The Impact of Prophylactic Negative Wound Pressure Treatment (NWPT) on Surgical Site Occurrences After Gynecologic Cancer Surgery: A Meta-Analysis of Randomized Controlled and Observational Cohort Studies
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Protocol and Registration
2.2. Study Types
2.3. Information Sources and Search
2.4. Study Data and Predefined Outcomes
2.5. Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
3.1. Included Studies
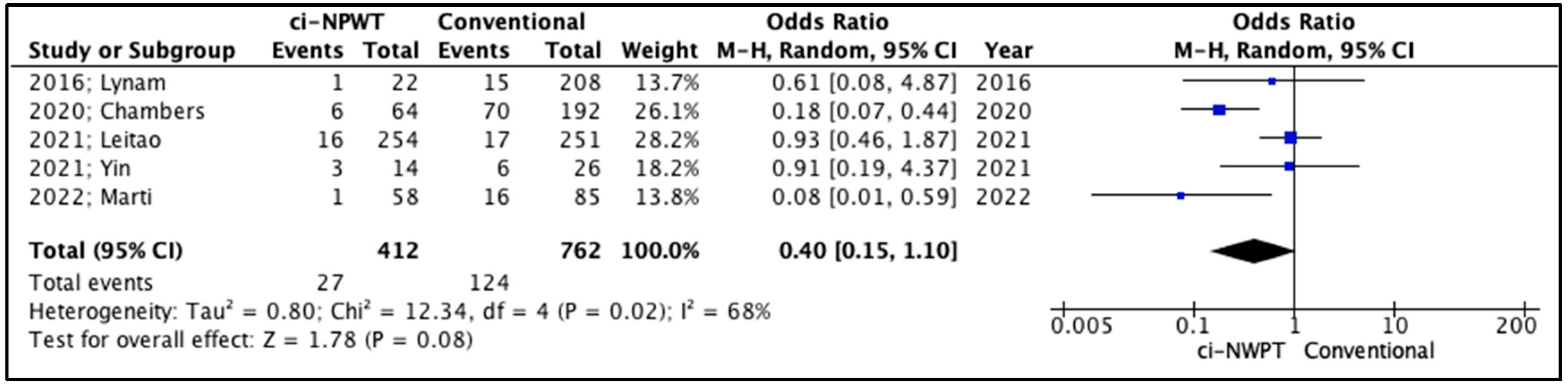
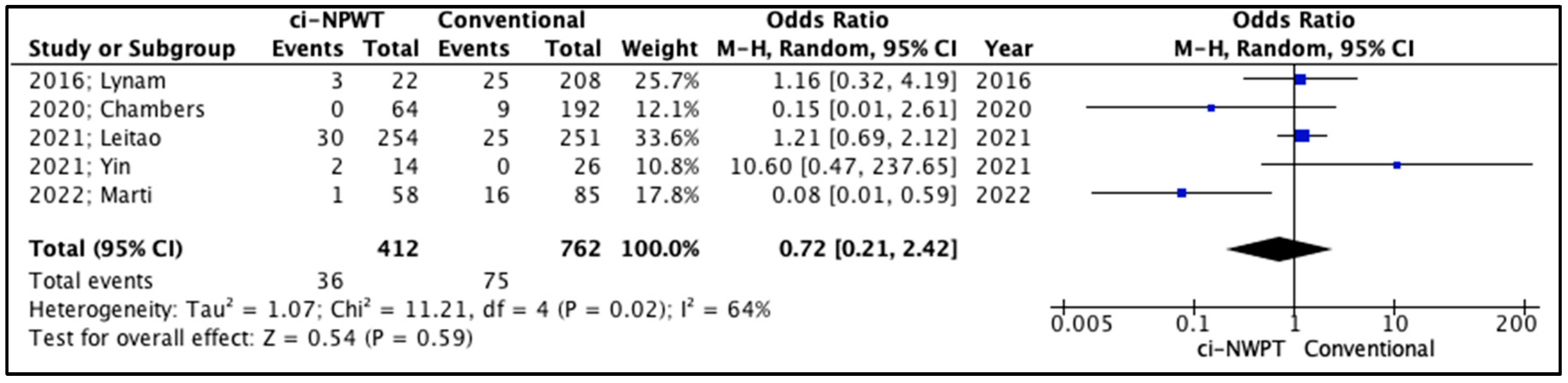
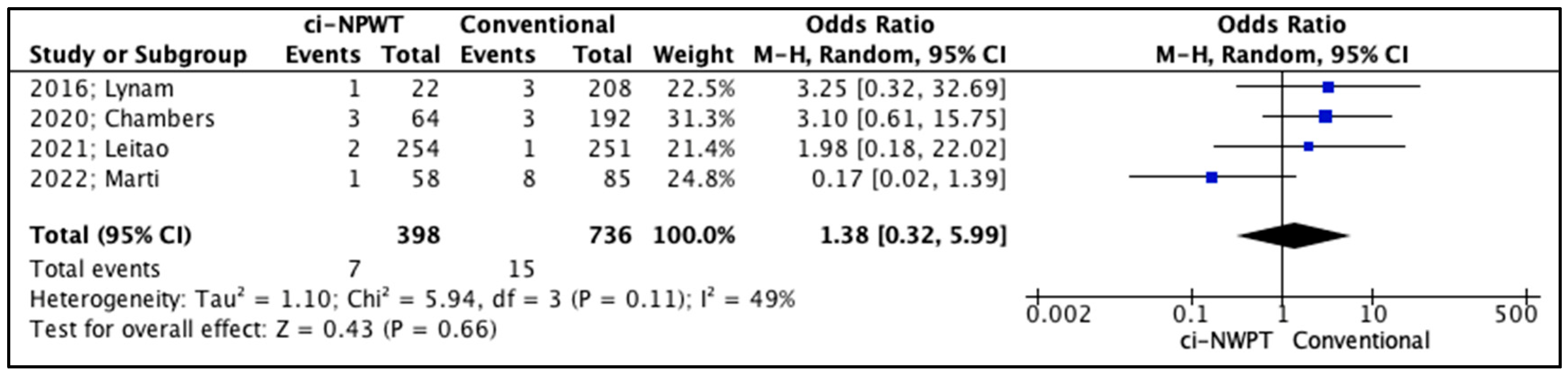
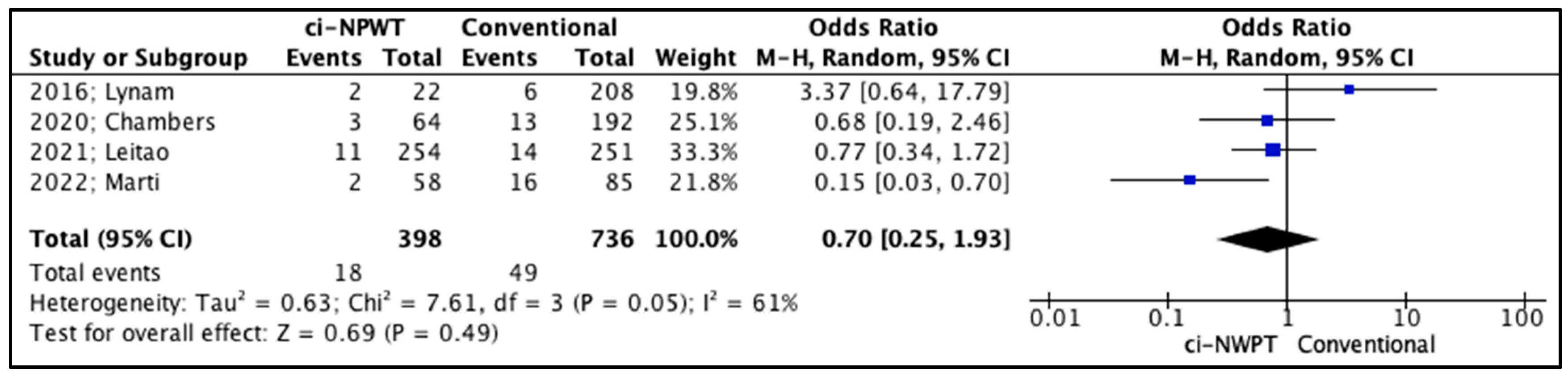
3.2. Quantitative Analysis
3.3. Qualitative Analysis
4. Discussion
4.1. Implications of Negative Pressure Wound Therapy
4.2. Strengths and Weaknesses
4.3. Clinical Practice and Future Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; He, W.; Zhang, Q.; Zhang, B.; Dong, L.; Li, L.; Liu, X. Evaluation and prediction analysis of 3-and 5-year relative survival rates of patients with cervical cancer: A model-based period analysis. Cancer Control 2024, 31, 10732748241232324. [Google Scholar] [CrossRef]
- Kwaan, M.R.; Weight, C.J.; Carda, S.J.; Mills-Hokanson, A.; Wood, E.; Rivard-Hunt, C.; Argenta, P.A. Abdominal closure protocol in colorectal, gynecologic oncology, and urology procedures: A randomized quality improvement trial. Am. J. Surg. 2016, 211, 1077–1083. [Google Scholar] [CrossRef]
- Bakkum-Gamez, J.N.; Dowdy, S.C.; Borah, B.J.; Haas, L.R.; Mariani, A.; Martin, J.R.; Weaver, A.L.; McGree, M.E.; Cliby, W.A.; Podratz, K.C. Predictors and costs of surgical site infections in patients with endometrial cancer. Gynecol. Oncol. 2013, 130, 100–106. [Google Scholar] [CrossRef]
- Iatrakis, G.; Sakellaropoulos, G.; Georgoulias, N.; Chadjithomas, A.; Kourounis, G.; Tsionis, C.; Ladopoulos, J.; Tzingounis, V. Gynecologic cancer and surgical infectious morbidity. Clin. Exp. Obstet. Gynecol. 1998, 25, 36–37. [Google Scholar] [PubMed]
