Survival Outcomes of Luminal Metastatic Breast Cancer Patients According to Changes in Molecular Subtype at Re-Biopsy: Insights from the GIM-13—AMBRA Study
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Objectives
2.3. Statistical Analysis
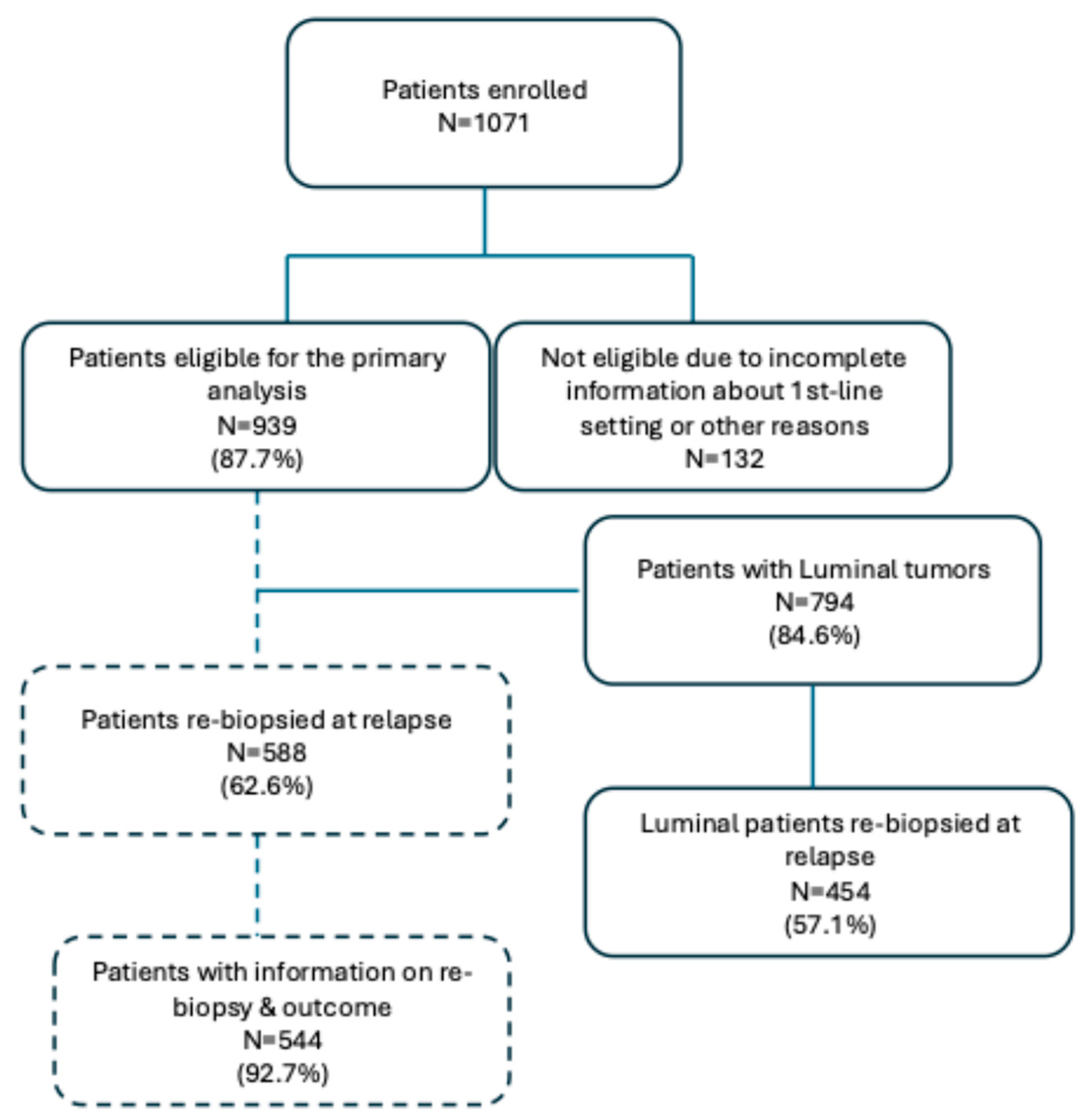
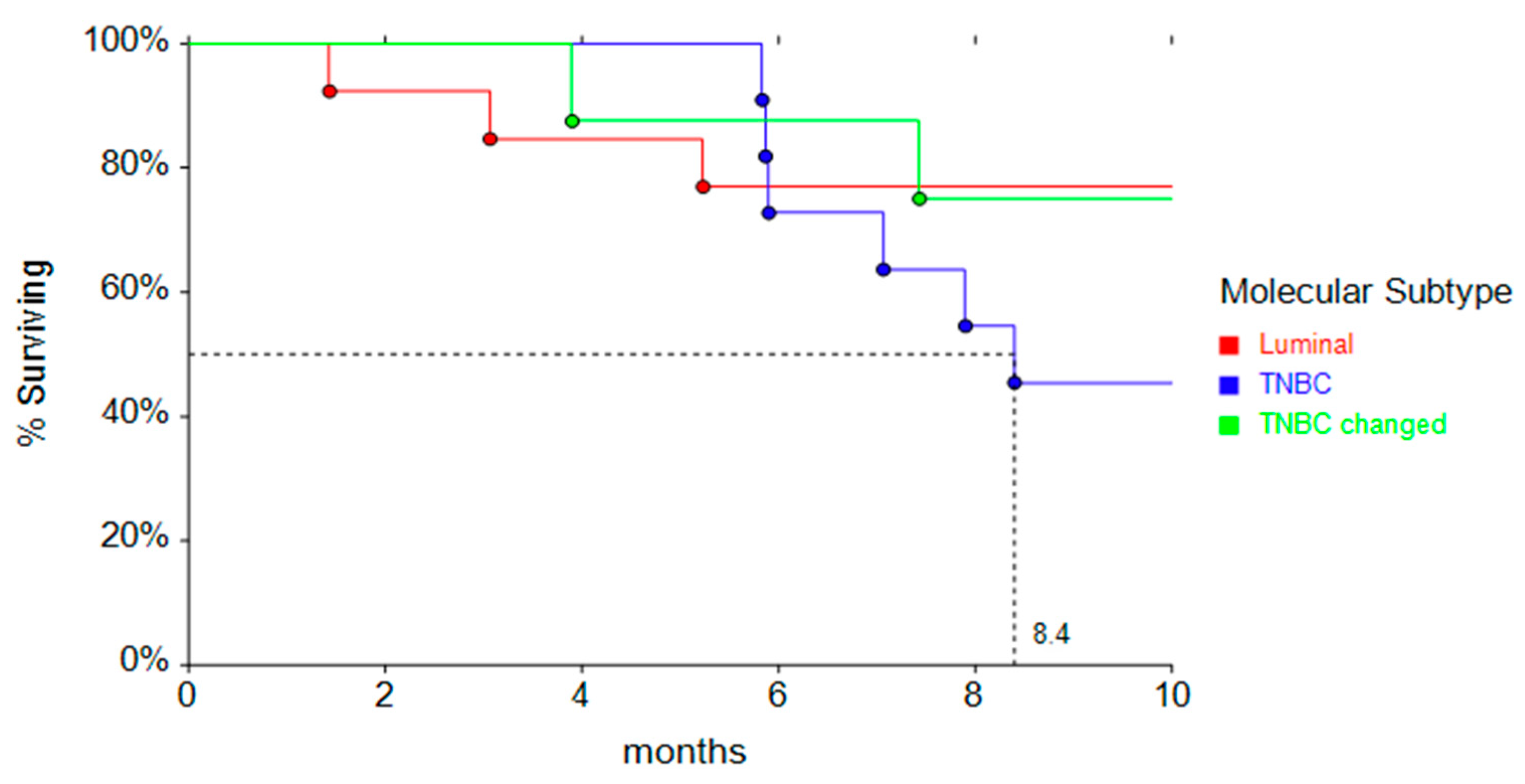
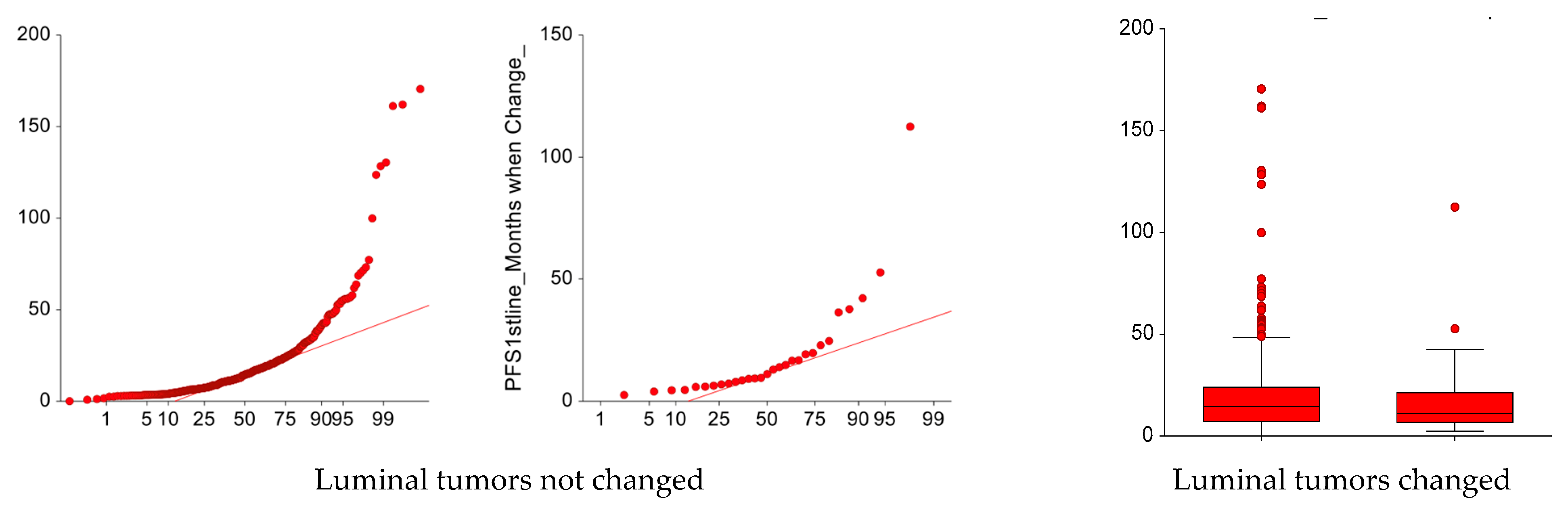
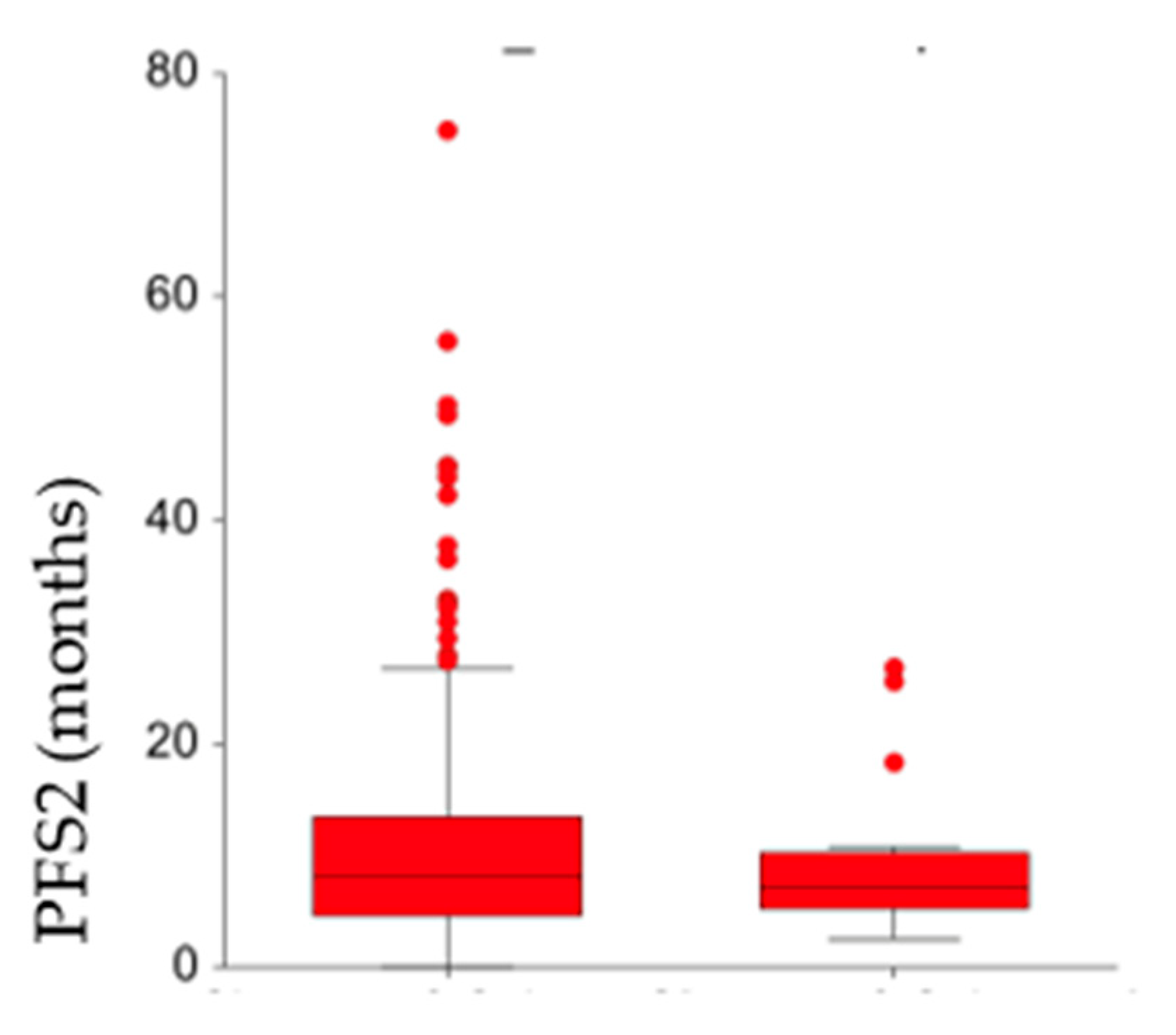
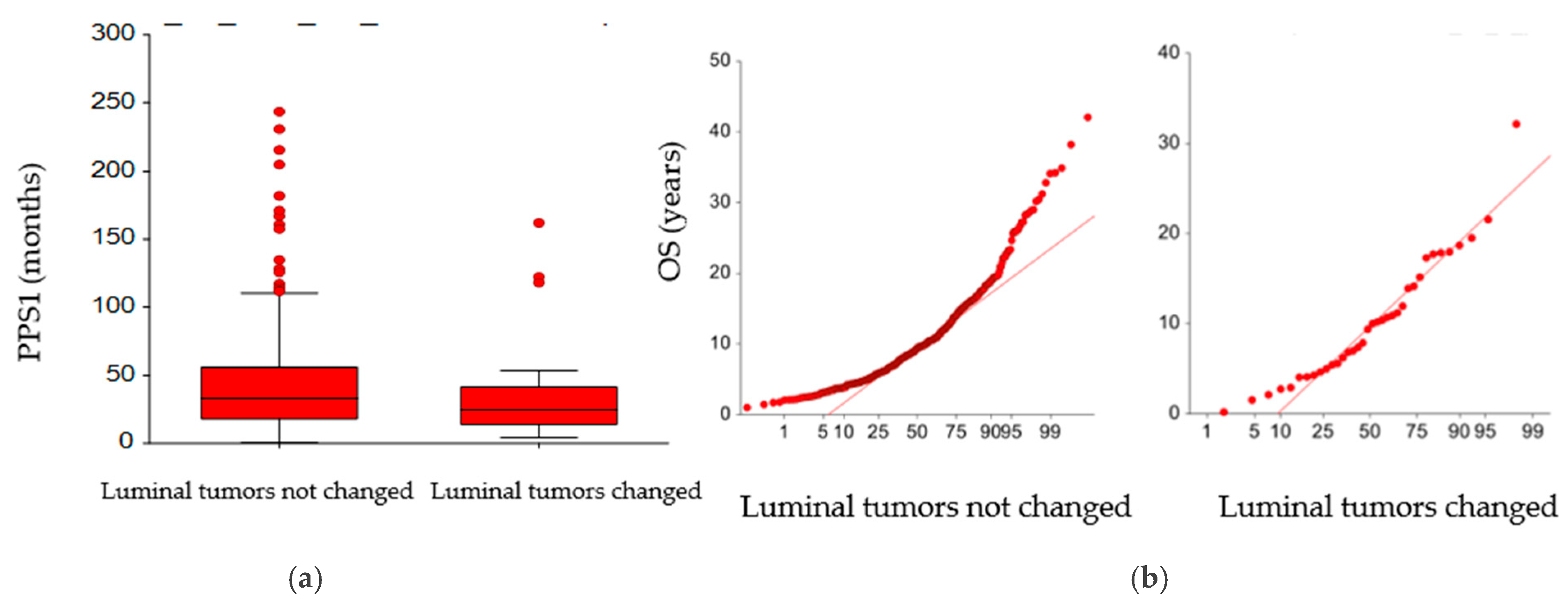
3. Results
Patients’ and Tumor Characteristics at First Relapse
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cos’è il Cancro|AIRC. Available online: https://www.airc.it/cancro/informazioni-tumori/cose-il-cancro/numeri-del-cancro (accessed on 4 April 2025).
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H. Prognostic and predictive parameters in breast pathology: A pathologist’s primer. Mod. Pathol. 2021, 34, 94–106. [Google Scholar] [CrossRef]
- Curigliano, G.; Burstein, H.J.; Winer, E.P.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.M.; Piccart-Gebhart, M.; Senn, H.J.; et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 2017, 28, 1700–1712. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.H.; Cortes, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer 5 behalf of the ESMO Guidelines Committee. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J. Clin. Oncol. 2022, 40, 3246–3256. [Google Scholar] [CrossRef]
