Active Surveillance in Non-Muscle Invasive Bladder Cancer: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Acquisition of Evidence
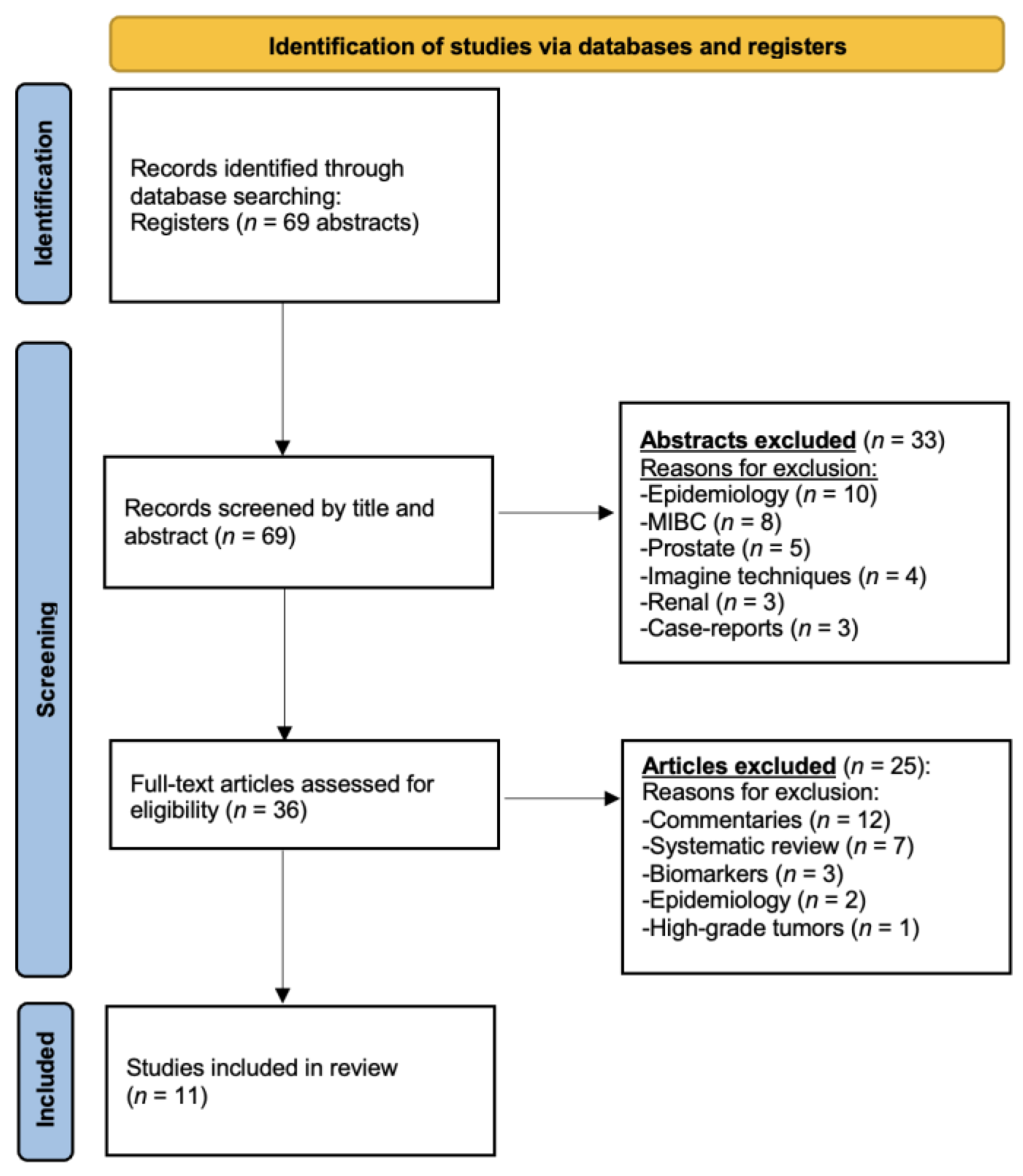
2.1. Search Strategy and Results
2.2. Quality Assessment
2.3. Data Extraction
3. Evidence Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NMIBC | Non-muscle-invasive bladder cancer |
| AS | Active surveillance |
| CIS | Carcinoma in situ |
| MIBC | Muscle-invasive bladder cancer |
References
- Cancer Today. Available online: https://gco.iarc.fr/today/en/dataviz/pie?mode=population (accessed on 15 January 2025).
