Clinical Characteristics and Prognostic Factors in Patients with Gestational Trophoblastic Neoplasia: A Single-Center Study Comparing Ultra-High-Risk and Other Risk Groups
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
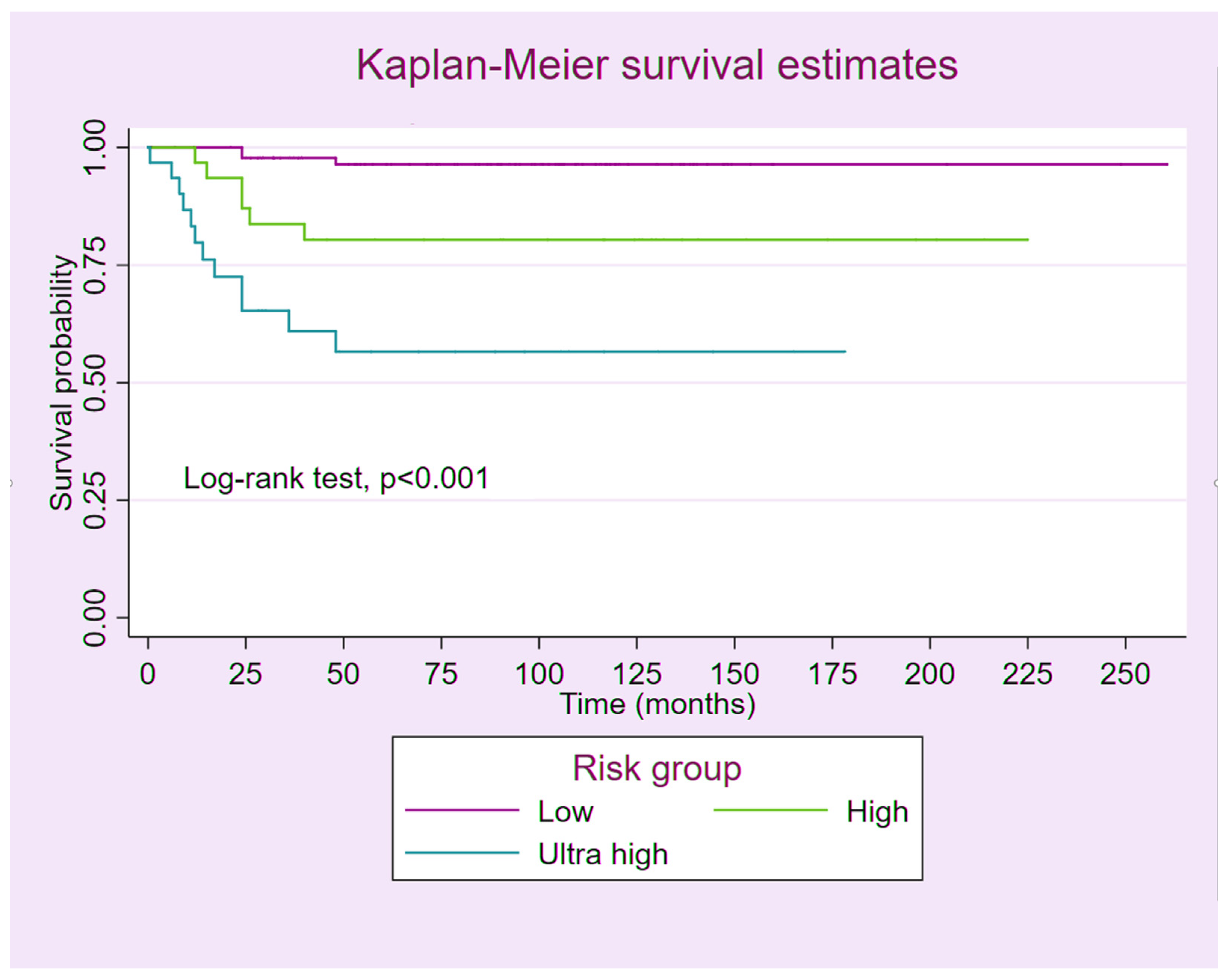
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lurain, J.R. Gestational trophoblastic disease I: Epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am. J. Obstet. Gynecol. 2010, 203, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Bruce, S.; Sorosky, J. Gestational Trophoblastic Disease. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ngan, H.Y.S.; Seckl, M.J.; Berkowitz, R.S.; Xiang, Y.; Golfier, F.; Sekharan, P.K.; Lurain, J.R.; Massuger, L. Update on the diagnosis and management of gestational trophoblastic disease. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 79–85. [Google Scholar] [CrossRef]
- Yamamoto, E.; Nishino, K.; Niimi, K.; Ino, K. Epidemiologic study on gestational trophoblastic diseases in Japan. J. Gynecol. Oncol. 2022, 33, e72. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.; Frijstein, M.; van Trommel, N. Clinical presentation and diagnosis of Gestational Trophoblastic Disease. Best. Pract. Res. Clin. Obstet. Gynaecol. 2021, 74, 42–52. [Google Scholar] [CrossRef]
- Seckl, M.J.; Sebire, N.J.; Berkowitz, R.S. Gestational trophoblastic disease. Lancet 2010, 376, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.A. Epidemiology of gestational trophoblastic disease. Am. J. Obstet. Gynecol. 1984, 150, 309–318. [Google Scholar] [CrossRef]
- Kohorn, E.I. The new FIGO 2000 staging and risk factor scoring system for gestational trophoblastic disease: Description and critical assessment. Int. J. Gynecol. Cancer 2001, 11, 73–77. [Google Scholar] [CrossRef]
- Ngan, H.Y.S.; Seckl, M.J.; Berkowitz, R.S.; Xiang, Y.; Golfier, F.; Sekharan, P.K.; Lurain, J.R.; Massuger, L. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. 1), 86–93. [Google Scholar] [CrossRef]
- Wang, K.L.; Yang, Y.C.; Wang, T.Y.; Cheng-Yen Lai, J.; Chen, T.C.; Chang, C.L. Treatment of gestational trophoblastic neoplasia according to the FIGO 2000 staging and scoring system: A 20 years’ experience. Acta Obstet. Gynecol. Scand. 2009, 88, 204–208. [Google Scholar] [CrossRef]
- Yun, B.S.; Park, E.H.; Ha, J.; Lee, J.Y.; Lee, K.H.; Lee, T.S.; Lee, K.J.; Kim, Y.J.; Jung, K.W.; Roh, J.W. Incidence and survival of gynecologic cancer including cervical, uterine, ovarian, vaginal, vulvar cancer and gestational trophoblastic neoplasia in Korea, 1999-2019: Korea Central Cancer Registry. Obstet. Gynecol. Sci. 2023, 66, 545–561. [Google Scholar] [CrossRef]
