The Prognostic Value of FOXL2 Mutant Circulating Tumor DNA in Adult Granulosa Cell Tumor Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
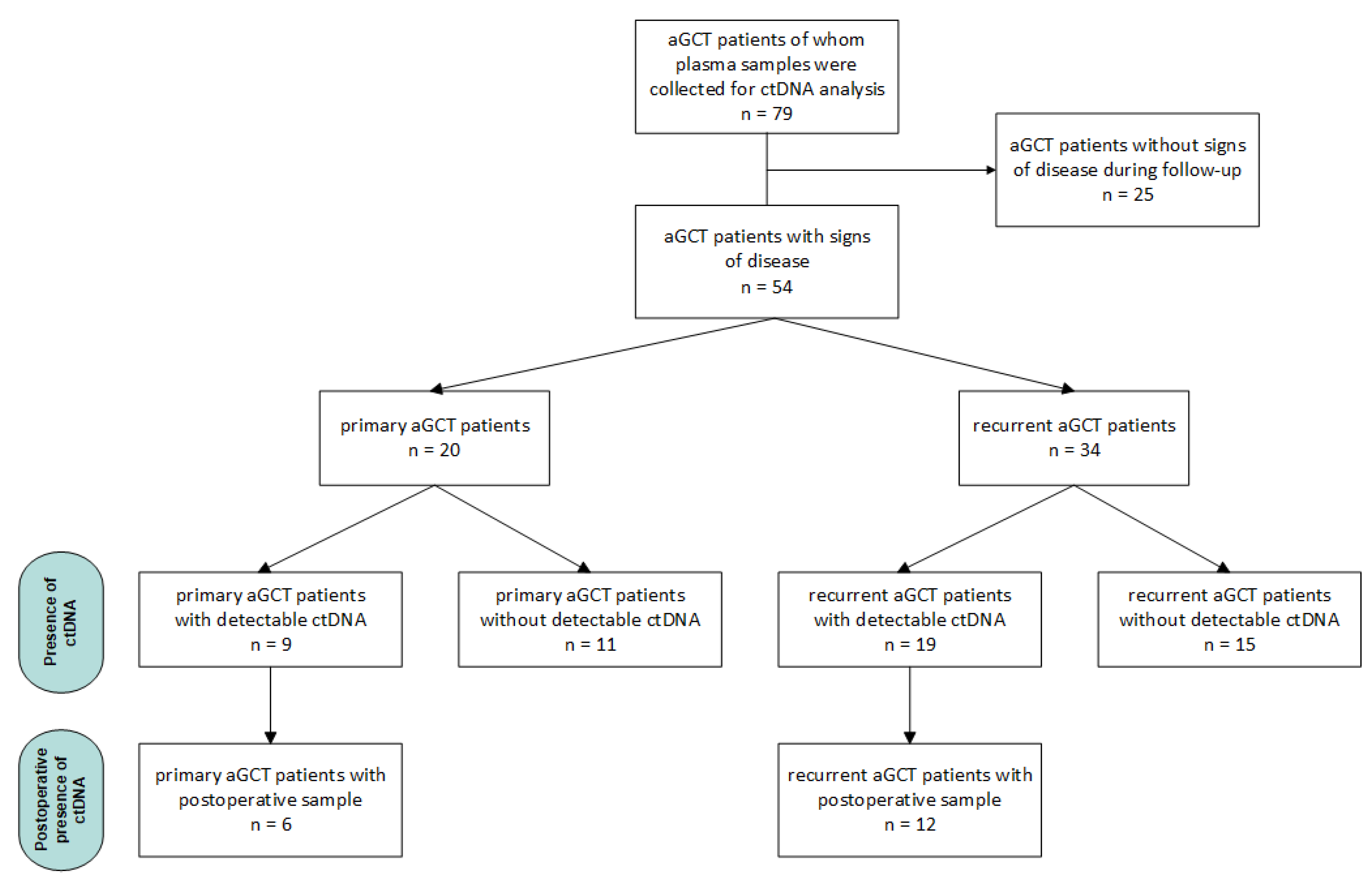
2.1. Samples and Patients
2.2. Sample Preparation and Digital Droplet PCR
2.3. Data and Statistical Analysis
3. Results
3.1. Primary aGCT Patients
3.1.1. Prognostic Value of the Presence of ctDNA
3.1.2. Postoperative Detection of ctDNA
3.2. Recurrent aGCT Patients
3.2.1. Prognostic Value of the Presence of ctDNA
3.2.2. Postoperative Detection of ctDNA
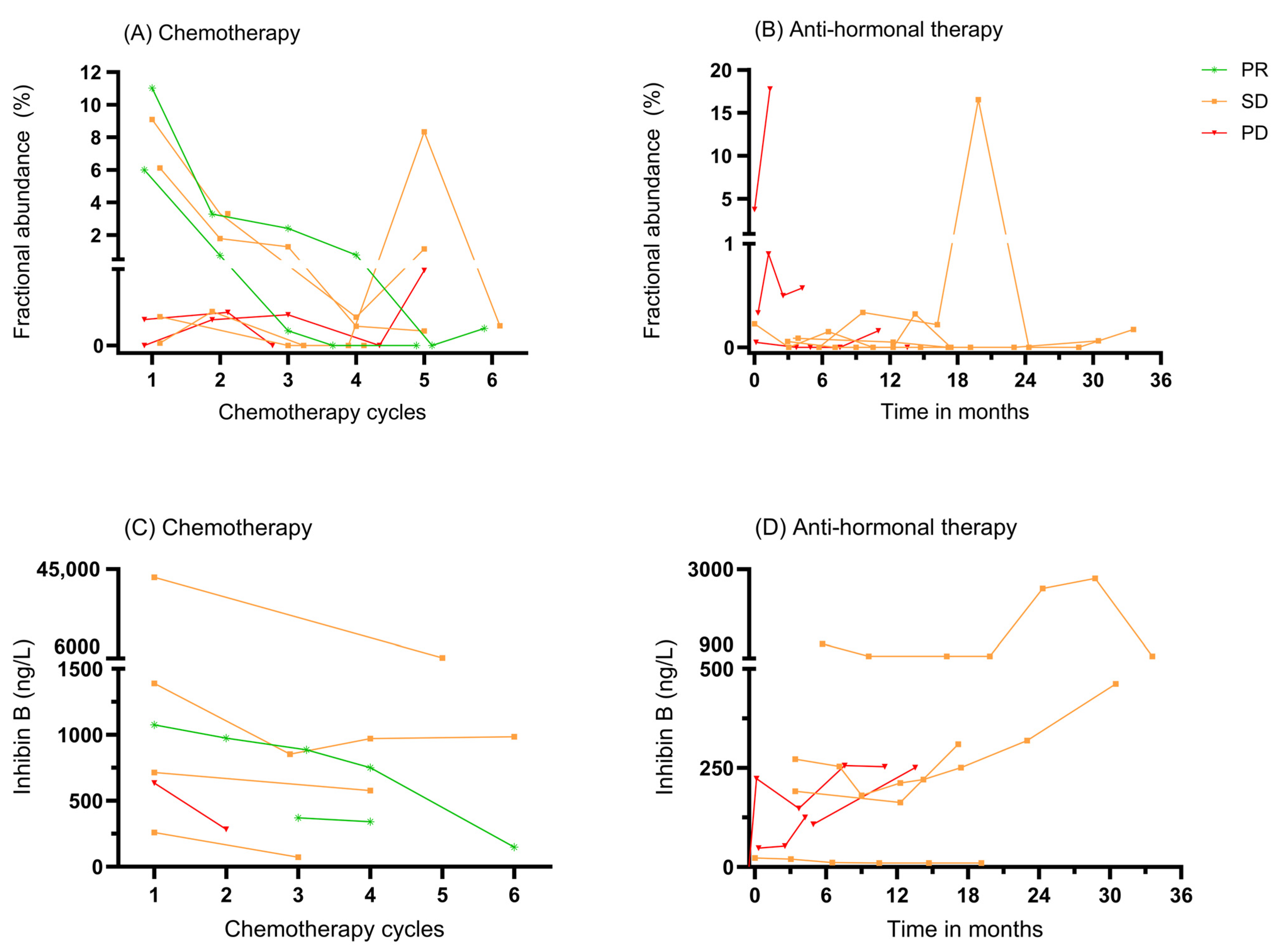
3.3. ctDNA as a Biomarker for Monitoring Systemic Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aGCT | Adult-type granulosa cell tumor |
| AMH | Anti-Müllerian hormone |
| AWD | Alive with disease |
| cfDNA | Cell-free DNA |
| CR | Complete response |
| ctDNA | Circulating tumor DNA |
| ddPCR | Digital droplet PCR |
| DOC | Died of other causes |
| DOD | Died of disease |
| IQR | Interquartile range |
| NED | No evidence of disease |
| OS | Overall survival |
| PD | Progressive disease |
| PR | Partial response |
| RFS | Recurrence-free survival |
| SD | Stable disease |
Appendix A. A Detailed Protocol of Sample Preparation and ddPCR Analysis
References
- Ferlay, J.E.M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.who.int/today (accessed on 11 June 2024).