- Babjuk, M.; Burger, M.; Zigeuner, R.; Shariat, S.F.; Van Rhijn, B.W.G.; Compérat, E.; Sylvester, R.J.; Kaasinen, E.; Böhle, A.; Palou Redorta, J.; et al. EAU Guidelines on Non–Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2013. Eur. Urol. 2013, 64, 639–653. [Google Scholar] [CrossRef] [PubMed]
- van Rhijn, B.W.; Burger, M.; Lotan, Y.; Solsona, E.; Stief, C.G.; Sylvester, R.J.; Witjes, J.A.; Zlotta, A.R. Recurrence and Progression of Disease in Non–Muscle-Invasive Bladder Cancer: From Epidemiology to Treatment Strategy. Eur. Urol. 2009, 56, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Fasulo, V.; Paciotti, M.; Lazzeri, M.; Contieri, R.; Casale, P.; Saita, A.; Lughezzani, G.; Diana, P.; Frego, N.; Avolio, P.P.; et al. Xpert Bladder Cancer Monitor May Avoid Cystoscopies in Patients Under “Active Surveillance” for Recurrent Bladder Cancer (BIAS Project): Longitudinal Cohort Study. Front. Oncol. 2022, 12, 832835. [Google Scholar] [CrossRef]
- Contieri, R.; Paciotti, M.; Lughezzani, G.; Buffi, N.M.; Frego, N.; Diana, P.; Fasulo, V.; Saita, A.; Casale, P.; Lazzeri, M.; et al. Long-term Follow-up and Factors Associated with Active Surveillance Failure for Patients with Non–muscle-invasive Bladder Cancer: The Bladder Cancer Italian Active Surveillance (BIAS) Experience. Eur. Urol. Oncol. 2022, 5, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.M.; Weingarten, J.; Murphy, W.M. Transitional cell neoplasms of the urinary bladder. Can biologic potential be predicted from histologic grading? Cancer 1987, 60, 2766–2774. [Google Scholar] [CrossRef]
- Kavalieris, L.; O’sullivan, P.; Frampton, C.; Guilford, P.; Darling, D.; Jacobson, E.; Suttie, J.; Raman, J.D.; Shariat, S.F.; Lotan, Y. Performance Characteristics of a Multigene Urine Biomarker Test for Monitoring for Recurrent Urothelial Carcinoma in a Multicenter Study. J. Urol. 2017, 197, 1419–1426. [Google Scholar] [CrossRef]
- Netto, G.J. Molecular biomarkers in urothelial carcinoma of the bladder: Are we there yet? Nat. Rev. Urol. 2012, 9, 41–51. [Google Scholar] [CrossRef]
- Catto, J.W.; Miah, S.; Owen, H.C.; Bryant, H.; Myers, K.; Dudziec, E.; Larré, S.; Milo, M.; Rehman, I.; Rosario, D.J.; et al. Distinct MicroRNA alterations characterize high- and low-grade bladder cancer. Cancer Res. 2009, 69, 8472–8481. [Google Scholar] [CrossRef]
- Gontero, P.; Birtle, A.; Compérat, E.M.; Dominguez-Escrig, J.L.; Liedberg, F.; Mariappan, P.; Masson-Lecomte, A.; Mostafid, A.H.; van Rhijn, B.W.G.; Seisen, T.; et al. Non-Muscle-Invasive Bladder Cancer (TaT1 and CIS) EAU Guidelines on. 2024; EAU Guidelines Office: Arnhem, The Netherlands, 2024. [Google Scholar]
- Malmström, P.-U.; Sylvester, R.J.; Crawford, D.E.; Friedrich, M.; Krege, S.; Rintala, E.; Solsona, E.; Di Stasi, S.M.; Witjes, J.A. An Individual Patient Data Meta-Analysis of the Long-Term Outcome of Randomised Studies Comparing Intravesical Mitomycin C versus Bacillus Calmette-Guérin for Non–Muscle-Invasive Bladder Cancer. Eur. Urol. 2009, 56, 247–256. [Google Scholar] [CrossRef]
