Advanced Therapies for Inflammatory Bowel Disease and Risk of Skin Cancer: What’s New?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. TNFα Inhibitors
| Author | Therapy | Type of Study | Subjects | Melanoma | NMSC | Increased Risk |
|---|---|---|---|---|---|---|
| Long MD et al. [15] | TNFα inhibitors and natalizumab | Retrospective | 10,879 IBD patients | OR: 1.88 (95% CI: 1.08–3.29) | OR: 1.14 (95% CI: 0.95–1.36) | Yes, only for melanoma |
| Nyboe Andersen N et al. [16] | TNFα inhibitors | Retrospective | 56,146 IBD patients | RR: 1.31 (95% CI: 0.63–2.74) | - | No |
| Esse S et al. [17] | TNFα inhibitors | Systematic review and meta-analysis | 7901 IBD patients | pRR: 1.20 (95% CI: 0.60–2.40) | No | |
| Osterman MT et al. [18] | Adalimumab Adalimumab + thiopurine or methotrexate | Comparative study | 1594 CD patients | - | SIR: 1.20 (95% CI: 0.39–2.80) RR: 3.46 (95% CI: 1.08–11.06 | No Yes |
| Muller M et al. [19] | TNFα inhibitors | Systematic review | 298,717 IBD patients | - | 123/692, 17.8% | No |
| Lichtenstein GR et al. [20] | Infliximab | Prospective | 6273 CD patients | SIR: 1.05 (95% CI 0.42, 2.16) | 0.16 events/100 PY | No |
| DʼHaens G et al. [21] | Adalimumab | Prospective | 5025 CD patients | <0.1 events/100 PY | 0.3 events/100 PY | No |
| Cohen RD et al. [24] | Vedolizumab | Retrospective | 32,752 IBD patients | 12 cases of skin malignancies | No | |
| Ghosh S et al. [25] | Ustekinumab | Pooled Safety Analysis of clinical trials | 2575 IBD patients | 0.11 events/ 100 PYs | 0.38 events/100 PY (95% CI: 0.22–0.59) | No |
| Ferrante M et al. [26] | Risankizumab | RCT | 1147 CD patients | - | 0.6 events/100 PY | No |
| Sands BE et al. [27] | Mirikizumab | RCT | 1162 UC patients | - | - | No |
| Rubin DT et al. [28] | Guselkumab | RCT | 701 UC patients | - | - | No |
| Olivera PA et al. [29] | Tofacitinib, filgotinib, baricitinib, and upadacitinib | Meta-analysis | 5987 IBD patients | RR: 1.21 (95% CI: 0.19–7.65) | No | |
| Panés J et al. [30] | Tofacitinib | RCT | 1157 UC patients | 2 cases of melanoma | IR: 0.71/100 PY(95% CI, 0.45–1.07). | No |
| Russell MD et al. [31] | Tofacitinib, baricitinib, upadacitinib, filgotinib, peficitinib | Meta-analysis | 82,366 PY of exposure (RA, PsA, PsO, axSpA, IBD or AD) | - | IRR: 1.93 (95% CI 1.19–3.12) | Yes |
| Siegel C et al. [32] | Ozanimod | RCT | 823 UC patients | - | EAIR: 0.1/100 PY OLEw94 EAIR: 0.04/100 PY OLEw142 | No |
| Vermeire S et al. [33] | Etrasimod | RCT | 956 UC patients | - | - | No |
3.2. Vedolizumab
3.3. Ustekinumab
3.4. Selective IL-23 Inhibitors (e.g., Risankizumab, Mirikizumab, Guselkumab)
3.5. JAK Inhibitors (e.g., Tofacitinib, Filgotinib and Upadacitinib)
3.6. S1P Receptor Modulators
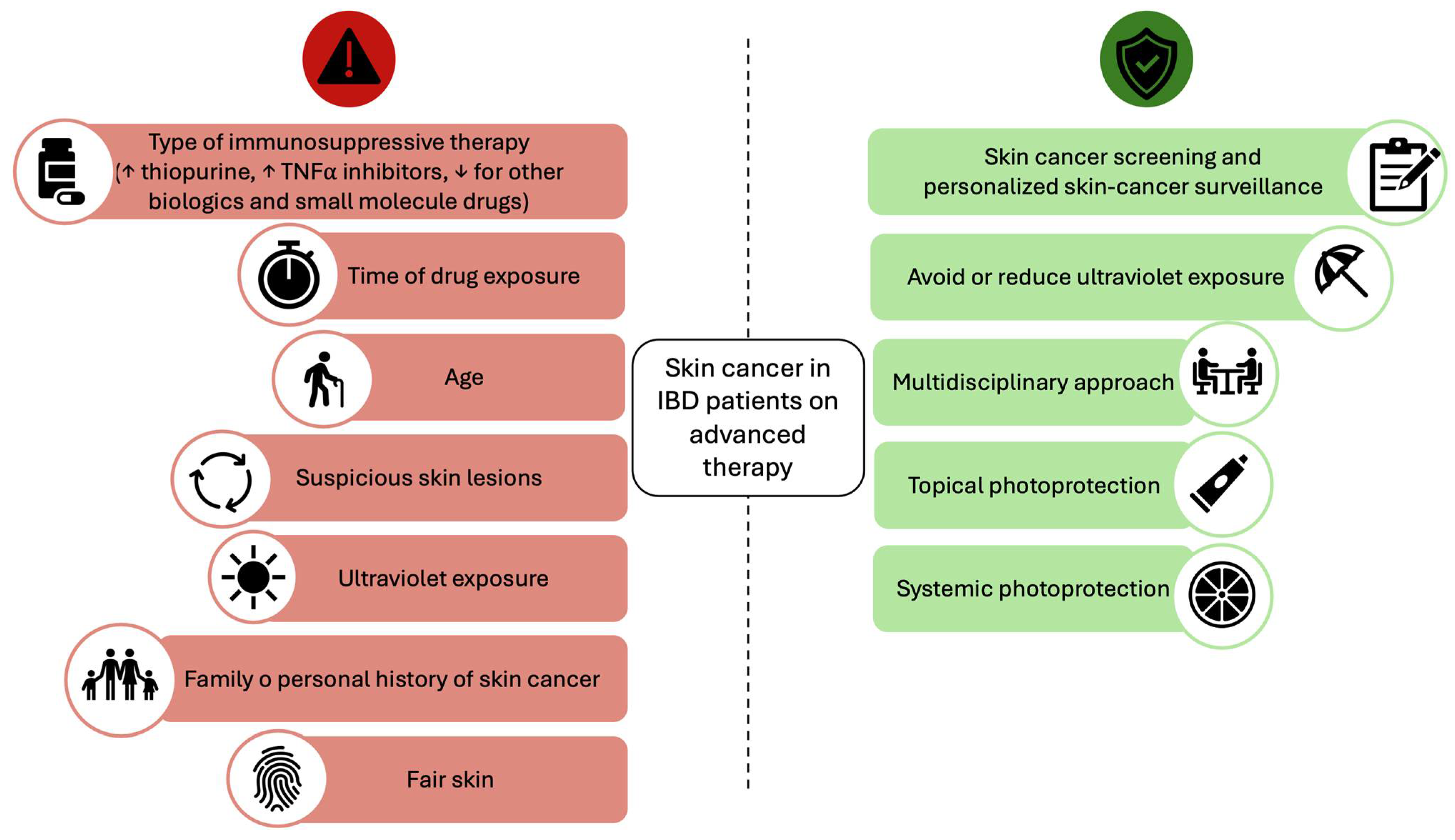
