Simple Summary
This review examines the therapeutic potential of targeting CXCL1 and its receptor, CXCR2, in cancer treatment. It discusses anti-CXCL1 antibodies and CXCR2 antagonists, including AZD5069, SB225002, SCH-479833, navarixin/SCH-527123, ladarixin/DF2156A, and reparixin, as well as strategies to enhance CXCR2 expression in lymphocytes during adoptive cell therapy to improve immunotherapy outcomes. Particular attention is given to the role of CXCL1 in treatment resistance, including resistance to chemotherapy, radiotherapy, and anti-angiogenic therapy.
Abstract
CXCL1 (Gro-α, MGSA) is a chemokine functionally similar to CXCL8/IL-8, as both activate the same receptor, CXCR2. CXCL1 levels are frequently elevated in tumors compared to healthy tissue, where they play a key role in promoting cancer cell migration, angiogenesis, and neutrophil recruitment. While the involvement of CXCL1 in tumor progression is well established, its relevance to cancer therapy remains underexplored. This review examines the therapeutic potential of targeting CXCL1 and its receptor, CXCR2, in cancer treatment. It discusses anti-CXCL1 antibodies and CXCR2 antagonists, including AZD5069, SB225002, SCH-479833, navarixin/SCH-527123, ladarixin/DF2156A, and reparixin, as well as strategies to enhance CXCR2 expression in lymphocytes during adoptive cell therapy to improve immunotherapy outcomes. Particular attention is given to the role of CXCL1 in treatment resistance, including resistance to chemotherapy, radiotherapy, and anti-angiogenic therapy. Cancer therapies often upregulate CXCL1 expression, which in turn drives treatment resistance. Additionally, this review explores the contribution of CXCL1 to therapy-induced side effects, such as chemotherapy-induced metastasis, neuropathy, nephrotoxicity, diarrhea, and cardiotoxicity. CXCR2 inhibitors are well tolerated by patients in clinical trials. However, the limited number of studies evaluating these agents in combination with standard chemotherapy precludes any definitive conclusions.
1. Introduction
CXC motif chemokine ligand 1 (CXCL1) is an α-chemokine characterized by a CXC motif at its N-terminus [1]. Previously known as growth-regulated gene-α (Gro-α) and melanoma growth-stimulatory activity (MGSA), CXCL1 primarily signals through the CXC motif chemokine receptor 2 (CXCR2). At higher concentrations, it can also activate CXCR1 [2]. Another receptor for CXCL1 is the atypical chemokine receptor 1 (ACKR1), also known as the Duffy antigen receptor for chemokines (DARC) [3]. However, binding to this receptor does not appear to induce significant signal transduction, though it may regulate CXCL1 availability [4].
CXCL1 plays a critical role in cancer progression by directly affecting tumor cells. It promotes cancer cell proliferation [5,6,7] and supports the self-renewal of cancer stem cells [8,9]. However, its effect on proliferation varies depending on the tumor type; in cholangiocarcinoma, CXCL1 has been shown to suppress cancer cell proliferation [10]. Additionally, CXCL1 exerts anti-apoptotic effects by modulating the expression of B-cell leukemia/lymphoma-2 (Bcl-2) family proteins [11,12]. It also enhances cancer cell migration [13,14,15] and contributes to immune evasion by upregulating programmed death-ligand 1 (PD-L1) expression in tumor cells [6].
Beyond its direct effects on cancer cells, CXCL1 influences the tumor microenvironment by acting on non-malignant cells. Due to the expression of CXCR2 on endothelial cells, CXCL1 promotes angiogenesis (Figure 1) [16,17,18]. Like other chemokines, it facilitates immune cell migration and contributes to the immunosuppressive tumor milieu. CXCL1 is involved in recruiting granulocytic myeloid-derived suppressor cells (G-MDSC) [19,20] and tumor-associated neutrophils (TAN) [21,22,23]. Moreover, CXCL1 influences tumor-associated macrophages (TAM) by promoting M2 polarization [24,25]. It also indirectly increases the presence of monocytic myeloid-derived suppressor cells (M-MDSC) in the tumor niche by stimulating their expansion in the bone marrow [26].
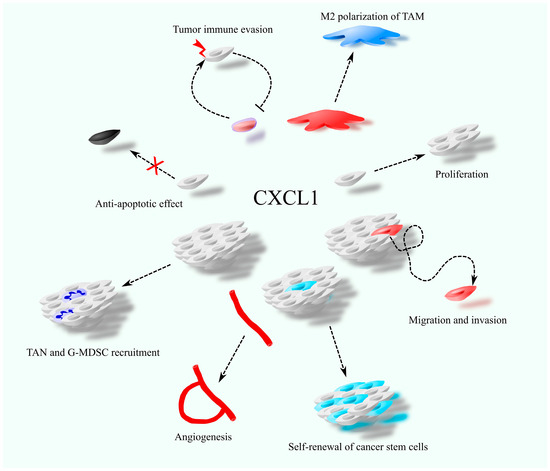
Figure 1.
The involvement of CXCL1 in cancer-related processes, including cancer cell proliferation and migration, self-renewal of cancer stem cells, anti-apoptotic effects, angiogenesis, recruitment of tumor-associated neutrophils (TAN), polarization of tumor-associated macrophages (TAM), and tumor immune evasion.
Although the role of CXCL1 in cancer progression is well documented, its impact on cancer therapy has received relatively little attention. This review aims to address this gap and highlight the potential of CXCL1 as a therapeutic target in combination with standard cancer treatments. The discussion focuses on CXCL1-mediated therapy resistance and its contribution to the adverse effects of cancer treatment.
2. Anticancer Therapy vs. CXCL1
2.1. CXCL1-CXCR2 Axis as a Therapeutic Target in Cancer Therapy
CXCL1 is a significant factor in cancer processes, inducing an autocrine stimulation of tumor cell proliferation [27,28] and migration [8,28]. CXCL1 chemokine induces angiogenesis [17,18,29,30] and recruitment of G-MDSCs [31,32] and neutrophils [33] into the tumor niche. Also, the expression of this chemokine is elevated relative to healthy tissues in many types of cancer [34,35,36,37,38,39,40,41,42,43]. For this reason, the CXCL1-CXCR2 axis represents a convenient therapeutic target for cancer treatment.
Many therapeutic approaches targeting the CXCL1-CXCR2 axis are currently under investigation. An example of this is HL2401, a monoclonal antibody anti-human CXCL1 [44] which inhibits the proliferation and migration of bladder and prostate cancer cells and inhibits the angiogenesis of the tested tumors. To date, no clinical trials have been conducted using HL2401.
However, a more effective therapeutic approach is the use of CXCR2 inhibitors. In addition to disrupting the CXCL1 function, this approach also targets all CXCR2 ligands. The best-known CXCR2 antagonists include SB225002: (N-(2-hydroxy-4-nitrophenyl)-N’-(2-bromophenyl)urea) [45], which has shown activity against glioma [46], androgen-independent prostate cancer [47], cervical cancer [7], chronic myeloid leukemia (CML) [48], nasopharyngeal carcinoma [49], oral squamous cell carcinoma [50], triple negative breast cancer [51], ovarian cancer [52], and cholangiocellular carcinoma [53]. At the same time, SB225002 not only decreases CXCR2 activity but also binds to β-tubulin [54,55], which leads to the disruption of microtubules, which may account in part for the anticancer properties of this compound. To date, no clinical trials have been conducted using SB225002.
Another CXCR2 inhibitor is AZD5069, a reversible antagonist of CXCR2 [56]. In vitro studies have demonstrated its antitumor activity against thyroid cancer cells [57]. In models of triple-negative breast cancer, it reverses doxorubicin resistance [58], likely by reducing the production of transforming growth factor (TGF)-β by cancer cells. AZD5069 also enhances the efficacy of anti-PD-L1 antibodies in an in vivo model of hepatocellular carcinoma [59]. AZD5069 is currently undergoing clinical evaluation [60] and has been shown to be well tolerated by patients [61]. It is being investigated for the treatment of asthma (ClinicalTrials.gov ID: NCT01704495) [62]; however, clinical trials have not demonstrated efficacy in this indication. It has also been tested in patients with chronic obstructive pulmonary disease (COPD) (ClinicalTrials.gov ID: NCT01233232) [61] and bronchiectasis (ClinicalTrials.gov ID: NCT01255592) [63]. While the compound was well tolerated, it did not lead to significant clinical improvement.
AZD5069 is also being evaluated as a potential anticancer agent. It is currently undergoing testing in combination with the anti-PD-L1 antibody durvalumab in patients with metastatic pancreatic ductal adenocarcinoma (PDAC) (ClinicalTrials.gov ID: NCT02583477), although the results of this trial have not yet been published. A similar ongoing study (ClinicalTrials.gov ID: NCT02499328) is assessing AZD5069 alone or in combination with durvalumab in patients with advanced head and neck squamous cell carcinoma (HNSCC).
Also being tested are dual antagonists for CXCR1 and CXCR2. These compounds inhibit the activity of a receptor for CXCL1 and also inhibit the activity of CXCR1, which means that they also reduce the activity of CXCL8/IL-8. Such a compound is SCH-479833 [64,65]. SCH-479833 has been shown to have anticancer properties for melanoma [64] and colon cancer [65]. However, this compound has not yet undergone clinical testing.
Another dual antagonist of CXCR1 and CXCR2 is navarixin (SCH-527123, MK-7123) [66,67], which also inhibits CCR7 [68]. Navarixin has demonstrated antitumor activity against several cancer types, including colorectal cancer [65,69] and melanoma [64,70]. It is currently being investigated in clinical trials as a potential treatment for COPD (ClinicalTrials.gov ID: NCT01068145, NCT00441701) and asthma (ClinicalTrials.gov ID: NCT01006161, NCT00632502), as well as for psoriasis (ClinicalTrials.gov ID: NCT00684593). Navarixin has also been evaluated in oncology (ClinicalTrials.gov ID: NCT03473925) [71]. In this trial, navarixin was administered in combination with pembrolizumab to 105 patients with various cancer types. Pembrolizumab is an anti-PD-1 antibody, and the treatment aimed to simultaneously block CXCR1/CXCR2 signaling and a key immune checkpoint involved in tumor immune evasion. However, the combination failed to produce a satisfactory therapeutic response. The most severe adverse events reported included neutropenia, hepatitis, and pneumonitis [71]. These results suggest that CXCR2 inhibitors, whether used alone or in combination with PD-1-targeted therapies, may lack sufficient therapeutic efficacy. One possible explanation is the off-target inhibition of CCR7 by navarixin [68]. CCR7 plays a critical role in directing lymphocyte trafficking to lymph nodes [72]; thus, its disruption may impair immune system function and counteract the effects of PD-1 blockade.
Another compound being tested is ladarixin/DF2156A [73,74,75], a non-competitive allosteric inhibitor of CXCR1 and CXCR2 receptors, which has demonstrated anti-tumor properties for melanoma [75] and PDAC [76]. Ladarixin is being evaluated in clinical trials as a potential treatment for type 1 diabetes (ClinicalTrials.gov IDs: NCT05368402, NCT02814838, NCT04899271, NCT04628481, NCT05035368). In these studies, it did not significantly prevent β-cell loss in most patients [77], although further investigations into its use for type 1 diabetes are ongoing [78]. Ladarixin is also being tested in combination with sotorasib—a targeted inhibitor of mutant K-RAS—in patients with KRAS G12C-mutant non-small-cell lung cancer (NSCLC) (ClinicalTrials.gov ID: NCT05815173). This trial is currently in the patient recruitment phase.
Another dual CXCR1/CXCR2 antagonist is reparixin (Figure 2), a non-competitive allosteric inhibitor that preferentially binds and stabilizes the inactive conformation of both receptors [79,80]. In vitro studies have shown that reparixin exhibits antitumor activity in thyroid cancer [81] and pancreatic cancer [82]. It has also demonstrated both in vitro and in vivo efficacy against breast cancer [83], at least in part by reducing the self-renewal capacity of cancer stem cells (CSCs), thereby enhancing the therapeutic effect of docetaxel. Reparixin is currently undergoing clinical evaluation as a potential anticancer agent. In a Phase I trial in patients with HER2-negative breast cancer (ClinicalTrials.gov ID: NCT01861054), the compound was well tolerated and exhibited minimal toxicity [84]. In another trial, reparixin was tested in combination with paclitaxel in 30 patients with HER2-negative metastatic breast cancer (ClinicalTrials.gov ID: NCT02001974) [85], where it maintained a favorable safety profile and was associated with a 30% response rate. However, in a separate clinical study involving patients with metastatic triple-negative breast cancer (ClinicalTrials.gov ID: NCT02370238), reparixin did not enhance the therapeutic efficacy of paclitaxel [86], though it continued to show low toxicity.
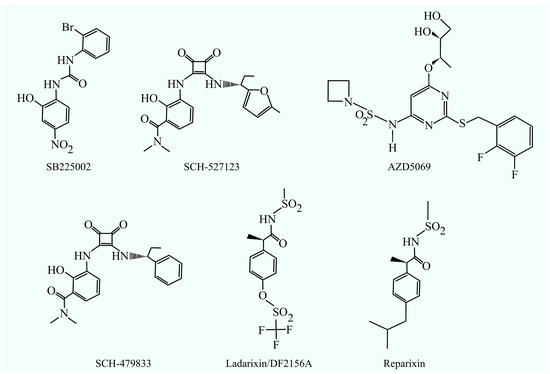
Figure 2.