- Braga, A.; Paiva, G.; Alevato, R.; Saldanha, P.; Elias, K.M.; Horowitz, N.S.; Berkowitz, R.S. Treatment of High-Risk Gestational Trophoblastic Neoplasia. Hematol. Oncol. Clin. N. Am. 2024, 38, 1245–1258. [Google Scholar] [CrossRef] [PubMed]
- Sharami, S.R.Y.; Saffarieh, E. A review on management of gestational trophoblastic neoplasia. J. Fam. Med. Prim. Care 2020, 9, 1287–1295. [Google Scholar] [CrossRef]
- Gueye, M.; Ndiaye-Gueye, M.D.; Kane-Gueye, S.M.; Gassama, O.; Diallo, M.; Moreau, J.C. Diagnosis, Treatment and Outcome of Gestational Trophoblastic Neoplasia in a Low Resource Income Country. Int. J. MCH AIDS 2016, 5, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Yang, J.; Jiang, F.; Zhao, J.; Ren, T.; Li, J.; Wang, X.; Feng, F.; Wan, X.; Xiang, Y. Clinical characteristics and prognosis of ultra high-risk gestational trophoblastic neoplasia patients: A retrospective cohort study. Gynecol. Oncol. 2017, 146, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Suprasert, P.; Manopunya, M. Outcomes of Non-Metastatic Gestational Trophoblastic Neoplasia: Twelve Year Experience from a Northern Thailand Tertiary Care Center. Asian Pac. J. Cancer Prev. 2015, 16, 5913–5916. [Google Scholar] [CrossRef]
- Suprasert, P.; Siriaree, S.; Manopunya, M. Outcomes of Metastatic Gestational Trophoblastic Neoplasia: Fourteen Year Experience from a Northern Thailand Tertiary Care Center. Asian Pac. J. Cancer Prev. 2016, 17, 1357–1362. [Google Scholar] [CrossRef]
- Yanaranop, M.; Potikul, C.; Tuipae, S. A 10-Year Clinical Experience of Gestational Trophoblastic Disease at Rajavithi Hospital, 2001–2010. J. Med. Assoc. Thai 2016, 99 (Suppl. 2), S17–S27. [Google Scholar]
- Oranratanaphan, S.; Lertkhachonsuk, R. Treatment of extremely high risk and resistant gestational trophoblastic neoplasia patients in King Chulalongkorn Memorial Hospital. Asian Pac. J. Cancer Prev. 2014, 15, 925–928. [Google Scholar] [CrossRef]
- Bolze, P.A.; Riedl, C.; Massardier, J.; Lotz, J.P.; You, B.; Schott, A.M.; Hajri, T.; Golfier, F. Mortality rate of gestational trophoblastic neoplasia with a FIGO score of ≥13. Am. J. Obstet. Gynecol. 2016, 214, 390.e1–398.e8. [Google Scholar] [CrossRef]
- Maestá, I.; de Freitas Segalla Moreira, M.; Rezende-Filho, J.; Bianconi, M.I.; Jankilevich, G.; Otero, S.; Correa Ramirez, L.A.; Sun, S.Y.; Elias, K.; Horowitz, N.; et al. Outcomes in the management of high-risk gestational trophoblastic neoplasia in trophoblastic disease centers in South America. Int. J. Gynecol. Cancer 2020, 30, 1366–1371. [Google Scholar] [CrossRef]
