Revealing New Patterns in Colorectal Cancer Screening with a Focus on a Younger Patient Population
Simple Summary
Abstract
1. Introduction
1.1. Background
1.2. Study Objectives
2. Materials and Methods
2.1. Study Design
2.2. Study Outcomes
2.3. Statistical Analysis
3. Results
3.1. Analysis of All Patients
3.2. Analysis of Patients Stratified by Age
4. Discussion
4.1. Racial Disparities in CRC Screening
4.2. Disparities in Employment Status
4.3. Disparities in Relationship Status
4.4. Disparities in Modifiable Risk Factors
4.5. Impact of Age on CRC Screening
4.6. Comparison to National Average
4.7. Strengths and Weaknesses
4.8. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| BMI | Body mass index |
| AI/AN | American Indian/Alaskan Native |
References
- SEER. Cancer of the Colon and Rectum—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 3 January 2025).
- US Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965. [Google Scholar] [CrossRef] [PubMed]
- Warren Andersen, S.; Blot, W.J.; Lipworth, L.; Steinwandel, M.; Murff, H.J.; Zheng, W. Association of Race and Socioeconomic Status with Colorectal Cancer Screening, Colorectal Cancer Risk, and Mortality in Southern US Adults. JAMA Netw. Open 2019, 2, e1917995. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Lundquist, M.; Ju, E.; Luo, X.; Townsend, A. Colorectal cancer screening disparities in Asian Americans and Pacific Islanders: Which groups are most vulnerable? Ethn. Health 2011, 16, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, T.B.; Jemal, A.; Miller, K.D.; Nash, S.; Wiggins, C.; Redwood, D.; Smith, R.; Siegel, R.L. Cancer statistics for American Indian and Alaska Native individuals, 2022: Including increasing disparities in early onset colorectal cancer. CA Cancer J. Clin. 2023, 73, 120–146. [Google Scholar] [CrossRef]
- Balan, N.; Petrie, B.A.; Chen, K.T. Racial Disparities in Colorectal Cancer Care for Black Patients: Barriers and Solutions. Am. Surg. 2022, 88, 2823–2830. [Google Scholar] [CrossRef]
- Carethers, J.M.; Doubeni, C.A. Causes of Socioeconomic Disparities in Colorectal Cancer and Intervention Framework and Strategies. Gastroenterology 2020, 158, 354–367. [Google Scholar] [CrossRef]
- Daly, M.C.; Jung, A.D.; Hanseman, D.J.; Shah, S.A.; Paquette, I.M. Surviving rectal cancer: Examination of racial disparities surrounding access to care. J. Surg. Res. 2017, 211, 100–106. [Google Scholar] [CrossRef]