- Mazzeo, R.; Sears, J.; Palmero, L.; Bolzonello, S.; Davis, A.A.; Gerratana, L.; Puglisi, F. Liquid biopsy in triple-negative breast cancer: Unlocking the potential of precision oncology. ESMO Open 2024, 9, 103700. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Diéras, V.; Rugo, H.S.; Harbeck, N.; Im, S.A.; Gelmon, K.A.; Lipatov, O.N.; Walshe, J.M.; Martin, M.; Chavez-MacGregor, M.; et al. Overall Survival With Palbociclib Plus Letrozole in Advanced Breast Cancer. J. Clin. Oncol. 2024, 42, 994–1000. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Huober, J.; Sohn, J.; Trédan, O.; Park, I.H.; Campone, M.; Chen, S.C.; Manso, L.M.; Paluch-Shimon, S.; et al. Abemaciclib plus a nonsteroidal aromatase inhibitor as initial therapy for HR+, HER2- advanced breast cancer: Final overall survival results of MONARCH 3. Ann. Oncol. 2024, 35, 718–727. [Google Scholar] [CrossRef]
- Lu, Y.S.; Im, S.A.; Colleoni, M.; Franke, F.; Bardia, A.; Cardoso, F.; Harbeck, N.; Hurvitz, S.; Chow, L.; Sohn, J.; et al. Updated Overall Survival of Ribociclib plus Endocrine Therapy versus Endocrine Therapy Alone in Pre- and Perimenopausal Patients with HR+/HER2- Advanced Breast Cancer in MONALEESA-7: A Phase III Randomized Clinical Trial. Clin. Cancer Res. 2022, 28, 851–859. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Schrijver, W.A.M.E.; Suijkerbuijk, K.P.M.; Van Gils, C.H.; Van Der Wall, E.; Moelans, C.B.; Van Diest, P.J. Receptor Conversion in Distant Breast Cancer Metastases: A Systematic Review and Meta-analysis. JNCI J. Natl. Cancer Inst. 2018, 110, 568–580. [Google Scholar] [CrossRef]
- Kolberg-Liedtke, C.; Wuerstlein, R.; Gluz, O.; Heitz, F.; Freudenberger, M.; Bensmann, E.; du Bois, A.; Nitz, U.; Pelz, E.; Warm, M.; et al. Phenotype Discordance between Primary Tumor and Metastasis Impacts Metastasis Site and Outcome: Results of WSG-DETECT-PriMet. Breast Care 2021, 16, 475–483. [Google Scholar] [CrossRef]
- Cazzaniga, M.E.; Pronzato, P.; Amoroso, D.; Bernardo, A.; Biganzoli, L.; Bisagni, G.; Blasi, L.; Bria, E.; Cognetti, F.; Crinò, L.; et al. Clinical Outcomes of HER2-Negative Metastatic Breast Cancer Patients in Italy in the Last Decade: Results of the GIM 13-AMBRA Study. Cancers 2024, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Adamo, B.; Cheang, M.C.U.; Anders, C.K.; Carey, L.A.; Perou, C.M. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist 2013, 18, 123–133. [Google Scholar] [CrossRef]
