Endometrial Stromal Sarcoma: An Update
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
- Sixty-two were original research articles (including retrospective and prospective cohort studies, molecular profiling studies, and clinical trials),
- Forty-one were review articles or meta-analyses,
- Twelve were expert consensus statements or clinical guidelines,
- Eight were selected case reports or case series offering unique diagnostic or therapeutic insights.
4. Discussion
4.1. Definition, Epidemiology, and Clinical Features
4.2. Clinical Presentation
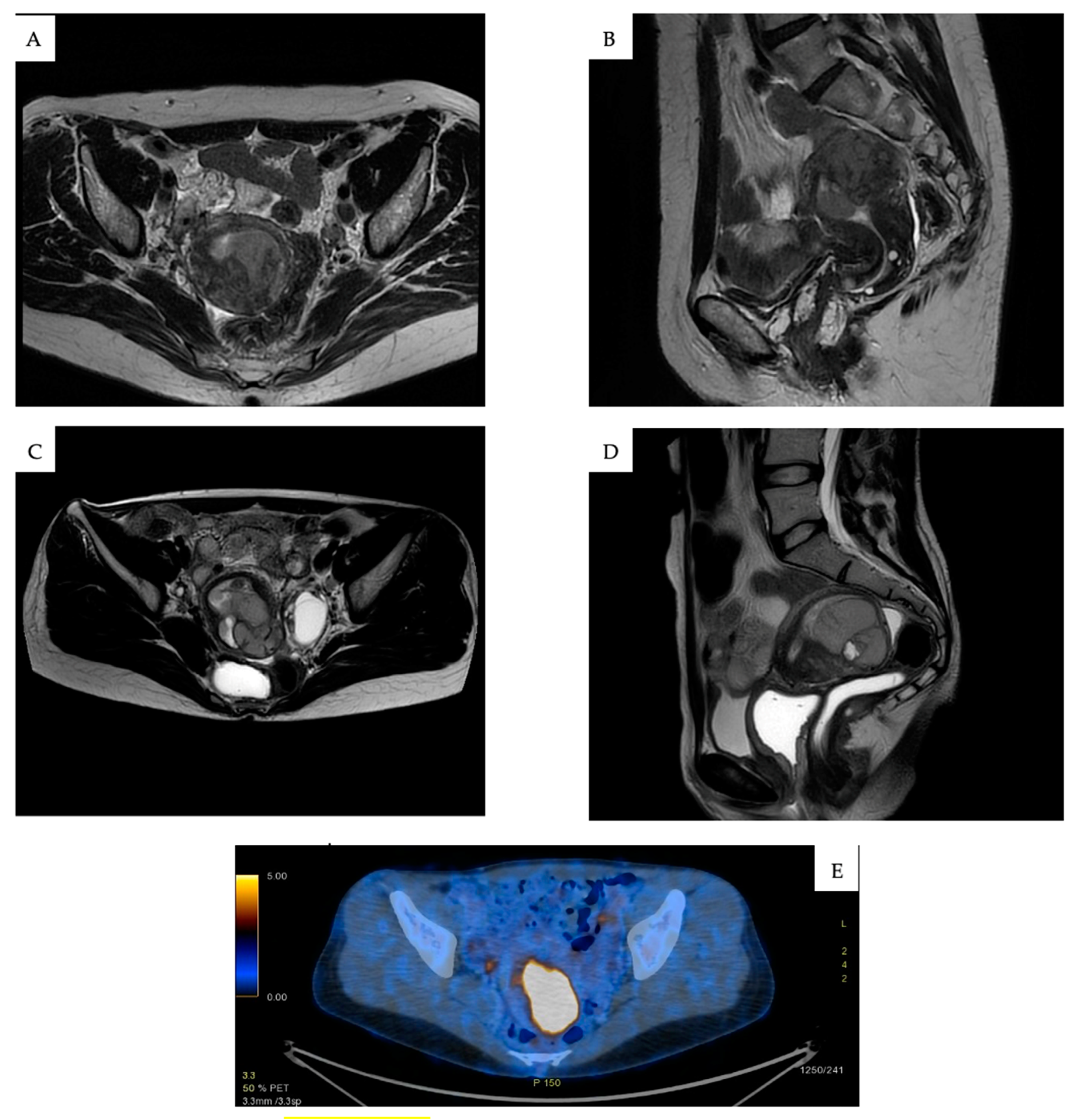
4.3. Diagnosis and Staging
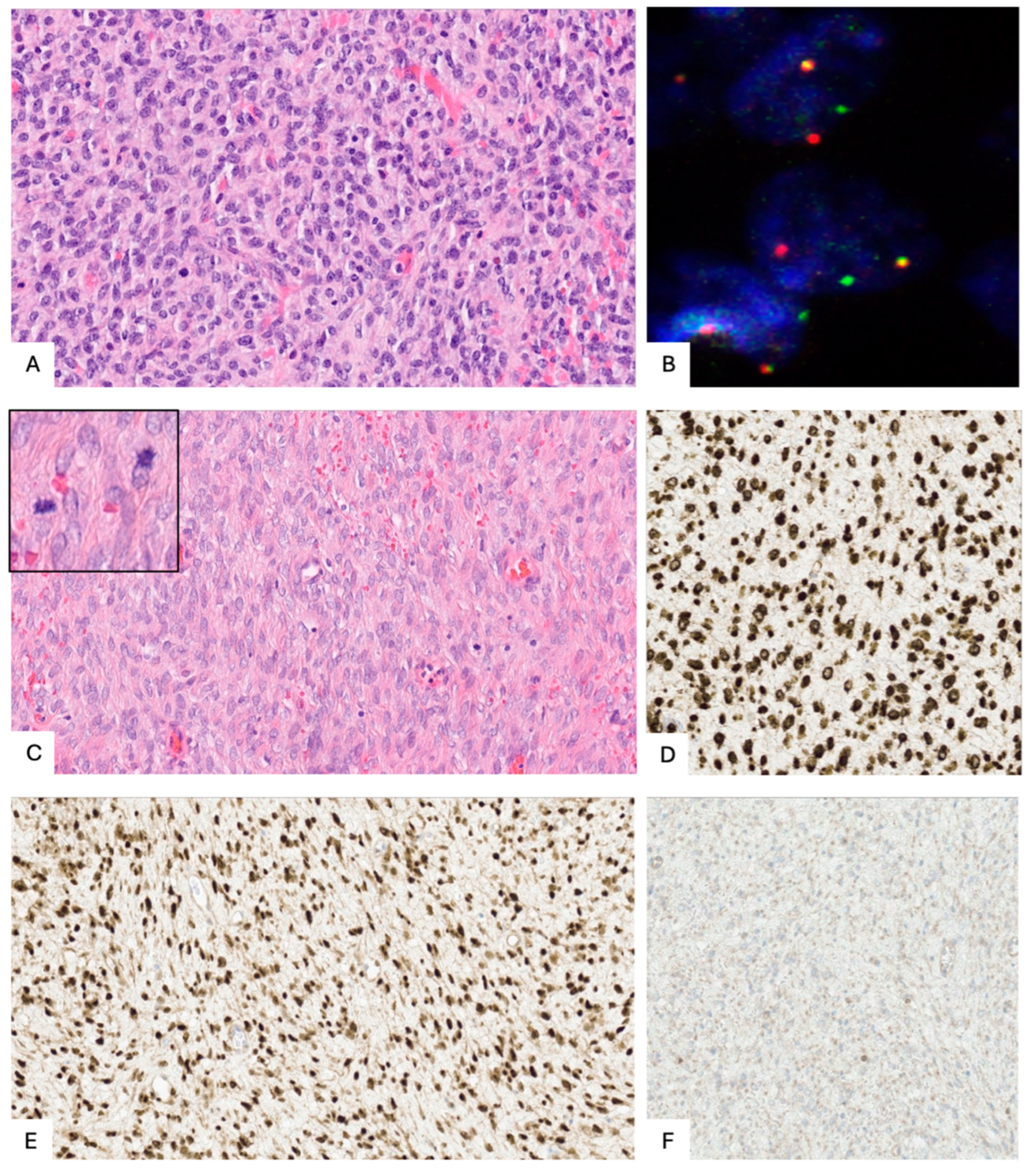
4.4. Histopathological and Molecular Characteristics
4.4.1. A. Low-Grade ESS (LG-ESS)
4.4.2. B. High-Grade ESS (HG-ESS)
4.4.3. HG-ESS with YWHAE-NUTM2A/B Fusion