- Di Leo, A.; Piffer, S.; Ricci, F.; Manzi, A.; Poggi, E.; Porretto, V.; Fambri, P.; Piccini, G.; Patrizia, T.; Fabbri, L. Surgical site infections in an italian surgical ward: A prospective study. Surg. Infect. 2009, 10, 533–538. [Google Scholar] [CrossRef]
- O’Donnell, R.L.; Angelopoulos, G.; Beirne, J.P.; Biliatis, I.; Bolton, H.; Bradbury, M.; Craig, E.; Gajjar, K.; Mackintosh, M.L.; MacNab, W.; et al. Impact of surgical site infection (ssi) following gynaecological cancer surgery in the uk: A trainee-led multicentre audit and service evaluation. BMJ Open 2019, 9, e024853. [Google Scholar] [CrossRef]
- Nasioudis, D.; Mastroyannis, S.A.; Ko, E.M.; Haggerty, A.F.; Cory, L.; Giuntoli, R.L., 2nd; Kim, S.H.; Morgan, M.A.; Latif, N.A. Delay in adjuvant chemotherapy administration for patients with figo stage i epithelial ovarian carcinoma is associated with worse survival; an analysis of the national cancer database. Gynecol. Oncol. 2022, 166, 263–268. [Google Scholar] [CrossRef]
- Gombert, A.; Babilon, M.; Barbati, M.E.; Keszei, A.; von Trotha, K.T.; Jalaie, H.; Kalder, J.; Kotelis, D.; Greiner, A.; Langer, S. Closed incision negative pressure therapy reduces surgical site infections in vascular surgery: A prospective randomised trial (aims trial). Eur. J. Vasc. Endovasc. Surg. 2018, 56, 442–448. [Google Scholar] [CrossRef]
- O’Leary, D.P.; Peirce, C.; Anglim, B.; Burton, M.; Concannon, E.; Carter, M.; Hickey, K.; Coffey, J.C. Prophylactic Negative Pressure Dressing Use in Closed Laparotomy Wounds Following Abdominal Operations: A Randomized, Controlled, Open-Label Trial: The Pico Trial. Ann. Surg. 2017, 265, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Willy, C.; Agarwal, A.; Andersen, C.A.; Santis, G.D.; Gabriel, A.; Grauhan, O.; Guerra, O.M.; Lipsky, B.A.; Malas, M.B.; Mathiesen, L.L. Closed incision negative pressure therapy: International multidisciplinary consensus recommendations. Int. Wound J. 2017, 14, 385–398. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Lynam, S.; Mark, K.S.; Temkin, S.M. Primary placement of incisional negative pressure wound therapy at time of laparotomy for gynecologic malignancies. Int. J. Gynecol. Cancer 2016, 26, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.M.; Morton, M.; Lampert, E.; Yao, M.; Debernardo, R.; Rose, P.G.; Vargas, R. Use of prophylactic closed incision negative pressure therapy is associated with reduced surgical site infections in gynecologic oncology patients undergoing laparotomy. Am. J. Obstet. Gynecol. 2020, 223, 731.e1–731.e9. [Google Scholar] [CrossRef] [PubMed]
- Leitao, M.M., Jr.; Zhou, Q.C.; Schiavone, M.B.; Cowan, R.A.; Smith, E.S.; Iasonos, A.; Veith, M.; Rafizadeh, M.; Curran, K.; Ramesh, B. Prophylactic negative pressure wound therapy after laparotomy for gynecologic surgery: A randomized controlled trial. Obstet. Gynecol. 2021, 137, 334–341. [Google Scholar] [CrossRef]
- Yin, L.; Lau, K.; Mehra, G.; Sayasneh, A. Closed-incision negative pressure wound management following midline laparotomy in gynecological oncology operations: A feasibility pilot study. Cureus 2021, 13, e19871. [Google Scholar] [CrossRef]
- Martí, M.T.C.; Fernandez-Gonzalez, S.; Martí, M.D.; Pla, M.J.; Barahona, M.; Ponce, J. Prophylactic incisional negative pressure wound therapy for gynaecologic malignancies. Int. Wound J. 2022, 19, 272–277. [Google Scholar] [CrossRef]
- Nejadghaderi, S.A.; Balibegloo, M.; Rezaei, N. The cochrane risk of bias assessment tool 2 (rob 2) versus the original rob: A perspective on the pros and cons. Health Sci. Rep. 2024, 7, e2165. [Google Scholar] [CrossRef]
- Igelström, E.; Campbell, M.; Craig, P.; Katikireddi, S.V. Cochrane’s risk of bias tool for non-randomized studies (robins-i) is frequently misapplied: A methodological systematic review. J. Clin. Epidemiol. 