- Jones, B.; Williams, J.L.; Komanduri, S.; Muthusamy, V.R.; Shaheen, N.J.; Wani, S. Racial Disparities in Adherence to Quality Indicators in Barrett’s Esophagus: An Analysis Using the GIQuIC National Benchmarking Registry. Am. J. Gastroenterol. 2021, 116, 1201–1210. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Gheorghe, G.; Bacalbasa, N.; Chiotoroiu, A.L.; Diaconu, C. Colorectal Cancer: From Risk Factors to Oncogenesis. Medicina 2023, 59, 1646. [Google Scholar] [CrossRef]
- Gomez, S.L.; Noone, A.-M.; Lichtensztajn, D.Y.; Scoppa, S.; Gibson, J.T.; Liu, L.; Morris, C.; Kwong, S.; Fish, K.; Wilkens, L.R.; et al. Cancer incidence trends among Asian American populations in the United States, 1990–2008. J. Natl. Cancer Inst. 2013, 105, 1096–1110. [Google Scholar] [CrossRef]
- McCracken, M.; Olsen, M.; Chen, M.S.; Jemal, A.; Thun, M.; Cokkinides, V.; Deapen, D.; Ward, E. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J. Clin. 2007, 57, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.-L.B.; Chen, J.J.; Braun, K.L. Colorectal Cancer Screening Compliance among Asian and Pacific Islander Americans. J. Immigr. Minor. Health 2018, 20, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Carney, P.A.; Lin, F.L.; Mongoue-Tchokote, S.; Mori, M.; Leung, H.; Lau, C.; Le, T.; Lieberman, D.A. Improving Colorectal Cancer Screening in Asian Americans: Results of a Randomized Intervention Study. Cancer 2014, 120, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.H.; McPhee, S.J.; Stewart, S.L.; Doan, H.T. Effectiveness of a controlled trial to promote colorectal cancer screening in Vietnamese Americans. Am. J. Public. Health 2010, 100, 870–876. [Google Scholar] [CrossRef]
- Tong, E.K.; Nguyen, T.T.; Lo, P.; Stewart, S.L.; Gildengorin, G.L.; Tsoh, J.Y.; Jo, A.M.; Kagawa-Singer, M.L.; Sy, A.U.; Cuaresma, C.; et al. Lay health educators increase colorectal cancer screening among Hmong Americans: A cluster randomized controlled trial. Cancer 2017, 123, 98–106. [Google Scholar] [CrossRef]
- Wang, C.P.; Lin, J.J.; Shah, S.C.; Kim, M.K. Colorectal Cancer Screening Working Group Geographic Variation in Colorectal Cancer Incidence Among Asian Americans: A Population-Based Analysis 2006-2016. Clin. Gastroenterol. Hepatol. 2023, 21, 543–545.e3. [Google Scholar] [CrossRef]
