An Activation Likelihood Estimation Meta-Analysis of Voxel-Based Morphometry Studies of Chemotherapy-Related Brain Volume Changes in Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Journal article of a comparative neuroimaging study.
- Focus on cognitive impairment associated with breast cancer chemotherapy.
- Population consists of human female breast cancer patients and/or survivors that have started or completed one or more chemotherapy regimen(s).
- Comparison of population to themselves over time, other chemotherapy groups, or a control group.
- Volume changes investigated with voxel-based morphometry.
- Data provided in MNI or Talairach coordinate systems.
- Reviews, abstracts, case studies, theses, books.
- Animal studies, cell line studies, fetal studies, or male cancer patients/survivors.
- Comparisons of treatments or mitigations
- Machine learning classifiers.
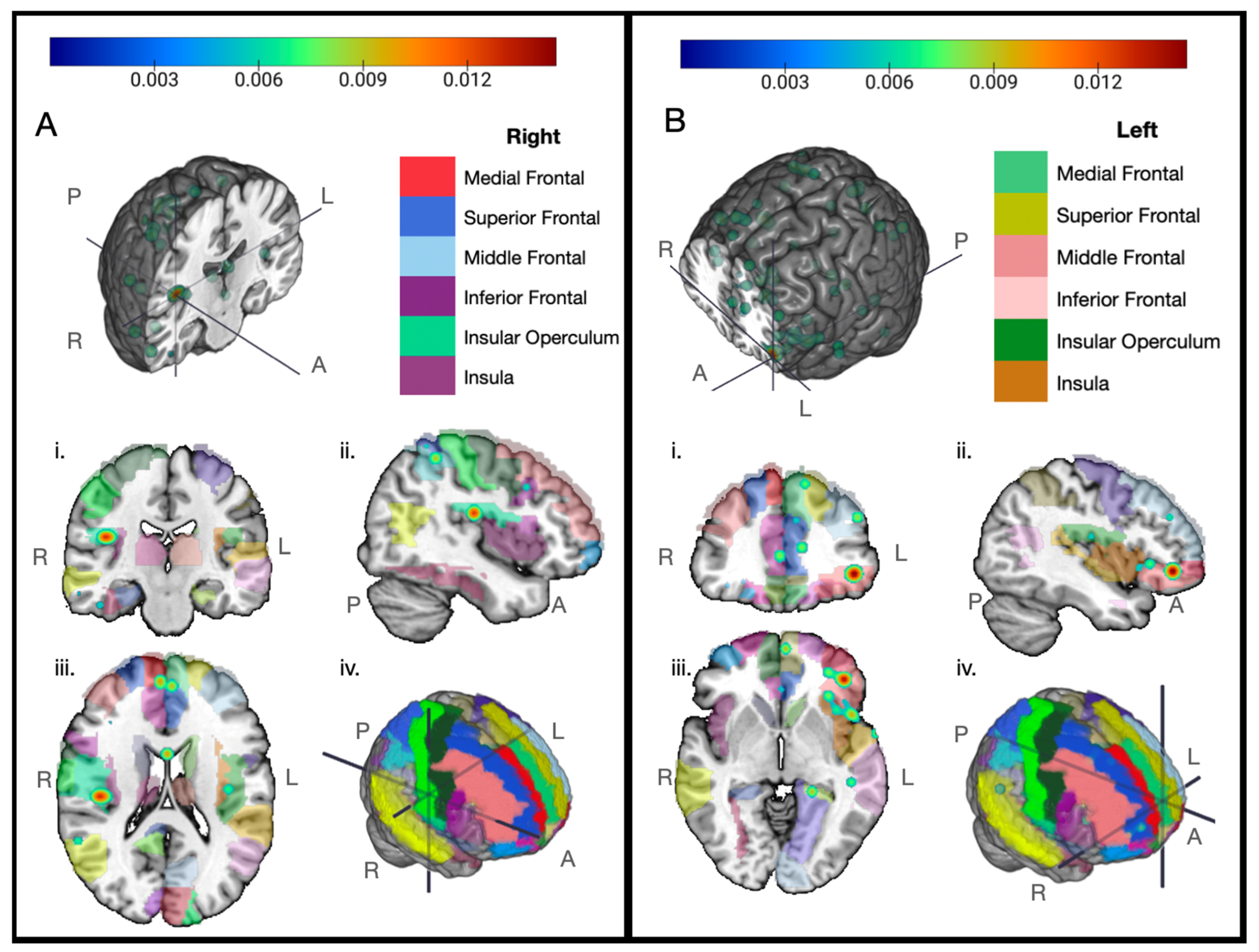
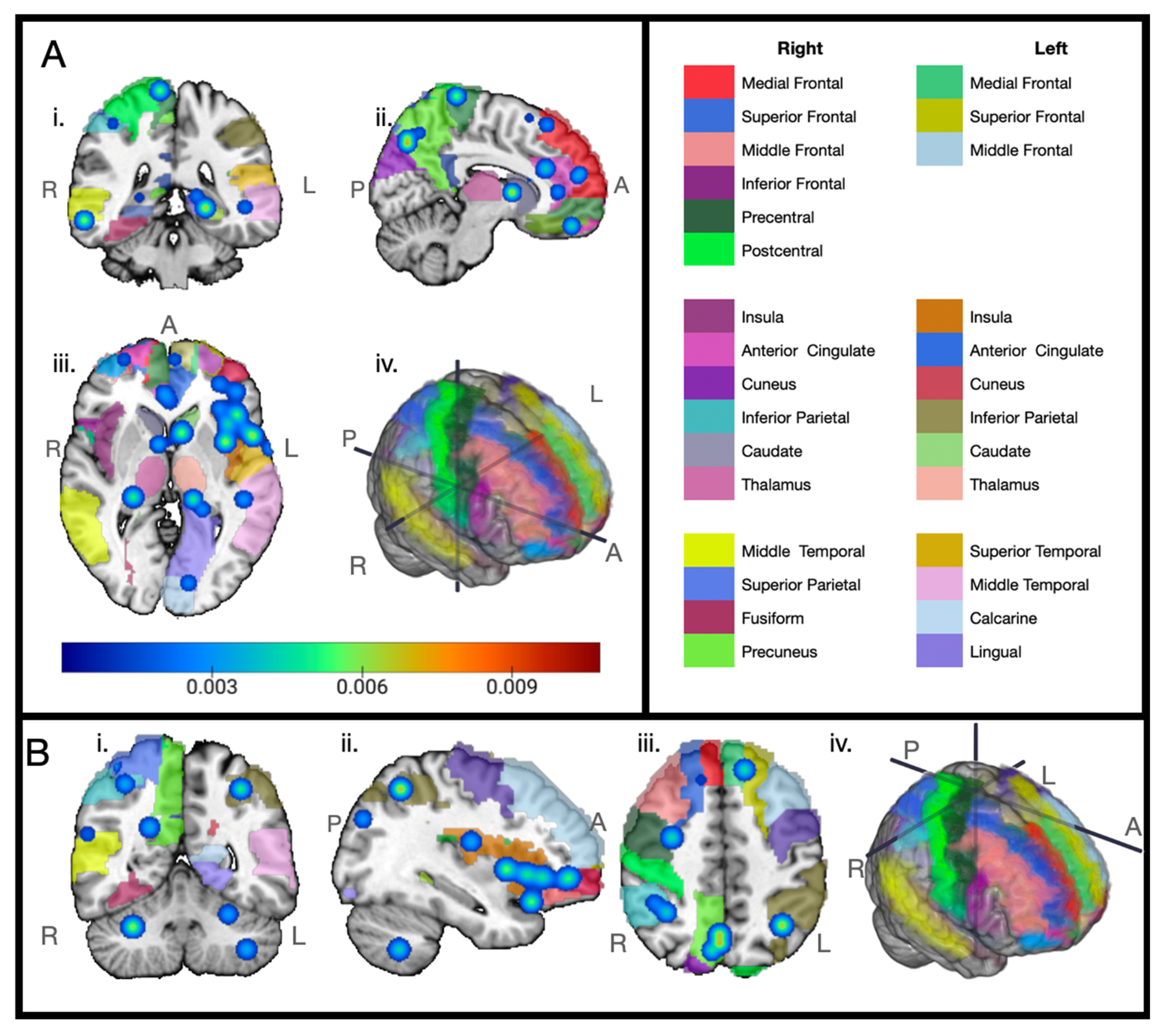
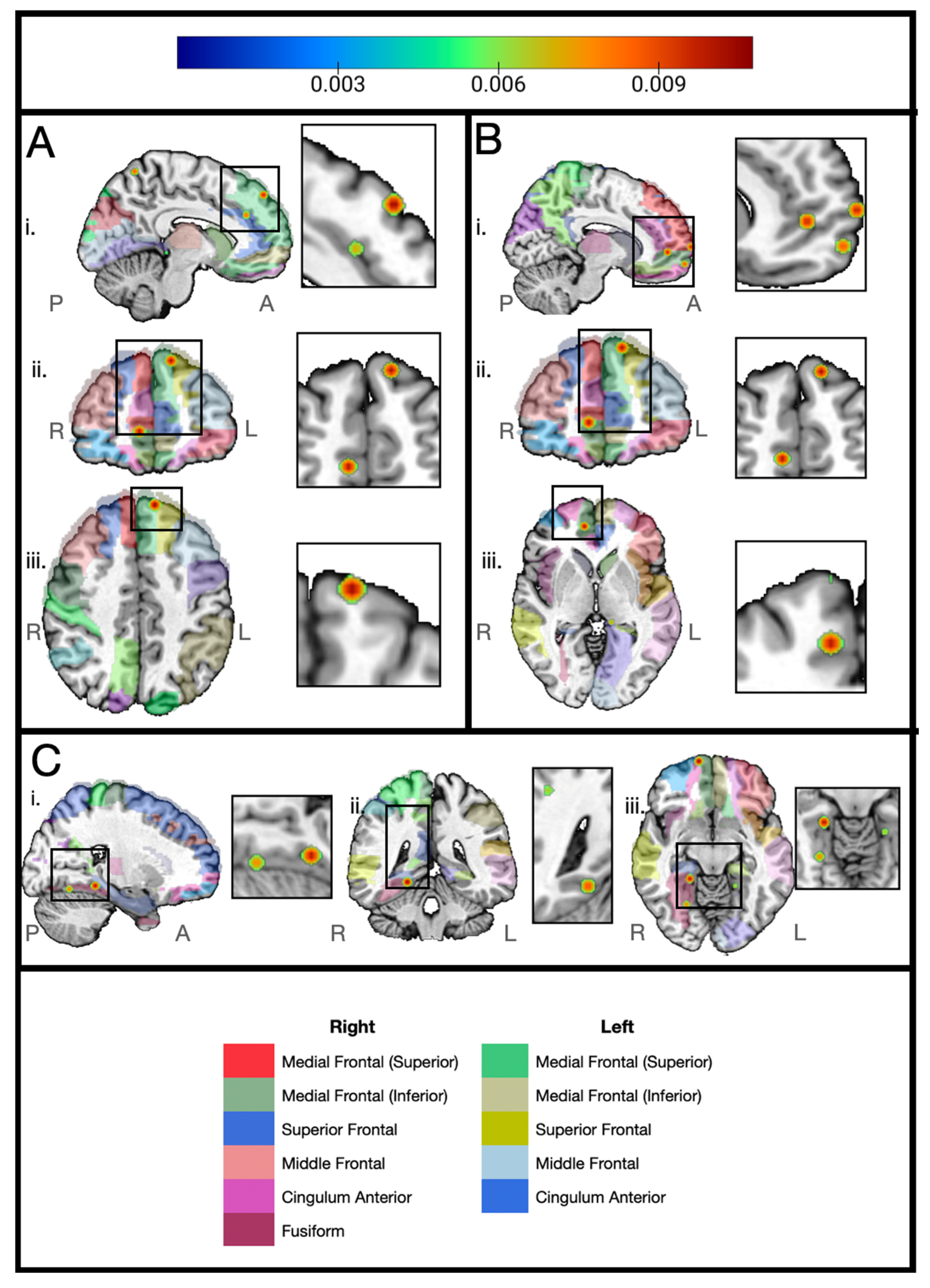
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, Y.; Wang, Y.F.; Zheng, L.J.; Shi, Z.; Huang, W.; Zhang, L.J. Network-Level Functional Connectivity Alterations in Chemotherapy Treated Breast Cancer Patients: A Longitudinal Resting State Functional MRI Study. Cancer Imaging 2020, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Chen, X.; Yan, Y.; He, X.; Hu, S.; Kong, J.; Wu, M.; Wei, Y.; Zhou, Y.; Wang, L.; et al. Functional Connectivity Change of Brain Default Mode Network in Breast Cancer Patients after Chemotherapy. Neuroradiology 2016, 58, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-Related Cognitive Impairment: An Update on State of the Art, Detection, and Management Strategies in Cancer Survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Kohli, S.; Mohile, S.G.; Usuki, K.; Ahles, T.A.; Morrow, G.R. An Update on Cancer- and Chemotherapy-Related Cognitive Dysfunction: Current Status. Semin. Oncol. 2011, 38, 431–438. [Google Scholar] [CrossRef]
- Yamada, T.H.; Denburg, N.L.; Beglinger, L.J.; Schultz, S.K. Neuropsychological Outcomes of Older Breast Cancer Survivors: Cognitive Features Ten or More Years After Chemotherapy. J. Neuropsychiatry Clin. Neurosci. 2010, 22, 48–54. [Google Scholar] [CrossRef]
- De Ruiter, M.B.; Reneman, L.; Boogerd, W.; Veltman, D.J.; Caan, M.; Douaud, G.; Lavini, C.; Linn, S.C.; Boven, E.; Van Dam, F.S.A.M.; et al. Late Effects of High-Dose Adjuvant Chemotherapy on White and Gray Matter in Breast Cancer Survivors: Converging Results from Multimodal Magnetic Resonance Imaging. Hum. Brain Mapp. 2012, 33, 2971–2983. [Google Scholar] [CrossRef]
