Simple Summary
Primary cutaneous peripheral T-cell lymphoma, not otherwise specified (pcPTCL-NOS), is a rare, aggressive, fatal type of cutaneous T-cell lymphoma that accounts for only approximately 2% of primary cutaneous lymphomas. This study examined the clinical manifestations, immunophenotypic characteristics, selection of treatment, and outcomes of patients with pcPTCL-NOS. Fifteen patients with pcPTCL-NOS were here identified. A detailed selection of treatments and combination applications of new drugs for these patients were described. All fifteen patients were treated with CHOP-based regimens as the initial treatment. Generally, pcPTCL-NOS requires early and active systemic treatment. However, for patients with T1 tumors, reducing the intensity of treatment with CHOP should be appropriately considered.
Abstract
Background: Primary cutaneous peripheral T-cell lymphoma, not otherwise specified (pcPTCL-NOS), is a rare and aggressive form of lymphoma. Its characteristics and treatment outcomes remain poorly understood. Methods: We identified 15 patients who were diagnosed with pcPTCL-NOS between January 2014 and August 2024 at Tianjin Medical University Cancer Institute and Hospital (TMUCIH) in this retrospective study. The clinical and immunophenotypic features, treatment regimens, and outcomes of these patients were investigated. Results: All patients (4 men, 11 women; median age 54 years) presented with skin lesions, including five stage T1, four stage T2 and six stage T3 lesions. pcPTCL-NOS manifests clinically either with solitary or disseminated rapidly growing nodules/tumors and papules and, less often, ulcers. The lesion sites in patients presenting with solitary/localized tumors (stage T1 and T2) were the head and limbs, and those in patients presenting with disseminated lesions (stage T3) were the trunk, head, and limbs. The CD4/CD8 immunophenotypic characteristics were as follows: CD4+/CD8− 53.33%; CD4+/CD8+ 26.67%; CD4−/CD8− 13.33%; and CD4−/CD8+ 6.67%. One patient had a T follicular helper (TFH) phenotype. Five patients had aberrant expression of the B-cell marker CD20 by tumor cells. All patients received CHOP or CHOP-like regimens as the initial treatment, with three patients undergoing complete lesion resection before chemotherapy, seven patients receiving treatment combined with chidamide (tucidinostat), two patients receiving treatment combined with brentuximab vedotin, two patients receiving treatment combined with mitoxantrone liposomes (Lipo-Mit), three patients receiving treatment combined with radiotherapy, and two patients receiving ASCT after the first-line treatment. The OS rates at 1 year, 2 years, and 3 years were 80%, 77.8%, and 77.8%, respectively; the PFS rates were 60%, 44.4%, and 33.3%, respectively. With a median follow-up of 40 months, the median PFS was 21 months, and the median OS was not reached. Univariate analyses revealed that patients with B symptoms and the CD4−/CD8− phenotype had inferior outcomes (p < 0.05). Age, sex, tumor stage, PIT score, Ki-67 index, elevated β2-MG levels, expression of CD20 or PD1, and treatment selection were not associated with the prognosis. A trend of a survival benefit in patients with solitary (T1) tumors compared with patients with disseminated (T2, T3) tumors was observed, suggesting that it is possible to reduce the intensity of treatment in patients with T1 tumors in the future. Conclusions: pcPTCL-NOS is an aggressive but poorly characterized lymphoma that may require early and active systemic treatment. However, for patients with T1 tumors, reducing the intensity of treatment with CHOP should be appropriately considered.