- Savage, P.; Winter, M.; Parker, V.; Harding, V.; Sita-Lumsden, A.; Fisher, R.A.; Harvey, R.; Unsworth, N.; Sarwar, N.; Short, D.; et al. Demographics, natural history and treatment outcomes of non-molar gestational choriocarcinoma: A UK population study. Bjog 2020, 127, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Albright, B.B.; Ellett, T.; Knochenhauer, H.E.; Goins, E.C.; Monuszko, K.A.; Kaplan, S.J.; Previs, R.A.; Moss, H.A.; Havrilesky, L.J.; Davidson, B.A. Treatments and outcomes in high-risk gestational trophoblastic neoplasia: A systematic review and meta-analysis. Bjog 2023, 130, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Batti, R.; Mokrani, A.; Rachdi, H.; Raies, H.; Touhami, O.; Ayadi, M.; Meddeb, K.; Letaief, F.; Yahiaoui, Y.; Chraiet, N.; et al. Gestational trophoblastic neoplasia: Experience at Salah Azaiez Institute. Pan Afr. Med. J. 2019, 33, 121. [Google Scholar] [CrossRef]
- Gulia, S.; Bajpai, J.; Gupta, S.; Maheshwari, A.; Deodhar, K.; Kerkar, R.A.; Seth, V.; Rekhi, B.; Menon, S. Outcome of gestational trophoblastic neoplasia: Experience from a tertiary cancer centre in India. Clin. Oncol. 2014, 26, 39–44. [Google Scholar] [CrossRef]
- Singhal, S.; Kumar, L.; Kumar, S.; Khurana, S.; Bhatla, N. Predictors of chemotherapy resistance & relapse in gestational trophoblastic neoplasia. Indian J. Med. Res. 2020, 152, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wan, X.R.; Xu, T.; Feng, F.Z.; Ren, T.; Yang, J.J.; Zhao, J.; Yang, T.; Xiang, Y. Evaluation and suggestions for improving the FIGO 2000 staging criteria for gestational trophoblastic neoplasia: A ten-year review of 1420 patients. Gynecol. Oncol. 2018, 149, 539–544. [Google Scholar] [CrossRef]
- Miller, D.S.; Lurain, J.R. Classification and staging of gestational trophoblastic tumors. Obstet. Gynecol. Clin. N. Am. 1988, 15, 477–490. [Google Scholar] [CrossRef]
- Soper, J.T.; Clarke-Pearson, D.; Hammond, C.B. Metastatic gestational trophoblastic disease: Prognostic factors in previously untreated patients. Obstet. Gynecol. 1988, 71, 338–343. [Google Scholar]
- Parazzini, F.; La Vecchia, C.; Pampallona, S. Parental age and risk of complete and partial hydatidiform mole. Br. J. Obstet. Gynaecol. 1986, 93, 582–585. [Google Scholar] [CrossRef]
- Gockley, A.A.; Melamed, A.; Joseph, N.T.; Clapp, M.; Sun, S.Y.; Goldstein, D.P.; Horowitz, N.S.; Berkowitz, R.S. The effect of adolescence and advanced maternal age on the incidence of complete and partial molar pregnancy. Gynecol. Oncol. 2016, 140, 470–473. [Google Scholar] [CrossRef]
| Risk Group | ||||