4.4.4. HG-ESS with BCOR Alterations (Including BCOR or BCORL1 Fusions or BCOR Internal Tandem Duplication (ITD))
4.4.5. ESS with KAT6B/A::KANSL1 Fusion
4.4.6. HG-ESS Not Otherwise Specified (NOS)
5. Surgical Management of Early-Stage ESS
Fertility-Sparing Treatment
6. Adjuvant Treatment
6.1. Low-Grade Endometrial Stromal Sarcoma (LG-ESS)
6.2. High-Grade Endometrial Stromal Sarcoma (HG-ESS)
7. Treatment of Advanced and Recurrent ESS
7.1. LG-ESS
7.2. HG-ESS
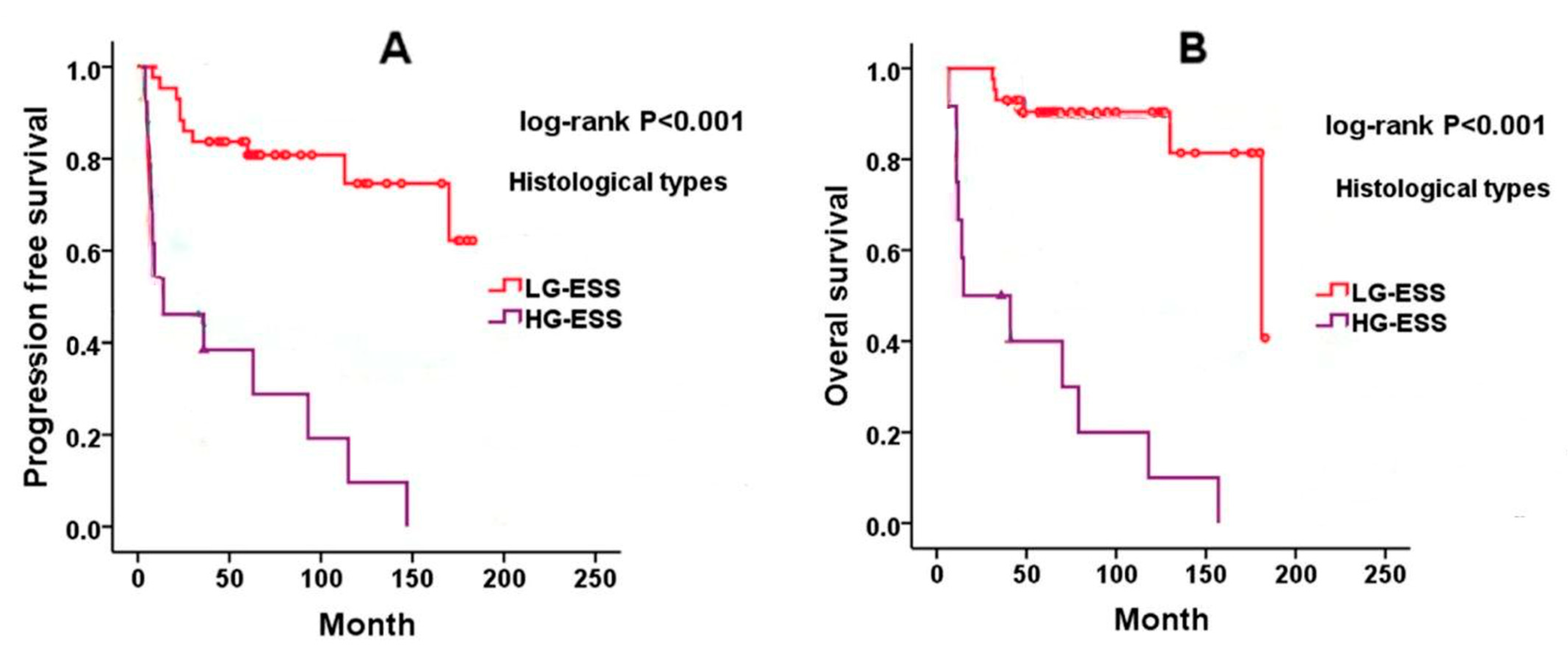
8. Prognosis of Endometrial Stromal Sarcoma
8.1. Low-Grade ESS (LG-ESS)
8.2. High-Grade ESS (HG-ESS)
9. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IARC Publications—WHO Classification of Tumours of Female Reproductive Organs. Fourth Edition. Available online: https://www.iarc.who.int/news-events/iarc-publications-who-classification-of-tumours-of-female-reproductive-organs-fourth-edition (accessed on 29 September 2024).