- Shah, S.P.; Köbel, M.; Senz, J.; Morin, R.D.; Clarke, B.A.; Wiegand, K.C.; Leung, G.; Zayed, A.; Mehl, E.; Kalloger, S.E. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N. Engl. J. Med. 2009, 360, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Schumer, S.T.; Cannistra, S.A. Granulosa cell tumor of the ovary. J. Clin. Oncol. 2003, 21, 1180–1189. [Google Scholar] [CrossRef]
- Färkkilä, A.; Koskela, S.; Bryk, S.; Alfthan, H.; Bützow, R.; Leminen, A.; Puistola, U.; Tapanainen, J.S.; Heikinheimo, M.; Anttonen, M. The clinical utility of serum anti-M üllerian hormone in the follow-up of ovarian adult-type granulosa cell tumors—A comparative study with inhibin B. Int. J. Cancer 2015, 137, 1661–1671. [Google Scholar] [CrossRef]
- Rey, R.A.; Lhommé, C.; Marcillac, I.; Lahlou, N.; Duvillard, P.; Josso, N.; Bidart, J.M. Antimüllerian hormone as a serum marker of granulosa cell tumors of the ovary: Comparative study with serum α-inhibin and estradiol. Am. J. Obstet. Gynecol. 1996, 174, 958–965. [Google Scholar] [CrossRef]
- Mom, C.H.; Engelen, M.J.; Willemse, P.H.; Gietema, J.A.; ten Hoor, K.A.; de Vries, E.G.; van der Zee, A.G. Granulosa cell tumors of the ovary: The clinical value of serum inhibin A and B levels in a large single center cohort. Gynecol. Oncol. 2007, 105, 365–372. [Google Scholar] [CrossRef]
- Geerts, I.; Vergote, I.; Neven, P.; Billen, J. The role of inhibins B and antimüllerian hormone for diagnosis and follow-up of granulosa cell tumors. Int. J. Gynecol. Cancer 2009, 19, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Boggess, J.F.; Soules, M.R.; Goff, B.A.; Greer, B.E.; Cain, J.M.; Tamimi, H.K. Serum inhibin and disease status in women with ovarian granulosa cell tumors. Gynecol. Oncol. 1997, 64, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Yamoto, M.; Shikone, T.; Minami, S.; Imai, M.; Nishimori, K.; Nakano, R. Production of inhibin A and inhibin B in human ovarian sex cord stromal tumors. Am. J. Obstet. Gynecol. 1997, 177, 1450–1457. [Google Scholar] [CrossRef]
- Petraglia, F.; Luisi, S.; Pautier, P.; Sabourin, J.-C.; Rey, R.; Lhomme, C.; Bidart, J.-M. Inhibin B is the major form of inhibin/activin family secreted by granulosa cell tumors. J. Clin. Endocrinol. Metab. 1998, 83, 1029–1032. [Google Scholar] [CrossRef]