- Böhle, A.; Bock, P. Intravesical bacille calmette-guérin versus mitomycin c in superficial bladder cancer: Formal meta-analysis of comparative studies on tumor progression. Urology 2004, 63, 682–686. [Google Scholar] [CrossRef]
- Brausi, M.; Witjes, J.A.; Lamm, D.; Persad, R.; Palou, J.; Colombel, M.; Buckley, R.; Soloway, M.; Akaza, H.; Böhle, A. A Review of Current Guidelines and Best Practice Recommendations for the Management of Nonmuscle Invasive Bladder Cancer by the International Bladder Cancer Group. J. Urol. 2011, 186, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.W.; Donat, S.M.; Dalbagni, G. Correlation of cystoscopy with histology of recurrent papillary tumors of the bladder. J. Urol. 2002, 168, 978–980. [Google Scholar] [CrossRef]
- Hu, H.; Zhou, M.; Yang, B.; Zhou, S.; Liu, Z.; Zhang, J. A Systematic Review on the Role of Repeat Transurethral Resection after Initial en Bloc Resection for Non-Muscle Invasive Bladder Cancer. J. Clin. Med. 2022, 11, 5049. [Google Scholar] [CrossRef] [PubMed]
- Naselli, A.; Hurle, R.; Paparella, S.; Buffi, N.M.; Lughezzani, G.; Lista, G.; Casale, P.; Saita, A.; Lazzeri, M.; Guazzoni, G. Role of Restaging Transurethral Resection for T1 Non–muscle invasive Bladder Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2018, 4, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.G.; Foerster, B.; Catto, J.W.; Kamat, A.M.; Kassouf, W.; Jubber, I.; Shariat, S.F.; Sylvester, R.J.; Gontero, P. Repeat Transurethral Resection in Non–muscle-invasive Bladder Cancer: A Systematic Review. Eur. Urol. 2018, 73, 925–933. [Google Scholar] [CrossRef]
- Bansal, A.; Sankhwar, S.; Goel, A.; Kumar, M.; Purkait, B.; Aeron, R. Grading of complications of transurethral resection of bladder tumor using Clavien–Dindo classification system. Indian J. Urol. 2016, 32, 232–237. [Google Scholar] [CrossRef]
- Kim, L.H.C.; Patel, M.I. Transurethral resection of bladder tumour (TURBT). Transl. Androl. Urol. 2020, 9, 3056–3072. [Google Scholar] [CrossRef]
- Tomiyama, E.; Fujita, K.; Hashimoto, M.; Uemura, H.; Nonomura, N. Urinary markers for bladder cancer diagnosis: A review of current status and future challenges. Int. J. Urol. 2023, 31, 208–219. [Google Scholar] [CrossRef]
- Bruinsma, S.M.; Bangma, C.H.; Carroll, P.R.; Leapman, M.S.; Rannikko, A.; Petrides, N.; Weerakoon, M.; Bokhorst, L.P.; Roobol, M.J. The Movember GAP3 consortium Active surveillance for prostate cancer: A narrative review of clinical guidelines. Nat. Rev. Urol. 2016, 13, 151–167. [Google Scholar] [CrossRef]
- Bahouth, Z.; Halachmi, S.; Meyer, G.; Avitan, O.; Moskovitz, B.; Nativ, O. The Natural History and Predictors for Intervention in Patients with Small Renal Mass Undergoing Active Surveillance. Adv. Urol. 2015, 2015, 1–5. [Google Scholar] [CrossRef]