- Kesler, S.R. Default Mode Network as a Potential Biomarker of Chemotherapy-Related Brain Injury. Neurobiol. Aging 2014, 35, S11–S19. [Google Scholar] [CrossRef]
- Silberfarb, P.M. Chemotherapy and Cognitive Defects in Cancer Patients. Annu. Rev. Med. 1983, 34, 35–46. [Google Scholar] [CrossRef]
- Rodríguez Martín, B.; Fernández Rodríguez, E.J.; Rihuete Galve, M.I.; Cruz Hernández, J.J. Study of Chemotherapy-Induced Cognitive Impairment in Women with Breast Cancer. Int. J. Environ. Res. Public Health 2020, 17, 8896. [Google Scholar] [CrossRef]
- Blommaert, J.; Schroyen, G.; Vandenbulcke, M.; Radwan, A.; Smeets, A.; Peeters, R.; Sleurs, C.; Neven, P.; Wildiers, H.; Amant, F.; et al. Age-dependent Brain Volume and Neuropsychological Changes after Chemotherapy in Breast Cancer Patients. Hum. Brain Mapp. 2019, 40, 4994–5010. [Google Scholar] [CrossRef]
- Wefel, J.S.; Vardy, J.; Ahles, T.; Schagen, S.B. International Cognition and Cancer Task Force Recommendations to Harmonise Studies of Cognitive Function in Patients with Cancer. Lancet Oncol. 2011, 12, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.; Hosseini, S.M.H.; Kesler, S. Altered Resting State Functional Brain Network Topology in Chemotherapy-Treated Breast Cancer Survivors. Neurobiol. Dis. 2012, 48, 329–338. [Google Scholar] [CrossRef]
- Kardan, O.; Reuter-Lorenz, P.A.; Peltier, S.; Churchill, N.W.; Misic, B.; Askren, M.K.; Jung, M.S.; Cimprich, B.; Berman, M.G. Brain Connectivity Tracks Effects of Chemotherapy Separately from Behavioral Measures. NeuroImage Clin. 2019, 21, 101654. [Google Scholar] [CrossRef]
- Bekele, B.M.; Luijendijk, M.; Schagen, S.B.; de Ruiter, M.; Douw, L. Fatigue and Resting-State Functional Brain Networks in Breast Cancer Patients Treated with Chemotherapy. Breast Cancer Res. Treat. 2021, 189, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.M.; Isquith, P.K.; Gioia, G.A. Assessment of Executive Functioning Using the Behavior Rating Inventory of Executive Function (BRIEF). In Handbook of Executive Functioning; Goldstein, S., Naglieri, J.A., Eds.; Springer: New York, NY, 2014; pp. 301–331. ISBN 978-1-4614-8105-8. [Google Scholar]
- Aghakhani, A.; Chan, E.K. Test Reviews: Bracken, B.A., & Howell, K. (2004). Clinical Assessment of Depression. Odessa, FL: Psychological Assessment Resources. J. Psychoeduc. Assess. 2007, 25, 416–422. [Google Scholar] [CrossRef]