CXCR2 inhibitors: SB225002, SCH-527123, AZD5069, SCH-479833, ladarixin (DF2156A), and reparixin.
The antibody LY3041658 has been developed to neutralize all known ligands of the CXCR2 receptor [87]. Rather than acting directly on the receptor, it reduces CXCR2 activation by inactivating its ligands. LY3041658 is currently undergoing early-stage clinical trials in healthy volunteers (ClinicalTrials.gov ID: NCT02148627, NCT04653168).
In addition to reducing the activation of the CXCL1-CXCR2 axis in tumors, this axis can be used as part of anti-cancer therapy using the conjugation of daunorubicin with CXCL1 [63], where CXCL1 serves as a transporter for daunorubicin (Table 1). Cancer cells have high expression of CXCR2, so CXCL1 will bind with high affinity to its receptor on the membrane of cancer cells. Along with this chemokine, the cancer cell will take up the conjugated drug. This approach has been investigated in an in vitro melanoma model [88].

Table 1.
Compounds targeting CXCL1 or its receptor in preclinical studies or clinical trials.
Inhibition of CXCR2 abolishes all tumor processes involving the CXCL1-CXCR2 axis. However, it is possible to transform pro-cancerous mechanisms into anti-cancerous ones. An example of this is the polarization of neutrophils [89], cells which have either N1 or N2 polarity depending on the factors to which they are exposed. Neutrophils with N2 polarity are pro-tumor cells that contribute to cancerous tumor growth. In contrast, neutrophils with N1 polarity are anti-cancer cells. By using pro-inflammatory compounds, it is possible to recruit neutrophils through the CXCL1-CXCR2 axis and then polarize these cells into anti-tumor neutrophils with N1 polarity. OM-174, which is a lipid A analog, can be used for this [90]; alternatively, such therapy can be combined with a standard chemotherapeutic agent, such as oxaliplatin or cisplatin.
None of the compounds targeting CXCL1 or CXCR2 listed above have been approved by the US Food and Drug Administration (FDA) as official anticancer drugs. However, they are being evaluated in in vitro and in vivo studies as well as in early-phase clinical trials.
2.2. Herbal Substances with Anticancer Activity Against CXCL1
Some anticancer drugs and therapeutic substances act against CXCL1. An example of this is curcumin, which decreases nuclear factor κB (NF-κB) activation and thus reduces the expression of genes dependent on this transcription factor, including CXCL1 [91]. Curcumin also increases the expression of miR-181b, which directly downregulates CXCL1 mRNA [92]. This effect also occurs when curcumin is combined with 5-fluorouracil, a drug in which some side effects are due to an increase in CXCL1 expression induced by this drug. The use of curcumin abolishes this increase and improves the effectiveness of 5-fluorouracil in cancer treatment [93].
Another compound of natural origin is ginsenoside panaxatriol, one of the main active ingredients of Asian ginseng (Panax ginseng) preparations [94]. Ginsenoside panaxatriol reduces the activation of NF-κB in breast cancer cells and thus decreases the expression of chemokines, such as CXCL1 and CXC motif chemokine ligand 8 (CXCL8)/interleukin-8 (IL-8), increasing the effectiveness of paclitaxel [94].
The XIAOPI formula consists of 10 herbs and 196 compounds [95]. One of the compounds identified in this drug is baohuoside I, which downregulates CXCL1 expression in TAM [96,97]. For this reason, the XIAOPI formula acts against breast cancer. In particular, the XIAOPI formula interferes with the function of cancer stem cells [98] and decreases autophagy in cancer cells [99], which increases chemosensitivity during the use of anticancer drugs such as paclitaxel.
2.3. Adoptive Cell Therapy
Adoptive cell therapy is a type of immunotherapy which involves taking lymphocytes from a patient, modifying them to fight the cancer more effectively, and then introducing them back into the patient’s body [100]. In the first protocols, lymphocytes were isolated from the cancerous tumor, that is, lymphocytes that had the ability to infiltrate the cancerous tumor and destroy cancer cells. These lymphocytes were then multiplied and introduced back into the patient’s body. Currently, researchers are testing lymphocytes with either modified T cell receptors (TCR) or expressed chimeric antigen receptors (CARs) recognizing specific cancer antigens.
The modified lymphocytes recognize tumor antigens and thus effectively destroy tumor cells. However, infiltration of lymphocytes into a solid tumor is hampered because such lymphocytes have low-level expression of receptors responsible for their migration into the tumor niche. An example of such a receptor is CXCR2 [101,102,103,104]—the most important receptor for CXCL1. Expression of CXCL1 is upregulated relative to healthy tissue in many cancerous tumors such as bladder cancer [39], colon cancer [38,42], gastric cancer [36], hepatocellular carcinoma [35], non-small-cell lung carcinoma [40], melanoma [34], ovarian cancer [37], PDAC [43], and renal cell carcinoma [105]. In breast cancer, CXCL1 expression is reduced relative to healthy tissue [41]. Conversely, it is elevated in triple-negative breast cancer [106].
Due to increased CXCL1 expression in many cancers, CXCR2 transduction into lymphocytes increases tumor infiltration by such modified cells, which was confirmed by experiments on T cells with increased CXCR2 expression, which specifically migrated to ovarian cells [102]. These modified T cells exhibit improved infiltration into tumors in in vivo models of melanoma [103,107] and colon cancer [107]. At the same time, CXCR2 activation on such lymphocytes caused an increase in interferon-γ (IFN-γ) secretion [101], a cytokine that enhances the anti-tumor immune response.
CXCR2 transduction, as an additional modification of lymphocytes, improves the effectiveness of therapy. An example of this is the transduction of CXCR2 into either CAR-T cells or T cells with modified TCR. Such additional modification induces such modified T cells to specifically accumulate in solid tumors, as confirmed by experiments on ovarian cancer [108], hepatocellular carcinoma [104], glioblastoma, and PDAC [108]. This efficacy can also be improved by the use of radiation therapy [108]. Ionizing radiation increases CXCL1 expression in many cancers, such as breast cancer [109], glioblastoma multiforme [108,110,111,112,113], and non-small-cell lung cancer [114]. This may increase the infiltration of T cells with increased CXCR2 expression.
In addition to T cells, natural killer (NK) cells are also being tested as an example of adoptive cell therapy (Table 2). Increasing CXCR2 expression on these cells causes tumor infiltration by these cells, giving an anti-tumor effect, as shown in experiments in vitro on renal cell carcinoma [115].

Table 2.
CXCR2 in anticancer adoptive cell therapy.
On the other hand, it should be mentioned that CXCL1 causes the recruitment of G-MDSCs into the tumor niche [116,117,118]—cells that inhibit the action of anti-tumor lymphocytes and thus reduce the effectiveness of immunotherapy. For this reason, to increase the effectiveness of adoptive cell therapy, one should use modified lymphocytes with drugs that target cancer immune evasion.
2.4. Photodynamic Therapy
Photodynamic therapy involves using a photosensitizer that accumulates in the tumor and then applying light that reacts with the compound [119]. This results in the production of ROS that damage the cancer cells. Photodynamic therapy also causes inflammation, which activates the immune system to destroy cancer cells. This is because fibroblasts secrete heat shock protein family A member 1B (Hspa1b) in response to photodynamic therapy [120]. Hspa1b, dependent on toll-like receptors (TLR)2 and TLR4, activates NF-κB in macrophages, which increases the expression of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), as well as CXCR2 ligands in these cells, as shown in experiments on mouse models.
Photodynamic therapy also increases the expression of CXCR2 ligands in tumorigenesis, as shown in experiments on breast cancer in mice [121]. In mice with murine colon carcinoma, it induces the accumulation of T helper type 17 (Th17) cells in tumor-draining lymph nodes [122], which increases the expression of macrophage inflammatory protein-2 (MIP-2) (but not keratinocyte-derived chemokine (KC)) that causes the infiltration of neutrophils into tumor-draining lymph nodes. Neutrophils in this location enhance the anti-tumor immune response.
In mouse models, MIP-2 (but not KC) plays an important role in neutrophil recruitment [121,122]. Studies in human models are lacking, and for this reason it is not known which of the CXCR2 ligands in humans have a significant role in the induction of the anti-tumor immune response.
3. Resistance to Therapy
3.1. Resistance to Chemotherapy
CXCL1 expression is increased by chemotherapeutics such as the histone deacetylase (HDAC) inhibitor Belinostat in breast cancer cells [123], paclitaxel in triple-negative breast cancer cells [94] and in melanoma cells [124], 5-fluorouracil in murine 4T1 triple-negative breast cancer cells [125], oxaliplatin in colorectal cancer cells [126] and metastatic castration-resistant prostate cancer cells [127], epidoxorubicin in bladder cancer cells [128], doxorubicin in triple-negative breast cancer cells [129], and carboplatin in murine B16-F10 melanoma cells [130]. Also, chemotherapeutics increase the expression of other CXCR2 ligands, including CXCL8/IL-8 [127]. However, not every anticancer drug induces the CXCL1-CXCR2 axis activation, and also not in every type of cancer. For example, taxanes cause a decrease in CXCR2 expression in metastatic castration-resistant prostate cancer [131], which reduces the action of CXCL1.
An increase in CXCL1 expression by chemotherapeutics is associated with NF-κB activation (Figure 3) [126,129], although this effect may depend on an increase in TNF-α production [132] and, at least in the case of doxorubicin, the activation of NF-κB depends on p53 deficiency [129]. Increased expression of CXCL1 as well as other CXCR2 ligands results in autocrine increases in the expression of these chemokines [133].
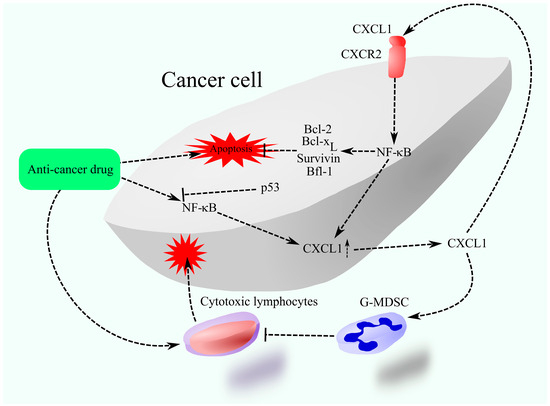
Figure 3.
CXCL1 as a mediator of treatment resistance. Chemotherapy elevates CXCL1 expression, which, through CXCR2 activation on cancer cells, enhances the expression of anti-apoptotic proteins such as those from the Bcl-2 family and survivin, thus inhibiting therapy-induced apoptosis. Moreover, CXCL1 facilitates the recruitment of G-MDSCs into the tumor microenvironment, obstructing the ability of therapy-activated cytotoxic lymphocytes to eliminate cancer cells.
CXCR2 activation is responsible for resistance to chemotherapy. In particular, it causes increased activation of NF-κB, which leads to the increased expression of Bcl-2 [126,127,131] and baculoviral IAP repeat containing 5 (BIRC5)/survivin [127]. NF-κB can also directly increase the expression of other anti-apoptotic Bcl-2 family proteins, for example, Bcl-2-related gene expressed in fetal liver-1 (Bfl-1)/A1 [134] and B-cell lymphoma-extra-large (Bcl-xL) [135]. Therefore, this may be the main mechanism of chemoresistance. Also, CXCR2 ligand-induced chemoresistance may be mediated by neutrophils and G-MDSCs [132,136]. G-MDSCs are recruited to the tumor niche by the CXCR2 ligands. CXCL1 present in extracellular vesicles derived from apoptotic cancer cells also contributes to tumor immune evasion [137]. It enhances PD-1 activity and promotes M2 polarization in TAMs. In addition, CXCL1 increases PD-L1 expression on cancer cells, thereby suppressing the activity of cytotoxic lymphocytes [138]. G-MDSCs and M2 TAM may inhibit the anti-cancer response of the immune system induced by the use of chemotherapeutics.
Another drug resistance mechanism involving CXCL1 is autophagy-mediated chemoresistance [139]. This process has been observed in experimental models of breast cancer and is specifically linked to resistance to paclitaxel.
Due to the pro-survival effects of CXCR2 ligands, it is possible to use a standard chemotherapeutic agent together with a CXCR2 inhibitor to increase the efficacy of the applied therapy. Therefore, blocking CXCR2 activity or inactivating CXCR2 ligands increases the efficacy of drugs such as 5-fluorouracil [140], paclitaxel [133,141], doxorubicin [133,141], oxaliplatin [69,126], and cisplatin [131,136] on in vitro models and in experimental animal tests.