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nicolò, S.; Faggiani, I.; Errico, C.; D’Amico, F.; Parigi, T.L.; Danese, S.; Ungaro, F. Translational Characterization of Immune Pathways in Inflammatory Bowel Disease: Insights for Targeted Treatments. Expert Rev. Clin. Immunol. 2025, 21, 55–72. [Google Scholar] [CrossRef]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Nikolopoulos, G.K.; Peyrin-Biroulet, L.; Danese, S. Biologic Therapies and Risk of Infection and Malignancy in Patients with Inflammatory Bowel Disease: A Systematic Review and Network Meta-Analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1385–1397.e10. [Google Scholar] [CrossRef]
- Fanizza, J.; Bencardino, S.; Allocca, M.; Furfaro, F.; Zilli, A.; Parigi, T.L.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S.; D’Amico, F. Inflammatory Bowel Disease and Colorectal Cancer. Cancers 2024, 16, 2943. [Google Scholar] [CrossRef]
- Lo, B.; Zhao, M.; Vind, I.; Burisch, J. The Risk of Extraintestinal Cancer in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Population-Based Cohort Studies. Clin. Gastroenterol. Hepatol. 2021, 19, 1117–1138.e19. [Google Scholar] [CrossRef]
- Beaugerie, L.; Itzkowitz, S.H. Cancers Complicating Inflammatory Bowel Disease. N. Engl. J. Med. 2015, 372, 1441–1452. [Google Scholar] [CrossRef]
- Long, M.D.; Kappelman, M.D.; Pipkin, C.A. Nonmelanoma Skin Cancer in Inflammatory Bowel Disease: A Review. Inflamm. Bowel Dis. 2011, 17, 1423–1427. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Khosrotehrani, K.; Carrat, F.; Bouvier, A.-M.; Chevaux, J.-B.; Simon, T.; Carbonnel, F.; Colombel, J.-F.; Dupas, J.-L.; Godeberge, P.; et al. Increased Risk for Nonmelanoma Skin Cancers in Patients Who Receive Thiopurines for Inflammatory Bowel Disease. Gastroenterology 2011, 141, 1621–1628.e5. [Google Scholar] [CrossRef]
- Gordon, H.; Biancone, L.; Fiorino, G.; Katsanos, K.H.; Kopylov, U.; Al Sulais, E.; Axelrad, J.E.; Balendran, K.; Burisch, J.; de Ridder, L.; et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J. Crohn’s Colitis 2023, 17, 827–854. [Google Scholar] [CrossRef]
- Singh, S.; Nagpal, S.J.S.; Murad, M.H.; Yadav, S.; Kane, S.V.; Pardi, D.S.; Talwalkar, J.A.; Loftus, E.V. Inflammatory Bowel Disease Is Associated with an Increased Risk of Melanoma: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 210–218. [Google Scholar] [CrossRef]
- Pedersen, N.; Duricova, D.; Elkjaer, M.; Gamborg, M.; Munkholm, P.; Jess, T. Risk of Extra-Intestinal Cancer in Inflammatory Bowel Disease: Meta-Analysis of Population-Based Cohort Studies. Am. J. Gastroenterol. 2010, 105, 1480–1487. [Google Scholar] [CrossRef]
- Bongartz, T.; Sutton, A.J.; Sweeting, M.J.; Buchan, I.; Matteson, E.L.; Montori, V. Anti-TNF Antibody Therapy in Rheumatoid Arthritis and the Risk of Serious Infections and Malignancies: Systematic Review and Meta-Analysis of Rare Harmful Effects in Randomized Controlled Trials. JAMA 2006, 295, 2275–2285. [Google Scholar] [CrossRef]