- Wagner, L.I. Examining and Managing “Chemobrain”. J. Support. Oncol. 2008, 6, 65–66. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Kesler, S.R.; Ahles, T.A.; Morrow, G.R. Prevalence, Mechanisms, and Management of Cancer-Related Cognitive Impairment. Int. Rev. Psychiatry 2014, 26, 102–113. [Google Scholar] [CrossRef]
- Ren, X.; Boriero, D.; Chaiswing, L.; Bondada, S.; St. Clair, D.K.; Butterfield, D.A. Plausible Biochemical Mechanisms of Chemotherapy-Induced Cognitive Impairment (“Chemobrain”), a Condition That Significantly Impairs the Quality of Life of Many Cancer Survivors. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2019, 1865, 1088–1097. [Google Scholar] [CrossRef]
- Cheng, H.; Li, W.; Gong, L.; Xuan, H.; Huang, Z.; Zhao, H.; Wang, L.S.; Wang, K. Altered Resting-State Hippocampal Functional Networks Associated with Chemotherapy-Induced Prospective Memory Impairment in Breast Cancer Survivors. Sci. Rep. 2017, 7, 45135. [Google Scholar] [CrossRef]
- Harrison, R.A.; Rao, V.; Kesler, S.R. The Association of Genetic Polymorphisms with Neuroconnectivity in Breast Cancer Patients. Sci. Rep. 2021, 11, 6169. [Google Scholar] [CrossRef] [PubMed]
- Schroyen, G.; Sleurs, C.; Bartsoen, E.; Smeets, D.; Van Weehaeghe, D.; Van Laere, K.; Smeets, A.; Deprez, S.; Sunaert, S. Neuroinflammation as Potential Precursor of Leukoencephalopathy in Early-Stage Breast Cancer Patients: A Cross-Sectional PET-MRI Study. Breast 2022, 62, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Dumas, J.A.; Makarewicz, J.; Schaubhut, G.J.; Devins, R.; Albert, K.; Dittus, K.; Newhouse, P.A. Chemotherapy Altered Brain Functional Connectivity in Women with Breast Cancer: A Pilot Study. Brain Imaging Behav. 2013, 7, 524–532. [Google Scholar] [CrossRef]
- Park, H.Y.; Lee, H.; Sohn, J.; An, S.K.; Namkoong, K.; Lee, E. Increased Resting-State Cerebellar-Cortical Connectivity in Breast Cancer Survivors with Cognitive Complaints after Chemotherapy. Sci. Rep. 2021, 11, 12105. [Google Scholar] [CrossRef]
- Müller, V.I.; Cieslik, E.C.; Laird, A.R.; Fox, P.T.; Radua, J.; Mataix-Cols, D.; Tench, C.R.; Yarkoni, T.; Nichols, T.E.; Turkeltaub, P.E.; et al. Ten Simple Rules for Neuroimaging Meta-Analysis. Neurosci. Biobehav. Rev. 2018, 84, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Scarpazza, C.; De Simone, M. Voxel-Based Morphometry: Current Perspectives. Neurosci. Neuroecon. 2016, 5, 19–35. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Eden, G.F.; Jones, K.M.; Zeffiro, T.A. Meta-Analysis of the Functional Neuroanatomy of Single-Word Reading: Method and Validation. NeuroImage 2002, 16, 765–780. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Laird, A.R.; Grefkes, C.; Wang, L.E.; Zilles, K.; Fox, P.T. Coordinate-based Activation Likelihood Estimation Meta-analysis of Neuroimaging Data: A Random-effects Approach Based on Empirical Estimates of Spatial Uncertainty. Hum. Brain Mapp. 2009, 30, 2907–2926. [Google Scholar] [CrossRef]
- Laird, A.R.; Fox, P.M.; Price, C.J.; Glahn, D.C.; Uecker, A.M.; Lancaster, J.L.; Turkeltaub, P.E.; Kochunov, P.; Fox, P.T. ALE Meta-Analysis: Controlling the False Discovery Rate and Performing Statistical Contrasts. Hum. Brain Mapp. 2005, 25, 155–164. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Eickhoff, S.B.; Laird, A.R.; Fox, M.; Wiener, M.; Fox, P. Minimizing Within-experiment and Within-group Effects in Activation Likelihood Estimation Meta-analyses. Hum. Brain Mapp. 2012, 33, 1–13. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Bzdok, D.; Laird, A.R.; Kurth, F.; Fox, P.T. Activation Likelihood Estimation Meta-Analysis Revisited. NeuroImage 2012, 59, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.C.; Janke, A.L.; Collins, D.L.; Baillet, S. Brain Templates and Atlases. NeuroImage 2012, 62, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. Voxel-Based Morphometry—The Methods. NeuroImage 2000, 11, 805–821. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.C.; Conroy, S.K.; Ahles, T.A.; West, J.D.; Saykin, A.J. Gray Matter Reduction Associated with Systemic Chemotherapy for Breast Cancer: A Prospective MRI Study. Breast Cancer Res. Treat. 2010, 123, 819–828. [Google Scholar] [CrossRef]
- McDonald, B.C.; Conroy, S.K.; Smith, D.J.; West, J.D.; Saykin, A.J. Frontal Gray Matter Reduction after Breast Cancer Chemotherapy and Association with Executive Symptoms: A Replication and Extension Study. Brain. Behav. Immun. 2013, 30, S117–S125. [Google Scholar] [CrossRef]
- Lepage, C.; Smith, A.M.; Moreau, J.; Barlow-Krelina, E.; Wallis, N.; Collins, B.; MacKenzie, J.; Scherling, C. A Prospective Study of Grey Matter and Cognitive Function Alterations in Chemotherapy-Treated Breast Cancer Patients. SpringerPlus 2014, 3, 444. [Google Scholar] [CrossRef]
- Jenkins, V.; Thwaites, R.; Cercignani, M.; Sacre, S.; Harrison, N.; Whiteley-Jones, H.; Mullen, L.; Chamberlain, G.; Davies, K.; Zammit, C.; et al. A Feasibility Study Exploring the Role of Pre-Operative Assessment When Examining the Mechanism of ‘Chemo-Brain’ in Breast Cancer Patients. SpringerPlus 2016, 5, 390. [Google Scholar] [CrossRef]