|---|---|---|---|---|
| Characteristics | Low (n = 98) | High (n = 31) | Ultra-High (n = 31) | p-Value |
| Age (yrs) | 35.50 (27.0–46.0) | 33.0 (30.0–44.0) | 35.0 (27.0–49.0) | 0.89 |
| Parity | 1.00 (0.00–2.00) | 1.00 (0.00–2.00) | 2.00 (1.00–3.00) | 0.07 |
| Pretreatment serum β-hCG (mIU/mL) | 2897 (441–18,591) | 82,476 (16,000–171,800) | 102,989 (26,180–529,916) | <0.01 |
| Antecedent pregnancy | <0.01 | |||
| 85 (86.7) | 17 (54.8) | 5 (16.1) | |
| 11 (11.2) | 0 (0.0) | 1 (3.2) | |
| 1 (1.0) | 7 (22.6) | 5 (16.1) | |
| Term | 1 (1.0) | 7 (22.6) | 20 (64.5) | |
| Interval from index pregnancy (months) | <0.01 | |||
| 87 (88.8) | 15 (48.4) | 4 (12.9) | |
| 4 (4.1) | 1 (3.2) | 2 (6.5) | |
| 2 (2.0) | 2 (6.5) | 3 (9.7) | |
| 5 (5.1) | 13 (41.9) | 22 (71.0) | |
| Sign and symptom * | ||||
| 92 (93.9) | 22 (71.0) | 17 (54.8) | <0.01 |
| 9 (9.2) | 6 (19.4) | 6 (19.4) | 0.14 |
| 8 (8.2) | 4 (12.9) | 5 (16.1) | 0.31 |
| 0 (0.0) | 3 (9.7) | 6 (19.4) | 0.01 |
| 0 (0.0) | 0 (0.0) | 4 (12.9) | 0.01 |
| 0 (0.0) | 1 (3.2) | 5 (16.1) | <0.01 |
| Histologic diagnosis | <0.01 | |||
| 9 (9.2) | 1 (3.2) | 2 (6.5) | |
| 7 (7.1) | 16 (51.6) | 23 (74.2) | |
| 82 (83.7) | 14 (45.2) | 6 (19.4) | |
| <0.01 | |||
| 81 (82.7) | 10 (32.3) | 2 (6.5) | |
| 9 (9.2) | 3 (9.7) | 4 (12.9) | |
| 8 (8.2) | 18 (58.1) | 25 (80.6) | |
| Sites of metastasis | ||||
| 11 (11.2) | 21 (67.7) | 25 (80.6) | <0.01 |
| 0 (0.0) | 1 (3.2) | 3 (9.7) | 0.01 |
| 1 (1.0) | 3 (9.7) | 1 (3.2) | 0.05 |
| 0 (0.0) | 1 (3.2) | 6 (19.4) | <0.01 |
| 0 (0.0) | 0 (0.0) | 11 (35.5) | <0.01 |
| 0 (0.0) | 0 (0.0) | 2 (6.5) | 0.38 |
| 0 (0.0) | 0 (0.0) | 3 (9.7) | 0.07 |
| 0 (0.0) | 0 (0.0) | 2 (6.5) | 0.38 |
| 0 (0.0) | 0 (0.0) | 1 (3.2) | 0.38 |
| Number of metastases | <0.01 | |||
| 87 (88.8) | 7 (22.6) | 1 (3.2) | |
| 9 (9.2) | 7 (22.6) | 5 (16.1) | |
| 0 (0.0) | 6 (19.4) | 3 (9.7) | |
| 2 (2.0) | 11 (35.5) | 22 (71.0) | |
| Risk score | 2 (1–4) | 9 (8–11) | 14 (13–16) | <0.01 |
| FIGO stage | <0.01 | |||
| 87 (88.8) | 8 (25.8) | 1 (3.2) | |
| 1 (1.0) | 2 (6.5) | 1 (3.2) | |
| 10 (10.2) | 20 (64.5) | 13 (41.9) | |
| 0 (0.0) | 1 (3.2) | 16 (51.6) | |
| Previous failed chemotherapy | 0.16 | |||
| 86 (87.8) | 26 (83.9) | 25 (80.6) | |
| 12 (12.2) | 4 (12.9) | 4 (12.9) | |
| 0 (0.0) | 1 (3.2) | 2 (6.5) |
| Characteristics | Low (n = 97) | High (n = 30) | Ultra-High (n = 30) | p-Value |
|---|---|---|---|---|
| Response after first line | 0.91 | |||
| 66 (68.0) | 20 (66.7) | 19 (63.3) | |
| 31 (32.0) | 10 (33.3) | 11 (36.7) | |
| Response after second line | 0.04 | |||
| 19 (61.3) | 4 (40.0) | 2 (18.2) | |