- Balkwill, F. Tumor Necrosis Factor or Tumor Promoting Factor? Cytokine Growth Factor Rev. 2002, 13, 135–141. [Google Scholar] [CrossRef]
- Long, M.D.; Martin, C.F.; Pipkin, C.A.; Herfarth, H.H.; Sandler, R.S.; Kappelman, M.D. Risk of Melanoma and Nonmelanoma Skin Cancer among Patients with Inflammatory Bowel Disease. Gastroenterology 2012, 143, 390–399.e1. [Google Scholar] [CrossRef]
- Nyboe Andersen, N.; Pasternak, B.; Basit, S.; Andersson, M.; Svanström, H.; Caspersen, S.; Munkholm, P.; Hviid, A.; Jess, T. Association between Tumor Necrosis Factor-α Antagonists and Risk of Cancer in Patients with Inflammatory Bowel Disease. JAMA 2014, 311, 2406–2413. [Google Scholar] [CrossRef]
- Esse, S.; Mason, K.J.; Green, A.C.; Warren, R.B. Melanoma Risk in Patients Treated with Biologic Therapy for Common Inflammatory Diseases: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2020, 156, 787–794. [Google Scholar] [CrossRef]
- Osterman, M.T.; Sandborn, W.J.; Colombel, J.-F.; Robinson, A.M.; Lau, W.; Huang, B.; Pollack, P.F.; Thakkar, R.B.; Lewis, J.D. Increased Risk of Malignancy with Adalimumab Combination Therapy, Compared with Monotherapy, for Crohn’s Disease. Gastroenterology 2014, 146, 941–949. [Google Scholar] [CrossRef]
- Muller, M.; D’Amico, F.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. TNF Inhibitors and Risk of Malignancy in Patients with Inflammatory Bowel Diseases: A Systematic Review. J. Crohn’s Colitis 2021, 15, 840–859. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Diamond, R.H.; Langholff, W.; Londhe, A.; Sandborn, W.J. Drug Therapies and the Risk of Malignancy in Crohn’s Disease: Results from the TREATTM Registry. Am. J. Gastroenterol. 2014, 109, 212–223. [Google Scholar] [CrossRef]
- DʼHaens, G.; Reinisch, W.; Panaccione, R.; Satsangi, J.; Petersson, J.; Bereswill, M.; Arikan, D.; Perotti, E.; Robinson, A.M.; Kalabic, J.; et al. Lymphoma Risk and Overall Safety Profile of Adalimumab in Patients with Crohn’s Disease with up to 6 Years of Follow-Up in the Pyramid Registry. Am. J. Gastroenterol. 2018, 113, 872–882. [Google Scholar] [CrossRef]
- Fidder, H.; Schnitzler, F.; Ferrante, M.; Noman, M.; Katsanos, K.; Segaert, S.; Henckaerts, L.; Van Assche, G.; Vermeire, S.; Rutgeerts, P. Long-Term Safety of Infliximab for the Treatment of Inflammatory Bowel Disease: A Single-Centre Cohort Study. Gut 2009, 58, 501–508. [Google Scholar] [CrossRef]
- Biancone, L.; Petruzziello, C.; Orlando, A.; Kohn, A.; Ardizzone, S.; Daperno, M.; Angelucci, E.; Castiglione, F.; D’Incà, R.; Zorzi, F.; et al. Cancer in Crohn’s Disease Patients Treated with Infliximab: A Long-Term Multicenter Matched Pair Study. Inflamm. Bowel Dis. 2011, 17, 758–766. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cohen, R.D.; Bhayat, F.; Blake, A.; Travis, S. The Safety Profile of Vedolizumab in Ulcerative Colitis and Crohn’s Disease: 4 Years of Global Post-Marketing Data. J. Crohn’s Colitis 2020, 14, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Feagan, B.G.; Ott, E.; Gasink, C.; Godwin, B.; Marano, C.; Miao, Y.; Ma, T.; Loftus, E.V., Jr.; Sandborn, W.J.; et al. Safety of Ustekinumab in Inflammatory Bowel Disease: Pooled Safety Analysis Through 5 Years in Crohn’s Disease and 4 Years in Ulcerative Colitis. J. Crohn’s Colitis 2024, 18, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, M.; Garre, A.; Iborra, M.; Sierra-Ausín, M.; Barreiro-de Acosta, M.; Fernández-Clotet, A.; de Castro, L.; Boscá-Watts, M.; Casanova, M.J.; López-García, A.; et al. Effectiveness and Safety of Ustekinumab in Ulcerative Colitis: Real-World Evidence from the ENEIDA Registry. J. Crohn’s Colitis 2021, 15, 1846–1851. [Google Scholar] [CrossRef]
- Chaparro, M.; Baston-Rey, I.; Fernández-Salgado, E.; González García, J.; Ramos, L.; Diz-Lois Palomares, M.T.; Argüelles-Arias, F.; Iglesias Flores, E.; Cabello, M.; Rubio Iturria, S.; et al. Long-Term Real-World Effectiveness and Safety of Ustekinumab in Crohn’s Disease Patients: The SUSTAIN Study. Inflamm. Bowel Dis. 2022, 28, 1725–1736. [Google Scholar] [CrossRef]
- Ferrante, M.; Feagan, B.G.; Panés, J.; Baert, F.; Louis, E.; Dewit, O.; Kaser, A.; Duan, W.R.; Pang, Y.; Lee, W.-J.; et al. Long-Term Safety and Efficacy of Risankizumab Treatment in Patients with Crohn’s Disease: Results from the Phase 2 Open-Label Extension Study. J. Crohn’s Colitis 2021, 15, 2001–2010. [Google Scholar] [CrossRef]
- Danese, S.; Panaccione, R.; Feagan, B.G.; Afzali, A.; Rubin, D.T.; Sands, B.E.; Reinisch, W.; Panés, J.; Sahoo, A.; Terry, N.A.; et al. Efficacy and Safety of 48 Weeks of Guselkumab for Patients with Crohn’s Disease: Maintenance Results from the Phase 2, Randomised, Double-Blind GALAXI-1 Trial. Lancet Gastroenterol. Hepatol. 2024, 9, 133–146. [Google Scholar] [CrossRef]
- Panaccione, R.; Hart, A.; Steinwurz, F.; Danese, S.; Hisamatsu, T.; Cao, Q.; Olurinde, M.; Vetter, M.; Yang, Z.; Wang, Y.; et al. S1052 Efficacy and Safety of Subcutaneous Guselkumab Induction Therapy in Patients with Moderately to Severely Active Crohn’s Disease: Results Through Week 48 From the Phase 3 GRAVITI Study. Off. J. Am. Coll. Gastroenterol.| ACG 2024, 119, S740. [Google Scholar] [CrossRef]
- Russell, M.D.; Stovin, C.; Alveyn, E.; Adeyemi, O.; Chan, C.K.D.; Patel, V.; Adas, M.A.; Atzeni, F.; Ng, K.K.H.; Rutherford, A.I.; et al. JAK Inhibitors and the Risk of Malignancy: A Meta-Analysis across Disease Indications. Ann. Rheum. Dis. 2023, 82, 1059–1067. [Google Scholar] [CrossRef]
- Lichtenstein, G.R. Safety of Long-Term Ozanimod Treatment Up to 5 Years in Patients with Moderately to Severely Active Ulcerative Colitis: An Interim Analysis of the True North Open-Label Extension. Gastroenterol. Hepatol. 2024, 20, 14–15. [Google Scholar]