- Chen, B.T.; Jin, T.; Patel, S.K.; Ye, N.; Sun, C.-L.; Ma, H.; Rockne, R.C.; Root, J.C.; Saykin, A.J.; Ahles, T.A.; et al. Gray Matter Density Reduction Associated with Adjuvant Chemotherapy in Older Women with Breast Cancer. Breast Cancer Res. Treat. 2018, 172, 363–370. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Lv, Y.; Chao, H.H.; Gong, L.; Li, C.-S.R.; Cheng, H. Diminished Gray Matter Density Mediates Chemotherapy Dosage-Related Cognitive Impairment in Breast Cancer Patients. Sci. Rep. 2018, 8, 13801. [Google Scholar] [CrossRef]
- Zhou, X.; Tan, Y.; Yu, H.; Liu, J.; Lan, X.; Deng, Y.; Yu, F.; Wang, C.; Chen, J.; Zeng, X.; et al. Early Alterations in Cortical Morphology after Neoadjuvant Chemotherapy in Breast Cancer Patients: A Longitudinal Magnetic Resonance Imaging Study. Hum. Brain Mapp. 2022, 43, 4513–4528. [Google Scholar] [CrossRef]
- Inagaki, M.; Yoshikawa, E.; Matsuoka, Y.; Sugawara, Y.; Nakano, T.; Akechi, T.; Wada, N.; Imoto, S.; Murakami, K.; Uchitomi, Y.; et al. Smaller Regional Volumes of Brain Gray and White Matter Demonstrated in Breast Cancer Survivors Exposed to Adjuvant Chemotherapy. Cancer 2007, 109, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Robertson, M.; Uecker, A.; Fox, P.T.; Eickhoff, S.B. Trends in the Sample Size, Statistics, and Contributions to the BrainMap Database of Activation Likelihood Estimation Meta-analyses: An Empirical Study of 10-year Data. Hum. Brain Mapp. 2023, 44, 1876–1887. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Rorden, C.; Brett, M. Stereotaxic Display of Brain Lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- López Zunini, R.A.; Scherling, C.; Wallis, N.; Collins, B.; MacKenzie, J.; Bielajew, C.; Smith, A.M. Differences in Verbal Memory Retrieval in Breast Cancer Chemotherapy Patients Compared to Healthy Controls: A Prospective fMRI Study. Brain Imaging Behav. 2013, 7, 460–477. [Google Scholar] [CrossRef]
- Henneghan, A.M.; Kesler, S.R. Subjective Cancer-Related Cognitive Impairments and Salience Network Connectivity in Breast Cancer Survivors. J. Cancer Surviv. 2022, 17, 967–973. [Google Scholar] [CrossRef]
- Harrison, N.A.; Brydon, L.; Walker, C.; Gray, M.A.; Steptoe, A.; Dolan, R.J.; Critchley, H.D. Neural Origins of Human Sickness in Interoceptive Responses to Inflammation. Biol. Psychiatry 2009, 66, 415–422. [Google Scholar] [CrossRef]