2021, 140, 22–32. [Google Scholar] [CrossRef]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.A.; Xu, C. The freeman-tukey double arcsine transformation for the meta-analysis of proportions: Recent criticisms were seriously misleading. J. Evid. Based Med. 2021, 14, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Groenen, H.; Jalalzadeh, H.; Buis, D.R.; Dreissen, Y.E.; Goosen, J.H.; Griekspoor, M.; Harmsen, W.J.; IJpma, F.F.; van der Laan, M.J.; Schaad, R.R. Incisional negative pressure wound therapy for the prevention of surgical site infection: An up-to-date meta-analysis and trial sequential analysis. EClinicalMedicine 2023, 62, 102105. [Google Scholar] [CrossRef]
- James, K.; Glasswell, A.; Costa, B. Single-use negative pressure wound therapy versus conventional dressings for the reduction of surgical site infections in closed surgical incisions: Systematic literature review and meta-analysis. Am. J. Surg. 2024, 228, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.J.; Singh, D.P.; Gabriel, A.; Mantyh, C.; Silverman, R.; Griffin, L. Closed incision negative pressure therapy versus standard of care in reduction of surgical site complications: A systematic review and meta-analysis. Plast. Reconstr. Surg.-Glob. Open 2023, 11, e4722. [Google Scholar] [CrossRef]
- Schimp, V.L.; Worley, C.; Brunello, S.; Levenback, C.C.; Wolf, J.K.; Sun, C.C.; Bodurka, D.C.; Ramirez, P.T. Vacuum-assisted closure in the treatment of gynecologic oncology wound failures. Gynecol. Oncol. 2004, 92, 586–591. [Google Scholar] [CrossRef]
- Lavoie, M.C.; MPlante; Lemieux, M.-C.; Roberge, C.; Renaud, M.-C.; Grégoire, J.; Roy, M.; Sebastianelli, A. Extensive adipose tissue necrosis following pfannenstiel incision for endometrial cancer. J. Obstet. Gynaecol. Can. 2014, 36, 253–257. [Google Scholar] [CrossRef]
- Miller, M.; McDaniel, C. Postsurgical post-hysterectomy abdominal wound dehiscence treated with negative pressure wound therapy. Int. J. Gynecol. Obstet. 2006, 93, 264–266. [Google Scholar] [CrossRef]
- Nissman, K.W.; Nissman, D.B.; Leighton, B.L.; Varaday, S.S.; Lockhart, E.M. Necrotizing fasciitis after cesarean delivery. Obstet. Anesth. Dig. 2012, 32, 252. [Google Scholar] [CrossRef]