- Meredith, R.F.; Eisert, D.R.; Kaka, Z.; Hodgson, S.E.; Johnston, G.A.; Boutselis, J.G. An Excess of Uterine Sarcomas after Pelvic Irradiation. Cancer 1986, 58, 2003–2007. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.W.; Buchanan, R.; Buckley, C.H. Uterine Stromal Sarcoma Following Tamoxifen Treatment. J. Clin. Pathol. 1995, 48, 596. [Google Scholar] [CrossRef] [PubMed]
- Press, M.F.; Scully, R.E. Endometrial “Sarcomas” Complicating Ovarian Thecoma, Polycystic Ovarian Disease and Estrogen Therapy. Gynecol. Oncol. 1985, 21, 135–154. [Google Scholar] [CrossRef]
- Gadducci, A.; Sartori, E.; Landoni, F.; Zola, P.; Maggino, T.; Urgesi, A.; Lissoni, A.; Losa, G.; Fanucchi, A. Endometrial Stromal Sarcoma: Analysis of Treatment Failures and Survival. Gynecol. Oncol. 1996, 63, 247–253. [Google Scholar] [CrossRef]
- Denschlag, D.; Ackermann, S.; Battista, M.J.; Cremer, W.; Egerer, G.; Fehr, M.; Follmann, M.; Haase, H.; Harter, P.; Hettmer, S.; et al. Sarcoma of the Uterus. Guideline of the DGGG, OEGGG and SGGG (S2k-Level, AWMF Registry No. 015/074, April 2021). Geburtshilfe Frauenheilkd. 2022, 82, 1337–1367. [Google Scholar] [CrossRef]
- Chang, K.L.; Crabtree, G.S.; Lim-Tan, S.K.; Kempson, R.L.; Hendrickson, M.R. Primary Uterine Endometrial Stromal Neoplasms. A Clinicopathologic Study of 117 Cases. Am. J. Surg. Pathol. 1990, 14, 415–438. [Google Scholar] [CrossRef]
- D’Angelo, E.; Espinosa, I.; Ali, R.; Gilks, C.B.; van de Rijn, M.; Lee, C.-H.; Prat, J. Uterine Leiomyosarcomas: Tumor Size, Mitotic Index, and Biomarkers Ki67, and Bcl-2 Identify Two Groups with Different Prognosis. Gynecol. Oncol. 2011, 121, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.; Oliva, E. Endometrial Stromal Tumours of the Uterus: A Practical Approach Using Conventional Morphology and Ancillary Techniques. J. Clin. Pathol. 2007, 60, 235–243. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Garg, K.; Diaz, J.P.; Soslow, R.A.; Hensley, M.L.; Alektiar, K.M.; Barakat, R.R.; Leitao, M.M. Incidence of Lymph Node and Adnexal Metastasis in Endometrial Stromal Sarcoma. Gynecol. Oncol. 2011, 121, 319–322. [Google Scholar] [CrossRef]
- Mbatani, N.; Olawaiye, A.B.; Prat, J. Uterine Sarcomas. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. S2), 51–58. [Google Scholar] [CrossRef]
- Ferrandina, G.; Aristei, C.; Biondetti, P.R.; Cananzi, F.C.M.; Casali, P.; Ciccarone, F.; Colombo, N.; Comandone, A.; Corvo’, R.; De Iaco, P.; et al. Italian Consensus Conference on Management of Uterine Sarcomas on Behalf of S.I.G.O. (Societa’ Italiana Di Ginecologia E Ostetricia). Eur. J. Cancer 1990 2020, 139, 149–168. [Google Scholar] [CrossRef]
- Lebreton, C.; Meeus, P.; Genestie, C.; Croce, S.; Guyon, F.; Moscardo, C.L.; Taieb, S.; Blay, J.-Y.; Bonvalot, S.; Bompas, E.; et al. Endometrial stromal sarcoma: French Guidelines from the French Sarcoma Group and the Rare Malignant Gynecologic Tumors Group. Bull. Cancer 2023, 110, 844–854. [Google Scholar] [CrossRef]
- Pérez-Fidalgo, J.A.; Ortega, E.; Ponce, J.; Redondo, A.; Sevilla, I.; Valverde, C.; Isern Verdum, J.; de Alava, E.; Galera López, M.; Marquina, G.; et al. Uterine Sarcomas: Clinical Practice Guidelines for Diagnosis, Treatment, and Follow-up, by Spanish Group for Research on Sarcomas (GEIS). Ther. Adv. Med. Oncol. 2023, 15, 17588359231157645. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Casali, P.G.; Croce, S.; Fennessy, F.M.; Fischerova, D.; Jones, R.; Sanfilippo, R.; Zapardiel, I.; Amant, F.; Blay, J.-Y.; et al. ESGO/EURACAN/GCIG Guidelines for the Management of Patients with Uterine Sarcomas. Int. J. Gynecol. Cancer 2024, 34, 1499–1521. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft Tissue and Visceral Sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Ludovisi, M.; Moro, F.; Pasciuto, T.; Di Noi, S.; Giunchi, S.; Savelli, L.; Pascual, M.A.; Sladkevicius, P.; Alcazar, J.L.; Franchi, D.; et al. Imaging in Gynecological Disease (15): Clinical and Ultrasound Characteristics of Uterine Sarcoma. Ultrasound Obstet. Gynecol. 2019, 54, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, B.; Lehtovirta, P.; Wahlström, T.; Ylöstalo, P. Ultrasound Findings in Uterine Mixed Mullerian Sarcomas and Endometrial Stromal Sarcomas. Gynecol. Oncol. 1989, 35, 290–293. [Google Scholar] [CrossRef]
- Park, G.E.; Rha, S.E.; Oh, S.N.; Lee, A.; Lee, K.H.; Kim, M.-R. Ultrasonographic Findings of Low-Grade Endometrial Stromal Sarcoma of the Uterus with a Focus on Cystic Degeneration. Ultrasonography 2016, 35, 124–130. [Google Scholar] [CrossRef]