- Lopez, C.; Drew, M.; Hernandez, G.; Brinton, R. 15th Conference Clinical Trials Alzheimer’s Disease, November 29–December 2, 2022, San Francisco, CA, USA: Posters (Clinical Trial Alzheimer’s Disease). J. Prev. Alzheimer’s Dis. 2022, 9 (Suppl. S1), 51–248. [Google Scholar] [CrossRef]
- Tao, L.; Wang, L.; Chen, X.; Liu, F.; Ruan, F.; Zhang, J.; Shen, L.; Yu, Y. Modulation of Interhemispheric Functional Coordination in Breast Cancer Patients Receiving Chemotherapy. Front. Psychol. 2020, 11, 1689. [Google Scholar] [CrossRef]
- D’Andrea, C.B.; Laumann, T.O.; Newbold, D.J.; Nelson, S.M.; Nielsen, A.N.; Chauvin, R.; Marek, S.; Greene, D.J.; Dosenbach, N.U.F.; Gordon, E.M. Substructure of the Brain’s Cingulo-Opercular Network. bioRxiv 2023. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Raichle, M.E.; Gordon, E.M. The Brain’s Action-Mode Network. Nat. Rev. Neurosci. 2025, 26, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, J.F.; Hardin, F.M.; Nicklaus, J.; Kallogjeri, D.; Wilson, M.; Ma, C.X.; Coalson, R.S.; Shimony, J.; Schlaggar, B.L. Cognitive Impairment after Chemotherapy Related to Atypical Network Architecture for Executive Control. Oncology 2015, 88, 360–368. [Google Scholar] [CrossRef]
- Weintraub, S.; Dikmen, S.S.; Heaton, R.K.; Tulsky, D.S.; Zelazo, P.D.; Bauer, P.J.; Carlozzi, N.E.; Slotkin, J.; Blitz, D.; Wallner-Allen, K.; et al. Cognition Assessment Using the NIH Toolbox. Neurology 2013, 80, S54–S64. [Google Scholar] [CrossRef]
- Chen, B.T.; Chen, Z.; Deng, F.; Patel, S.K.; Sedrak, M.S.; Root, J.C.; Ahles, T.A.; Razavi, M.; Kim, H.; Sun, C.-L.; et al. Signal Variability and Cognitive Function in Older Long-Term Survivors of Breast Cancer with Exposure to Chemotherapy: A Prospective Longitudinal Resting-State fMRI Study. Brain Sci. 2022, 12, 1283. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.T.; Jin, T.; Patel, S.K.; Ye, N.; Ma, H.; Wong, C.W.; Rockne, R.C.; Root, J.C.; Saykin, A.J.; Ahles, T.A.; et al. Intrinsic Brain Activity Changes Associated with Adjuvant Chemotherapy in Older Women with Breast Cancer: A Pilot Longitudinal Study. Breast Cancer Res. Treat. 2019, 176, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.R.; Blayney, D.W. Neurotoxic Effects of Anthracycline- vs Nonanthracycline-Based Chemotherapy on Cognition in Breast Cancer Survivors. JAMA Oncol. 2016, 2, 185. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Chen, V.C.-H.; Yeh, D.-C.; Huang, S.-L.; Zhang, X.-R.; Chai, J.-W.; Huang, Y.-H.; Chou, M.-C.; Weng, J.-C. Association of Functional Dorsal Attention Network Alterations with Breast Cancer and Chemotherapy. Sci. Rep. 2019, 9, 104. [Google Scholar] [CrossRef]