Importantly, the increase in the expression of CXCL1 and other CXCR2 ligands may not be a mechanism of chemoresistance but only a marker of chemoresistance in some cancers associated with the induction of TNF-α expression by chemotherapeutics, e.g., docetaxel [142]. There may be two types of receptors for TNF-α on a tumor cell: tumor necrosis factor receptor (TNFR)1 and TNFR2. TNFR1 has pro-apoptotic properties, while TNFR2 exerts a pro-survival effect, causing the activation of NF-κB and increasing the expression of CXCL1. This means that an increase in CXCL1 expression can be a marker of TNFR2 expression on a tumor cell and decreased TNFR1 expression. In this way, CXCL1 is a marker of the lack of pro-apoptotic effect of TNF-α, whose expression is induced by chemotherapeutics [142].
3.2. Resistance to Radiotherapy
Radiation therapy is currently one of the most significant therapeutic approaches in cancer treatment [143]. It involves the use of ionizing radiation to damage cellular DNA and inhibit the division of rapidly dividing cancer cells, resulting in their destruction. Nevertheless, cancers have developed many radioresistant mechanisms. One such example is the cancer stem cells, which divide infrequently and exhibit an enhanced ability to repair DNA and protect against reactive oxygen species (ROS). Other mechanisms of radioresistance involve growth factors in the tumor microenvironment that have a pro-survival effect, for example, CXCL1, which activates extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) and thus has a pro-survival effect on cancer cells exposed to ionizing radiation [144]. CXCL1 also decreases the expression of superoxide dismutase 1 (SOD1) in cancer cells, as shown by experiments on esophageal squamous cell carcinoma cells, which results in increased levels of ROS in the tumor cell and thus increased activity of DNA damage repair enzymes [144]. Finally, CXCL1 induces the recruitment of neutrophils, which can induce radioresistance, into the tumor niche [145].
CXCL1 expression is at a high level in the tumors of many cancers [34,35,36,37,38,39,40,41,42,43]. An increase in CXCL1 expression in the tumor is also observed under the influence of ionizing radiation, mainly due to an increase in the expression of this chemokine and CXCR2 in cancer-associated fibroblasts (CAF), as shown by experiments on esophageal squamous cell carcinoma [144]. At the same time, the increase in CXCL1 expression may be the result of ionizing radiation. The fact that 1 to 5 centigrays (cGy) increase CXCL1 expression in normal human HFLIII fibroblasts [146] indicates that ionizing radiation may lead to radioresistance. Also, ionizing radiation increases CXCL1 expression in breast cancer cells [109], glioblastoma multiforme cells [108,110,111,112,113], and non-small-cell lung cancer cells [114], resulting in a decreased susceptibility of tumor cells to ionizing radiation. Studies on glioblastoma multiforme cells have shown that the increase in CXCL1 expression by ionizing radiation is casein kinase 1 alpha 1 (CK1α) dependent [113] and dependent on an increase in inhibitor of NF-κBζ (IκBζ) expression [110]. The increase in CXCL1 expression following ionizing radiation can persist for up to 35 days [111]. These effects of ionizing radiation on cancer cells may also be lineage dependent. A study on squamous cell carcinoma of head and neck cells showed that only 1 out of the 16 tested lines showed increased CXCL1 expression under ionizing radiation, and 3 of the 16 tested lines showed decreased expression of this chemokine [147].
Ionizing radiation increases the expression of other CXCR2 ligands, including CXCL2 and CXCL8/IL-8 [108]. The expression of other ligands may be higher than CXCL1. In glioblastoma multiforme cells, there was a much greater increase in CXCL8/IL-8 expression compared to CXCL1 [108].
The increase in CXCL1 expression following radiotherapy induces radioresistance and thus reduces the efficacy of the applied treatment. Also, CXCL1 causes the migration of tumor cells. For this reason, radiotherapy can also induce metastasis and cancer tumor growth, as confirmed by studies on non-small-cell lung cancer A549 cells [114]. Studies on human umbilical vein endothelial cells (HUVEC) have shown that ionizing radiation increases CXCL1 expression in these cells [148], which indicates that radiation therapy can induce tumor angiogenesis and thus tumor growth.
Blood CXCL1 levels after radiotherapy can be used as a diagnostic tool. For example, patients with hepatocellular carcinoma who had low blood CXCL1 levels after stereotactic body radiotherapy were more likely to have liver toxicity 3 months following the therapy [149].
3.3. Resistance to Anti-Vascular Endothelial Growth Factor Therapy
Cancer treatment also involves the use of drugs targeting angiogenesis. The earliest drug developed in anti-angiogenic therapy was bevacizumab, a humanized monoclonal antibody that inactivates vascular endothelial growth factor (VEGF)-A [150]. Currently, this drug has been approved for the treatment of many types of cancer. However, the tumor microenvironment contains several other pro-angiogenic factors which can take over the function of VEGF when it is missing.
These alternative pro-angiogenic factors include CXCL1 and other CXCR2 ligands [17,18,29,30]. CXCL1 also induces the recruitment of endothelial progenitor cells (EPC) into the tumor niche, as shown in experiments on glioblastoma multiforme [151,152,153]. CXCL1 induces the proliferation and formation of new blood vessels by recruiting EPC. In addition, glioblastoma multiforme tumors contain CXCR2+ cancer stem cells [154], cells that exhibit vascular mimicry due to their ability to incorporate into forming blood vessels. For this reason, high CXCL1 expression in the tumor is a factor that enhances resistance to anti-angiogenic therapies. The expression of CXCR2 ligands can also be increased by anti-angiogenic drugs, which abrogates the effect of the anti-angiogenic therapy. An example of this is vatalanib, a tyrosine kinase inhibitor of vascular endothelial growth factor receptor (VEGFR) used in experiments on mice with glioblastoma multiforme tumors [154].
The aforementioned properties of CXCR2 ligands are independent of VEGF and are the source of resistance to anti-angiogenic therapies [153]. For this reason, the use of CXCR2 inhibitors in combination with a drug targeting VEGF yields better results, as shown by experiments on glioblastoma multiforme [155] and ovarian cancer [156].
3.4. Resistance to Immunotherapy
CXCR2 ligands, including CXCL1, play a role in tumor immune evasion. This effect is partly mediated by the recruitment of G-MDSCs to the tumor microenvironment [21,22,23,157], where they exert immunosuppressive functions. CXCL1 also promotes M2 polarization of TAMs [24,25], a phenotype associated with immune suppression. In addition, CXCR2 ligands such as CXCL1 can induce autophagy-driven degradation of MHC class I molecules, as observed in colorectal cancer models [158], reducing the ability of immune cells to recognize and eliminate tumor cells. CXCL1 further contributes to immune evasion by upregulating PD-L1 expression on cancer cells, thereby inhibiting cytotoxic lymphocyte activity [138].
These mechanisms collectively contribute to resistance to immunotherapy. For example, elevated blood levels of CXCL8 in patients with esophageal cancer have been associated with poor outcomes following immunotherapy [159]. Similar findings were reported in biliary tract cancers, where high serum concentrations of CXCL1 and CXCL5 correlated with treatment failure in patients receiving camrelizumab (an anti-PD-1 antibody) in combination with gemcitabine and oxaliplatin [160]. In vivo studies in animal models have shown that neutralizing antibodies targeting CXCR2 ligands can enhance the efficacy of anti-PD-L1 therapy in triple-negative breast cancer [161] and glioma [162].
Several clinical trials are exploring combination therapies that target both PD-L1 and the CXCR2 signaling axis. One such trial is evaluating AZD5069 in combination with durvalumab in patients with advanced HNSCC (ClinicalTrials.gov ID: NCT02499328). Another study tested the combination of navarixin with pembrolizumab in patients with various malignancies [71], but the results were disappointing—navarixin failed to enhance the therapeutic activity of pembrolizumab.
Tumor immune evasion involves multiple redundant pathways and mechanisms; inhibiting a single pathway may not be sufficient to reverse immune suppression. While CXCR2 ligands contribute to this process, they may not be critical for maintaining immune evasion in all tumor types.
4. Side Effect of Chemotherapy
4.1. Metastasis as a Side Effect of Chemotherapy
Drugs used in chemotherapy may not so much treat cancer but also contribute to the development of cancer by inducing the migration of cancer cells and thus metastasis. This has been found in the use of drugs such as topoisomerase inhibitors [163], 5-fluorouracil [125], epidoxorubicin [128], as well as paclitaxel and carboplatin [130]. This effect depends on the type of tumor, as 5-fluorouracil induces metastasis on a 4T1 mouse triple-negative breast cancer cell line model [125] but not on a murine B16-F10 melanoma cell model [130].
The induction of tumor cell migration by chemotherapy drugs is related in part to CXCL1 and other CXCR2 ligands. Different drugs have different mechanisms of action leading to metastasis. 5-fluorouracil activates NF-κB in murine 4T1 triple-negative breast cancer cells, resulting in the increased expression of CXCR2 ligands in the tumor [125]. The same mechanism of increased CXCL1 expression has been observed for epidoxorubicin acting on bladder cancer cells [128]. Also, paclitaxel and carboplatin increase the expression of CXCR2 ligands, as found in a murine B16-F10 melanoma cell model [130]. Anticancer drugs can increase CXCL1 expression independently of NF-κB. Topoisomerase inhibitors increase ROS levels in the cell [163], which leads to the activation of Janus tyrosine kinase 2 (JAK2)-signal transducer and activator of transcription 1 (STAT1) and thus elevated CXCL1 expression, as shown in experiments on many types of cancer [163].
CXCL1, through its receptor CXCR2, induces the activation of Snail, which leads to an epithelial-to-mesenchymal transition (EMT) and the migration of tumor cells [128]. Also, CXCL1 causes the recruitment of neutrophils into the tumor niche; these cells secrete prokineticin-2, which causes tumor cell migration and subsequent metastasis. Anticancer drugs, such as 5-fluorouracil, also increase the expression of CXCR2 ligands in the lung, which results in metastasis in this organ [125].
CXCR2 ligands are an important factor in the process where chemotherapy drugs cause the migration of cancer cells and thus metastasis. For this reason, the use of anticancer drugs in combination with a CXCR2 inhibitor, such as SB265610, prevents the formation of metastasis following treatment [125,130]. It is also important to note that other factors may contribute to therapy-induced metastasis. One example is CCL2, which recruits M-MDSCs to the lungs, promoting the formation of metastases in this organ by breast cancer cells [164].
4.2. Chemotherapy vs. Neuropathy
Chemotherapy induces neuropathy. It is estimated that approximately 30–68% of patients treated for cancer have chemotherapy-induced peripheral neuropathy (CIPN) as a side effect of treatment [165,166]. Drugs that induce this side effect include paclitaxel, platinum compounds, and vinca alkaloids.
CIPN is partly associated with neuroinflammation of neural tissue in the dorsal root ganglia [167]. An important component of CIPN is CXCL1. Studies in mice have shown that paclitaxel increases the levels of CXCR2 ligands in the blood [167] and in the dorsal root ganglion and spinal cord [168]. In part, the expression of CXCR2 ligands in the dorsal root ganglion is caused by the infiltration of this part of the nervous system by macrophages, which produce and secrete those ligands [167]. Infiltration by macrophages is caused by an increase in CC motif chemokine ligand 2 (CCL2)/monocyte chemoattractant protein 1 (MCP-1) expression by neurons in the dorsal root ganglion [169]. CXCR2 receptor ligands, as well as the CXCR2 receptor itself, are important components of CIPN. For this reason, the use of a CXCR2 antagonist (SB225002) negates paclitaxel-induced CIPN [167].
Chemotherapy drugs can also cause neuroinflammation in the brain, resulting in cognitive impairments in treated patients [170]. An example of such a drug is doxorubicin, which in rats caused an increase in the expression of interleukin-6 (IL-6) and CXCR2 ligands (but not TNF-α) in the brain. As a consequence, the rats showed behavioral changes, although it is not known whether this was associated with a change in the expression of CXCR2 ligands in the brain, and if so, which chemokine in the treated animals was responsible for the cognitive impairments.
4.3. Nephrotoxicity of Chemotherapy
Current chemotherapy has many side effects. An example of this is chemotherapy-induced peripheral nephropathy after using paclitaxel, oxaliplatin, or cisplatin. This side effect is estimated to affect 70% of patients treated with such chemotherapeutics [171,172]. In the case of cisplatin, it is related to the accumulation of this drug in the kidneys, more precisely in the proximal and distal tubules [173,174]. Cisplatin damages mitochondria, resulting in the apoptosis of kidney cells. It also causes the activation of NF-κB in kidney cells and expression of pro-inflammatory cytokines in these cells [174], which results in inflammatory reactions in the kidneys that damage these organs.
One of the components of cisplatin’s effect on the kidneys is CXCL1. Cisplatin, through the activation of p38 MAPK and NF-κB, increases CXCL1 expression [175,176]. In particular, an increase in the expression of CXCL1 occurs in endothelial or tubular epithelium cells. Also, the source of CXCL1 in the kidney may be CD4+ T cells [177]. An increase in CXCL1 expression leads to the infiltration of the kidney by neutrophils, which are involved in inflammatory reactions that cause chemotherapy-induced peripheral neuropathy. At the same time, the infiltration of the kidneys by neutrophils during chemotherapy with cisplatin may also be mediated by leukotriene B4 (LTB4) [178].