1. Introduction
Cutaneous T-cell lymphomas (CTCLs) are a heterogeneous group of cutaneous lymphoproliferative disorders (LPDs) characterized by the neoplastic proliferation of clonal T-cells in the skin [1,2]. CTCL represents approximately 75–80% of all primary cutaneous lymphomas. The World Health Organization–European Organization for Research and Treatment of Cancer (WHO–EORTC) provides a classification of cutaneous lymphomas based on the clinical, pathological, and molecular characteristics, and mycosis fungoides (MF) and CD30+ LPD are the most frequent entities. All other cases, which cannot be assigned to other specific lymphoma categories, are referred to as primary cutaneous peripheral T-cell lymphoma, not otherwise specified (pcPTCL-NOS) or unspecified. With the increasing number of non-mycosis fungoides cutaneous T-cell lymphomas (non-MF CTCLs) observed in recent years, the frequency of pcPTCL-NOS has decreased. pcPTCL-NOS is a rare, aggressive, fatal type of cutaneous T-cell lymphoma (CTCL) that accounts for only approximately 2% of primary cutaneous lymphomas [3,4]. The clinical presentation of pcPTCL-NOS is characterized by solitary, localized or, more frequently, generalized plaques, nodules, or tumors. The 5-year survival rate for patients with pcPTCL-NOS is less than 20% [5,6], which makes both a timely diagnosis and effective treatment important measures to improve outcomes.
Owing to the rarity of pcPTCL-NOS, a paucity of data regarding its clinicopathological and immunophenotypic features, clinical course, and outcomes exists. In accordance with the World Health Organization–European Organization for Research and Treatment of Cancer (WHO–EORTC, 2018) and the World Health Organization classification (5th edition, 2022) [4,7], we conducted a real-world study to examine the clinical manifestations, immunophenotypic characteristics, selection of treatment, and outcomes of patients with pcPTCL-NOS.
2. Methods
2.1. Patients and Data Collection
Patients who were diagnosed with pcPTCL-NOS according to the World Health Organization–European Organization for Research and Treatment of Cancer (WHO–EORTC, 2018), as well as the World Health Organization classification (5th edition, 2022), between January 2014 and August 2024 at Tianjin Medical University Cancer Institute and Hospital (TMUCIH), were included in this study. Patients with extracutaneous involvement at the time of diagnosis were excluded; by definition, they represented secondary cutaneous involvement of nodal PTCL-NOS. Two pathologists reviewed the disease pathology for all patients. In this retrospective chart review, data on the baseline demographic characteristics, laboratory parameters at diagnosis, treatment regimens, and patient follow-up were collected.
Staging was performed according to the instructions of the International Society for Cutaneous Lymphomas/European Organization of Research and Treatment of Cancer (ISCL/EORTC) Proposal on the TNM Classification of Cutaneous Lymphomas other than MF/SS [8]. The responses to the therapy were evaluated according to the 2014 Lugano criteria. The overall response rate (ORR) was defined as the proportion of patients who achieved a complete response (CR) or partial response (PR), and the complete response rate (CRR) was the proportion of patients who achieved a CR. Disease relapse or progression was defined as the detection of a new lesion or the progression of an existing lesion according to a physical or radiographic examination. Progression-free survival (PFS) was defined as the time from the initial treatment to disease recurrence, disease progression, the last follow-up, or death. OS was defined as the time from the initial treatment until death from any cause or the last follow-up date. Patients were followed by referring to medical records and by telephone, and the last follow-up date was August 2024.
Approval for this study was granted by the institutional review board at TMUCIH, and this study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients.
2.2. Statistical Analysis
Statistical analyses were performed with SPSS 26.0 and R4.1.0 software. Continuous variables are presented as medians, interquartile ranges (IQRs), and ranges; categorical variables are presented as frequency counts and percentages. Owing to the generally skewed distributions of all the variables, nonparametric tests were performed. PFS and OS were evaluated by means of the Kaplan–Meier method with the log-rank test. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Statistical significance was set at p < 0.05.
3. Results
Fifteen patients with pathologically confirmed pcPTCL-NOS between January 2014 and August 2024 at TMUCIH were identified. The total clinicopathological characteristics of the 15 patients with pcPTCL-NOS are summarized in Supplementary Table S1.