- Portuesi, R.; Loppini, A.; Mancari, R.; Filippi, S.; Colombo, N. Role of inhibin B in detecting recurrence of granulosa cell tumors of the ovary in postmenopausal patients. Int. J. Gynecol. Cancer 2021, 31, 893–898. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Lee, S.-W.; Lee, Y.-J.; Lee, H.-Y.; Lee, J.-E.; Choi, E.-K. Prospective study of the efficacy and utility of TP53 mutations in circulating tumor DNA as a non-invasive biomarker of treatment response monitoring in patients with high-grade serous ovarian carcinoma. J. Gynecol. Oncol. 2019, 30, e32. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.G.; Malapelle, U.; André, F.; Paz-Ares, L.; Schuler, M.; Thomas, D.M.; Vainer, G.; Yoshino, T.; Rolfo, C. Practical considerations for the use of circulating tumor DNA in the treatment of patients with cancer: A narrative review. JAMA Oncol. 2022, 8, 1830–1839. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.W.; Selenica, P.; Patel, J.; Wu, M.; Nincevic, J.; Lakhman, Y.; Zhou, Q.; Shah, R.H.; Berger, M.F.; Da Cruz Paula, A. High-sensitivity mutation analysis of cell-free DNA for disease monitoring in endometrial cancer. Clin. Cancer Res. 2023, 29, 410–421. [Google Scholar] [CrossRef]
- Hou, J.Y.; Chapman, J.S.; Kalashnikova, E.; Pierson, W.; Smith-McCune, K.; Pineda, G.; Vattakalam, R.M.; Ross, A.; Mills, M.; Suarez, C.J. Circulating tumor DNA monitoring for early recurrence detection in epithelial ovarian cancer. Gynecol. Oncol. 2022, 167, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.; Wang, J.H.; O’Leary, D.P.; Corrigan, M.; Redmond, H.P. Association of preoperative and postoperative circulating tumour DNA (ctDNA) with PIK3CA gene mutation with risk of recurrence in patients with non-metastatic breast cancer. Surg. Oncol. 2024, 54, 102060. [Google Scholar] [CrossRef]
- Recio, F.; Scalise, C.B.; Loar, P.; Lumish, M.; Berman, T.; Peddada, A.; Kalashnikova, E.; Rivero-Hinojosa, S.; Beisch, T.; Nicosia, B. Post-surgical ctDNA-based molecular residual disease detection in patients with stage I uterine malignancies. Gynecol. Oncol. 2024, 182, 63–69. [Google Scholar] [CrossRef]
- Färkkilä, A.; McConechy, M.K.; Yang, W.; Talhouk, A.; Ng, Y.; Lum, A.; Morin, R.D.; Bushell, K.; Riska, A.; McAlpine, J.N. FOXL2 402C> G mutation can be identified in the circulating tumor DNA of patients with adult-type granulosa cell tumor. J. Mol. Diagn. 2017, 19, 126–136. [Google Scholar] [CrossRef]
- Groeneweg, J.W.; Roze, J.F.; Peters, E.D.; Sereno, F.; Brink, A.G.; Paijens, S.T.; Nijman, H.W.; van Meurs, H.S.; van Lonkhuijzen, L.R.; Piek, J.M. FOXL2 and TERT promoter mutation detection in circulating tumor DNA of adult granulosa cell tumors as biomarker for disease monitoring. Gynecol. Oncol. 2021, 162, 413–420. [Google Scholar] [CrossRef]