4.4. Diarrhea as a Side Effect of Chemotherapy
Another side effect of chemotherapy is diarrhea. For example, 5-fluorouracil reduces the expression of aquaporin 4 (AQP4) and aquaporin 8 (AQP8) in the colon [179], which leads to diarrhea. The exact mechanism of this side effect is dependent on CXCR2 ligands, as shown by experiments in mice [93,179,180]. These chemokines induce the recruitment of neutrophils to the colon, where these cells are an important factor in reducing the expression of the aforementioned AQP4 and AQP8.
4.5. Cardiotoxicity as a Side Effect of Chemotherapy
Chemotherapy can also cause cardiotoxicity, for example when using doxorubicin [181]. CXCL1 can be a marker of cardiotoxicity caused by doxorubicin, as shown in a study of breast cancer patients who were treated with this chemotherapeutic agent. Cardiotoxicity was observed in those who had reduced CXCL1 levels following the first cycle of chemotherapy with doxorubicin, relative to pre-treatment levels [181]. Nevertheless, the association between reduced CXCL1 levels and cardiotoxicity is unclear. Patients with heart failure [182] and chronic ischemic heart disease [183] have elevated levels of CXCL1 in their blood, which indicates that increased, not decreased, blood levels of CXCL1 should be a marker of cardiotoxicity.
5. Conclusions
CXCL1 and the broader CXCR2 axis play a key role in tumor progression. They are also implicated in many of the side effects associated with standard chemotherapy and radiotherapy, as well as in resistance to anticancer treatment. A number of compounds targeting CXCL1 or its receptor CXCR2 have already been developed, most of which exhibit low toxicity. Given this, these agents represent promising candidates for combination with conventional cancer therapies. Such an approach should be further investigated for its potential to overcome therapeutic resistance and alleviate treatment-related side effects.
Author Contributions
Conceptualization, J.K. and I.B.-B.; investigation, M.B., M.P., M.K., I.W., P.D. and D.C.; writing—original draft preparation, J.K. and I.B.-B.; writing—review and editing, M.B., I.B.-B. and J.K.; editing: D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the statutory budget of the Department of Biochemistry and Medical Chemistry at Pomeranian Medical University in Szczecin, Poland, and the Institute of Biology, University of Szczecin, Poland.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, S.K.; Murphy, P.M. The CXC Chemokines Growth-regulated Oncogene (GRO) α, GROβ, GROγ, Neutrophil-activating Peptide-2, and Epithelial Cell-derived Neutrophil-activating Peptide-78 Are Potent Agonists for the Type B, but Not the Type A, Human Interleukin-8 Receptor. J. Biol. Chem. 1996, 271, 20545–20550. [Google Scholar] [CrossRef]
- Neote, K.; Mak, J.Y.; Kolakowski, L.F., Jr.; Schall, T.J. Functional and biochemical analysis of the cloned Duffy antigen: Identity with the red blood cell chemokine receptor. Blood 1994, 84, 44–52. [Google Scholar] [CrossRef]
- Dawson, T.C.; Lentsch, A.B.; Wang, Z.; Cowhig, J.E.; Rot, A.; Maeda, N.; Peiper, S.C. Exaggerated response to endotoxin in mice lacking the Duffy antigen/receptor for chemokines (DARC). Blood 2000, 96, 1681–1684. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Varney, M.L.; Singh, R.K. Constitutive expression of growth regulated oncogene (gro) in human colon carcinoma cells with different metastatic potential and its role in regulating their metastatic phenotype. Clin. Exp. Metast. 2005, 21, 571–579. [Google Scholar] [CrossRef]
- Han, D.N.; Zhang, N.; Zhao, S.; Liu, H.; Wang, X.; Yang, M.; Wang, S.; Li, Y.; Liu, Z.; Teng, L. AKIP1 promotes glioblastoma viability, mobility and chemoradiation resistance via regulating CXCL1 and CXCL8 mediated NF-κB and AKT pathways. Am. J. Cancer Res. 2021, 11, 1185–1205. [Google Scholar] [PubMed]
- Sun, J.; Yuan, J. Chemokine (C-X-C motif) ligand 1/chemokine (C-X-C motif) receptor 2 autocrine loop contributes to cellular proliferation, migration and apoptosis in cervical cancer. Bioengineered 2022, 13, 7579–7591. [Google Scholar] [CrossRef]
- Bajetto, A.; Pattarozzi, A.; Corsaro, A.; Barbieri, F.; Daga, A.; Bosio, A.; Gatti, M.; Pisaturo, V.; Sirito, R.; Florio, T. Different Effects of Human Umbilical Cord Mesenchymal Stem Cells on Glioblastoma Stem Cells by Direct Cell Interaction or Via Released Soluble Factors. Front. Cell. Neurosci. 2017, 11, 312. [Google Scholar] [CrossRef]
- Ciummo, S.L.; D’antonio, L.; Sorrentino, C.; Fieni, C.; Lanuti, P.; Stassi, G.; Todaro, M.; Di Carlo, E. The C-X-C Motif Chemokine Ligand 1 Sustains Breast Cancer Stem Cell Self-Renewal and Promotes Tumor Progression and Immune Escape Programs. Front. Cell Dev. Biol. 2021, 9, 689286. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sugimoto, A.; Maruo, K.; Tsujio, G.; Sera, T.; Kushiyama, S.; Nishimura, S.; Kuroda, K.; Togano, S.; Eguchi, S.; et al. CXCR2 signaling might have a tumor-suppressive role in patients with cholangiocarcinoma. PLoS ONE 2022, 17, e0266027. [Google Scholar] [CrossRef]
- Yang, G.; Rosen, D.G.; Liu, G.; Yang, F.; Guo, X.; Xiao, X.; Xue, F.; Mercado-Uribe, I.; Huang, J.; Lin, S.-H.; et al. CXCR2 Promotes Ovarian Cancer Growth through Dysregulated Cell Cycle, Diminished Apoptosis, and Enhanced Angiogenesis. Clin. Cancer Res. 2010, 16, 3875–3886. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lin, F.; Wang, Z.; Yang, L.; Meng, J.; Ou, Z.; Shao, Z.-M.; Di, G.; Yang, G. CXCR2 promotes breast cancer metastasis and chemoresistance via suppression of AKT1 and activation of COX2. Cancer Lett. 2018, 412, 69–80. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Liu, Q.; Bell, R.; Muruve, D.A.; Forsyth, P.; Arcellana-Panlilio, M.; Robbins, S.; Yong, V. The chemokine GRO-α (CXCL1) confers increased tumorigenicity to glioma cells. Carcinogenesis 2005, 26, 2058–2068. [Google Scholar] [CrossRef]
- Kawanishi, H.; Matsui, Y.; Ito, M.; Watanabe, J.; Takahashi, T.; Nishizawa, K.; Nishiyama, H.; Kamoto, T.; Mikami, Y.; Tanaka, Y.; et al. Secreted CXCL1 Is a Potential Mediator and Marker of the Tumor Invasion of Bladder Cancer. Clin. Cancer Res. 2008, 14, 2579–2587. [Google Scholar] [CrossRef]
- Man, X.; Yang, X.; Wei, Z.; Tan, Y.; Li, W.; Jin, H.; Wang, B. High expression level of CXCL1/GROα is linked to advanced stage and worse survival in uterine cervical cancer and facilitates tumor cell malignant processes. BMC Cancer 2022, 22, 712. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Burdick, M.D.; Reckamp, K.; Pantuck, A.; Figlin, R.A.; Strieter, R.M. The Role of CXCR2/CXCR2 Ligand Biological Axis in Renal Cell Carcinoma. J. Immunol. 2005, 175, 5351–5357. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Goodison, S.; Urquidi, V.; Giacoia, E.G.; Rosser, C.J. Expression of CXCL1 in human endothelial cells induces angiogenesis through the CXCR2 receptor and the ERK1/2 and EGF pathways. Mod. Pathol. 2013, 93, 768–778. [Google Scholar] [CrossRef]
- Ma, C.; Liu, G.; Liu, W.; Xu, W.; Li, H.; Piao, S.; Sui, Y.; Feng, W. CXCL1 stimulates decidual angiogenesis via the VEGF-A pathway during the first trimester of pregnancy. Mol. Cell. Biochem. 2021, 476, 2989–2998. [Google Scholar] [CrossRef]
- Tannenbaum, C.S.; Rayman, P.A.; Pavicic, P.G.; Kim, J.S.; Wei, W.; Polefko, A.; Wallace, W.; Rini, B.I.; Morris-Stiff, G.; Allende, D.S.; et al. Mediators of Inflammation-Driven Expansion, Trafficking, and Function of Tumor-Infiltrating MDSCs. Cancer Immunol. Res. 2019, 7, 1687–1699. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, D.; Liu, H.; Ou, X.; Zhang, C.; Zhao, Z.; Zhao, S.; Peng, J.; Cai, S.; He, Y.; et al. PMN-MDSCs accumulation induced by CXCL1 promotes CD8+ T cells exhaustion in gastric cancer. Cancer Lett. 2022, 532, 215598. [Google Scholar] [CrossRef]
- Ma, X.; Aoki, T.; Tsuruyama, T.; Narumiya, S. Definition of Prostaglandin E2–EP2 Signals in the Colon Tumor Microenvironment That Amplify Inflammation and Tumor Growth. Cancer Res. 2015, 75, 2822–2832. [Google Scholar] [CrossRef] [PubMed]
- Nywening, T.M.; Belt, B.A.; Cullinan, D.R.; Panni, R.Z.; Han, B.J.; E Sanford, D.; Jacobs, R.C.; Ye, J.; Patel, A.A.; Gillanders, W.E.; et al. Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 2017, 67, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Dong, B.; Xu, F.; Xu, Y.; Pan, J.; Song, J.; Zhang, J.; Huang, Y.; Xue, W. CXCL1-LCN2 paracrine axis promotes progression of prostate cancer via the Src activation and epithelial-mesenchymal transition. Cell Commun. Signal. 2019, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Di Mitri, D.; Mirenda, M.; Vasilevska, J.; Calcinotto, A.; Delaleu, N.; Revandkar, A.; Gil, V.; Boysen, G.; Losa, M.; Mosole, S.; et al. Re-education of Tumor-Associated Macrophages by CXCR2 Blockade Drives Senescence and Tumor Inhibition in Advanced Prostate Cancer. Cell Rep. 2019, 28, 2156–2168.e5. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, Q.; Kong, L.-Y.; Wang, J.; Yan, J.; Xia, X.; Jia, Z.; Heimberger, A.B.; Li, S. Regulation of tumor immune suppression and cancer cell survival by CXCL1/2 elevation in glioblastoma multiforme. Sci. Adv. 2021, 7, eabc2511. [Google Scholar] [CrossRef]
- Han, X.; Shi, H.; Sun, Y.; Shang, C.; Luan, T.; Wang, D.; Ba, X.; Zeng, X. CXCR2 expression on granulocyte and macrophage progenitors under tumor conditions contributes to mo-MDSC generation via SAP18/ERK/STAT3. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Richmond, A.; Lawson, D.H.; Nixon, D.W.; Chawla, R.K. Characterization of autostimulatory and transforming growth factors from human melanoma cells. Cancer Res. 1985, 45, 6390–6394. [Google Scholar]
- Kuo, P.-L.; Shen, K.-H.; Hung, S.-H.; Hsu, Y.-L. CXCL1/GROα increases cell migration and invasion of prostate cancer by decreasing fibulin-1 expression through NF-κB/HDAC1 epigenetic regulation. Carcinogenesis 2012, 33, 2477–2487. [Google Scholar] [CrossRef]
- Strieter, R.M.; Polverini, P.J.; Kunkel, S.L.; Arenberg, D.A.; Burdick, M.D.; Kasper, J.; Dzuiba, J.; Van Damme, J.; Walz, A.; Marriott, D.; et al. The Functional Role of the ELR Motif in CXC Chemokine-mediated Angiogenesis. J. Biol. Chem. 1995, 270, 27348–27357. [Google Scholar] [CrossRef]
- Addison, C.L.; Daniel, T.O.; Burdick, M.D.; Liu, H.; Ehlert, J.E.; Xue, Y.Y.; Buechi, L.; Walz, A.; Richmond, A.; Strieter, R.M. The CXC Chemokine Receptor 2, CXCR2, Is the Putative Receptor for ELR+ CXC Chemokine-Induced Angiogenic Activity. J. Immunol. 2000, 165, 5269–5277. [Google Scholar] [CrossRef]