3.1. Clinical and Pathological Features
The median age at diagnosis was 54 years (range: 31–72 years), and 66.67% of patients were under 60 years old. A preponderance of females was observed, with 11/15 (73.33%) being women and 4/15 (26.67%) being men. At the time of diagnosis, 13.33% (n = 2) of the patients had B symptoms, and elevated beta 2-microglobulin and lactate dehydrogenase (LDH) levels were found in 26.67% (n = 4) of the patients. Five out of fifteen patients (33.33%) presented with a solitary nodule/tumor according to the TNM stage (stage T1), and four out of fifteen patients (26.67%) presented with localized papules and nodules (stage T2), whereas six out of fifteen patients (40%) presented with disseminated papules and nodular lesions (stage T3). The lesion sites in the patients who presented with a solitary nodule/tumor (T1) were the head (three out of five patients), arm (one out of five patients), and leg (one out of five patients), whereas those in the patients with localized papules and nodules (T2) were the arms (two out of four patients), head (one out of four patients), and hand (one out of four patients). In patients with disseminated disease, the lesions were mostly located on the trunk and extremities. The clinical features of each patient are summarized in Table 1.

Table 1.
Clinical features of each patient with pcPTCL-NOS.
Among the 15 patients, the details of the CD4/CD8 immunophenotypic characteristics were as follows: CD4+/CD8− in 53.33% (8/15 patients), CD4+/CD8+ in 26.67% (4/15 patients), CD4−/CD8− in 13.33% (2/15 patients), and CD4−/CD8+ in 6.67% (1/15 patients). The phenotypes varied across stages as follows: stage T1, CD4+/CD8− in 20% (1/5 cases), CD4+/CD8+ in 40% (2/5 cases), CD4−/CD8− in 20% (1/5 cases), and CD4−/CD8+ in 20% (1/5 cases); stage T2, CD4+/CD8− in 75% (3/4 cases) and CD4+/CD8+ in 25% (1/4 cases); and stage T3, CD4+/CD8− in 66.67% (4/6 cases), CD4+/CD8+ in 16.67% (1/6 cases), and CD4−/CD8− in 16.67% (1/6 cases). In this group, only one patient with a stage T1 tumor had a CD4−/CD8+ phenotype (Figure 1). Ki67 staining revealed proliferative fractions of ≥80% and <80% in 40% (6/15 patients) and 60% (9/15 patients), respectively. The percentages of CD30- and CD20-positive patients were 40% (6/15 patients) and 33.33% (5/15 patients), respectively. The percentage of PD-1-positive patients was 20% (3/15 patients). One patient with a stage T3 tumor had a T follicular helper (TFH) cell phenotype of CD10+, CXCL13+, PD1+, and BCL6+. The presence of EBV, as assessed by in situ hybridization for EBER, was negative in all patients, with one patient with a stage T3 tumor testing positive for EBV-DNA. The histological and immunophenotypic characteristics of each patient are summarized in Table 2.
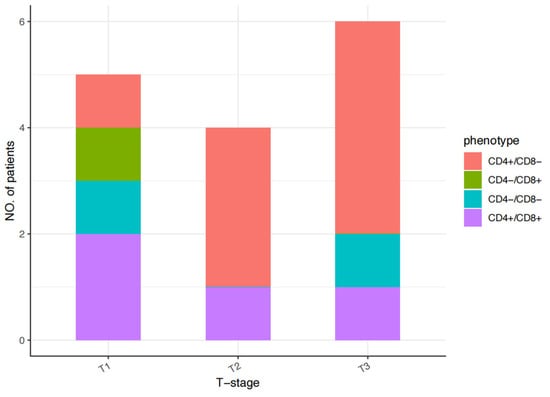
Figure 1.
CD4/CD8 immunophenotypic characteristics of each patient with pcPTCL-NOS.

Table 2.
Histological and immunophenotypic features of each patient with pcPTCL-NOS.
3.2. Frontline Treatments and Responses
All fifteen patients with pcPTCL-NOS received systemic chemotherapy as the initial treatment. The effects of combination therapy as a first-line treatment are shown in Figure 2. The frontline treatment was the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like regimens. Three patients with stage T1 tumors underwent complete lesion resection before chemotherapy, seven patients (four with both stage T1 and T2 tumors and three with stage T3 tumors) received CHOP combined with chidamide (tucidinostat), two patients with stage T3 tumors received brentuximab vedotin, two patients received CHOP combined with mitoxantrone liposomes (Lipo-Mit) (one patient with a stage T1 tumor and the other with a stage T3 tumor), three patients received CHOP combined with radiotherapy (two patients with stage T1 tumors and one patient with a stage T3 tumor), and two patients (both with stage T3 tumors) received ASCT after the first-line treatment. The initial response to treatment was complete remission (CR) in nine patients (60%) and partial remission (PR) in four patients (26.67%), and the ORR was 86.67%.