- Siegel, C.; Danese, S.; Rubin, D.T.; Sabino, J.; Long, M.D.; Cross, R.K.; Armuzzi, A.; Blumenstein, I.; Kobayashi, T.; Lama, S.; et al. DOP16 Safety of Long-Term Ozanimod Treatment for up to 4 Years in Patients with Moderately to Severely Active Ulcerative Colitis: An Interim Analysis of the True North Open-Label Extension. J. Crohn’s Colitis 2024, 18, i100–i101. [Google Scholar] [CrossRef]
- Nguyen, G.C. First Do No Harm: Is It Safe to Use Immunosuppressants in Inflammatory Bowel Disease Patients with Prior Cancer? Gastroenterology 2016, 151, 22–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, N.; Vallarino, C.; Lissoos, T.; Darr, U.; Luo, M. Risk of Malignancy in a Nationwide Cohort of Elderly Inflammatory Bowel Disease Patients. Drugs Aging 2017, 34, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, U.; Hanzel, J.; Liefferinckx, C.; De Marco, D.; Imperatore, N.; Plevris, N.; Baston-Rey, I.; Harris, R.J.; Truyens, M.; Domislovic, V.; et al. Effectiveness of Ustekinumab Dose Escalation in Crohn’s Disease Patients with Insufficient Response to Standard-Dose Subcutaneous Maintenance Therapy. Aliment. Pharmacol. Ther. 2020, 52, 135–142. [Google Scholar] [CrossRef]
- Ferrante, M.; Panaccione, R.; Colombel, J.F.; Dubinsky, M.; Hisamatsu, T.; Lindsay, J.O.; Song, A.; Neimark, E.; Zhang, Y.; Kligys, K.; et al. DOP53 Long-Term Efficacy and Safety of Risankizumab in Patients with Moderate to Severe Crohn’s Disease up to 3 Years of Treatment: Results From the FORTIFY Open-Label Long-Term Extension. J. Crohn’s Colitis 2024, 18, i168–i170. [Google Scholar] [CrossRef]
- Ferrante, M.; Panaccione, R.; Baert, F.; Bossuyt, P.; Colombel, J.-F.; Danese, S.; Dubinsky, M.; Feagan, B.G.; Hisamatsu, T.; Lim, A.; et al. Risankizumab as Maintenance Therapy for Moderately to Severely Active Crohn’s Disease: Results from the Multicentre, Randomised, Double-Blind, Placebo-Controlled, Withdrawal Phase 3 FORTIFY Maintenance Trial. Lancet 2022, 399, 2031–2046. [Google Scholar] [CrossRef]
- Sands, B.E.; D’Haens, G.; Clemow, D.B.; Irving, P.M.; Johns, J.T.; Hunter Gibble, T.; Abreu, M.T.; Lee, S.; Hisamatsu, T.; Kobayashi, T.; et al. Two-Year Efficacy and Safety of Mirikizumab Following 104 Weeks of Continuous Treatment for Ulcerative Colitis: Results From the LUCENT-3 Open-Label Extension Study. Inflamm. Bowel Dis. 2024, 30, 2245–2258. [Google Scholar] [CrossRef]
- Rubin, D.T.; Allegretti, J.R.; Panés, J.; Shipitofsky, N.; Yarandi, S.S.; Huang, K.-H.G.; Germinaro, M.; Wilson, R.; Zhang, H.; Johanns, J.; et al. Guselkumab in Patients with Moderately to Severely Active Ulcerative Colitis (QUASAR): Phase 3 Double-Blind, Randomised, Placebo-Controlled Induction and Maintenance Studies. Lancet 2025, 405, 33–49. [Google Scholar] [CrossRef]