- Highfill, S.L.; Cui, Y.; Giles, A.J.; Smith, J.P.; Zhang, H.; Morse, E.; Kaplan, R.N.; Mackall, C.L. Disruption of CXCR2-Mediated MDSC Tumor Trafficking Enhances Anti-PD1 Efficacy. Sci. Transl. Med. 2014, 6, 237ra67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, C.; Duan, Y.; Heinrich, B.; Rosato, U.; Diggs, L.P.; Ma, L.; Roy, S.; Fu, Q.; Brown, Z.J.; et al. Gut Microbiome Directs Hepatocytes to Recruit MDSCs and Promote Cholangiocarcinoma. Cancer Discov. 2020, 11, 1248–1267. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, L.; Yan, J.; Zhen, Z.-J.; Ji, Y.; Liu, C.-Q.; Lau, W.Y.; Zheng, L.; Xu, J. CXCR2–CXCL1 axis is correlated with neutrophil infiltration and predicts a poor prognosis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 129. [Google Scholar] [CrossRef]
- Haqq, C.; Nosrati, M.; Sudilovsky, D.; Crothers, J.; Khodabakhsh, D.; Pulliam, B.L.; Federman, S.; Miller, J.R.; Allen, R.E.; Singer, M.I.; et al. The gene expression signatures of melanoma progression. Proc. Natl. Acad. Sci. USA 2005, 102, 6092–6097. [Google Scholar] [CrossRef]
- Cao, Z.; Fu, B.; Deng, B.; Zeng, Y.; Wan, X.; Qu, L. Overexpression of Chemokine (C-X-C) ligand 1 (CXCL1) associated with tumor progression and poor prognosis in hepatocellular carcinoma. Cancer Cell Int. 2014, 14, 1–7. [Google Scholar] [CrossRef]
- Chen, X.; Jin, R.; Chen, R.; Huang, Z. Complementary action of CXCL1 and CXCL8 in pathogenesis of gastric carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 1036–1045. [Google Scholar]
- Li, W.; Ma, J.-A.; Sheng, X.; Xiao, C. Screening of CXC chemokines in the microenvironment of ovarian cancer and the biological function of CXCL10. World J. Surg. Oncol. 2021, 19, 1–16. [Google Scholar] [CrossRef]
- Yang, X.; Wei, Y.; Sheng, F.; Xu, Y.; Liu, J.; Gao, L.; Yang, J.; Sun, X.; Huang, J.; Guo, Q. Comprehensive analysis of the prognosis and immune infiltration for CXC chemokines in colorectal cancer. Aging 2021, 13, 17548–17567. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, Q.; Zhang, L.; Chen, J.; Zhang, X. Exploration of prognostic biomarkers and therapeutic targets in the microenvironment of bladder cancer based on CXC chemokines. Math. Biosci. Eng. 2021, 18, 6262–6287. [Google Scholar] [CrossRef]
- Unver, N. Identification of the dominant angiogenic CXCL class chemokines associated with non-small cell lung cancer via bioinformatics tools. Med. Oncol. 2021, 38, 1–11. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, C.; Wu, H.-Z.; Liu, B.; Yang, Y.-F. Bioinformatics, Molecular Docking and Experiments In Vitro Analyze the Prognostic Value of CXC Chemokines in Breast Cancer. Front. Oncol. 2021, 11, 665080. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Niu, T.; Li, Z. MicroRNA miR-145-5p regulates cell proliferation and cell migration in colon cancer by inhibiting chemokine (C-X-C motif) ligand 1 and integrin α2. Bioengineered 2021, 12, 9909–9917. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Molczyk, C.; Purohit, A.; Ehrhorn, E.; Goel, P.; Prajapati, D.R.; Atri, P.; Kaur, S.; Grandgenett, P.M.; Hollingsworth, M.A.; et al. Differential expression profile of CXC-receptor-2 ligands as potential biomarkers in pancreatic ductal adenocarcinoma. Am. J. Cancer Res. 2022, 12, 68–90. [Google Scholar] [PubMed]
- Miyake, M.; Furuya, H.; Onishi, S.; Hokutan, K.; Anai, S.; Chan, O.; Shi, S.; Fujimoto, K.; Goodison, S.; Cai, W.; et al. Monoclonal Antibody against CXCL1 (HL2401) as a Novel Agent in Suppressing IL6 Expression and Tumoral Growth. Theranostics 2019, 9, 853–867. [Google Scholar] [CrossRef]
- White, J.R.; Lee, J.M.; Young, P.R.; Hertzberg, R.P.; Jurewicz, A.J.; Chaikin, M.A.; Widdowson, K.; Foley, J.J.; Martin, L.D.; Griswold, D.E.; et al. Identification of a Potent, Selective Non-peptide CXCR2 Antagonist That Inhibits Interleukin-8-induced Neutrophil Migration. J. Biol. Chem. 1998, 273, 10095–10098. [Google Scholar] [CrossRef]
- Acker, G.; Zollfrank, J.; Jelgersma, C.; Nieminen-Kelhä, M.; Kremenetskaia, I.; Mueller, S.; Ghori, A.; Vajkoczy, P.; Brandenburg, S. The CXCR2/CXCL2 signalling pathway—An alternative therapeutic approach in high-grade glioma. Eur. J. Cancer 2020, 126, 106–115. [Google Scholar] [CrossRef]
- Xu, M.; Jaing, H.; Wang, H.; Liu, J.; Liu, B.; Guo, Z. SB225002 inhibits prostate cancer invasion and attenuates the expression of BSP, OPN and MMP-2. Oncol. Rep. 2018, 40, 726–736. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, S.-J.; Kang, K.-W.; Lee, B.-H.; Park, Y.; Kim, B.-S. CXCR2, a novel target to overcome tyrosine kinase inhibitor resistance in chronic myelogenous leukemia cells. Biochem. Pharmacol. 2021, 190, 114658. [Google Scholar] [CrossRef]
- Liu, X.; Lan, T.; Mo, F.; Yang, J.; Wei, Y.; Wei, X. Antitumor and Radiosensitization Effects of a CXCR2 Inhibitor in Nasopharyngeal Carcinoma. Front. Cell Dev. Biol. 2021, 9, 689613. [Google Scholar] [CrossRef]
- Romanini, J.; Mielcke, T.R.; Leal, P.C.; Figueiredo, C.P.; Calixto, J.B.; Morrone, F.B.; Batista, E.L.; Campos, M.M. The role of CXCR2 chemokine receptors in the oral squamous cell carcinoma. Investig. New Drugs 2011, 30, 1371–1378. [Google Scholar] [CrossRef]
- Jeong, Y.; Yoon, S.Y.; Jung, S.P.; Nam, S.J.; Lee, J.E.; Kim, S. Inhibition of IL-8/CXCR2 signaling axis prevents tumor growth and metastasis in triple negative breast cancer cells. Pharmacology 2025, 1–17. [Google Scholar] [CrossRef]
- Du, M.; Qiu, Q.; Gruslin, A.; Gordon, J.; He, M.; Chan, C.C.; Li, D.; Tsang, B.K. SB225002 Promotes Mitotic Catastrophe in Chemo-Sensitive and -Resistant Ovarian Cancer Cells Independent of p53 Status In Vitro. PLoS ONE 2013, 8, e54572. [Google Scholar] [CrossRef]
- Sueoka, H.; Hirano, T.; Uda, Y.; Iimuro, Y.; Yamanaka, J.; Fujimoto, J. Blockage of CXCR2 suppresses tumor growth of intrahepatic cholangiocellular carcinoma. Surgery 2014, 155, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Goda, A.E.; Koyama, M.; Sowa, Y.; Elokely, K.M.; Yoshida, T.; Kim, B.-Y.; Sakai, T. Molecular mechanisms of the antitumor activity of SB225002: A novel microtubule inhibitor. Biochem. Pharmacol. 2013, 85, 1741–1752. [Google Scholar] [CrossRef]
- Goda, A.E.; Sakai, T. Molecular insights into the microtubules depolymerizing activity of the IL-8 receptor B antagonist SB225002. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3726–3734. [Google Scholar] [PubMed]
- Nicholls, D.J.; Wiley, K.; Dainty, I.; MacIntosh, F.; Phillips, C.; Gaw, A.; Mårdh, C.K. Pharmacological Characterization of AZD5069, a Slowly Reversible CXC Chemokine Receptor 2 Antagonist. J. Pharmacol. Exp. Ther. 2015, 353, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Greco, A.; Petrosino, E.; Croce, L.; Teliti, M.; Marchesi, N.; Pascale, A.; Calì, B.; Pignatti, P.; Magri, F.; et al. Selective anti-CXCR2 receptor blockade by AZD5069 inhibits CXCL8-mediated pro-tumorigenic activity in human thyroid cancer cells in vitro. J. Endocrinol. Investig. 2024, 48, 53–65. [Google Scholar] [CrossRef]
- Ghallab, A.M.; Eissa, R.A.; El Tayebi, H.M. CXCR2 Small-Molecule Antagonist Combats Chemoresistance and Enhances Immunotherapy in Triple-Negative Breast Cancer. Front. Pharmacol. 2022, 13, 862125. [Google Scholar] [CrossRef]
- Kwong, T.T.; Xiong, Z.; Zhang, Y.; Wu, H.; Cao, J.; Wong, P.P.-C.; Liu, X.; Wang, J.; Wong, C.H.; Tse, G.M.-K.; et al. Overcoming immunotherapy resistance in hepatocellular carcinoma by targeting myeloid IL-8/CXCR2 signaling. Mol. Ther. 2025, 33, 1659–1673. [Google Scholar] [CrossRef]
- Cullberg, M.; Arfvidsson, C.; Larsson, B.; Malmgren, A.; Mitchell, P.; Hamrén, U.W.; Wray, H. Pharmacokinetics of the Oral Selective CXCR2 Antagonist AZD5069: A Summary of Eight Phase I Studies in Healthy Volunteers. Drugs RD 2018, 18, 149–159. [Google Scholar] [CrossRef]
- Kirsten, A.; Förster, K.; Radeczky, E.; Linnhoff, A.; Balint, B.; Watz, H.; Wray, H.; Salkeld, L.; Cullberg, M.; Larsson, B. The safety and tolerability of oral AZD5069, a selective CXCR2 antagonist, in patients with moderate-to-severe COPD. Pulm. Pharmacol. Ther. 2015, 31, 36–41. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, P.M.; Metev, H.; Puu, M.; Richter, K.; Keen, C.; Uddin, M.; Larsson, B.; Cullberg, M.; Nair, P. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2016, 4, 797–806. [Google Scholar] [CrossRef] [PubMed]
- De Soyza, A.; Pavord, I.; Elborn, J.S.; Smith, D.; Wray, H.; Puu, M.; Larsson, B.; Stockley, R. A randomised, placebo-controlled study of the CXCR2 antagonist AZD5069 in bronchiectasis. Eur. Respir. J. 2015, 46, 1021–1032. [Google Scholar] [CrossRef]
- Singh, S.; Sadanandam, A.; Nannuru, K.C.; Varney, M.L.; Mayer-Ezell, R.; Bond, R.; Singh, R.K. Small-Molecule Antagonists for CXCR2 and CXCR1 Inhibit Human Melanoma Growth by Decreasing Tumor Cell Proliferation, Survival, and Angiogenesis. Clin. Cancer Res. 2009, 15, 2380–2386. [Google Scholar] [CrossRef] [PubMed]
- Varney, M.L.; Singh, S.; Li, A.; Mayer-Ezell, R.; Bond, R.; Singh, R.K. Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases. Cancer Lett. 2011, 300, 180–188. [Google Scholar] [CrossRef]
- Dwyer, M.P.; Yu, Y.; Chao, J.; Aki, C.; Chao, J.; Biju, P.; Girijavallabhan, V.; Rindgen, D.; Bond, R.; Mayer-Ezel, R.; et al. Discovery of 2-Hydroxy-N,N-dimethyl-3-{2-[[(R)-1-(5- methylfuran-2-yl)propyl]amino]-3,4-dioxocyclobut-1-enylamino}benzamide (SCH 527123): A Potent, Orally Bioavailable CXCR2/CXCR1 Receptor Antagonist. J. Med. Chem. 2006, 49, 7603–7606. [Google Scholar] [CrossRef]
- Gonsiorek, W.; Fan, X.; Hesk, D.; Fossetta, J.; Qiu, H.; Jakway, J.; Billah, M.; Dwyer, M.; Chao, J.; Deno, G.; et al. Pharmacological Characterization of Sch527123, a Potent Allosteric CXCR1/CXCR2 Antagonist. J. Pharmacol. Exp. Ther. 2007, 322, 477–485. [Google Scholar] [CrossRef]
- Leser, F.S.; Júnyor, F.d.S.; Pagnoncelli, I.B.; Delgado, A.B.; Medeiros, I.; Nóbrega, A.C.C.; Andrade, B.d.S.; de Lima, M.N.; da Silva, N.E.; Jacob, L.; et al. CCL21-CCR7 blockade prevents neuroinflammation and degeneration in Parkinson’s disease models. J. Neuroinflamm. 2025, 22, 1–23. [Google Scholar] [CrossRef]
- Ning, Y.; Labonte, M.J.; Zhang, W.; Bohanes, P.O.; Gerger, A.; Yang, D.; Benhaim, L.; Paez, D.; Rosenberg, D.O.; Venkata, K.C.N.; et al. The CXCR2 Antagonist, SCH-527123, Shows Antitumor Activity and Sensitizes Cells to Oxaliplatin in Preclinical Colon Cancer Models. Mol. Cancer Ther. 2012, 11, 1353–1364. [Google Scholar] [CrossRef]