- Soloway, M.S.; Bruck, D.S.; Kim, S.S. Expectant Management of Small, Recurrent, Noninvasive Papillary Bladder Tumors. J. Urol. 2003, 170, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Matulay, J.T.; Soloway, M.; Witjes, J.A.; Buckley, R.; Persad, R.; Lamm, D.L.; Boehle, A.; Palou, J.; Colombel, M.; Brausi, M.; et al. Risk-adapted management of low-grade bladder tumours: Recommendations from the International Bladder Cancer Group (IBCG). BJU Int. 2020, 125, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Contieri, R.; Lazzeri, M.; Hurle, R. When and How To Perform Active Surveillance for Low-risk Non–muscle-invasive Bladder Cancer. Eur. Urol. Focus 2023, 9, 564–566. [Google Scholar] [CrossRef]
- Fan, X.; He, W.; Huang, J. Bladder-sparing approaches for muscle invasive bladder cancer: A narrative review of current evidence and future perspectives. Transl. Androl. Urol. 2023, 12, 802–808. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting Recurrence and Progression in Individual Patients with Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients from Seven EORTC Trials. Eur. Urol. 2006, 49, 466–477. [Google Scholar] [CrossRef]
- Kikuchi, E.; Fujimoto, H.; Mizutani, Y.; Okajima, E.; Koga, H.; Hinotsu, S.; Shinohara, N.; Oya, M.; Miki, T.; Cancer Registration Committee of the Japanese Urological Association. Clinical outcome of tumor recurrence for Ta, T1 non-muscle invasive bladder cancer from the data on registered bladder cancer patients in Japan: 1999-2001 report from the Japanese Urological Association. Int. J. Urol. 2009, 16, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Kim, W.; Wilbur, W.J. Evaluation of query expansion using MeSH in PubMed. Inf. Retr. 2008, 12, 69–80. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. The PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu Symp. Proc. 2006, 2006, 359–363. [Google Scholar]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Pruthi, R.S.; Baldwin, N.; Bhalani, V.; Wallen, E.M. Conservative Management of Low Risk Superficial Bladder Tumors. J. Urol. 2008, 179, 87–90. [Google Scholar] [CrossRef]
- Gofrit, O.N.; Pode, D.; Lazar, A.; Katz, R.; Shapiro, A. Watchful Waiting Policy in Recurrent Ta G1 Bladder Tumors. Eur. Urol. 2006, 49, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Hernández, V.; Alvarez, M.; de la Peña, E.; Amaruch, N.; Martín, M.D.; de la Morena, J.; Gómez, V.; Llorente, C. Safety of Active Surveillance Program for Recurrent Nonmuscle-invasive Bladder Carcinoma. Urology 2009, 73, 1306–1310. [Google Scholar] [CrossRef]
- Hernández, V.; Llorente, C.; de la Peña, E.; Pérez-Fernández, E.; Guijarro, A.; Sola, I. Long-term oncological outcomes of an active surveillance program in recurrent low grade Ta bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 165.e19–165.e23. [Google Scholar] [CrossRef]
- Hurle, R.; Pasini, L.; Lazzeri, M.; Colombo, P.; Buffi, N.; Lughezzani, G.; Casale, P.; Morenghi, E.; Peschechera, R.; Zandegiacomo, S.; et al. Active surveillance for low-risk non-muscle-invasive bladder cancer: Mid-term results from the Bladder cancer Italian Active Surveillance (BIAS) project. BJU Int. 2016, 118, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Hurle, R.; Lazzeri, M.; Vanni, E.; Lughezzani, G.; Buffi, N.; Casale, P.; Saita, A.; Morenghi, E.; Forni, G.; Cardone, P.; et al. Active Surveillance for Low Risk Nonmuscle