- TREMFYA® (Guselkumab) Receives U.S. FDA Approval for Adults with Moderately to Severely Active Ulcerative Colitis, Strengthening Johnson & Johnson’s Leadership in Inflammatory Bowel Disease. Available online: https://www.jnj.com/media-center/press-releases/tremfya-guselkumab-receives-u-s-fda-approval-for-adults-with-moderately-to-severely-active-ulcerative-colitis-strengthening-johnson-johnsons-leadership-in-inflammatory-bowel-disease (accessed on 29 January 2025).
- Olivera, P.A.; Lasa, J.S.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. Safety of Janus Kinase Inhibitors in Patients with Inflammatory Bowel Diseases or Other Immune-Mediated Diseases: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1554–1573.e12. [Google Scholar] [CrossRef]
- Panés, J.; D’Haens, G.R.; Sands, B.E.; Ng, S.C.; Lawendy, N.; Kulisek, N.; Guo, X.; Wu, J.; Vranic, I.; Panaccione, R.; et al. Analysis of Tofacitinib Safety in Ulcerative Colitis from the Completed Global Clinical Developmental Program up to 9.2 Years of Drug Exposure. United Eur. Gastroenterol. J. 2024, 12, 793–801. [Google Scholar] [CrossRef]
- Vermeire, S.; Peyrin-Biroulet, L.; Panés, J.; Regueiro, M.; Kotze, P.G.; Charabaty, A.; Goetsch, M.; Shan, K.; Wu, J.; McDonnell, A.; et al. P490 Etrasimod for the Treatment of Ulcerative Colitis: Up to 2.5 Years of Pooled Safety Data from Global Clinical Trials. J. Crohn’s Colitis 2023, 17, i619–i620. [Google Scholar] [CrossRef]
- Psaty, E.L.; Scope, A.; Halpern, A.C.; Marghoob, A.A. Defining the Patient at High Risk for Melanoma. Int. J. Dermatol. 2010, 49, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Attwood, J. Common Skin Cancers and Their Precursors. Surg. Clin. N. Am. 2009, 89, 703–712. [Google Scholar] [CrossRef]
- Nissen, L.H.C.; Pierik, M.; Derikx, L.A.A.P.; de Jong, E.; Kievit, W.; van den Heuvel, T.R.A.; van Rosendael, A.R.; Plasmeijer, E.I.; Dewint, P.; Verhoeven, R.H.A.; et al. Risk Factors and Clinical Outcomes in Patients with IBD with Melanoma. Inflamm. Bowel Dis. 2017, 23, 2018–2026. [Google Scholar] [CrossRef]
- Scott, F.I.; Mamtani, R.; Brensinger, C.M.; Haynes, K.; Chiesa-Fuxench, Z.C.; Zhang, J.; Chen, L.; Xie, F.; Yun, H.; Osterman, M.T.; et al. Risk of Nonmelanoma Skin Cancer Associated with the Use of Immunosuppressant and Biologic Agents in Patients with a History of Autoimmune Disease and Nonmelanoma Skin Cancer. JAMA Dermatol. 2016, 152, 164–172. [Google Scholar] [CrossRef]
- Khan, N.; Patel, D.; Trivedi, C.; Kavani, H.; Medvedeva, E.; Pernes, T.; Xie, D.; Lewis, J.; Yang, Y.-X. Repeated Occurrences of Basal Cell Cancer in Patients with Inflammatory Bowel Disease Treated with Immunosuppressive Medications. Am. J. Gastroenterol. 2020, 115, 1246–1252. [Google Scholar] [CrossRef]
- Laredo, V.; García-Mateo, S.; Martínez-Domínguez, S.J.; López de la Cruz, J.; Gargallo-Puyuelo, C.J.; Gomollón, F. Risk of Cancer in Patients with Inflammatory Bowel Diseases and Keys for Patient Management. Cancers 2023, 15, 871. [Google Scholar] [CrossRef]