- Koyama, T.; Togashi, K.; Konishi, I.; Kobayashi, H.; Ueda, H.; Kataoka, M.L.; Kobayashi, H.; Itoh, T.; Higuchi, T.; Fujii, S.; et al. MR Imaging of Endometrial Stromal Sarcoma: Correlation with Pathologic Findings. Am. J. Roentgenol. 1999, 173, 767–772. [Google Scholar] [CrossRef]
- Ueda, M.; Otsuka, M.; Hatakenaka, M.; Sakai, S.; Ono, M.; Yoshimitsu, K.; Honda, H.; Torii, Y. MR Imaging Findings of Uterine Endometrial Stromal Sarcoma: Differentiation from Endometrial Carcinoma. Eur. Radiol. 2001, 11, 28–33. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Ueng, S.-H.; Chen, K.; Huang, Y.-T.; Lu, H.-Y.; Ng, K.-K.; Chang, T.-C.; Lai, C.-H.; Lin, G. Utility of Diffusion-Weighted and Contrast-Enhanced Magnetic Resonance Imaging in Diagnosing and Differentiating between High- and Low-Grade Uterine Endometrial Stromal Sarcoma. Cancer Imaging 2019, 19, 63. [Google Scholar] [CrossRef]
- Fujiishi, K.; Nagata, S.; Kano, R.; Kubo, C.; Shirayanagi, M.; Ozaki, M.; Yamamoto, T.; Nakanishi, K.; Kamiura, S.; Nakatsuka, S. JAZF1–SUZ12 Endometrial Stromal Sarcoma Forming Subserosal Masses with Extraordinary Uptake of Fluorodeoxyglucose on Positron Emission Tomography: A Case Report. Diagn. Pathol. 2019, 14, 110. [Google Scholar] [CrossRef]
- FIGO Committee on Gynecologic Oncology. Corrigendum to “FIGO Staging for Uterine Sarcomas” [International Journal of Gynecology and Obstetrics (2009) 104:179]. Int. J. Gynecol. Obstet. 2009, 106, 277. [Google Scholar] [CrossRef]
- Baek, M.-H.; Park, J.-Y.; Rhim, C.C.; Park, Y.; Kim, K.-R.; Kim, J.-H.; Nam, J.-H. Immunohistochemical Characterization of Histone Deacetylase as a Potential Prognostic Marker and Therapeutic Target in Endometrial Stromal Sarcoma. Anticancer Res. 2016, 36, 2527–2534. [Google Scholar]
- Zou, Y.; Turashvili, G.; Soslow, R.A.; Park, K.J.; Croce, S.; McCluggage, W.G.; Stewart, C.J.; Oda, Y.; Oliva, E.; Young, R.H.; et al. High-Grade Transformation of Low-Grade Endometrial Stromal Sarcomas Lacking YWHAE and BCOR Genetic Abnormalities. Mod. Pathol. 2020, 33, 1861. [Google Scholar] [CrossRef]
- Lee, C.-H.; Mariño-Enriquez, A.; Ou, W.; Zhu, M.; Ali, R.H.; Chiang, S.; Amant, F.; Gilks, C.B.; van de Rijn, M.; Oliva, E.; et al. The Clinicopathologic Features of YWHAE-FAM22 Endometrial Stromal Sarcomas: A Histologically High-Grade and Clinically Aggressive Tumor. Am. J. Surg. Pathol. 2012, 36, 641–653. [Google Scholar] [CrossRef]
- Lin, D.I.; Huang, R.S.P.; Mata, D.A.; Decker, B.; Danziger, N.; Lechpammer, M.; Hiemenz, M.; Ramkissoon, S.H.; Ross, J.S.; Elvin, J.A. Clinicopathological and Genomic Characterization of BCORL1-Driven High-Grade Endometrial Stromal Sarcomas. Mod. Pathol. 2021, 34, 2200–2210. [Google Scholar] [CrossRef]
- Agaimy, A.; Clarke, B.A.; Kolin, D.L.; Lee, C.-H.; Lee, J.-C.; McCluggage, W.G.; Pöschke, P.; Stoehr, R.; Swanson, D.; Turashvili, G.; et al. Recurrent KAT6B/A::KANSL1 Fusions Characterize a Potentially Aggressive Uterine Sarcoma Morphologically Overlapping with Low-Grade Endometrial Stromal Sarcoma. Am. J. Surg. Pathol. 2022, 46, 1298–1308. [Google Scholar] [CrossRef]
- Solomon, J.P.; Linkov, I.; Rosado, A.; Mullaney, K.; Rosen, E.Y.; Frosina, D.; Jungbluth, A.A.; Zehir, A.; Benayed, R.; Drilon, A.; et al. NTRK Fusion Detection across Multiple Assays and 33,997 Cases: Diagnostic Implications and Pitfalls. Mod. Pathol. 2019, 33, 38. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.; Yashar, C.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; et al. Uterine Neoplasms, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.-Y.; Honoré, C.; Stoeckle, E.; Meeus, P.; Jafari, M.; Gouin, F.; Anract, P.; Ferron, G.; Rochwerger, A.; Ropars, M.; et al. Surgery in Reference Centers Improves Survival of Sarcoma Patients: A Nationwide Study. Ann. Oncol. 2019, 30, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.P.; Bryant, C.S.; Kumar, S.; Ali-Fehmi, R.; Malone, J.M.; Morris, R.T. Lymphadenectomy and Ovarian Preservation in Low-Grade Endometrial Stromal Sarcoma. Obstet. Gynecol. 2008, 112, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Hua, K.; Malpica, A.; Zhou, X.; Baak, J.P.A. Stages I to II WHO 2003-Defined Low-Grade Endometrial Stromal Sarcoma: How Much Primary Therapy Is Needed and How Little Is Enough? Int. J. Gynecol. Cancer 2013, 23, 488–493. [Google Scholar] [CrossRef]