- GlobalSurg Collaborative. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: A prospective, international, multicentre cohort study. Lancet Infect Dis. 2018, 18, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.; Edmiston, C. World health organization: Global guidelines for the prevention of surgical site infection. J. Hosp. Infect. 2017, 95, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.P.; Apostolides, J.; Chatterjee, A.; Dardano, A.N.; Fearmonti, R.M.; Gabriel, A.; Grant, R.T.; Johnson, O.N., III; Koneru, S.; Kuang, A.A. The use of closed incision negative pressure therapy for incision and surrounding soft tissue management: Expert panel consensus recommendations. Int. Wound J. 2022, 19, 643–655. [Google Scholar] [CrossRef] [PubMed]

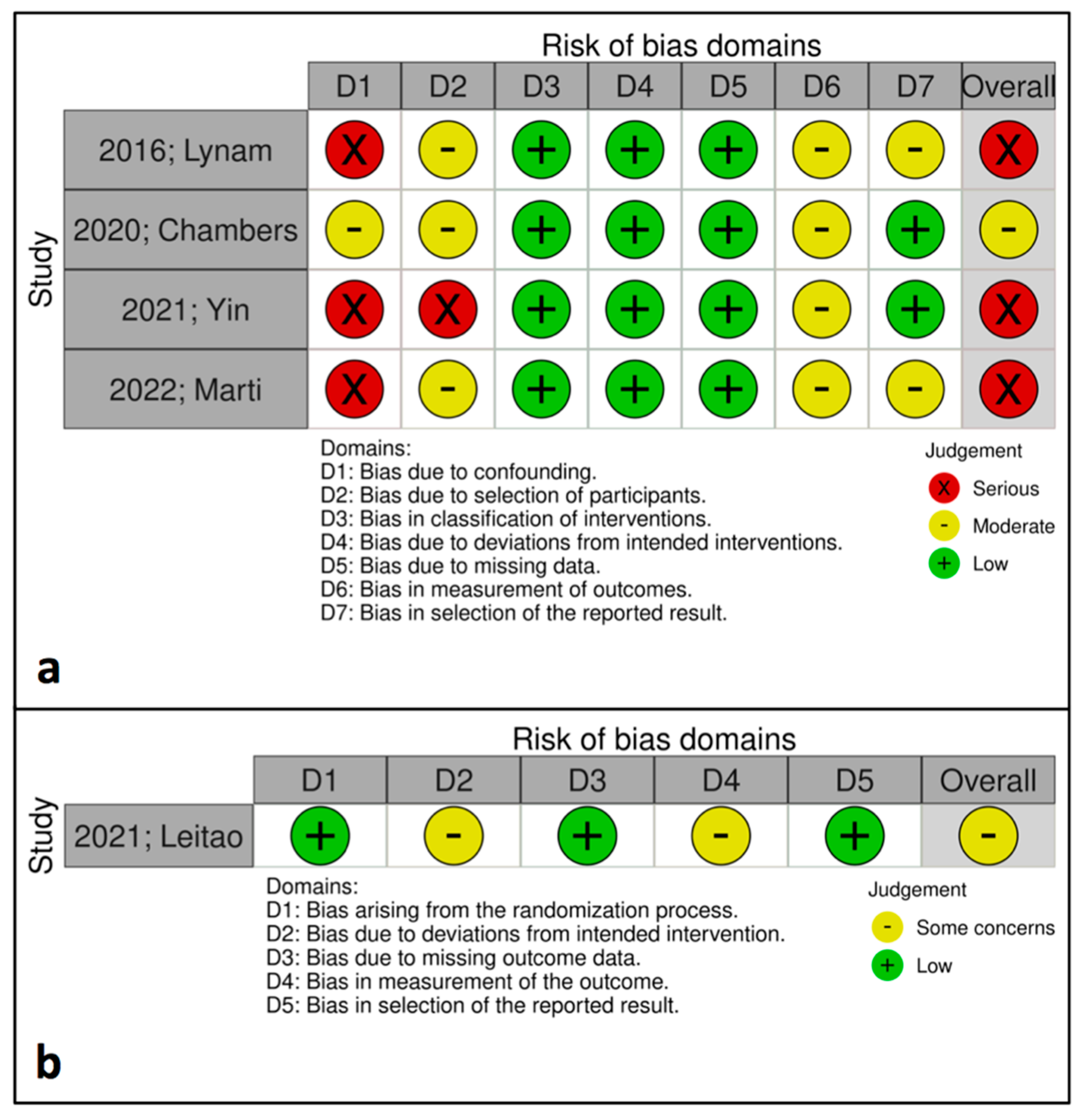
| Study Characteristics (ciNWPT vs. Conventional Gauze) | ||||||
|---|---|---|---|---|---|---|
| Year; Author | Type of Study | No. of Patients (ciNWPT vs. Conventional Gauze) | Study Origin | Inclusion Criteria | Exclusion Criteria | Postoperative Follow-up |
| 2016; Lynam [14] | Retrospective case–control study | 230 (22 vs. 208) | USA | High-risk for wound complications | Lost to follow-up | 90 days |
| 2020; Chambers [15] | Retrospective multicenter case–control study | 256 (64 vs. 192) | USA | Surgeons’ estimation of high risk | Laparotomy for tissue extraction | 30 days |
| 2021; Leitao [16] | Randomized single blinded clinical trial | 505 (254 vs. 251) | USA |
| Open abdomen | 30 days |
| 2021; Yin [17] | Observational cohort study | 40 (14 vs. 26) | UK |
|
| 30 days |
| 2022; Marti [18] | Retrospective cohort study | 143 (58 vs. 85) | Spain |
|
| 30 days |
| Patient Characteristics (ciNWPT vs. Conventional Gauze) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year; Author | Age (Years) | BMI (kg/m2) | Smoking | Diabetes | Cardiovascular Disease | Steroids | Previous Surgery | Performance Status (ASA Score) | |
| ciNWPT | Conventional Gauze | ||||||||