- Eden, C.M.; Johnson, J.; Syrnioti, G.; Malik, M.; Ju, T. The Landmark Series: The Breast Cancer Burden of the Asian American Population and the Need for Disaggregated Data. Ann. Surg. Oncol. 2023, 30, 2121–2127. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Vilorio, D.; Bureau of Labor Statistics. Education Matters. Available online: https://www.bls.gov/careeroutlook/2016/data-on-display/education-matters.htm (accessed on 9 January 2025).
- Brinton, R.D.; Bernard, M.A. Advisory Committee to the Director Working Group on Diversity Subgroup on Individuals with Disabilities Report, December 1, 2022; National Institutes of Health: Bethesda, MD, USA, 2022. [Google Scholar]
- Ricciardi, G.E.; Cuciniello, R.; De Ponti, E.; Lunetti, C.; Pennisi, F.; Signorelli, C.; Renzi, C. Disability and Participation in Colorectal Cancer Screening: A Systematic Review and Meta-Analysis. Curr. Oncol. 2024, 31, 7023–7039. [Google Scholar] [CrossRef]
- Karahalios, A.; English, D.R.; Simpson, J.A. Weight change and risk of colorectal cancer: A systematic review and meta-analysis. Am. J. Epidemiol. 2015, 181, 832–845. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working. Group Body Fatness and Cancer--Viewpoint of the IARC Working Group. New Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; Iodice, S.; Bagnardi, V.; Raimondi, S.; Lowenfels, A.B.; Maisonneuve, P. Smoking and colorectal cancer: A meta-analysis. JAMA 2008, 300, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.E.; Lai, A.Y.; Gupta, A.; Nguyen, A.M.; Berry, C.A.; Shelley, D.R. Rapid Transition to Telehealth and the Digital Divide: Implications for Primary Care Access and Equity in a Post-COVID Era. Milbank Q. 2021, 99, 340–368. [Google Scholar] [CrossRef]
- Kowalkowski, H.; Austin, G.; Guo, Y.; Miller-Wilson, L.-A.; DaCosta Byfield, S. Patterns of colorectal cancer screening and adherence rates among an average-risk population enrolled in a national health insurance provider during 2009–2018 in the United States. Prev. Med. Rep. 2023, 36, 102497. [Google Scholar] [CrossRef]
| Patient Characteristics | Age 50–55 N (%) | Age 56+ N (%) |
|---|---|---|
| Race | ||
| Pacific Islander | 40 (0.2%) | 68 (0.1%) |
| East Asian | 1328 (7.6%) | 2908 (4.7%) |
| White | 10,016 (57.5%) | 40,880 (66.2%) |
| American Indian or Alaskan Native (AI/AN) | 46 (0.3%) | 165 (0.3%) |
| Black or African American (B/AA) | 5640 (32.4%) | 17,080 (27.7%) |
| Asian Indian | 359 (2.1%) | 611 (1.0%) |
| Gender Identity | ||