- Shang, F.-M.; Li, J. A small-molecule antagonist of CXCR1 and CXCR2 inhibits cell proliferation, migration and invasion in melanoma via PI3K/AKT pathway. Med. Clin. 2018, 152, 425–430. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Geva, R.; Chung, H.C.; Lemech, C.; Miller, W.H.; Hansen, A.R.; Lee, J.-S.; Tsai, F.; Solomon, B.J.; Kim, T.M.; et al. CXCR2 antagonist navarixin in combination with pembrolizumab in select advanced solid tumors: A phase 2 randomized trial. Investig. New Drugs 2024, 42, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; De Giovanni, M.; Xu, Y.; An, J.; Kirthivasan, N.; Lu, E.; Jiang, K.; Brooks, S.; Ranucci, S.; Yang, J.; et al. Inflammation switches the chemoattractant requirements for naive lymphocyte entry into lymph nodes. Cell 2024, 188, 1019–1035.e22. [Google Scholar] [CrossRef]
- Garau, A.; Bertini, R.; Mosca, M.; Bizzarri, C.; Anacardio, R.; Triulzi, S.; Allegretti, M.; Ghezzi, P.; Villa, P. Development of a systemically-active dual CXCR1/CXCR2 allosteric inhibitor and its efficacy in a model of transient cerebral ischemia in the rat. Eur Cytokine Netw. 2006, 17, 35–41. [Google Scholar]
- Bertini, R.; Barcelos, L.; Beccari, A.; Cavalieri, B.; Moriconi, A.; Bizzarri, C.; Di Benedetto, P.; Di Giacinto, C.; Gloaguen, I.; Galliera, E.; et al. Receptor binding mode and pharmacological characterization of a potent and selective dual CXCR1/CXCR2 non-competitive allosteric inhibitor. Br. J. Pharmacol. 2011, 165, 436–454. [Google Scholar] [CrossRef]
- Kemp, D.M.; Pidich, A.; Larijani, M.; Jonas, R.; Lash, E.; Sato, T.; Terai, M.; De Pizzol, M.; Allegretti, M.; Igoucheva, O.; et al. Ladarixin, a dual CXCR1/2 inhibitor, attenuates experimental melanomas harboring different molecular defects by affecting malignant cells and tumor microenvironment. Oncotarget 2017, 8, 14428–14442. [Google Scholar] [CrossRef]
- Piro, G.; Carbone, C.; Agostini, A.; Esposito, A.; De Pizzol, M.; Novelli, R.; Allegretti, M.; Aramini, A.; Caggiano, A.; Granitto, A.; et al. CXCR1/2 dual-inhibitor ladarixin reduces tumour burden and promotes immunotherapy response in pancreatic cancer. Br. J. Cancer 2022, 128, 331–341. [Google Scholar] [CrossRef]
- Piemonti, L.; Keymeulen, B.; Gillard, P.; Linn, T.; Bosi, E.; Rose, L.; Pozzilli, P.; Giorgino, F.; Cossu, E.; Daffonchio, L.; et al. Ladarixin, an inhibitor of the interleukin-8 receptors CXCR1 and CXCR2, in new-onset type 1 diabetes: A multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 2022, 24, 1840–1849. [Google Scholar] [CrossRef] [PubMed]
- Sordi, V.; Monti, P.; Lampasona, V.; Melzi, R.; Pellegrini, S.; Keymeulen, B.; Gillard, P.; Linn, T.; Bosi, E.; Rose, L.; et al. Post hoc analysis of a randomized, double-blind, prospective trial evaluating a CXCR1/2 inhibitor in new-onset type 1 diabetes: Endo-metabolic features at baseline identify a subgroup of responders. Front. Endocrinol. 2023, 14, 1175640. [Google Scholar] [CrossRef] [PubMed]
- Bertini, R.; Allegretti, M.; Bizzarri, C.; Moriconi, A.; Locati, M.; Zampella, G.; Cervellera, M.N.; Di Cioccio, V.; Cesta, M.C.; Galliera, E.; et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: Prevention of reperfusion injury. Proc. Natl. Acad. Sci. USA 2004, 101, 11791–11796. [Google Scholar] [CrossRef]
- Casilli, F.; Bianchini, A.; Gloaguen, I.; Biordi, L.; Alesse, E.; Festuccia, C.; Cavalieri, B.; Strippoli, R.; Cervellera, M.N.; Di Bitondo, R.; et al. Inhibition of interleukin-8 (CXCL8/IL-8) responses by repertaxin, a new inhibitor of the chemokine receptors CXCR1 and CXCR2. Biochem. Pharmacol. 2005, 69, 385–394. [Google Scholar] [CrossRef]
- Liotti, F.; De Pizzol, M.; Allegretti, M.; Prevete, N.; Melillo, R.M. Multiple anti-tumor effects of Reparixin on thyroid cancer. Oncotarget 2017, 8, 35946–35961. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Chen, X.; Lin, H.-J.; Lin, J. Inhibition of interleukin 8/C-X-C chemokine receptor�1,/2 signaling reduces malignant features in human pancreatic cancer cells. Int. J. Oncol. 2018, 53, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, L.; Cristiano, L.; Fidoamore, A.; De Pizzol, M.; Di Giacomo, E.; Florio, T.M.; Confalone, G.; Galante, A.; Cinque, B.; Benedetti, E.; et al. Targeting CXCR1 on breast cancer stem cells: Signaling pathways and clinical application modelling. Oncotarget 2015, 6, 43375–43394. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.J.; Perez, R.P.; Yardley, D.; Han, L.K.; Reuben, J.M.; Gao, H.; McCanna, S.; Butler, B.; Ruffini, P.A.; Liu, Y.; et al. A window-of-opportunity trial of the CXCR1/2 inhibitor reparixin in operable HER-2-negative breast cancer. Breast Cancer Res. 2020, 22, 1–9. [Google Scholar]
- Schott, A.F.; Goldstein, L.J.; Cristofanilli, M.; Ruffini, P.A.; McCanna, S.; Reuben, J.M.; Perez, R.P.; Kato, G.; Wicha, M. Phase Ib Pilot Study to Evaluate Reparixin in Combination with Weekly Paclitaxel in Patients with HER-2–Negative Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5358–5365. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Mansutti, M.; Levy, C.; Chang, J.C.; Henry, S.; Fernandez-Perez, I.; Prausovà, J.; Staroslawska, E.; Viale, G.; Butler, B.; et al. A randomized, placebo-controlled phase 2 study of paclitaxel in combination with reparixin compared to paclitaxel alone as front-line therapy for metastatic triple-negative breast cancer (fRida). Breast Cancer Res. Treat. 2021, 190, 265–275. [Google Scholar] [CrossRef]
- Boyles, J.S.; Beidler, C.B.; Strifler, B.A.; Girard, D.S.; Druzina, Z.; Durbin, J.D.; Swearingen, M.L.; Lee, L.N.; Kikly, K.; Chintharlapalli, S.; et al. Discovery and characterization of a neutralizing pan-ELR plus CXC chemokine monoclonal antibody. mAbs 2020, 12, 1831880. [Google Scholar] [CrossRef]
- Roby, P.; Page, M. Melanoma-specific cytotoxicity of a human MGSA/GRO alpha C-terminal peptide conjugated to daunorubicin. Oncol. Rep. 1996, 3, 175–179. [Google Scholar] [CrossRef]
- Ohms, M.; Möller, S.; Laskay, T. An Attempt to Polarize Human Neutrophils Toward N1 and N2 Phenotypes in vitro. Front. Immunol. 2020, 11, 532. [Google Scholar] [CrossRef]
- Seignez, C.; Martin, A.; Rollet, C.-E.; Racoeur, C.; Scagliarini, A.; Jeannin, J.-F.; Bettaieb, A.; Paul, C. Senescence of tumor cells induced by oxaliplatin increases the efficiency of a lipid A immunotherapy via the recruitment of neutrophils. Oncotarget 2014, 5, 11442–11451. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Mohrenz, I.V.; Mirisola, V.; Schleicher, E.; Romeo, F.; Höhneke, C.; Jochum, M.; Nerlich, A.G.; Pfeffer, U. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFκB. Carcinogenesis 2007, 29, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Kronski, E.; Fiori, M.E.; Barbieri, O.; Astigiano, S.; Mirisola, V.; Killian, P.H.; Bruno, A.; Pagani, A.; Rovera, F.; Pfeffer, U.; et al. miR181b is induced by the chemopreventive polyphenol curcumin and inhibits breast cancer metastasis via down-regulation of the inflammatory cytokines CXCL1 and -2. Mol. Oncol. 2014, 8, 581–595. [Google Scholar] [CrossRef]
- Sakai, H.; Kai, Y.; Oguchi, A.; Kimura, M.; Tabata, S.; Yaegashi, M.; Saito, T.; Sato, K.; Sato, F.; Yumoto, T.; et al. Curcumin Inhibits 5-Fluorouracil-induced Up-regulation of CXCL1 and CXCL2 of the Colon Associated with Attenuation of Diarrhoea Development. Basic Clin. Pharmacol. Toxicol. 2016, 119, 540–547. [Google Scholar] [CrossRef]
- Wang, P.; Song, D.; Wan, D.; Li, L.; Mei, W.; Li, X.; Han, L.; Zhu, X.; Yang, L.; Cai, Y.; et al. Ginsenoside panaxatriol reverses TNBC paclitaxel resistance by inhibiting the IRAK1/NF-κB and ERK pathways. PeerJ. 2020, 8, e9281. [Google Scholar] [CrossRef]
- Wang, N.; Yang, B.; Zhang, J.; Zheng, Y.; Wang, S.; Zhang, X.; Situ, H.; Lin, Y.; Wang, Z. Metabolite profiling of traditional Chinese medicine XIAOPI formula: An integrated strategy based on UPLC-Q-Orbitrap MS combined with network pharmacology analysis. Biomed. Pharmacother. 2020, 121, 109569. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Huang, R.; Zheng, Y.; Wang, N.; Yang, B.; Situ, H.; Lin, Y.; Wang, Z. XIAOPI Formula Inhibits Breast Cancer Stem Cells via Suppressing Tumor-Associated Macrophages/C-X-C Motif Chemokine Ligand 1 Pathway. Front. Pharmacol. 2019, 10, 1371. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, N.; Wang, S.; Yang, B.; Situ, H.; Zhong, L.; Lin, Y.; Wang, Z. XIAOPI formula inhibits the pre-metastatic niche formation in breast cancer via suppressing TAMs/CXCL1 signaling. Cell Commun. Signal. 2020, 18, 1–17. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, Y.; Gu, J.; Cai, Y.; Wang, S.; Zhang, F.; Chen, J.; Situ, H.; Lin, Y.; Wang, Z. Network-pharmacology-based validation of TAMS/CXCL-1 as key mediator of XIAOPI formula preventing breast cancer development and metastasis. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Wang, N.; Yang, B.; Muhetaer, G.; Wang, S.; Zheng, Y.; Lu, J.; Li, M.; Zhang, F.; Situ, H.; Lin, Y.; et al. XIAOPI formula promotes breast cancer chemosensitivity via inhibiting CXCL1/HMGB1-mediated autophagy. Biomed. Pharmacother. 2019, 120, 109519. [Google Scholar] [CrossRef]
- Met, Ö.; Jensen, K.M.; Chamberlain, C.A.; Donia, M.; Svane, I.M. Principles of adoptive T cell therapy in cancer. Semin. Immunopathol. 2018, 41, 49–58. [Google Scholar] [CrossRef]
- Kershaw, M.H.; Wang, G.; Westwood, J.A.; Pachynski, R.K.; Tiffany, H.L.; Marincola, F.M.; Wang, E.; Young, H.A.; Murphy, P.M.; Hwu, P. Redirecting Migration of T Cells to Chemokine Secreted from Tumors by Genetic Modification with CXCR2. Hum. Gene Ther. 2002, 13, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Idorn, M.; Olsen, M.; Halldórsdóttir, H.R.; Skadborg, S.K.; Pedersen, M.; Høgdall, C.; Høgdall, E.; Met, Ö.; Straten, P.T. Improved migration of tumor ascites lymphocytes to ovarian cancer microenvironment by CXCR2 transduction. OncoImmunology 2017, 7, e1412029. [Google Scholar] [CrossRef] [PubMed]
- Idorn, M.; Skadborg, S.K.; Kellermann, L.; Halldórsdóttir, H.R.; Olofsson, G.H.; Met, Ö.; Straten, P.T. Chemokine receptor engineering of T cells with CXCR2 improves homing towards subcutaneous human melanomas in xenograft mouse model. OncoImmunology 2018, 7, e1450715. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Rui, W.; Zheng, H.; Huang, D.; Yu, F.; Zhang, Y.; Dong, J.; Zhao, X.; Lin, X. CXCR2-modified CAR-T cells have enhanced trafficking ability that improves treatment of hepatocellular carcinoma. Eur. J. Immunol. 2020, 50, 712–724. [Google Scholar] [CrossRef]