| 2016; Lynam [14] | 54.9 vs. 53.2 | 41.29 vs. 30.67 | 5 (22.73%) vs. 30 (14.42%) | 7 (31.82%) vs. 35 (16.83%) | N/A | 0 vs. 6 (2.88%) | 12 (54.54%) vs. 70 (33.65%) | N/A | N/A |
| 2020; Chambers [15] | 59.0 ± 11.8 vs. 60.9 ± 11.8 | <30: 14 (21.9%) vs. 51 (26.6%) 31–40: 27 (42.2%) vs. 85 (44.3%) 41–50: 19 (71.9%) vs. 52 (27.1%) >51: 4 (6.3%) vs. 4 (2.1%) | Current: 4 (6.2%) vs. 16 (0.8%) Historical: 19 (29.2%) vs. 40 (20.9%) None: 42 (64.6%) vs. 135 (70.7%) | 25 (39%) vs. 75 (39.1%) | CAD: 7 (10.9%) vs. 22 (11.5%) Prior MI: 1 (1.6%) vs. 2 (1.0%) Prior Stroke: 0 vs. 8 (4.2%) Prior VTE: 11 (17.2%) vs. 25 (13%) | 2 (3.1%) vs. 3 (1.6%) | N/A | (1) 7 (10.9%) (2) 20 (31.3%) (3) 36 (56.3%) (4) 1 (1.6%) | (1) 1 (0.5%) (2) 40 (20.8%) (3) 141 (73.4%) (4) 10 (5.2%) |
| 2021; Leitao [16] | 60 (20–85) vs. 61 (23–87) | 26 (range, 18–60) vs. 26 (range, 17–56) | Never: 143 (57%)/152 (61%) Current: 10 (4%)/11 (4%) Former: 97 (39%)/87 (35%) | 36 (14%) vs. 0 | Hypertension: 85 (34%) vs. 86 (35%) Vascular disease: 7 (2.8%) vs. 12 (5%) | 175 (70%) vs. 168 (68%) | N/A | N/A | |
| 2021; Yin [17] | 59.6 vs. 57.6 | ≥30: 5 (36%) vs. 4 (15%) | Active smoking 5 (36%)/4 (15%) | 3 (21%) vs. 1 (4%) | N/A | N/A | ASA grade ≥ 3:10 (71%) | ASA grade ≥ 3:6 (23%) | |
| 2022; Marti [18] | 63.28 vs. 61.51 | 28.59 vs. 27.59 | N/A | 12 (20.7%) vs. 19 (22.4%) | N/A | N/A | 31 (53.4%) vs. 50 (58.8%) | N/A | N/A |
| Operative Parameters (ciNWPT vs. Conventional Gauze) | ||||||
|---|---|---|---|---|---|---|
| Year; Author | Blood Loss (mL) | Operative Duration (min) | Bowel Resection | Blood Transfusion | Staple Closure | Wound Classification (CDC) |
| 2016; Lynam [14] | 656 vs. 394 | 138 vs. 137 | 0 vs. 34 (16.34%) | 6 (27.27%) vs. 61 (29.19%) | 19 (86.36%) vs. 106 (51%) | N/A |
| 2020; Chambers [15] | N/A | 233.0 (range, 136.5–311.5) vs. 211.0 (range, 150–313.0) | N/A | N/A | 53 (82.8%) vs. 147 (76.6%) | Mean 2.0 vs. 2.0 |
| 2021; Leitao [16] | 400 (range, 5–3200) vs. 300 (range, 5–3300) | 291 (range, 56–701) vs. 256 (range, 60–786) | 92 (37%) vs. 92 (37%) | 46 (18%) vs. 31 (12%) | 254 (100%) vs. 251 (100%) | Clean 16 (6%) vs. 11 (4%) Clean-contaminated 229 (91%) vs. 236 (94%) Contaminated/Dirty 6 (2.4%) vs. 3 (1.2%) |