| Female | 11204 (58.4%) | 37540 (56.9%) |
| Male | 7974 (41.6%) | 28466 (43.1%) |
| Insurance Status | ||
| Insured | 18,817 (99.5%) | 65,231 (99.7%) |
| Uninsured | 100 (0.5%) | 229 (0.3%) |
| Occupational Status * | ||
| Employed (Full time) | 14,315 (84.4%) | 35,637 (79.3%) |
| Unemployed | 2150 (12.7%) | 6677 (14.9%) |
| Disabled | 457 (2.7%) | 2350 (5.2%) |
| Student (Full time) | 42 (0.2%) | 251 (0.6%) |
| Relationship Status | ||
| In a Relationship | 5788 (29.8%) | 21,702 (32.9%) |
| Single | 13,643 (70.2%) | 44,305 (67.12%) |
| Tobacco Smoking Status | ||
| Never Smokers | 15,290 (92.5%) | 54,215 (91.6%) |
| Current or past smokers | 1243 (7.5%) | 4981 (8.4%) |
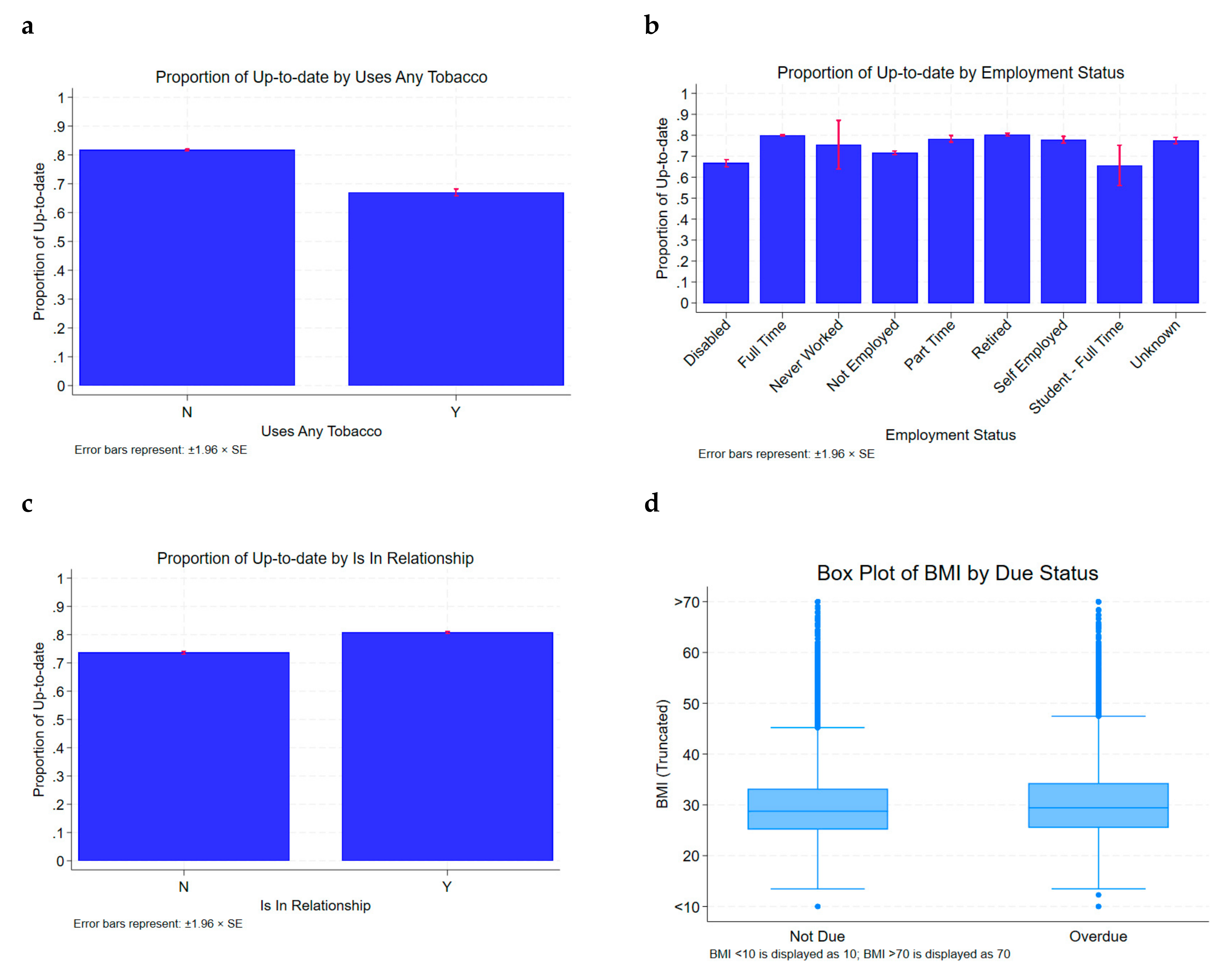
| Patient Characteristics | Up to Date on CRC Screening N (%) | Overdue (Not Up to Date on CRC Screening) N (%) | p Value |
|---|---|---|---|
| Race | |||
| Pacific Islander | 92 (85.2) | 16 (14.8) | <0.05 |
| East Asian | 3488 (82.3) | 748 (17.7) | |
| White | 40,250 (79.1) | 10,646 (20.9) | |
| American Indian or Alaskan Native (AI/AN) | 166 (78.7) | 223 (23) | |
| Black or African American (B/AA) | 17,523 (77.1) | 5197 (22.9) | |
| Asian Indian | 727 (74.9) | 243 (25.1) | |
| Occupational Status * | |||
| Employed (Full time) | 39,950 (79.9) | 10,002 (20.1) | <0.05 |
| Unemployed | 6321(71.6) | 2506 (28.4) | |
| Disabled | 1872 (66.7) | 935 (33.3) | |
| Student (Full time) | 61 (65.6) | 32 (34.4) | |
| Relationship Status | |||
| In a Relationship | 46,819 (80.8) | 11,129 (19.2) | <0.05 |
| Single | 20,055 (73.6) | 7182 (26.4) | |
| Tobacco Smoking Status | |||
| Never Smokers | 56,785 (81.7) | 12,720 (18.3) | <0.05 |
| Current or past smokers | 4170 (67) | 2054 (23) | |
| Mean BMI (kg/m2) | 29.5 kg/m2 | 30.5 kg/m2 | <0.05 |
| (a) | |||||||
|---|---|---|---|---|---|---|---|
| Age 50–55 | Age 56–75 | p Value | |||||
| Up-to-Date N (%) | Overdue N (%) | Up-to-Date N (%) | Overdue N (%) | ||||
| Overall n (%) | 14,910 (77.7) | 4268 (22.3) | 51,964 (78.7) | 14,043 (21.2) | p < 0.05 | ||
| Form of CRC screening chosen * | |||||||
| Cologuard | 2588 (24.1) | N/A | 7275 (14.0) | N/A | p < 0.05 | ||
| Colonoscopy | 10,456 (70.1) | N/A | 41,255 (79.4) | N/A | p < 0.05 | ||
| (b) | |||||||
| Age 50–55 | Age 56–75 | ||||||
| Up-to-Date N (%) | Overdue N (%) | p Value | Up-to-Date N (%) | Overdue N (%) | p Value | ||
| Race | |||||||
| Pacific Islander | 35 (87.5) | 5 (12.5) | p < 0.05 | 57 (83.8) | 11 (18.8) | p < 0.05 | |