- Brink, G.J.; Groeneweg, J.W.; Sickinghe, A.A.; Nijman, H.W.; van Lonkhuijzen, L.R.; Lok, C.A.; Piek, J.M.; Roes, E.M.; de Kroon, C.D.; Hofhuis, W. Is it time to abandon staging surgery and prolonged follow-up in patients with primary adult-type granulosa cell tumor? J. Ovarian Res. 2025, 18, 37. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Ogasawara, A.; Hihara, T.; Shintani, D.; Yabuno, A.; Ikeda, Y.; Tai, K.; Fujiwara, K.; Watanabe, K.; Hasegawa, K. Evaluation of circulating tumor DNA in patients with ovarian cancer harboring somatic PIK3CA or KRAS mutations. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2020, 52, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Kim, Y.-N.; Shin, S.; Lee, K.; Lee, J.-H.; Lee, Y.J.; Choi, Z.; Park, J.; Min, S.; Kim, S.W. Serial Circulating Tumor DNA Analysis with a Tumor-Naïve Next-Generation Sequencing Panel Detects Minimal Residual Disease and Predicts Outcome in Ovarian Cancer. Cancer Res. 2024, 84, 468–478. [Google Scholar] [CrossRef] [PubMed]
- van Rees, J.M.; Wullaert, L.; Grüter, A.A.J.; Derraze, Y.; Tanis, P.J.; Verheul, H.M.W.; Martens, J.W.M.; Wilting, S.M.; Vink, G.; van Vugt, J.L.A.; et al. Circulating tumour DNA as biomarker for rectal cancer: A systematic review and meta-analyses. Front. Oncol. 2023, 13, 1083285. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Dong, Z.; Song, Q.; Wang, Z. A meta-analysis of circulating tumor DNA as a survival indicator in small cell lung cancer patients. Clin. Exp. Med. 2023, 23, 3935–3945. [Google Scholar] [CrossRef]
- Nakamura, Y.; Watanabe, J.; Akazawa, N.; Hirata, K.; Kataoka, K.; Yokota, M.; Kato, K.; Kotaka, M.; Kagawa, Y.; Yeh, K.-H.; et al. ctDNA-based molecular residual disease and survival in resectable colorectal cancer. Nat. Med. 2024, 30, 3272–3283. [Google Scholar] [CrossRef]
- Dickinson, K.; Sharma, A.; Agnihotram, R.-K.V.; Altuntur, S.; Park, M.; Meterissian, S.; Burnier, J.V. Circulating Tumor DNA and Survival in Metastatic Breast Cancer: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2024, 7, e2431722. [Google Scholar] [CrossRef]
- Tie, J.; Wang, Y.; Tomasetti, C.; Li, L.; Springer, S.; Kinde, I.; Silliman, N.; Tacey, M.; Wong, H.-L.; Christie, M.; et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 2016, 8, 346ra392. [Google Scholar] [CrossRef]
- Moding, E.J.; Nabet, B.Y.; Alizadeh, A.A.; Diehn, M. Detecting Liquid Remnants of Solid Tumors: Circulating Tumor DNA Minimal Residual Disease. Cancer Discov. 2021, 11, 2968–2986. [Google Scholar] [CrossRef] [PubMed]
- Pellini, B.; Chaudhuri, A.A. Circulating Tumor DNA Minimal Residual Disease Detection of Non–Small-Cell Lung Cancer Treated With Curative Intent. J. Clin. Oncol. 2022, 40, 567–575. [Google Scholar] [CrossRef]