- Wertheimer, T.; Zwicky, P.; Rindlisbacher, L.; Sparano, C.; Vermeer, M.; de Melo, B.M.S.; Haftmann, C.; Rückert, T.; Sethi, A.; Schärli, S.; et al. IL-23 Stabilizes an Effector Treg Cell Program in the Tumor Microenvironment. Nat. Immunol. 2024, 25, 512–524. [Google Scholar] [CrossRef]
- Hyeraci, M.; Papanikolau, E.S.; Grimaldi, M.; Ricci, F.; Pallotta, S.; Monetta, R.; Minafò, Y.A.; Di Lella, G.; Galdo, G.; Abeni, D.; et al. Systemic Photoprotection in Melanoma and Non-Melanoma Skin Cancer. Biomolecules 2023, 13, 1067. [Google Scholar] [CrossRef]
- Chen, A.C.; Martin, A.J.; Choy, B.; Fernández-Peñas, P.; Dalziell, R.A.; McKenzie, C.A.; Scolyer, R.A.; Dhillon, H.M.; Vardy, J.L.; Kricker, A.; et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N. Engl. J. Med. 2015, 373, 1618–1626. [Google Scholar] [CrossRef]
- Parrado, C.; Philips, N.; Gilaberte, Y.; Juarranz, A.; González, S. Oral Photoprotection: Effective Agents and Potential Candidates. Front. Med. 2018, 5, 188. [Google Scholar] [CrossRef] [PubMed]
- Goessens, L.; Colombel, J.-F.; Outtier, A.; Ferrante, M.; Sabino, J.; Judge, C.; Saeidi, R.; Rabbitt, L.; Armuzzi, A.; Domenech, E.; et al. Safety and Efficacy of Combining Biologics or Small Molecules for Inflammatory Bowel Disease or Immune-Mediated Inflammatory Diseases: A European Retrospective Observational Study. United Eur. Gastroenterol. J. 2021, 9, 1136–1147. [Google Scholar] [CrossRef]
| Melanoma | NMSC | |
|---|---|---|
| TNFα inhibitor | Yes | No |
| Ustekinumab | No | No |
| Vedolizumab | No | No |
| Selective IL-23 inhibitors | Unknown * | Unknown * |
| JAK inhibitors | No ** | No ** |
| S1P modulators | Unknown * | Unknown * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bencardino, S.; Bernardi, F.; Allocca, M.; Zilli, A.; Furfaro, F.; Peyrin-Biroulet, L.; Danese, S.; D’Amico, F. Advanced Therapies for Inflammatory Bowel Disease and Risk of Skin Cancer: What’s New? Cancers 2025, 17, 1710. https://doi.org/10.3390/cancers17101710
Bencardino S, Bernardi F, Allocca M, Zilli A, Furfaro F, Peyrin-Biroulet L, Danese S, D’Amico F. Advanced Therapies for Inflammatory Bowel Disease and Risk of Skin Cancer: What’s New? Cancers. 2025; 17(10):1710. https://doi.org/10.3390/cancers17101710
Chicago/Turabian StyleBencardino, Sarah, Francesca Bernardi, Mariangela Allocca, Alessandra Zilli, Federica Furfaro, Laurent Peyrin-Biroulet, Silvio Danese, and Ferdinando D’Amico. 2025. "Advanced Therapies for Inflammatory Bowel Disease and Risk of Skin Cancer: What’s New?" Cancers 17, no. 10: 1710. https://doi.org/10.3390/cancers17101710
APA StyleBencardino, S., Bernardi, F., Allocca, M., Zilli, A., Furfaro, F., Peyrin-Biroulet, L., Danese, S., & D’Amico, F. (2025). Advanced Therapies for Inflammatory Bowel Disease and Risk of Skin Cancer: What’s New? Cancers, 17(10), 1710. https://doi.org/10.3390/cancers17101710