- Zeng, Q.; Sun, S.; Li, Y.; Li, X.; Li, Z.; Liang, H. Identification of Therapeutic Targets and Prognostic Biomarkers Among CXC Chemokines in the Renal Cell Carcinoma Microenvironment. Front. Oncol. 2020, 9, 1555. [Google Scholar] [CrossRef]
- Ignacio, R.M.C.; Gibbs, C.R.; Lee, E.-S.; Son, D.-S. The TGFα-EGFR-Akt signaling axis plays a role in enhancing proinflammatory chemokines in triple-negative breast cancer cells. Oncotarget 2018, 9, 29286–29303. [Google Scholar] [CrossRef]
- Peng, W.; Ye, Y.; Rabinovich, B.A.; Liu, C.; Lou, Y.; Zhang, M.; Whittington, M.; Yang, Y.; Overwijk, W.W.; Lizée, G.; et al. Transduction of Tumor-Specific T Cells with CXCR2 Chemokine Receptor Improves Migration to Tumor and Antitumor Immune Responses. Clin. Cancer Res. 2010, 16, 5458–5468. [Google Scholar] [CrossRef]
- Jin, L.; Tao, H.; Karachi, A.; Long, Y.; Hou, A.Y.; Na, M.; Dyson, K.A.; Grippin, A.J.; Deleyrolle, L.P.; Zhang, W.; et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Sprung, C.N.; Yang, Y.; Forrester, H.B.; Li, J.; Zaitseva, M.; Cann, L.; Restall, T.; Anderson, R.L.; Crosbie, J.C.; Rogers, P.A.W. Genome-Wide Transcription Responses to Synchrotron Microbeam Radiotherapy. Radiat. Res. 2012, 178, 249–259. [Google Scholar] [CrossRef]
- Brennenstuhl, H.; Armento, A.; Braczysnki, A.K.; Mittelbronn, M.; Naumann, U. IκBζ, an atypical member of the inhibitor of nuclear factor kappa B family, is induced by γ-irradiation in glioma cells, regulating cytokine secretion and associated with poor prognosis. Int. J. Oncol. 2015, 47, 1971–1980. [Google Scholar] [CrossRef]
- McDonald, J.T.; Gao, X.; Steber, C.; Breed, J.L.; Pollock, C.; Ma, L.; Hlatky, L. Host mediated inflammatory influence on glioblastoma multiforme recurrence following high-dose ionizing radiation. PLoS ONE 2017, 12, e0178155. [Google Scholar] [CrossRef]
- Alafate, W.; Li, X.; Zuo, J.; Zhang, H.; Xiang, J.; Wu, W.; Xie, W.; Bai, X.; Wang, M.; Wang, J. Elevation of CXCL1 indicates poor prognosis and radioresistance by inducing mesenchymal transition in glioblastoma. CNS Neurosci. Ther. 2020, 26, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, H.; Zhang, W.; Yu, J.; Zhang, X.; Wu, R.; Niu, M.; Liu, X.; Yu, R. Csnk1a1 inhibition modulates the inflammatory secretome and enhances response to radiotherapy in glioma. J. Cell. Mol. Med. 2021, 25, 7395–7406. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, D.-M.; Han, R.; Yu, Y.; Deng, S.-H.; Liu, T.; Zhang, T.; Xu, Y. Low-Dose Radiation Promotes Invasion and Migration of A549 Cells by Activating the CXCL1/NF-κB Signaling Pathway. OncoTargets Ther. 2020, 13, 3619–3629. [Google Scholar] [CrossRef]
- Kremer, V.; Ligtenberg, M.A.; Zendehdel, R.; Seitz, C.; Duivenvoorden, A.; Wennerberg, E.; Colón, E.; Scherman-Plogell, A.-H.; Lundqvist, A. Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. J. Immunother. Cancer 2017, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Novitskiy, S.V.; Pickup, M.W.; Gorska, A.E.; Owens, P.; Chytil, A.; Aakre, M.; Wu, H.; Shyr, Y.; Moses, H.L. TGF-β Receptor II Loss Promotes Mammary Carcinoma Progression by Th17-Dependent Mechanisms. Cancer Discov. 2011, 1, 430–441. [Google Scholar] [CrossRef]
- Zhu, H.; Gu, Y.; Xue, Y.; Yuan, M.; Cao, X.; Liu, Q. CXCR2+ MDSCs promote breast cancer progression by inducing EMT and activated T cell exhaustion. Oncotarget 2017, 8, 114554–114567. [Google Scholar] [CrossRef]
- Sun, L.; Clavijo, P.E.; Robbins, Y.; Patel, P.; Friedman, J.; Greene, S.; Das, R.; Silvin, C.; Van Waes, C.; Horn, L.A.; et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. J. Clin. Investig. 2019, 4, e126853. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, C.Y.; Gao, J.; Wang, Z. Recent advances in photodynamic therapy for cancer and infectious diseases. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1560. [Google Scholar] [CrossRef]
- Zulaziz, N.; Azhim, A.; Himeno, N.; Tanaka, M.; Satoh, Y.; Kinoshita, M.; Miyazaki, H.; Saitoh, D.; Shinomiya, N.; Morimoto, Y. Photodynamic therapy mediates innate immune responses via fibroblast–macrophage interactions. Hum. Cell 2015, 28, 159–166. [Google Scholar] [CrossRef]
- Gollnick, S.O.; Evans, S.S.; Baumann, H.; Owczarczak, B.; Maier, P.; Vaughan, L.; Wang, W.C.; Unger, E.; Henderson, B.W. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br. J. Cancer 2003, 88, 1772–1779. [Google Scholar] [CrossRef]
- Brackett, C.M.; Muhitch, J.B.; Evans, S.S.; O Gollnick, S. IL-17 Promotes Neutrophil Entry into Tumor-Draining Lymph Nodes following Induction of Sterile Inflammation. J. Immunol. 2013, 191, 4348–4357. [Google Scholar] [CrossRef]
- Han, X.-L.; Du, J.; Zheng, Y.-D.; Dai, J.-J.; Lin, S.-W.; Zhang, B.-Y.; Zhong, F.-B.; Lin, Z.-G.; Jiang, S.-Q.; Wei, W.; et al. CXCL1 Clone Evolution Induced by the HDAC Inhibitor Belinostat Might Be a Favorable Prognostic Indicator in Triple-Negative Breast Cancer. BioMed Res. Int. 2021, 2021, 5089371. [Google Scholar] [CrossRef] [PubMed]
- Taxman, D.J.; MacKeigan, J.P.; Clements, C.; Bergstralh, D.T.; Ting, J.P.-Y. Transcriptional profiling of targets for combination therapy of lung carcinoma with paclitaxel and mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor. Cancer Res. 2003, 63, 5095–5104. [Google Scholar]
- Sasaki, S.; Baba, T.; Muranaka, H.; Tanabe, Y.; Takahashi, C.; Matsugo, S.; Mukaida, N. Involvement of Prokineticin 2–expressing Neutrophil Infiltration in 5-Fluorouracil–induced Aggravation of Breast Cancer Metastasis to Lung. Mol. Cancer Ther. 2018, 17, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- de Porras, V.R.; Bystrup, S.; Martínez-Cardús, A.; Pluvinet, R.; Sumoy, L.; Howells, L.; James, M.I.; Iwuji, C.; Manzano, J.L.; Layos, L.; et al. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-κB signalling pathway. Sci. Rep. 2016, 6, 24675. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Purcell, C.; Seaton, A.; Oladipo, O.; Maxwell, P.J.; O’sullivan, J.M.; Wilson, R.H.; Johnston, P.G.; Waugh, D.J.J. Chemotherapy-Induced CXC-Chemokine/CXC-Chemokine Receptor Signaling in Metastatic Prostate Cancer Cells Confers Resistance to Oxaliplatin through Potentiation of Nuclear Factor-κB Transcription and Evasion of Apoptosis. J. Pharmacol. Exp. Ther. 2008, 327, 746–759. [Google Scholar] [CrossRef]
- Chen, L.; Pan, X.-W.; Huang, H.; Gao, Y.; Yang, Q.-W.; Wang, L.-H.; Cui, X.-G.; Xu, D.-F. Epithelial-mesenchymal transition induced by GRO-α-CXCR2 promotes bladder cancer recurrence after intravesical chemotherapy. Oncotarget 2017, 8, 45274–45285. [Google Scholar] [CrossRef]
- Dalmases, A.; González, I.; Menendez, S.; Arpí, O.; Corominas, J.M.; Servitja, S.; Tusquets, I.; Chamizo, C.; Rincón, R.; Espinosa, L.; et al. Deficiency in p53 is required for doxorubicin induced transcriptional activation of NF-κB target genes in human breast cancer. Oncotarget 2013, 5, 196–210. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Y.; Qi, F.; Jia, L.; Lu, X.; He, T.; Fu, Y.; Li, L.; Luo, Y. Specific chemotherapeutic agents induce metastatic behaviour through stromal- and tumour-derived cytokine and angiogenic factor signalling. J. Pathol. 2015, 237, 190–202. [Google Scholar] [CrossRef]
- de Porras, V.R.; Wang, X.C.; Palomero, L.; Marin-Aguilera, M.; Solé-Blanch, C.; Indacochea, A.; Jimenez, N.; Bystrup, S.; Bakht, M.; Conteduca, V.; et al. Taxane-induced Attenuation of the CXCR2/BCL-2 Axis Sensitizes Prostate Cancer to Platinum-based Treatment. Eur. Urol. 2021, 79, 722–733. [Google Scholar] [CrossRef]
- Acharyya, S.; Oskarsson, T.; Vanharanta, S.; Malladi, S.; Kim, J.; Morris, P.G.; Manova-Todorova, K.; Leversha, M.; Hogg, N.; Seshan, V.E.; et al. A CXCL1 Paracrine Network Links Cancer Chemoresistance and Metastasis. Cell 2012, 150, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Nawandar, D.M.; Nannuru, K.C.; Varney, M.L.; Singh, R.K. Targeting CXCR2 Enhances Chemotherapeutic Response, Inhibits Mammary Tumor Growth, Angiogenesis, and Lung Metastasis. Mol. Cancer Ther. 2013, 12, 799–808. [Google Scholar] [CrossRef]
- Zong, W.-X.; Edelstein, L.C.; Chen, C.; Bash, J.; Gélinas, C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappa B that blocks TNFalpha -induced apoptosis. Genes Dev. 1999, 13, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Edelstein, L.C.; Gélinas, C. The Rel/NF-κB Family Directly Activates Expression of the Apoptosis Inhibitor Bcl-xL. Mol. Cell. Biol. 2000, 20, 2687–2695. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Mo, F.; Li, Q.; Han, X.; Shi, H.; Chen, S.; Wei, Y.; Wei, X. Targeting CXCR2 inhibits the progression of lung cancer and promotes therapeutic effect of cisplatin. Mol. Cancer 2021, 20, 1–21. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Xu, S.; Wang, N.; Pan, B.; Yang, B.; Zheng, Y.; Zhang, J.; Peng, F.; Peng, C.; et al. Baohuoside I chemosensitises breast cancer to paclitaxel by suppressing extracellular vesicle/CXCL1 signal released from apoptotic cells. J. Extracell. Vesicles 2024, 13, e12493. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, S.; Wang, L.; Zhao, L.; Li, T.; Zhao, X.; Zhang, L. M2 macrophages promote PD-L1 expression in triple-negative breast cancer via secreting CXCL1. Pathol.-Res. Pr. 2024, 260, 155458. [Google Scholar] [CrossRef]
- Yang, B.; Li, G.; Wang, S.; Zheng, Y.; Zhang, J.; Pan, B.; Wang, N.; Wang, Z. Tumor-associated macrophages/C-X-C motif chemokine ligand 1 promotes breast cancer autophagy-mediated chemoresistance via IGF1R/STAT3/HMGB1 signaling. Cell Death Dis. 2024, 15, 1–15. [Google Scholar] [CrossRef]
- Wang, J.; Hu, W.; Wang, K.; Yu, J.; Luo, B.; Luo, G.; Wang, W.; Wang, H.; Li, J.; Wen, J. Repertaxin, an inhibitor of the chemokine receptors CXCR1 and CXCR2, inhibits malignant behavior of human gastric cancer MKN45 cells in vitro and in vivo and enhances efficacy of 5-fluorouracil. Int. J. Oncol. 2016, 48, 1341–1352. [Google Scholar] [CrossRef]
- Sharma, B.; Varney, M.L.; Saxena, S.; Wu, L.; Singh, R.K. Induction of CXCR2 ligands, stem cell-like phenotype, and metastasis in chemotherapy-resistant breast cancer cells. Cancer Lett. 2016, 372, 192–200. [Google Scholar] [CrossRef]
- Edwardson, D.W.; Boudreau, J.; Mapletoft, J.; Lanner, C.; Kovala, A.T.; Parissenti, A.M. Inflammatory cytokine production in tumor cells upon chemotherapy drug exposure or upon selection for drug resistance. PLoS ONE 2017, 12, e0183662. [Google Scholar] [CrossRef]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yue, J.; Jiang, Z.; Zhou, R.; Xie, R.; Xu, Y.; Wu, S. CAF-secreted CXCL1 conferred radioresistance by regulating DNA damage response in a ROS-dependent manner in esophageal squamous cell carcinoma. Cell Death Dis. 2017, 8, e2790. [Google Scholar] [CrossRef]