| 12 (38.7) | 6 (60.0) | 9 (81.8) | |
| Response after salvage therapy | <0.01 | |||
| 11 (91.7) | 1 (16.7) | 2 (22.2) | |
| 1 (8.3) | 5 (83.3) | 7 (77.8) | |
| Vital status | <0.01 | |||
| 94 (96.9) | 24 (80.0) | 19 (63.3) | |
| 3 (3.1) | 6 (20.0) | 11 (36.7) |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p-Value | aHR (95% CI) | p-Value |
| Age (years) | 1.02 (0.98–1.06) | 0.27 | ||
| Antecedent pregnancy | <0.01 | <0.01 | ||
| 1 | 1 | ||
| 2.21 (0.25–19.80) | 2.31 (0.26–20.69) | ||
| 6.46 (1.45–28.86) | 4.02 (0.84–19.22) | ||
| 15.99 (5.20–49.14) | 11.50 (3.56–37.22) | ||
| Pretreatment β-hCG (mIU/mL) | 0.72 | |||
| 1 | |||
| 1.20 (0.44–3.27) | |||
| Previous failed chemotherapy | 0.51 | |||
| 1 | |||
| 1.19 (0.35–4.06) | |||
| 3.27 (0.44–24.62) | |||
| Liver metastasis | 0.24 | |||
| 1 | |||
| 2.39 (0.56–10.25) | |||
| Brain metastasis | <0.01 | <0.01 | ||
| 1 | 1 | ||
| 11.43 (4.59–28.48) | 4.61 (1.73–12.28) | ||
| Renal metastasis | 0.07 | |||
| 1 | |||
| 6.58 (0.88–49.10) | |||
| Spleen metastasis | <0.01 | |||
| 1 | |||
| 12.93 (2.99–55.85) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruengsaen, A.; Sethasathien, S.; Tantipalakorn, C.; Charoenkwan, K.; Suprasert, P.; Srisomboon, J.; Tongsong, T. Clinical Characteristics and Prognostic Factors in Patients with Gestational Trophoblastic Neoplasia: A Single-Center Study Comparing Ultra-High-Risk and Other Risk Groups. Cancers 2025, 17, 1655. https://doi.org/10.3390/cancers17101655
Ruengsaen A, Sethasathien S, Tantipalakorn C, Charoenkwan K, Suprasert P, Srisomboon J, Tongsong T. Clinical Characteristics and Prognostic Factors in Patients with Gestational Trophoblastic Neoplasia: A Single-Center Study Comparing Ultra-High-Risk and Other Risk Groups. Cancers. 2025; 17(10):1655. https://doi.org/10.3390/cancers17101655
Chicago/Turabian StyleRuengsaen, Atita, Sethawat Sethasathien, Charuwan Tantipalakorn, Kittipat Charoenkwan, Prapaporn Suprasert, Jatupol Srisomboon, and Theera Tongsong. 2025. "Clinical Characteristics and Prognostic Factors in Patients with Gestational Trophoblastic Neoplasia: A Single-Center Study Comparing Ultra-High-Risk and Other Risk Groups" Cancers 17, no. 10: 1655. https://doi.org/10.3390/cancers17101655
APA StyleRuengsaen, A., Sethasathien, S., Tantipalakorn, C., Charoenkwan, K., Suprasert, P., Srisomboon, J., & Tongsong, T. (2025). Clinical Characteristics and Prognostic Factors in Patients with Gestational Trophoblastic Neoplasia: A Single-Center Study Comparing Ultra-High-Risk and Other Risk Groups. Cancers, 17(10), 1655. https://doi.org/10.3390/cancers17101655