- Signorelli, M.; Fruscio, R.; Dell’Anna, T.; Buda, A.; Giuliani, D.; Ceppi, L.; Milani, R. Lymphadenectomy in Uterine Low-Grade Endometrial Stromal Sarcoma: An Analysis of 19 Cases and a Literature Review. Int. J. Gynecol. Cancer 2010, 20, 1363–1366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, N.; Wang, W.; Yao, H.; An, J.; Li, N.; Sun, Y.; Wu, L. Long-Term Impact of Lymphadenectomies in Patients with Low-Grade, Early-Stage Uterine Endometrial Stroma Sarcoma. J. Obstet. Gynaecol. Res. 2020, 46, 654–662. [Google Scholar] [CrossRef]
- Nasioudis, D.; Mastroyannis, S.A.; Latif, N.A.; Ko, E.M.; Haggerty, A.F.; Kim, S.H.; Morgan, M.A.; Giuntoli, R.L. Role of Lymphadenectomy for Apparent Early Stage Uterine Sarcoma; a Comprehensive Analysis of the National Cancer Database. Surg. Oncol. 2021, 38, 101589. [Google Scholar] [CrossRef]
- Si, M.; Jia, L.; Song, K.; Zhang, Q.; Kong, B. Role of Lymphadenectomy for Uterine Sarcoma: A Meta-Analysis. Int. J. Gynecol. Cancer 2017, 27, 109–116. [Google Scholar] [CrossRef]
- Nasioudis, D.; Ko, E.M.; Kolovos, G.; Vagios, S.; Kalliouris, D.; Giuntoli, R.L. Ovarian Preservation for Low-Grade Endometrial Stromal Sarcoma: A Systematic Review of the Literature and Meta-Analysis. Int. J. Gynecol. Cancer 2019, 29, 126–132. [Google Scholar] [CrossRef]
- Nasioudis, D.; Mastroyannis, S.A.; Latif, N.A.; Ko, E.M.; Haggerty, A.F.; Kim, S.H.; Morgan, M.A.; Giuntoli, R.L. Effect of Bilateral Salpingo-Oophorectomy on the Overall Survival of Premenopausal Patients with Stage I Low-Grade Endometrial Stromal Sarcoma; a National Cancer Database Analysis. Gynecol. Oncol. 2020, 157, 634–638. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Li, Y.; Qin, M.; Cai, Y.; Jin, Y.; Pan, L.-Y. High-Grade Endometrial Stromal Sarcoma: A Retrospective Study of Factors Influencing Prognosis. Cancer Manag. Res. 2019, 11, 831–837. [Google Scholar] [CrossRef]
- Gadducci, A.; Multinu, F.; De Vitis, L.A.; Cosio, S.; Carinelli, S.; Aletti, G.D. Endometrial Stromal Tumors of the Uterus: Epidemiology, Pathological and Biological Features, Treatment Options and Clinical Outcomes. Gynecol. Oncol. 2023, 171, 95–105. [Google Scholar] [CrossRef]
- Bai, H.; Yang, J.; Cao, D.; Huang, H.; Xiang, Y.; Wu, M.; Cui, Q.; Chen, J.; Lang, J.; Shen, K. Ovary and Uterus-Sparing Procedures for Low-Grade Endometrial Stromal Sarcoma: A Retrospective Study of 153 Cases. Gynecol. Oncol. 2014, 132, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Cao, D.; Yang, J.; Jiang, X.; Shen, K.; Pan, L.; Huang, H.; Lang, J.; You, Y.; Chen, J. Fertility-Sparing Surgery for Patients with Low-Grade Endometrial Stromal Sarcoma. Oncotarget 2016, 8, 10602. [Google Scholar] [CrossRef]
- Morimoto, A.; Tsubamoto, H.; Inoue, K.; Ikeda, Y.; Hirota, S. Fatal Case of Multiple Recurrences of Endometrial Stromal Sarcoma after Fertility-Sparing Management. J. Obstet. Gynaecol. Res. 2015, 41, 162–166. [Google Scholar] [CrossRef]
- Zheng, Y.; Yin, Q.; Yang, X.; Dong, R. Fertility-Sparing Management of Low-Grade Endometrial Stromal Sarcoma: Analysis of an Institutional Series, a Population-Based Analysis and Review of the Literature. Ann. Transl. Med. 2020, 8, 1358. [Google Scholar] [CrossRef]
- Laurelli, G.; Falcone, F.; Scaffa, C.; Messalli, E.M.; Del Giudice, M.; Losito, S.; Greggi, S. Fertility-Sparing Management of Low-Grade Endometrial Stromal Sarcoma: Analysis of an Institutional Series and Review of the Literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 195, 61–66. [Google Scholar] [CrossRef]
- Dondi, G.; Porcu, E.; De Palma, A.; Damiano, G.; De Crescenzo, E.; Cipriani, L.; Dirodi, M.; Ravegnini, G.; De Leo, A.; Nannini, M.; et al. Uterine Preservation Treatments in Sarcomas: Oncological Problems and Reproductive Results: A Systematic Review. Cancers 2021, 13, 5808. [Google Scholar] [CrossRef]
- Amant, F.; De Knijf, A.; Van Calster, B.; Leunen, K.; Neven, P.; Berteloot, P.; Vergote, I.; Van Huffel, S.; Moerman, P. Clinical Study Investigating the Role of Lymphadenectomy, Surgical Castration and Adjuvant Hormonal Treatment in Endometrial Stromal Sarcoma. Br. J. Cancer 2007, 97, 1194–1199. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakayama, K.; Ishikawa, M.; Ishikawa, N.; Katagiri, H.; Katagiri, A.; Ishibashi, T.; Sato, E.; Iida, K.; Sultana, R.; et al. Letrozole as Second-Line Hormonal Treatment for Recurrent Low-Grade Endometrial Stromal Sarcoma: A Case Report and Review of the Literature. Oncol. Lett. 2016, 12, 3856. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.M.; Baigrie, C.F.; Manek, S. Tamoxifen and the Endometrium: Review of 102 Cases and Comparison with HRT-Related and Non-HRT-Related Endometrial Pathology. Int. J. Gynecol. Pathol. 1999, 18, 130–137. [Google Scholar] [PubMed]