- Meegdes, M.; Ibragimova, K.I.; Lobbezoo, D.J.; Vriens, I.J.; Kooreman, L.F.; Erdkamp, F.L.; Dercksen, M.W.; Vriens, B.E.; Aaldering, K.N.; Pepels, M.J.; et al. The initial hormone receptor/HER2 subtype is the main determinator of subtype discordance in advanced breast cancer: A study of the SONABRE registry. Breast Cancer Res. Treat. 2022, 192, 331–342. [Google Scholar] [CrossRef]
- Mellouli, M.; Graja, S.; Kridis, W.B.; Ayed, H.B.; Makni, S.; Triki, M.; Charfi, S.; Khanfir, A.; Boudawara, T.S.; Kallel, R. Discordance in receptor status between primary and metastatic breast cancer and overall survival: A single-center analysis. Ann. Diagn. Pathol. 2022, 61, 152044. [Google Scholar] [CrossRef]
- Grinda, T.; Joyon, N.; Lusque, A.; Lefèvre, S.; Arnould, L.; Penault-Llorca, F.; Macgrogan, G.; Treilleux, I.; Vincent-Salomon, A.; Haudebourg, J.; et al. Phenotypic discordance between primary and metastatic breast cancer in the large-scale real-life multicenter French ESME cohort. NPJ Breast Cancer 2021, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhang, Z.; Zhao, D.; Zhao, J.; Mao, F.; Sun, Q. Discordance in ER, PR, HER2, and