Invasive Bladder Cancer: A Confirmatory and Resource Consumption Study from the BIAS Project. J. Urol. 2018, 199, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Hurle, R.; Colombo, P.; Lazzeri, M.; Lughezzani, G.; Buffi, N.M.; Saita, A.; Elefante, G.M.; Morenghi, E.; Forni, G.; Cardone, P.; et al. Pathological Outcomes for Patients Who Failed To Remain Under Active Surveillance for Low-risk Non–muscle-invasive Bladder Cancer: Update and Results from the Bladder Cancer Italian Active Surveillance Project. Eur. Urol. Oncol. 2018, 1, 437–442. [Google Scholar] [CrossRef]
- Lokeshwar, S.; Rahman, S.; Press, B.; Khan, A.; Soloway, M. Surveillance and office management of low-grade Ta bladder tumors. Actas Urol. Esp. 2022, 46, 613–618. [Google Scholar] [CrossRef]
- Tan, W.S.; Contieri, R.; Buffi, N.M.; Lughezzani, G.; Grajales, V.; Soloway, M.; Casale, P.; Hurle, R.; Kamat, A.M. International Bladder Cancer Group Intermediate-risk Nonmuscle-invasive Bladder Cancer Scoring System Predicts Outcomes of Patients on Active Surveillance. J. Urol. 2023, 210, 763–770. [Google Scholar] [CrossRef]
- Gofrit, O.N.; Shapiro, A. Active surveillance of low grade bladder tumors. Arch Ital Urol Androl. 2008, 80, 132–135. [Google Scholar]
- Finocchiaro, A.; Paciotti, M.; Contieri, R.; Fasulo, V.; Saita, A.; Lughezzani, G.; Buffi, N.M.; Lazzeri, M.; Hurle, R.; Casale, P. Assessing long-term upgrade risks in recurrent low-grade non-muscle-invasive bladder cancer, can we deintensify the treatment? BJU Int. 2024, 134, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.C.; Lopez, J.I.; Grignon, D.J.; Sanchez-Chapado, M. Muscularis mucosa differentiates two populations with different prognosis in Stage T1 bladder cancer. Urology 1995, 45, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Pavlica, P.; Gaudiano, C.; Barozzi, L. Sonography of the bladder. World J. Urol. 2004, 22, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Holzbeierlein, J.M.; Bixler, B.R.; Buckley, D.I.; Chang, S.S.; Holmes, R.; James, A.C.; Kirkby, E.; McKiernan, J.M.; Schuckman, A.K. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment. J. Urol. 2024, 211, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Hurle, R.; Casale, P.; Saita, A.; Colombo, P.; Elefante, G.M.; Lughezzani, G.; Fasulo, V.; Paciotti, M.; Domanico, L.; Bevilacqua, G.; et al. Clinical performance of Xpert Bladder Cancer (BC) Monitor, a mRNA-based urine test, in active surveillance (AS) patients with recurrent non-muscle-invasive bladder cancer (NMIBC): Results from the Bladder Cancer Italian Active Surveillance (BIAS) project. World J. Urol. 2019, 38, 2215–2220. [Google Scholar] [CrossRef]
- Masson-Lecomte, A.; Gontero, P.; Birtle, A.; Compérat, E.M.; Dominguez-Escrig, J.L.; Liedberg, F.; Mariappan, P.; Mostafid, A.H.; van Rhijn, B.W.G.; Seisen, T.; et al. Upper Urinary Tract Urothelial Carcinoma EAU Guidelines on. 2024; EAU Guidelines Office: Arnhem, The Netherlands, 2024. [Google Scholar]
- Witjes, J.A.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. [Google Scholar] [CrossRef]