- Deshmukh, U.; Black, J.; Perez-Irizarry, J.; Passarelli, R.; Levy, K.; Rostkowski, A.; Hui, P.; Rutherford, T.J.; Santin, A.D.; Azodi, M.; et al. Adjuvant Hormonal Therapy for Low-Grade Endometrial Stromal Sarcoma. Reprod. Sci. Thousand Oaks Calif. 2019, 26, 600–608. [Google Scholar] [CrossRef]
- Beck, T.L.; Singhal, P.K.; Ehrenberg, H.M.; Rose, P.G.; Lele, S.B.; Krivak, T.C.; McBee, W.C. Endometrial Stromal Sarcoma: Analysis of Recurrence Following Adjuvant Treatment. Gynecol. Oncol. 2012, 125, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.C.; Mor, G.; Lim, C.; Zheng, W.; Parkash, V.; Schwartz, P.E. Low-Grade Endometrial Stromal Sarcoma: Hormonal Aspects. Gynecol. Oncol. 2003, 90, 170–176. [Google Scholar] [CrossRef]
- Comert, G.K.; Turkmen, O.; Kar, I.; Yucel, O.; Kilic, C.; Boran, N.; Basaran, D.; Karalok, A.; Turan, T. Hormone Therapy Following Surgery in Low-Grade Endometrial Stromal Sarcoma: Is It Related to a Decrease in Recurrence Rate? J. Chin. Med. Assoc. 2019, 82, 385. [Google Scholar] [CrossRef]
- Leath, C.A.; Huh, W.K.; Hyde, J.; Cohn, D.E.; Resnick, K.E.; Taylor, N.P.; Powell, M.A.; Mutch, D.G.; Bradley, W.H.; Geller, M.A.; et al. A Multi-Institutional Review of Outcomes of Endometrial Stromal Sarcoma. Gynecol. Oncol. 2007, 105, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Cao, G.; Bai, H.; Zhang, Z. The Clinical Benefits of Hormonal Treatment for LG-ESS: A Meta-Analysis. Arch. Gynecol. Obstet. 2019, 300, 1167–1175. [Google Scholar] [CrossRef]
- Berchuck, A.; Rubin, S.C.; Hoskins, W.J.; Saigo, P.E.; Pierce, V.K.; Lewis, J.L. Treatment of Endometrial Stromal Tumors. Gynecol. Oncol. 1990, 36, 60–65. [Google Scholar] [CrossRef]
- Weitmann, H.D.; Knocke, T.H.; Kucera, H.; Pötter, R. Radiation Therapy in the Treatment of Endometrial Stromal Sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 739–748. [Google Scholar] [CrossRef]
- Wang, W.; Sun, S.; Miao, Z.; Hou, X.; Zhang, F.; Hu, K. Adjuvant Radiotherapy Improved Survival in Stage I to II Low-Grade Endometrial Stromal Sarcoma: A Retrospective Study of 152 Cases. Front. Oncol. 2020, 10, 608152. [Google Scholar] [CrossRef]
- Barney, B.; Tward, J.D.; Skidmore, T.; Gaffney, D.K. Does Radiotherapy or Lymphadenectomy Improve Survival in Endometrial Stromal Sarcoma? Int. J. Gynecol. Cancer 2009, 19, 1232–1238. [Google Scholar] [CrossRef]
- Sampath, S.; Schultheiss, T.E.; Ryu, J.K.; Wong, J.Y.C. The Role of Adjuvant Radiation in Uterine Sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Reed, N.S.; Mangioni, C.; Malmström, H.; Scarfone, G.; Poveda, A.; Pecorelli, S.; Tateo, S.; Franchi, M.; Jobsen, J.J.; Coens, C.; et al. Phase III Randomised Study to Evaluate the Role of Adjuvant Pelvic Radiotherapy in the Treatment of Uterine Sarcomas Stages I and II: An European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (Protocol 55874). Eur. J. Cancer 1990 2008, 44, 808–818. [Google Scholar] [CrossRef]
- Seagle, B.-L.L.; Shilpi, A.; Buchanan, S.; Goodman, C.; Shahabi, S. Low-Grade and High-Grade Endometrial Stromal Sarcoma: A National Cancer Database Study. Gynecol. Oncol. 2017, 146, 254–262. [Google Scholar] [CrossRef]
- Malouf, G.G.; Lhommé, C.; Duvillard, P.; Morice, P.; Haie-Meder, C.; Pautier, P. Prognostic Factors and Outcome of Undifferentiated Endometrial Sarcoma Treated by Multimodal Therapy. Int. J. Gynaecol. Obstet. 2013, 122, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Meurer, M.; Floquet, A.; Ray-Coquard, I.; Bertucci, F.; Auriche, M.; Cordoba, A.; Piperno-Neumann, S.; Salas, S.; Delannes, M.; Chevalier, T.; et al. Localized High Grade Endometrial Stromal Sarcoma and Localized Undifferentiated Uterine Sarcoma: A Retrospective Series of the French Sarcoma Group. Int. J. Gynecol. Cancer 2019, 29, 691–698. [Google Scholar] [CrossRef]
- Cabrera, S.; Bebia, V.; Acosta, U.; Franco-Camps, S.; Mañalich, L.; García-Jiménez, A.; Gil-Moreno, A. Survival Outcomes and Prognostic Factors of Endometrial Stromal Sarcoma and Undifferentiated Uterine Sarcoma. Clin. Transl. Oncol. 2021, 23, 1210–1219. [Google Scholar] [CrossRef]
- Ferron, G.; Bataillon, G.; Martinez, A.; Chibon, F.; Valentin, T. Gynecological Sarcomas, Surgical Management: Primary, Metastatic, and Recurrent Disease. Int. J. Gynecol. Cancer 2024, 34, 393–402. [Google Scholar] [CrossRef]