- Kallio, H.M.; Savolainen, K.; Virtanen, T.; Ryyppö, L.; Selin, H.; Martikainen, P.; Staff, S.; Kivinummi, K.; Sipola, J.; Vuorinen, J. Sensitive circulating tumor DNA–based residual disease detection in epithelial ovarian cancer. Life Sci. Alliance 2024, 7, e202402658. [Google Scholar] [CrossRef]
- Shu, T.; Liang, Y.; Zhang, S.; Sun, T.; Gao, Y.; Guo, C.; Li, Z.; Gao, M.; Zhang, N.; Song, N. The prognostic value of tumor-informed minimal residual disease detection using circulating tumor DNA in first-line treatment of ovarian cancer. Gynecol. Oncol. 2025, 192, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, Y.; Xiao, X.; Xu, S.; Zhu, S.; Cao, D.; Yu, M.; Peng, P.; Wang, J.; Wang, Y. Circulating tumor DNA detection improves relapse prediction in epithelial ovarian cancer. BMC Cancer 2024, 24, 1565. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, A.W.; Litiere, S.; Bidard, F.-C.; Cabel, L.; Dyrskjøt, L.; Karlovich, C.A.; Pantel, K.; Petrie, J.; Philip, R.; Andrews, H.S.; et al. Plasma ctDNA as a Treatment Response Biomarker in Metastatic Cancers: Evaluation by the RECIST Working Group. Clin. Cancer Res. 2024, 30, 5034–5041. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Schuuring, E.; Vessies, D.C.L.; van der Leest, P.; Geerlings, M.J.; Rozendal, P.; Lanfermeijer, M.; Linders, T.C.; van Kempen, L.C.; Fijneman, R.J.A.; et al. A Micro-Costing Framework for Circulating Tumor DNA Testing in Dutch Clinical Practice. J. Mol. Diagn. 2023, 25, 36–45. [Google Scholar] [CrossRef]
- Kramer, A.; Rubio-Alarcón, C.; van den Broek, D.; Vessies, D.C.L.; Van’t Erve, I.; Meijer, G.A.; Vink, G.R.; Schuuring, E.; Fijneman, R.J.A.; Coupé, V.M.H.; et al. A scenario-drafting study to explore potential future implementation pathways of circulating tumor DNA testing in oncology. Mol. Oncol. 2024, 18, 2730–2742. [Google Scholar] [CrossRef]
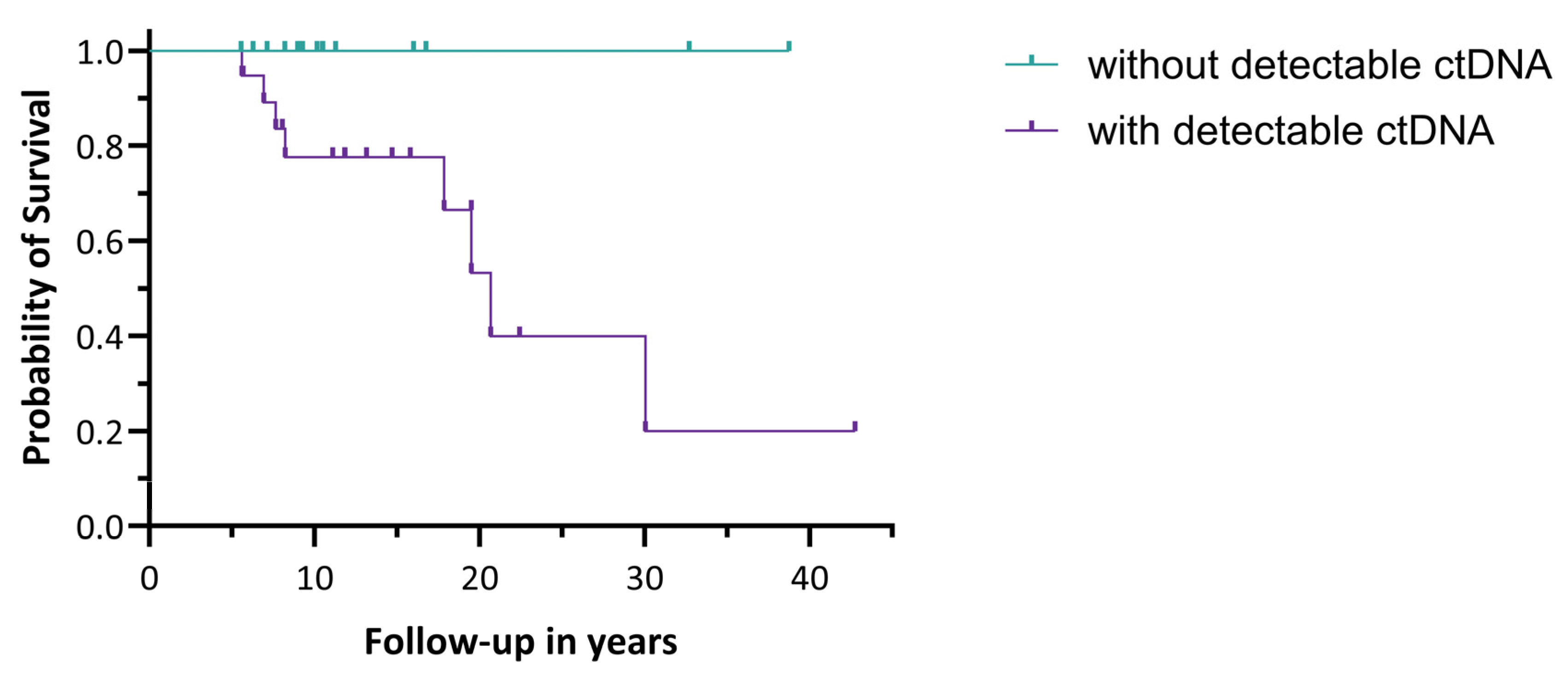
| Characteristics | Total | Detectable ctDNA | Non-Detectable ctDNA | p-Value |
|---|---|---|---|---|
| Patients | 54 | 28 | 26 | |
| Year of diagnosis | 2015 (2010–2021) | 2013 (2006–2018) | 2017 (2014–2021) | 0.046 |
| Age at diagnosis in years | 49 (42–62) | 49 (44–56) | 50 (39–67) | 0.808 |
| Tumor stage at diagnosis | 0.610 | |||
| IA | 23 (43) | 11 (39) | 12 (46) | |
| IB | 1 (2) | 1 (4) | 0 (0) | |
| IC | 26 (48) | 15 (53) | 11 (42) | |
| II | 3 (5) | 1 (4) | 2 (8) | |
| III | 1 (2) | 0 (0) | 1 (4) | |
| Follow-up time in years | 8.2 (3.1–15.0) | 10.0 (5.6–17.7) | 6.7 (2.1–10.5) | 0.087 |
| Recurrence | 0.366 | |||
| Yes | 39 (72) | 22 (79) | 17 (65) | |
| No | 15 (28) | 6 (21) | 9 (35) | |
| Disease status | 0.005 | |||
| No evidence of disease | 19 (35) | 7 (25) | 12 (46) | |