- Russo, G.I.; Sholklapper, T.N.; Cocci, A.; Broggi, G.; Caltabiano, R.; Smith, A.B.; Lotan, Y.; Morgia, G.; Kamat, A.M.; Witjes, J.A.; et al. Performance of Narrow Band Imaging (NBI) and Photodynamic Diagnosis (PDD) Fluorescence Imaging Compared to White Light Cystoscopy (WLC) in Detecting Non-Muscle Invasive Bladder Cancer: A Systematic Review and Lesion-Level Diagnostic Meta-Analysis. Cancers 2021, 13, 4378. [Google Scholar] [CrossRef]
| Selection | Comparability | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|
| Study | Representativeness of exposed cohort | Selection of non-exposed cohort | Ascertai-nment of exposure | Demonstration that outcome of interest was not present at start | Design or analysis | Ascertainment of outcome | Adequacy of follow-up | Adequacy of follow-up of cohorts |
| Soloway, 2003 [23] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (-) |
| Pruthi, 2008 [33] | (+) | (-) | (+) | (+) | (-) | (+) | (+) | (-) |
| Gofrit, 2008 [34] | (+) | (-) | (+) | (+) | (-) | (+) | (+) | (+) |
| Hernández, 2009 [35] | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (+) |
| Hernández, 2016 [36] | (+) | (-) | (+) | (+) | (-) | (+) | (+) | (-) |
| Hurle, 2016 [37] | (+) | (-) | (+) | (+) | (-) | (+) | (+) | (-) |
| Hurle, 2017 [38] | (+) | (-) | (+) | (+) | (-) | (+) | (+) | (-) |
| Hurle, 2018 [39] | (+) | (-) | (+) | (+) | (-) | (+) | (+) | (-) |
| Contieri, 2022 [25] | (+) | (-) | (+) | (+) | (+) | (+) | (+) | (+) |
| Lokeshwar, 2022 [40] | (+) | (-) | (+) | (+) | (+) | (+) | (+) | (+) |
| Tan, 2023 [41] | (+) | (-) | (+) | (+) | (+) | (+) | (+) | (+) |
| Author, Year | Patients/ AS Periods | Type of Study | Inclusion Criteria | Pathological Finding Before AS | Previous Intravesical Therapy (%) | Median Follow-Up (Months) | Median AS (Months) | AS Failure Rate (%) | Grade Progression n (%) | Stage Progression n (%) | Progression to MIBC (%) | Follow-Up | Discontinuation AS | Exclusion Criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soloway MS, 2003 [23] | 32/56 | Retrospective | Small (no size reported), <4 lesions | Ta/G1/G2/G3/T1/CIS | 53 | 38 | 10.1 | 100 | 9.4 | 6.3 | 0 | Cystoscopy every 3–5 months | ||
| Pruthi R, 2008 [33] | 22/32 | Retrospective | LG or HG papillary urothelial carcinoma or CIS | Ta/T1/CIS/G1/G2/G3 | 68.8 | 25 | 31.8 | 4.5 | 9 | 0 | Cystoscopy every 3 months for 2 years and every 6 months until 5 years and then annually | Urothelial papilloma, PULMP | ||
| Gofrit O, 2008 [34] | 31/43 | Retrospective | 10 mm, negative UC | Ta/G1/G2 | 58.1 | 16.1 | 81.4 | 3.1% | 3.1 | 0 | Increase in number and/or size (62.8%), patient’s request (16.3%) | |||
| Hernández V, 2009 [35] | 64/70 | Prospective | <10 mm <5 tumors and negative UC | Ta/T1/G1/G2 | NA | 38.6 | 10.3 | 75.8 | 16.2 | 6.5 | 0 | UC + cystoscopy every 4 months for 1 year, then alternating US and cystoscopy + UC every 6 months | Increase in number and/or size (58.6%), hematuria (4.3%), positive UC (2.9%) | History of HG carcinoma (G3), CIS or positive UC |