| East Asian | 1073 (80.7) | 255 (19.2) | 2415 (83) | 493 (17) | |||
| White | 7865 (78.5) | 2151 (21.4) | 32,385 (79.2) | 8495 (20.8) | |||
| AI/AN ** | 32 (69.6) | 14 (30.4) | 134 (81.2) | 21 (18.8) | |||
| B/AA ** | 4352 (77.1) | 1288 (22.8) | 13,171 (77.1) | 3909 (22.9) | |||
| Asian Indian | 258 (71.9) | 101 (28.1) | 469 (76.8) | 142 (23.2) | |||
| Employment Status | |||||||
| Employed (Full time) | 11,334 (79.2) | 2981 (20.8) | p < 0.05 | 28,616 (80.3) | 7021 (19.7) | p < 0.05 | |
| Unemployed | 1517 (70.6) | 633 (29.4) | 4804 (71.9) | 1873 (28.1) | |||
| Disabled | 299 (65.4) | 158 (34.6) | 1573 (66.9) | 777 (33.1) | |||
| Student (Full time) | 24 (57.1) | 18 (42.9) | p > 0.05 | 237 (72.5) | 14 (27.5) | ||
| Relationship Status | |||||||
| Single | 4268 (73.7) | 1520 (26.3) | p < 0.05 | 16,040 (73.9) | 5662 (26.1) | p < 0.05 | |
| In a relationship | 10,895 (79.9) | 2748 (20.1%) | 35,924 (81.1) | 8381 (18.9) | |||
| Tobacco Smoking Status | |||||||
| Never Smokers | 12,446 (81.3) | 2844 (18.6) | p < 0.05 | 44,339 (81.8) | 9876 (18.2) | p < 0.05 | |
| Current or past smokers | 817 (65.7) | 426 (32.7) | 3353 (67.3) | 1628 (32.7) | |||
| Mean BMI | |||||||
| Mean BMI (kg/m2)) | 30.4 | 31.7 | p < 0.05 *** | 29.2 | 30.2 | p < 0.05 *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sequeira, L.; Vaidya, D.; Ma, J.; Bansal, A.; Huang, S.; Nimgaonkar, A.; Gupta, E. Revealing New Patterns in Colorectal Cancer Screening with a Focus on a Younger Patient Population. Cancers 2025, 17, 1686. https://doi.org/10.3390/cancers17101686
Sequeira L, Vaidya D, Ma J, Bansal A, Huang S, Nimgaonkar A, Gupta E. Revealing New Patterns in Colorectal Cancer Screening with a Focus on a Younger Patient Population. Cancers. 2025; 17(10):1686. https://doi.org/10.3390/cancers17101686
Chicago/Turabian StyleSequeira, Lynette, Dhananjay Vaidya, Jianqiao Ma, Aarav Bansal, Shanshan Huang, Ashish Nimgaonkar, and Ekta Gupta. 2025. "Revealing New Patterns in Colorectal Cancer Screening with a Focus on a Younger Patient Population" Cancers 17, no. 10: 1686. https://doi.org/10.3390/cancers17101686
APA StyleSequeira, L., Vaidya, D., Ma, J., Bansal, A., Huang, S., Nimgaonkar, A., & Gupta, E. (2025). Revealing New Patterns in Colorectal Cancer Screening with a Focus on a Younger Patient Population. Cancers, 17(10), 1686. https://doi.org/10.3390/cancers17101686