Ki-67 Expression Between Primary and Recurrent/Metastatic Lesions in Patients with Primary Early Stage Breast Cancer and the Clinical Significance: Retrospective Analysis of 75 Cases. Pathol. Oncol. Res. 2021, 27, 599894. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.L.; Neven, P.; Casalnuovo, M.L.; Kim, S.B.; Tokunaga, E.; Aftimos, P.; Saura, C.; O’shaughnessy, J.; Harbeck, N.; Carey, L.A.; et al. Imlunestrant with or without Abemaciclib in Advanced Breast Cancer. N. Engl. J. Med. 2024, 392. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Im, S.A.; Saura, C.; Juric, D.; Loibl, S.; Kalinsky, K.; Schmid, P.; Loi, S.; Sunpaweravong, P.; Musolino, A.; et al. Inavolisib-Based Therapy in PIK3CA-Mutated Advanced Breast Cancer. N. Engl. J. Med. 2024, 391, 1584–1596. [Google Scholar] [CrossRef]
| Luminal A | Luminal B | TNBC |
|---|---|---|
| 234/386 | 254/408 | 100/145 |
| 60.6% | 62.3% | 68.9% |
| Rebiopsed Patients (N = 488) | Non-Rebiopsed Patients (N = 351) | |||
|---|---|---|---|---|
| Sites of relapse | ||||
| N | % | N | % | |
| Bone | 102 | 20.9 | 122 | 34.7 |
| Bone + soft tissue | 28 | 5.7 | 18 | 5.1 |
| Soft tissue | 91 | 18.6 | 36 | 10.3 |
| Visceral | 114 | 23.4 | 82 | 23.4 |
| Visceral + soft tissue | 42 | 8.6 | 23 | 6.6 |