| 2021; Yin [17] | N/A | N/A | N/A | N/A | 14 (100%) vs. N/A | N/A |
| 2022; Marti [18] | N/A | N/A | N/A | N/A | N/A | N/A |
| Cancer Characteristics | ||||||
|---|---|---|---|---|---|---|
| Year; Author | Cancer Site | Disease Stage (FIGO) | Neoadjuvant Therapy | |||
| ciNWPT | Conventional Gauze | ciNWPT | Conventional Gauze | ciNWPT | Conventional Gauze | |
| 2016; Lynam [14] | Cervix 0 Uterine corpus 9 (41%) Ovarian 6 (27%) | Cervix 19 (9%) Uterine corpus 53 (25%) Ovarian 54 (26%) | I 5 (23%) II 1 (5%) III 5 (23%) IV 5 (23%) | I 49 (24%) II 9 (4%) III 5 34 (16%) IV 23 (11%) | 1 (5%) | 16 (8%) |
| 2020; Chambers [15] | N/A | N/A | I 17 (27%) II 4 (6%) III 24 (38%) IV 12 (19%) | I 39 (20%) II 7 (4%) III 90 (47%) IV 37 (19%) | Neoadjuvant Chemotherapy 14 (22%) | Neoadjuvant Chemotherapy 44 (23%) |
| 2021; Leitao [16] | Ovary/fallopian tube/peritoneal cancer 203 (80%) Uterine cancer 37 (15%) Cervical cancer 4 (2%) Other 5 (2%) | Ovary/fallopian tube/peritoneal cancer 207 (82%) Uterine cancer 32 (13%) Cervical cancer 2 (1%) Other 5 (2%) | N/A | N/A | Prior radiation therapy exposure 7 (3%) Prior chemotherapy exposure 85 (33%) | Prior radiation therapy exposure 8 (3%) Prior chemotherapy exposure 79 (31%) |
| 2021; Yin [17] | N/A | N/A | N/A | N/A | N/A | N/A |
| 2022; Marti [18] | Ovarian cancer 33 (57%) Endometrial cancer 20 (34%) | Ovarian cancer 61 (72%) Cervical cancer 1 (1%) Endometrial cancer 20 (24%) Vulvar cancer 1 (1%) | I 11 (19%) II 4 (7%) III 25 (43%) IV 3 (5%) | I 13 (15%) II 10 (12%) III 44 (52%) IV 9 (11%) | N/A | N/A |
| Postoperative Outcomes (ciNWPT vs. Conventional Gauze) | |||||
|---|---|---|---|---|---|
| Year; Author | SSI | Dehiscence | Seroma | Hematoma | Length of Stay (Mean Days) |
| 2016; Lynam [14] | 1 (5%) vs. 15 (7%) | 3 (14%) vs. 25 (12%) | 2 (9%) vs. 6 (3%) | 1 (5%) vs. 3 (1%) | 6.22 vs. 5.25 |