- Li, Y.; Ren, H.; Wang, J. Outcome of Adjuvant Radiotherapy after Total Hysterectomy in Patients with Uterine Leiomyosarcoma or Carcinosarcoma: A SEER-Based Study. BMC Cancer 2019, 19, 697. [Google Scholar] [CrossRef]
- Mahdavi, A.; Monk, B.J.; Ragazzo, J.; Hunter, M.I.; Lentz, S.E.; Vasilev, S.A.; Tewari, K.S. Pelvic Radiation Improves Local Control after Hysterectomy for Uterine Leiomyosarcoma: A 20-Year Experience. Int. J. Gynecol. Cancer 2009, 19, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Kortmann, B.; Reimer, T.; Gerber, B.; Klautke, G.; Fietkau, R. Concurrent Radiochemotherapy of Locally Recurrent or Advanced Sarcomas of the Uterus. Strahlenther. Onkol. 2006, 182, 318–324. [Google Scholar] [CrossRef]
- Friedman, C.F.; Manning-Geist, B.L.; Zhou, Q.; Soumerai, T.; Holland, A.; Da Cruz Paula, A.; Green, H.; Ozsoy, M.A.; Iasonos, A.; Hollmann, T.; et al. Nivolumab for Mismatch-Repair-Deficient or Hypermutated Gynecologic Cancers: A Phase 2 Trial with Biomarker Analyses. Nat. Med. 2024, 30, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Thanopoulou, E.; Aleksic, A.; Thway, K.; Khabra, K.; Judson, I. Hormonal Treatments in Metastatic Endometrial Stromal Sarcomas: The 10-Year Experience of the Sarcoma Unit of Royal Marsden Hospital. Clin. Sarcoma Res. 2015, 5, 8. [Google Scholar] [CrossRef]
- Dahhan, T.; Fons, G.; Buist, M.R.; Ten Kate, F.J.W.; van der Velden, J. The Efficacy of Hormonal Treatment for Residual or Recurrent Low-Grade Endometrial Stromal Sarcoma. A Retrospective Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, 80–84. [Google Scholar] [CrossRef]
- Ioffe, Y.J.; Li, A.J.; Walsh, C.S.; Karlan, B.Y.; Leuchter, R.; Forscher, C.; Cass, I. Hormone Receptor Expression in Uterine Sarcomas: Prognostic and Therapeutic Roles. Gynecol. Oncol. 2009, 115, 466–471. [Google Scholar] [CrossRef]
- Friedlander, M.; Benson, C.; O’Connell, R.L.; Reed, N.; Clamp, A.; Lord, R.; Millan, D.; Nottley, S.; Amant, F.; Steer, C.; et al. Phase 2 Study of Anastrozole in Patients with Estrogen Receptor/Progesterone Receptor Positive Recurrent Low-Grade Endometrial Stromal Sarcomas: The PARAGON Trial (ANZGOG 0903). Gynecol. Oncol. 2021, 161, 160–165. [Google Scholar] [CrossRef]
- Pink, D.; Lindner, T.; Mrozek, A.; Kretzschmar, A.; Thuss-Patience, P.C.; Dörken, B.; Reichardt, P. Harm or Benefit of Hormonal Treatment in Metastatic Low-Grade Endometrial Stromal Sarcoma: Single Center Experience with 10 Cases and Review of the Literature. Gynecol. Oncol. 2006, 101, 464–469. [Google Scholar] [CrossRef]
- Trozzi, R.; Tuyaerts, S.; Annibali, D.; Herreros Pomares, A.; Boog, L.; Van Dam, P.; Leunen, K.; Deroose, C.; Trum, H.; Amant, F. An Open-Label, Single-Arm, Prospective, Multi-Center, Tandem Two-Stage Designed Phase II Study to Evaluate the Efficacy of Fulvestrant in Women with Recurrent/Metastatic Estrogen Receptor-Positive Gynecological Malignancies (FUCHSia Study). Int. J. Gynecol. Cancer 2024, 34, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Dessources, K.; Miller, K.M.; Kertowidjojo, E.; Da Cruz Paula, A.; Zou, Y.; Selenica, P.; da Silva, E.M.; Benayed, R.; Ashley, C.W.; Abu-Rustum, N.R.; et al. ESR1 Hotspot Mutations in Endometrial Stromal Sarcoma with High-Grade Transformation and Endocrine Treatment. Mod. Pathol. 2022, 35, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Leitao, M.M.; Zivanovic, O.; Chi, D.S.; Hensley, M.L.; O’Cearbhaill, R.; Soslow, R.A.; Barakat, R.R. Surgical Cytoreduction in Patients with Metastatic Uterine Leiomyosarcoma at the Time of Initial Diagnosis. Gynecol. Oncol. 2012, 125, 409–413. [Google Scholar] [CrossRef]
- Díaz-Montes, T.P.; El-Sharkawy, F.; Lynam, S.; Harper, A.; Sittig, M.; MacDonald, R.; Gushchin, V.; Sardi, A. Efficacy of Hyperthermic Intraperitoneal Chemotherapy and Cytoreductive Surgery in the Treatment of Recurrent Uterine Sarcoma. Int. J. Gynecol. Cancer 2018, 28, 1130–1137. [Google Scholar] [CrossRef]
- Sardi, A.; Sipok, A.; Baratti, D.; Deraco, M.; Sugarbaker, P.; Salti, G.; Yonemura, Y.; Sammartino, P.; Glehen, O.; Bakrin, N.; et al. Multi-Institutional Study of Peritoneal Sarcomatosis from Uterine Sarcoma Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Eur. J. Surg. Oncol. 2017, 43, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.-Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin Alone versus Intensified Doxorubicin plus Ifosfamide for First-Line Treatment of Advanced or Metastatic Soft-Tissue Sarcoma: A Randomised Controlled Phase 3 Trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Pautier, P.; Floquet, A.; Chevreau, C.; Penel, N.; Guillemet, C.; Delcambre, C.; Cupissol, D.; Selle, F.; Isambert, N.; Piperno-Neumann, S.; et al. Trabectedin in Combination with Doxorubicin for First-Line Treatment of Advanced Uterine or Soft-Tissue Leiomyosarcoma (LMS-02): A Non-Randomised, Multicentre, Phase 2 Trial. Lancet Oncol. 2015, 16, 457–464. [Google Scholar] [CrossRef]