| Visceral + bone | 100 | 20.5 | 58 | 16.5 |
| Visceral + bone + soft tissue | 5 | 10.2 | 0 | - |
| Other (non-visceral) | 3 | 0.6 | 4 | 1.1 |
| Not known | 3 | 0.6 | 8 | 2.3 |
| Type of treatment at relapse | ||||
| Endocrine Therapy (ET) | 178 | 36.5 | 156 | 44.4 |
| Chemotherapy | 159 | 32.6 | 112 | 31.9 |
| Chemo → ET | 151 | 30.9 | 83 | 23.6 |
| Luminal Tumors not Changed | Luminal Tumors Changed | |||
|---|---|---|---|---|
| N | % | N | % | |
| Luminal A (N = 217) | Luminal A → Luminal B (N = 2) | |||
| ET | 89 | 41.0 | 0 | 0.0 |
| Chemotherapy | 57 | 26.3 | 2 | 100.0 |
| Chemo → ET | 71 | 32.7 | 0 | 0 |
| Luminal B (N = 238) | Luminal A&B → TNBC (N = 31) | |||
| ET | 84 | 35.3 | 5 | 16.2 |
| Chemotherapy | 76 | 31.9 | 24 | 77.4 |
| Chemo → ET | 78 | 32.8 | 2 | 64.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzaniga, M.E.; Pronzato, P.; Amoroso, D.; Arpino, G.; Atzori, F.; Beano, A.; Biganzoli, L.; Bisagni, G.; Blasi, L.; Capello, C.; et al. Survival Outcomes of Luminal Metastatic Breast Cancer Patients According to Changes in Molecular Subtype at Re-Biopsy: Insights from the GIM-13—AMBRA Study. Cancers 2025, 17, 1715. https://doi.org/10.3390/cancers17101715