| Hernández V, 2016 [36] | 186/252 | Prospective | Ta/T1/G1/G2 | 43 | 72 | 13.4 | 80.6 | 20.7 | 13.6 | 2.15 | UC + cystoscopy every 4 months for 2 years, followed by every 6 months (alternating cystoscopy and US) | Increase in number and/or size (61.9%), increase in number and/or size and positive UC (8.7%), positive UC (7.1%) | ||
| Hurle R, 2016 [37] BIAS project | 55/70 | Prospective | Ta/T1a/G1/G2 | 96.4 | 53 | 12.5 | 51 | 18 | 13.2 | 0 | UC + cystoscopy every 3 months for 1 year, then every 6 months | Increase in number and/or size (53.8%), hematuria (32%), positive UC (14.3%) | ||
| Hurle R, 2017 [38] BIAS project | 122/146 | Prospective | Ta/T1a/G1/G2 | 41.0 | 11.9 | 11 | 37.7 | 13.1 | 7.4 | 0 | Increase in number and/or size (64.4%), hematuria (18.6%), positive UC (16.9%) | History of HG carcinoma (G3), CIS or positive UC | ||
| Hurle R, 2018 [39] BIAS project | 167/181 | Prospective | Ta/T1a/G1/G2 | 36.5% | 13 | 12 | 36.5 | NA | NA | 0 | Increase in number and/or size (22.1%), hematuria (6.1%), positive UC (5.5%) | |||
| Contieri R, 2022 [25] BIAS project | 214/251 | Prospective | Ta/T1a/G1/G2 | 35.8% | 38.8 | 13 | 51.8 | NA | NA | 0.7 | Increase in number and/or size (87.7%), hematuria (2.3%), positive UC (8.5%) | |||
| Lokeshwar SD, 2022 [40] | 45 | Retrospective | <5 lesions, <2 cm in prior TURBT, LGTa appearance | Ta/T1/G1/G2 | 58 | 62 | NA | 89 | 11 | 9 | 2.2 | Cystoscopy every 3–6 months | CIS or MIBC | |
| Tan WS, 2023 [41] BIAS project | 163/208 | Prospective | ≤5 lesions, <1 cm in prior TURBT, LGTa appearance | Ta/T1/G1/G2 | NA | 33 | 66.9 | 6.1 | 2.5 | 0 | UC + cystoscopy every 3 months for 1 year, then every 6 months | Increase in number and/or size (81.7%), positive UC (6.3%) and hematuria (5.8%) |
| Criterion | Characteristics for AS |
|---|---|
| Tumor size | ≤3 cm |
| Number of tumors | ≤5 tumors |
| Tumor appearance | Papillary |
| Urinary cytology | Negative |
| Previous pathology | No history of high-grade disease (G3) or carcinoma in situ (CIS) |
| Symptoms | Absence of gross hematuria |
| Follow-up compliance | Willing and able to adhere to cystoscopy follow-up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campistol, M.; Lozano, F.; Carrion, A.; Raventós, C.X.; Morote, J.; Trilla, E. Active Surveillance in Non-Muscle Invasive Bladder Cancer: A Systematic Review. Cancers 2025, 17, 1714. https://doi.org/10.3390/cancers17101714
Campistol M, Lozano F, Carrion A, Raventós CX, Morote J, Trilla E. Active Surveillance in Non-Muscle Invasive Bladder Cancer: A Systematic Review. Cancers. 2025; 17(10):1714. https://doi.org/10.3390/cancers17101714
Chicago/Turabian StyleCampistol, Míriam, Fernando Lozano, Albert Carrion, Carles Xavier Raventós, Juan Morote, and Enrique Trilla. 2025. "Active Surveillance in Non-Muscle Invasive Bladder Cancer: A Systematic Review" Cancers 17, no. 10: 1714. https://doi.org/10.3390/cancers17101714
APA StyleCampistol, M., Lozano, F., Carrion, A., Raventós, C. X., Morote, J., & Trilla, E. (2025). Active Surveillance in Non-Muscle Invasive Bladder Cancer: A Systematic Review. Cancers, 17(10), 1714. https://doi.org/10.3390/cancers17101714





