.; Yee, K.; Paguio, J.A.; Eala, M.A.B.; Robredo, J.P.G.; Ng, K.; Lim, J.; Pyone, K.T.; Peralta, C.A.; et al. Cancer disparities in Southeast Asia: Intersectionality and a call to action. Lancet Reg. Health West. Pac. 2023, 41, 100971. [Google Scholar] [CrossRef]
- Ioerger, P.; Mills, K.; Wagoner, S.F.; Lawrence, A.; Alapati, R.; Nallani, R.; Hamill, C.S.; Adjei Boakye, E.; Sykes, K.J. Inequities Associated with Advanced Stage at Presentation of Head and Neck Cancer: A Systematic Review. JAMA Otolaryngol. Head Neck Surg. 2024, 150, 727. [Google Scholar] [CrossRef]
- Hoehn, R.S.; Rieser, C.J.; Winters, S.; Stitt, L.; Hogg, M.E.; Bartlett, D.L.; Lee, K.K.; Paniccia, A.; Ohr, J.P.; Gorantla, V.C.; et al. A Pancreatic Cancer Multidisciplinary Clinic Eliminates Socioeconomic Disparities in Treatment and Improves Survival. Ann. Surg. Oncol. 2021, 28, 2438–2446. [Google Scholar] [CrossRef]
- Tripepi, M.; Pizzocaro, E.; Giardino, A.; Frigerio, I.; Guglielmi, A.; Butturini, G. Telemedicine and Pancreatic Cancer: A Systematic Review. Telemed. E-Health 2023, 29, 352–360. [Google Scholar] [CrossRef]
- Freeman, H.P.; Rodriguez, R.L. History and principles of patient navigation. Cancer 2011, 117, 3537–3540. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, V.S.; Falk, D.; Cheatham, C.; Cullen, J.; Hoehn, R. Patient Navigation in Cancer Treatment: A Systematic Review. Curr. Oncol. Rep. 2024, 26, 504–537. [Google Scholar] [CrossRef] [PubMed]
- Rosowicz, A.; Hewitt, D.B. Disparities in Cancer Screening Among the Foreign-Born Population in the United States: A Narrative Review. Cancers 2025, 17, 576. [Google Scholar] [CrossRef] [PubMed]
- Bazargan, M.; Cobb, S.; Assari, S. Discrimination and Medical Mistrust in a Racially and Ethnically Diverse Sample of California Adults. Ann. Fam. Med. 2021, 19, 4–15. [Google Scholar] [CrossRef]
- Bellhouse, S.; McWilliams, L.; Firth, J.; Yorke, J.; French, D.P. Are community-based health worker interventions an effective approach for early diagnosis of cancer? A systematic review and meta-analysis. Psycho-Oncology 2018, 27, 1089–1099. [Google Scholar] [CrossRef]
- Williams, O.; Ting, T.; Matthews, L.; Block, G.; Block, T.; Teresi, J.; Eimicke, J.; Kong, J.; Silver, S.; Ravenell, J.; et al. Community Health workers United to Reduce Colorectal cancer and cardiovascular disease among people at Higher risk (CHURCH): Study protocol for a randomized controlled trial. Trials 2024, 25, 283. [Google Scholar] [CrossRef]
- Munigala, S.; Gardner, T.B.; O’Reilly, E.M.; Castillo, C.F.; Ko, A.H.; Pleskow, D.; Mills, J.B.; Vollmer, C.M.; Searle, N.A.; Alsante, M.; et al. Understanding Pancreatic Diseases Using Animated Pancreas Patient: Informing Patients for Better Health Outcomes with Visual Formats of Learning. Pancreas 2018, 47, 1256–1261. [Google Scholar] [CrossRef]
- Morey, B.N.; Valencia, C.; Lee, S. The Influence of Asian Subgroup and Acculturation on Colorectal Cancer Screening Knowledge and Attitudes Among Chinese and Korean Americans. J. Cancer Educ. 2022, 37, 1806–1815. [Google Scholar] [CrossRef]
- Gustavell, T.; Langius-Eklöf, A.; Wengström, Y.; Segersvärd, R.; Sundberg, K. Development and Feasibility of an Interactive Smartphone App for Early Assessment and Management of Symptoms Following Pancreaticoduodenectomy. Cancer Nurs. 2019, 42, E1–E10. [Google Scholar] [CrossRef]
- Loehrer, A.P.; Chang, D.C.; Hutter, M.M.; Song, Z.; Lillemoe, K.D.; Warshaw, A.L.; Ferrone, C.R. Health Insurance Expansion and Treatment of Pancreatic Cancer: Does Increased Access Lead to Improved Care? J. Am. Coll. Surg. 2015, 221, 1015–1022. [Google Scholar] [CrossRef]
| Social Determinants | Disparities | Possible Interventions |
|---|---|---|
| SES Race Education Level Insurance Status | Delayed diagnosis Delayed treatment Lack of treatment (surgery, chemotherapy, radiation) Longer LOS Perioperative complications Decreased survival | Treatment at MDC Safety-net hospitals Telemedicine Patient navigators Community health workers Culturally competent care Simplified communication Expanding insurance access Broadening clinical trial eligibility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.N.; Habib, J.R.; Hewitt, D.B.; Kluger, M.D.; Morgan, K.; Javed, A.A.; Wolfgang, C.L.; Sacks, G.D. The Impact of Social Determinants on Pancreatic Cancer Care in the United States. Cancers 2025, 17, 1898. https://doi.org/10.3390/cancers17121898
Patel SN, Habib JR, Hewitt DB, Kluger MD, Morgan K, Javed AA, Wolfgang CL, Sacks GD. The Impact of Social Determinants on Pancreatic Cancer Care in the United States. Cancers. 2025; 17(12):1898. https://doi.org/10.3390/cancers17121898
Chicago/Turabian StylePatel, Shreeja N., Joseph R. Habib, Daniel Brock Hewitt, Michael D. Kluger, Katherine Morgan, Ammar A. Javed, Christopher L. Wolfgang, and Greg D. Sacks. 2025. "The Impact of Social Determinants on Pancreatic Cancer Care in the United States" Cancers 17, no. 12: 1898. https://doi.org/10.3390/cancers17121898
APA StylePatel, S. N., Habib, J. R., Hewitt, D. B., Kluger, M. D., Morgan, K., Javed, A. A., Wolfgang, C. L., & Sacks, G. D. (2025). The Impact of Social Determinants on Pancreatic Cancer Care in the United States. Cancers, 17(12), 1898. https://doi.org/10.3390/cancers17121898