Cazzaniga ME, Pronzato P, Amoroso D, Arpino G, Atzori F, Beano A, Biganzoli L, Bisagni G, Blasi L, Capello C, et al. Survival Outcomes of Luminal Metastatic Breast Cancer Patients According to Changes in Molecular Subtype at Re-Biopsy: Insights from the GIM-13—AMBRA Study. Cancers. 2025; 17(10):1715. https://doi.org/10.3390/cancers17101715
Chicago/Turabian StyleCazzaniga, Marina Elena, Paolo Pronzato, Domenico Amoroso, Grazia Arpino, Francesco Atzori, Alessandra Beano, Laura Biganzoli, Giancarlo Bisagni, Livio Blasi, Cristina Capello, and et al. 2025. "Survival Outcomes of Luminal Metastatic Breast Cancer Patients According to Changes in Molecular Subtype at Re-Biopsy: Insights from the GIM-13—AMBRA Study" Cancers 17, no. 10: 1715. https://doi.org/10.3390/cancers17101715
APA StyleCazzaniga, M. E., Pronzato, P., Amoroso, D., Arpino, G., Atzori, F., Beano, A., Biganzoli, L., Bisagni, G., Blasi, L., Capello, C., Chiari, R., D’Alonzo, A., De Laurentiis, M., Denaro, A., Fabi, A., Farci, D., Ferraù, F., Fiorio, E., Gennari, A., ... Mustacchi, G. (2025). Survival Outcomes of Luminal Metastatic Breast Cancer Patients According to Changes in Molecular Subtype at Re-Biopsy: Insights from the GIM-13—AMBRA Study. Cancers, 17(10), 1715. https://doi.org/10.3390/cancers17101715