- Pautier, P.; Italiano, A.; Piperno-Neumann, S.; Chevreau, C.; Penel, N.; Firmin, N.; Boudou-Rouquette, P.; Bertucci, F.; Balleyguier, C.; Lebrun-Ly, V.; et al. Doxorubicin Alone versus Doxorubicin with Trabectedin Followed by Trabectedin Alone as First-Line Therapy for Metastatic or Unresectable Leiomyosarcoma (LMS-04): A Randomised, Multicentre, Open-Label Phase 3 Trial. Lancet Oncol. 2022, 23, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, C. LG-ESSs and HG-ESSs: Underlying Molecular Alterations and Potential Therapeutic Strategies. J. Zhejiang Univ. Sci. B 2021, 22, 633–646. [Google Scholar] [CrossRef]
- Wang, F.; Dai, X.; Chen, H.; Hu, X.; Wang, Y. Clinical Characteristics and Prognosis Analysis of Uterine Sarcoma: A Single-Institution Retrospective Study. BMC Cancer 2022, 22, 1050. [Google Scholar] [CrossRef]
- Hardell, E.; Josefson, S.; Ghaderi, M.; Skeie-Jensen, T.; Westbom-Fremer, S.; Cheek, E.H.; Bell, D.; Selling, J.; Schoolmeester, J.K.; Måsbäck, A.; et al. Validation of a Mitotic Index Cutoff as a Prognostic Marker in Undifferentiated Uterine Sarcomas. Am. J. Surg. Pathol. 2017, 41, 1231–1237. [Google Scholar] [CrossRef]
- Gremel, G.; Liew, M.; Hamzei, F.; Hardell, E.; Selling, J.; Ghaderi, M.; Stemme, S.; Pontén, F.; Carlson, J.W. A Prognosis Based Classification of Undifferentiated Uterine Sarcomas: Identification of Mitotic Index, Hormone Receptors and YWHAE-FAM22 Translocation Status as Predictors of Survival. Int. J. Cancer 2015, 136, 1608–1618. [Google Scholar] [CrossRef]
- Dickson, B.C.; Lum, A.; Swanson, D.; Bernardini, M.Q.; Colgan, T.J.; Shaw, P.A.; Yip, S.; Lee, C.-H. Novel EPC1 Gene Fusions in Endometrial Stromal Sarcoma. Genes Chromosomes Cancer 2018, 57, 598–603. [Google Scholar] [CrossRef]
- Akaev, I.; Yeoh, C.C.; Rahimi, S. Update on Endometrial Stromal Tumours of the Uterus. Diagnostics 2021, 11, 429. [Google Scholar] [CrossRef]
- Kang, N.; Zhang, Y.; Guo, S.; Chen, R.; Kong, F.; Wang, S.; Yuan, M.; Chen, R.; Shen, D.; Wang, J. Genomic and Transcriptomic Characterization Revealed the High Sensitivity of Targeted Therapy and Immunotherapy in a Subset of Endometrial Stromal Sarcoma. Cancer Res. Treat. 2023, 55, 978–991. [Google Scholar] [CrossRef] [PubMed]

| Stage | Description |
|---|---|
| I stage | Tumor confined to the uterus |
| IA | Tumor limited to endometrium/endocervix stroma with no myometrial invasion |
| IB | Less than or equal to half myometrial invasion |
| IC | More than half myometrial invasion |
| II stage | Tumor extends to the pelvis |
| IIA | Involvement of adnexa (ovaries or fallopian tubes) |
| IIB | Tumor extends to extrauterine pelvic tissue |
| III stage | Tumor infiltrates abdominal tissues (not just protruding into the abdomen) |
| IIIA | One site |
| IIIB | More than one site |
| IIIC | Metastasis to pelvic and/or para-aortic lymph nodes |
| IV stage | Tumor invades bladder/rectum or shows distant metastasis |
| IVA | Direct invasion of bladder and/or rectum |
| IVB | Distant metastases (e.g., lungs, liver, bones) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricotta, G.; Russo, S.A.; Fagotti, A.; Martinez, A.; Gauroy, E.; Del, M.; Thibaud, V.; Guillaume, B.; Ferron, G. Endometrial Stromal Sarcoma: An Update. Cancers 2025, 17, 1893. https://doi.org/10.3390/cancers17111893
Ricotta G, Russo SA, Fagotti A, Martinez A, Gauroy E, Del M, Thibaud V, Guillaume B, Ferron G. Endometrial Stromal Sarcoma: An Update. Cancers. 2025; 17(11):1893. https://doi.org/10.3390/cancers17111893
Chicago/Turabian StyleRicotta, Giulio, Silvio Andrea Russo, Anna Fagotti, Alejandra Martinez, Elodie Gauroy, Mathilde Del, Valentin Thibaud, Bataillon Guillaume, and Gwenaël Ferron. 2025. "Endometrial Stromal Sarcoma: An Update" Cancers 17, no. 11: 1893. https://doi.org/10.3390/cancers17111893
APA StyleRicotta, G., Russo, S. A., Fagotti, A., Martinez, A., Gauroy, E., Del, M., Thibaud, V., Guillaume, B., & Ferron, G. (2025). Endometrial Stromal Sarcoma: An Update. Cancers, 17(11), 1893. https://doi.org/10.3390/cancers17111893