- Liu, Q.; Hao, Y.; Du, R.; Hu, D.; Xie, J.; Zhang, J.; Deng, G.; Liang, N.; Tian, T.; Käsmann, L.; et al. Radiotherapy programs neutrophils to an antitumor phenotype by inducing mesenchymal-epithelial transition. Transl. Lung Cancer Res. 2021, 10, 1424–1443. [Google Scholar] [CrossRef]
- Fujimori, A.; Okayasu, R.; Ishihara, H.; Yoshida, S.; Eguchi-Kasai, K.; Nojima, K.; Ebisawa, S.; Takahashi, S. Extremely Low Dose Ionizing Radiation Up-regulates CXC Chemokines in Normal Human Fibroblasts. Cancer Res. 2005, 65, 10159–10163. [Google Scholar] [CrossRef] [PubMed]
- Wolff, H.A.; Rolke, D.; Rave-Fränk, M.; Schirmer, M.; Eicheler, W.; Doerfler, A.; Hille, A.; Hess, C.F.; Matthias, C.; Rödel, R.M.W.; et al. Analysis of chemokine and chemokine receptor expression in squamous cell carcinoma of the head and neck (SCCHN) cell lines. Radiat. Environ. Biophys. 2010, 50, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Lerman, O.Z.; Thanik, V.D.; Scharf, C.L.; Greives, M.R.; Schneider, R.J.; Formenti, S.C.; Saadeh, P.B.; Warren, S.M.; Levine, J.P. Dose-dependent effect of radiation on angiogenic and angiostatic CXC chemokine expression in human endothelial cells. Cytokine 2009, 48, 295–302. [Google Scholar] [CrossRef]
- Ng, S.S.W.; Zhang, H.; Wang, L.; Citrin, D.; Dawson, L.A. Association of pro-inflammatory soluble cytokine receptors early during hepatocellular carcinoma stereotactic radiotherapy with liver toxicity. npj Precis. Oncol. 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Li, A.; Cheng, X.J.; Moro, A.; Singh, R.K.; Hines, O.J.; Eibl, G. CXCR2-Dependent Endothelial Progenitor Cell Mobilization in Pancreatic Cancer Growth. Transl. Oncol. 2011, 4, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Kanzler, I.; Tuchscheerer, N.; Steffens, G.; Simsekyilmaz, S.; Konschalla, S.; Kroh, A.; Simons, D.; Asare, Y.; Schober, A.; Bucala, R.; et al. Differential roles of angiogenic chemokines in endothelial progenitor cell-induced angiogenesis. Basic Res. Cardiol. 2012, 108, 1–14. [Google Scholar] [CrossRef]
- Fang, J.; Chen, X.; Wang, S.; Xie, T.; Du, X.; Liu, H.; Wang, S.; Li, X.; Chen, J.; Zhang, B.; et al. The expression of P2X7 receptors in EPCs and their potential role in the targeting of EPCs to brain gliomas. Cancer Biol. Ther. 2015, 16, 498–510. [Google Scholar] [CrossRef]
- Angara, K.; Borin, T.F.; Rashid, M.H.; Lebedyeva, I.; Ara, R.; Lin, P.-C.; Iskander, A.; Bollag, R.J.; Achyut, B.R.; Arbab, A.S. CXCR2-Expressing Tumor Cells Drive Vascular Mimicry in Antiangiogenic Therapy–Resistant Glioblastoma. Neoplasia 2018, 20, 1070–1082. [Google Scholar] [CrossRef]
- Carbone, C.; Tamburrino, A.; Piro, G.; Boschi, F.; Cataldo, I.; Zanotto, M.; Mina, M.M.; Zanini, S.; Sbarbati, A.; Scarpa, A.; et al. Combined inhibition of IL1, CXCR1/2, and TGFβ signaling pathways modulates in-vivo resistance to anti-VEGF treatment. Anti-Cancer Drugs 2016, 27, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Devapatla, B.; Sharma, A.; Woo, S. CXCR2 Inhibition Combined with Sorafenib Improved Antitumor and Antiangiogenic Response in Preclinical Models of Ovarian Cancer. PLoS ONE 2015, 10, e0139237. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.T.; Orlandella, R.M.; Turbitt, W.J.; Behring, M.; Manne, U.; Sorge, R.E.; Norian, L.A. Obesity-Associated Myeloid-Derived Suppressor Cells Promote Apoptosis of Tumor-Infiltrating CD8 T Cells and Immunotherapy Resistance in Breast Cancer. Front. Immunol. 2020, 11, 590794. [Google Scholar] [CrossRef]
- Kong, J.; Xu, S.; Zhang, P.; Zhao, Y. CXCL1 promotes immune escape in colorectal cancer by autophagy-mediated MHC-I degradation. Hum. Immunol. 2023, 84, 110716. [Google Scholar] [CrossRef]
- Gao, M.; Wu, X.; Jiao, X.; Hu, Y.; Wang, Y.; Zhuo, N.; Dong, F.; Wang, Y.; Wang, F.; Cao, Y.; et al. Prognostic and predictive value of angiogenesis-associated serum proteins for immunotherapy in esophageal cancer. J. Immunother. Cancer 2024, 12, e006616. [Google Scholar] [CrossRef]
- Cui, S.; Zheng, H.; Xu, Y.; Wu, Q.; Liu, W.; Cai, Y.; Fan, L.; Tian, Y.; Qian, H.; Ding, Y.; et al. Plasma proteomic biomarkers predict therapeutic responses in advanced biliary tract cancer patients receiving Camrelizumab plus the GEMOX treatment. npj Precis. Oncol. 2025, 9, 1–12. [Google Scholar] [CrossRef]
- Huang, R.; Wang, Z.; Hong, J.; Wu, J.; Huang, O.; He, J.; Chen, W.; Li, Y.; Chen, X.; Shen, K. Targeting cancer-associated adipocyte-derived CXCL8 inhibits triple-negative breast cancer progression and enhances the efficacy of anti-PD-1 immunotherapy. Cell Death Dis. 2023, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, Q.; Tan, L.; Wu, X.; Huang, R.; Zuo, Y.; Chen, L.; Yang, J.; Zhang, Z.-X.; Ruan, W.; et al. Neutralizing IL-8 potentiates immune checkpoint blockade efficacy for glioma. Cancer Cell 2023, 41, 693–710.e8. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, L.; Meng, L.; Shou, C. Topoisomerase inhibitors promote cancer cell motility via ROS-mediated activation of JAK2-STAT1-CXCL1 pathway. J. Exp. Clin. Cancer Res. 2019, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, Z.; Zhang, Z.; Cui, L.; Zhang, L.; Qiu, G.; Song, X.; Song, S. Local radiotherapy for murine breast cancer increases risk of metastasis by promoting the recruitment of M-MDSCs in lung. Cancer Cell Int. 2023, 23, 1–16. [Google Scholar] [CrossRef]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. PAIN. 2014, 155, 2461–2470. [Google Scholar] [CrossRef]
- Luo, X.; Huh, Y.; Bang, S.; He, Q.; Zhang, L.; Matsuda, M.; Ji, R.-R. Macrophage Toll-like Receptor 9 Contributes to Chemotherapy-Induced Neuropathic Pain in Male Mice. J. Neurosci. 2019, 39, 6848–6864. [Google Scholar] [CrossRef]
- Manjavachi, M.N.; Passos, G.F.; Trevisan, G.; Araújo, S.B.; Pontes, J.P.; Fernandes, E.S.; Costa, R.; Calixto, J.B. Spinal blockage of CXCL1 and its receptor CXCR2 inhibits paclitaxel-induced peripheral neuropathy in mice. Neuropharmacology 2019, 151, 136–143. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; de Carvalho-Barbosa, M.; Kavelaars, A.; Heijnen, C.J.; Albrecht, P.J.; Dougherty, P.M. Dorsal Root Ganglion Infiltration by Macrophages Contributes to Paclitaxel Chemotherapy-Induced Peripheral Neuropathy. J. Pain 2016, 17, 775–786. [Google Scholar] [CrossRef]
- Cardoso, C.V.; de Barros, M.P.; Bachi, A.L.L.; Bernardi, M.M.; Kirsten, T.B.; Martins, M.d.F.M.; Rocha, P.R.D.; Rodrigues, P.d.S.; Bondan, E.F. Chemobrain in rats: Behavioral, morphological, oxidative and inflammatory effects of doxorubicin administration. Behav. Brain Res. 2020, 378, 112233. [Google Scholar] [CrossRef]
- Angeles, M.A.; Quenet, F.; Vieille, P.; Gladieff, L.; Ruiz, J.; Picard, M.; Migliorelli, F.; Chaltiel, L.; Martínez-Gómez, C.; Martinez, A.; et al. Predictive risk factors of acute kidney injury after cytoreductive surgery and cisplatin-based hyperthermic intra-peritoneal chemotherapy for ovarian peritoneal carcinomatosis. Int. J. Gynecol. Cancer 2019, 29, 382–391. [Google Scholar] [CrossRef] [PubMed]
- McMahon, K.R.; Rassekh, S.R.; Schultz, K.R.; Blydt-Hansen, T.; Cuvelier, G.D.E.; Mammen, C.; Pinsk, M.; Carleton, B.C.; Tsuyuki, R.T.; Ross, C.J.D.; et al. Epidemiologic Characteristics of Acute Kidney Injury During Cisplatin Infusions in Children Treated for Cancer. JAMA Netw. Open 2020, 3, e203639. [Google Scholar] [CrossRef]
- Uozumi, J.; Ueda, T.; Yasumasu, T.; Koikawa, Y.; Naito, S.; Kumazawa, J.; Kamura, T.; Nakano, H.; Sueishi, K. Platinum Accumulation in the Kidney and Changes in Creatinine Clearance following Chemotherapy with Cisplatin in Humans. Urol. Int. 1993, 51, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, P.D.; López-Hernández, F.J.; López-Novoa, J.M.; Morales, A.I. An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit. Rev. Toxicol. 2011, 41, 803–821. [Google Scholar] [CrossRef]
- Ozkok, A.; Ravichandran, K.; Wang, Q.; Ljubanovic, D.; Edelstein, C.L. NF-κB transcriptional inhibition ameliorates cisplatin-induced acute kidney injury (AKI). Toxicol. Lett. 2016, 240, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, X.; Lv, W.; Xu, Z. Inhibition of CXCL1-CXCR2 axis ameliorates cisplatin-induced acute kidney injury by mediating inflammatory response. Biomed. Pharmacother. 2020, 122, 109693. [Google Scholar] [CrossRef]
- Akcay, A.; Nguyen, Q.; He, Z.; Turkmen, K.; Lee, D.W.; Hernando, A.A.; Altmann, C.; Toker, A.; Pacic, A.; Ljubanovic, D.G.; et al. IL-33 Exacerbates Acute Kidney Injury. J. Am. Soc. Nephrol. 2011, 22, 2057–2067. [Google Scholar] [CrossRef]
- Deng, B.; Lin, Y.; Ma, S.; Zheng, Y.; Yang, X.; Li, B.; Yu, W.; Xu, Q.; Liu, T.; Hao, C.; et al. The leukotriene B4–leukotriene B4 receptor axis promotes cisplatin-induced acute kidney injury by modulating neutrophil recruitment. Kidney Int. 2017, 92, 89–100. [Google Scholar] [CrossRef]
- Sakai, H.; Sagara, A.; Matsumoto, K.; Jo, A.; Hirosaki, A.; Takase, K.; Sugiyama, R.; Sato, K.; Ikegami, D.; Horie, S.; et al. Neutrophil recruitment is critical for 5-fluorouracil-induced diarrhea and the decrease in aquaporins in the colon. Pharmacol. Res. 2014, 87, 71–79. [Google Scholar] [CrossRef]
- Sakai, H.; Sagara, A.; Matsumoto, K.; Hasegawa, S.; Sato, K.; Nishizaki, M.; Shoji, T.; Horie, S.; Nakagawa, T.; Tokuyama, S.; et al. 5-Fluorouracil Induces Diarrhea with Changes in the Expression of Inflammatory Cytokines and Aquaporins in Mouse Intestines. PLoS ONE 2013, 8, e54788. [Google Scholar] [CrossRef]
- Yu, L.-R.; Cao, Z.; Makhoul, I.; Daniels, J.R.; Klimberg, S.; Wei, J.Y.; Bai, J.P.; Li, J.; Lathrop, J.T.; Beger, R.D.; et al. Immune response proteins as predictive biomarkers of doxorubicin-induced cardiotoxicity in breast cancer patients. Exp. Biol. Med. 2017, 243, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Pordel, S.; Khanian, M.S.; Karimi, M.H.; Nikoo, H.; Doroudchi, M. Plasma CXCL1 levels and TRAF3IP2 variants in patients with myocardial infarction. J. Clin. Lab. Anal. 2018, 32, e22402. [Google Scholar] [CrossRef] [PubMed]
- Keeley, E.C.; Moorman, J.R.; Liu, L.; Gimple, L.W.; Lipson, L.C.; Ragosta, M.; Taylor, A.M.; Lake, D.E.; Burdick, M.D.; Mehrad, B.; et al. Plasma Chemokine Levels Are Associated with the Presence and Extent of Angiographic Coronary Collaterals in Chronic Ischemic Heart Disease. PLoS ONE 2011, 6, e21174. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).