- Deprez, S.; Billiet, T.; Sunaert, S.; Leemans, A. Diffusion Tensor MRI of Chemotherapy-Induced Cognitive Impairment in Non-CNS Cancer Patients: A Review. Brain Imaging Behav. 2013, 7, 409–435. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Tao, L.; Cheng, H.; Li, J.; Zhang, J.; Qiu, B.; Yu, Y.; Wang, K. The Attention Network Changes in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy: Evidence from an Arterial Spin Labeling Perfusion Study. Sci. Rep. 2017, 7, 42684. [Google Scholar] [CrossRef]
- Boedhoe, P.S.W.; Heymans, M.W.; Schmaal, L.; Abe, Y.; Alonso, P.; Ameis, S.H.; Anticevic, A.; Arnold, P.D.; Batistuzzo, M.C.; Benedetti, F.; et al. An Empirical Comparison of Meta- and Mega-Analysis with Data from the ENIGMA Obsessive-Compulsive Disorder Working Group. Front. Neuroinform. 2019, 12, 102. [Google Scholar] [CrossRef]
- Schuster, A.L.R.; Hampel, H.; Paskett, E.D.; Bridges, J.F.P. Rethinking Patient Engagement in Cancer Research. Patient—Patient-Centered Outcomes Res. 2023, 16, 89–93. [Google Scholar] [CrossRef] [PubMed]

| Repository | Terms | Date Performed | Filters |
|---|---|---|---|
| Google Scholar | “functional connectivity” and “chemotherapy” and “breast” | 6 April 2023 | First 100 results, Last 10 years |
| Google Scholar | “breast cancer” “cognitive impairment” “coordinates” | 27 July 2023 | First 100 results |
| Google Scholar | “neuroimaging” “breast cancer” “cognitive impairment” | 27 July 2023 | First 100 results |
| Google Scholar | (“matter volume” “breast cancer” “chemotherapy”) | 3 September 2023 | First 100 results |
| Web of Science | (‘chemotherapy) AND (‘breast cancer’) AND (‘neuroimaging’) AND (‘cognitive decline’ OR ‘cognitive decline’ OR ‘cognitive dysfunction’ OR ‘cognitive impairment’) AND (“gr$y matter” OR ‘white matter OR ‘brain volume’ or ‘volume’ or ‘volumetric’) | 6 October 2023 | |
| Embase | ‘vbm’ AND (‘breast cancer’/exp OR ‘breast cancer’) AND (‘chemotherapy’/exp OR ‘chemotherapy’) | 13 October 2023 | |
| Embase | (‘breast cancer’/exp OR ‘breast cancer’) AND (‘chemotherapy’/exp OR ‘chemotherapy’) AND (‘neuroimaging’/exp OR ‘neuroimaging’) AND ‘cognitive defect’ AND [embase]/lim NOT ([embase]/lim AND [medline]/lim) | 13 October 2023 | Embase only |
| Embase | (‘breast cancer’/exp OR ‘breast cancer’) AND (‘brain volume’/exp OR ‘brain volume’) | 1 November 2023 |