| Alive with disease | 26 (48) | 12 (43) | 14 (54) | |
| Dead of disease | 9 (17) | 9 (32) | 0 (0) | |
| Highest fractional abundance | 1.34 (0.33–9.00) |
| Patient ID | Weeks After Surgery | Preop FA% | Postop FA% | Inhibin B (ng/L) | Recurrence During Follow-Up | Duration FU (Years) |
|---|---|---|---|---|---|---|
| P1 | 7 | n/a | 0.12 | 10 | − | 6 |
| P2 | 4 | 7.90 | 0.44 | 10 | + | 1 |
| P3 | 3 | n/a | 0.05 | 10 | + | 4 |
| P4 | 7 | 0.92 | 0.00 | 10 | − | 3 |
| P5 | 2 | 8.74 | 0.00 | n/a | − | 3 |
| P6 | 5 | 0.26 | 0.00 | n/a | − | 1 |
| Patient ID | Number of Recurrences | Weeks After Surgery | Preop FA% | Postop FA% | Inhibin B (ng/L) | New Recurrence During Follow-Up |
|---|---|---|---|---|---|---|
| P7 | 3 | 7 | 18.1 | 0.70 | 62 | + |
| P8 | 3 | 7 | 0.15 | 1.86 | 84 | + |
| P9 * | 4 | 13 | 7.54 | 0.15 | 87 | − |
| P10 | 5 | 7 | n/a | 0.06 | 101 | + |
| P11 | 2 | 13 | 0.22 | 0.00 | 10 | + |
| P12 | 1 | 10 | n/a | 0.00 | 133 | + |
| P13 | 4 | 6 | 0.00 | 0.11 | 246 | + |
| P14 | 2 | 7 | n/a | 0.06 | 11 | + |
| P15 | 2 | 7 | 1.44 | 0.22 | 25 | + |
| P16 | 4 | 6 | n/a | 0.00 | 208 | + |
| P17 | 1 | 9 | 0.02 | 0.12 | 10 | + |
| P18 | 1 | 5 | 0.43 | 0.23 | 833 | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brink, G.J.; Hami, N.; Nijman, H.W.; Piek, J.M.J.; van Lonkhuijzen, L.R.C.W.; Roes, E.M.; Hofhuis, W.; Lok, C.A.R.; de Kroon, C.D.; Gort, E.H.; et al. The Prognostic Value of FOXL2 Mutant Circulating Tumor DNA in Adult Granulosa Cell Tumor Patients. Cancers 2025, 17, 1894. https://doi.org/10.3390/cancers17111894
Brink GJ, Hami N, Nijman HW, Piek JMJ, van Lonkhuijzen LRCW, Roes EM, Hofhuis W, Lok CAR, de Kroon CD, Gort EH, et al. The Prognostic Value of FOXL2 Mutant Circulating Tumor DNA in Adult Granulosa Cell Tumor Patients. Cancers. 2025; 17(11):1894. https://doi.org/10.3390/cancers17111894
Chicago/Turabian StyleBrink, Geertruid J., Nizar Hami, Hans W. Nijman, Jurgen M. J. Piek, Luc R. C. W. van Lonkhuijzen, Eva Maria Roes, Ward Hofhuis, Christianne A. R. Lok, Cor D. de Kroon, Eelke H. Gort, and et al. 2025. "The Prognostic Value of FOXL2 Mutant Circulating Tumor DNA in Adult Granulosa Cell Tumor Patients" Cancers 17, no. 11: 1894. https://doi.org/10.3390/cancers17111894
APA StyleBrink, G. J., Hami, N., Nijman, H. W., Piek, J. M. J., van Lonkhuijzen, L. R. C. W., Roes, E. M., Hofhuis, W., Lok, C. A. R., de Kroon, C. D., Gort, E. H., Witteveen, P. O., Zweemer, R. P., & Groeneweg, J. W. (2025). The Prognostic Value of FOXL2 Mutant Circulating Tumor DNA in Adult Granulosa Cell Tumor Patients. Cancers, 17(11), 1894. https://doi.org/10.3390/cancers17111894