| 2020; Chambers [15] | 6 (9%) vs. 70 (36%) | 0 (0%) vs. 9 (5%) | 3 (5%) vs. 13 (7%) | 3 (5%) vs. 3 (2%) | N/A |
| 2021; Leitao [16] | 16 (6%) vs. 17 (7%) | 30 (12%) vs. 25 (10%) | 11 (4%) vs. 14 (6%) | 2 (1%) vs. 1 (0.4%) | N/A |
| 2021; Yin [17] | 3 (21%) vs. 6 (23%) | 2 (14%) vs. 0 (0%) | N/A | N/A | N/A |
| 2022; Marti [18] | 1 (2%) vs. 16 (19%) | 1 (2%) vs. 16 (19%) | 2 (3%) vs. 16 (19%) | 1 (2%) vs. 8 (9%) | 6.16 vs. 8.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frountzas, M.; Karavolias, I.; Nikolaou, C.; Toutouza, O.; Pergialiotis, V.; Toutouzas, K.G. The Impact of Prophylactic Negative Wound Pressure Treatment (NWPT) on Surgical Site Occurrences After Gynecologic Cancer Surgery: A Meta-Analysis of Randomized Controlled and Observational Cohort Studies. Cancers 2025, 17, 1717. https://doi.org/10.3390/cancers17101717
Frountzas M, Karavolias I, Nikolaou C, Toutouza O, Pergialiotis V, Toutouzas KG. The Impact of Prophylactic Negative Wound Pressure Treatment (NWPT) on Surgical Site Occurrences After Gynecologic Cancer Surgery: A Meta-Analysis of Randomized Controlled and Observational Cohort Studies. Cancers. 2025; 17(10):1717. https://doi.org/10.3390/cancers17101717
Chicago/Turabian StyleFrountzas, Maximos, Ioannis Karavolias, Christina Nikolaou, Orsalia Toutouza, Vasilios Pergialiotis, and Konstantinos G. Toutouzas. 2025. "The Impact of Prophylactic Negative Wound Pressure Treatment (NWPT) on Surgical Site Occurrences After Gynecologic Cancer Surgery: A Meta-Analysis of Randomized Controlled and Observational Cohort Studies" Cancers 17, no. 10: 1717. https://doi.org/10.3390/cancers17101717
APA StyleFrountzas, M., Karavolias, I., Nikolaou, C., Toutouza, O., Pergialiotis, V., & Toutouzas, K. G. (2025). The Impact of Prophylactic Negative Wound Pressure Treatment (NWPT) on Surgical Site Occurrences After Gynecologic Cancer Surgery: A Meta-Analysis of Randomized Controlled and Observational Cohort Studies. Cancers, 17(10), 1717. https://doi.org/10.3390/cancers17101717