| Authors | BCC+ | Mean Age Mean (SD) | Coordinate Count | Chemotherapy Agent |
|---|---|---|---|---|
| McDonald 2010 [35] | 17 | 52.4 (8.5) | 24 | AC-paclitaxel 12 TAC 2 AC 3 * |
| McDonald 2013 [36] | 27 | 49.9 (7.6) | 2 | AC-T 9 TC 9 Tb 5 TAC 1 Paclitaxel 1 Not available 1 ** |
| Lepage 2014 [37] | 19 | 50.2 (8.6) | 14 | FEC-T 13 * TC 4 AC 1 AC-paclitaxel 1 |
| Jenkins 2016 [38] | 8 | 52.6 (3.9) | 3 | AC 1 FEC 2 FEC-T |
| Chen 2018 [39] | 16 | 67(5.39) | 9 | TC 7 TbHP 1 paclitaxel/ trastuzumab 4 Carboplatin/paclitaxel 1 ddAC-paclitaxel 1 TAC 1 |
| Li 2018 [40] | 28 | 49.21 (8.15) | 5 | Doxorubicin and paclitaxel |
| Blommaert 2019 [10] | 72 | Younger group 43.7 (5.7) Older group 63.8 (3.4) | 21 | FEC 16 FEC-T 39 |
| Zhou 2022 [41] | 45 | 50.45 (9.08) | 13 | AC-T 11 EC-T 1 TAC 15 AT 1 TC 1 TbHP 11 TbHB 5 |
| Authors | Subjects | Coordinate Count | Chemotherapy Agent |
|---|---|---|---|
| Inagaki 2007 [42] | 169 | 9 | AC 3 CMF: 40 EC 2 Paclitaxel 2 tegafur/uracil 5 |
| McDonald 2013 [36] | 51 | 1 | AC-T 9 TC 9 Tb 5 TAC 1 Paclitaxel 1 Not available 1 * |
| Chen 2018 [39] | 31 | 9 | TC 7 TbHP 1 paclitaxel/ trastuzumab 4 Carboplatin/paclitaxel 1 ddAC-paclitaxel 1 TAC 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utecht, S.; Gomez-Acevedo, H.; Bona, J.; van der Plas, E.; Prior, F.; Larson-Prior, L.J. An Activation Likelihood Estimation Meta-Analysis of Voxel-Based Morphometry Studies of Chemotherapy-Related Brain Volume Changes in Breast Cancer. Cancers 2025, 17, 1684. https://doi.org/10.3390/cancers17101684
Utecht S, Gomez-Acevedo H, Bona J, van der Plas E, Prior F, Larson-Prior LJ. An Activation Likelihood Estimation Meta-Analysis of Voxel-Based Morphometry Studies of Chemotherapy-Related Brain Volume Changes in Breast Cancer. Cancers. 2025; 17(10):1684. https://doi.org/10.3390/cancers17101684
Chicago/Turabian StyleUtecht, Sonya, Horacio Gomez-Acevedo, Jonathan Bona, Ellen van der Plas, Fred Prior, and Linda J. Larson-Prior. 2025. "An Activation Likelihood Estimation Meta-Analysis of Voxel-Based Morphometry Studies of Chemotherapy-Related Brain Volume Changes in Breast Cancer" Cancers 17, no. 10: 1684. https://doi.org/10.3390/cancers17101684
APA StyleUtecht, S., Gomez-Acevedo, H., Bona, J., van der Plas, E., Prior, F., & Larson-Prior, L. J. (2025). An Activation Likelihood Estimation Meta-Analysis of Voxel-Based Morphometry Studies of Chemotherapy-Related Brain Volume Changes in Breast Cancer. Cancers, 17(10), 1684. https://doi.org/10.3390/cancers17101684