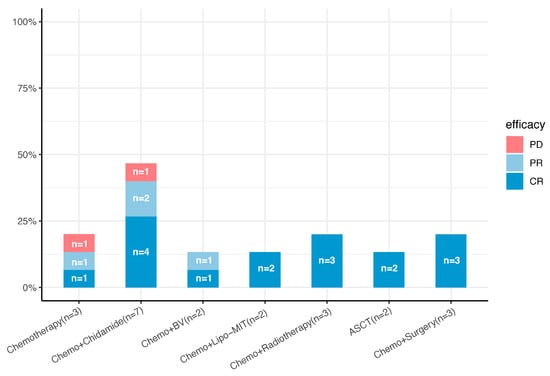
Figure 2.
Front-line treatment plans and efficacy for 15 patients with pcPTCL-NOS (n, number; Chemo, chemotherapy; Lipo-MIT, mitoxantrone liposomes; BV, brentuximab vedotin; ASCT, autologous stem cell transplantation; CR, complete response; PR, partial response; PD, progressive disease).
Among the nine patients who achieved a CR after the first-line treatment, five had stage T1 tumors, four had stage T3 tumors, and none had stage T2 tumors. Notably, two patients received the CMOPE regimen, in which mitoxantrone liposomes (Lipo-Mit) replaced anthracyclines in the CHOPE regimen, one of whom had a stage T1 tumor and the other had a stage T3 tumor; both achieved a CR after the first-line treatment, and, currently, they are still in CR. Four patients (two each with stage T1 and T3 tumors) received CHOP or CHOP-like regimens combined with chidamide, and one (with a stage T3 tumor) received chidamide combined with brentuximab vedotin.
Among the four patients who achieved a PR after first-line treatment, three had stage T2 disease, one had stage T3 disease, and none had stage T1 disease. Two patients with stage T2 disease received CHOP or a CHOP-like regimen alone, one patient with stage T2 disease received CHOP combined with chidamide, and another patient with stage T3 disease received CHOP combined with chidamide and BV simultaneously.
Two patients experienced disease progression after the first-line treatment, with one patient with stage T2 disease progressing after receiving six cycles of chidamide and the CHOPE regimen and the other patient with stage T3 disease progressing after receiving only two cycles of the CHOPE regimen. The detailed first-line treatments and responses of each patient are listed in Table 3.

Table 3.
First-line and salvage treatments, responses, and survival of each patient with pcPTCL-NOS.
3.3. Salvage Treatments and Responses
In total, eight patients relapsed and received salvage treatment, including 20% of the patients (1/5) with stage T1 tumors, 100% of the patients (4/4) with stage T2 tumors, and 50% of the patients (3/6) with stage T3 tumors. Various attempts were made to treat patients who experienced progression or relapse. In addition to second-line chemotherapy, such as the GDP, GEMOX, DICE, and DHAP regimens, patients chose immunochemotherapy, targeted therapy, or participation in clinical trials (involving agents such as chidamide, brentuximab vedotin, mitoxantrone liposomes, PD1 inhibitors, JAK1 inhibitors, and azacitidine). However, salvage treatment failed in three patients (two patients with stage T2 tumors and one with a stage T3 tumor), and the patients died due to disease progression. Ultimately, the response to salvage treatment was a CR in three patients (3/8, 37.5%) and PR in one patient (1/8, 12.5%), and the ORR was 50% (4/8).
Two patients received treatment with the combination of chidamide and azacitidine: one patient with a stage T1 tumor achieved a CR again, and the other patient with a stage T3 tumor died due to disease progression. One patient with a stage T2 tumor previously received a six-cycle CHOPE regimen as the first-line chemotherapy and then a one-cycle GDP regimen as a second-line treatment, but the disease had not been effectively controlled; salvage therapy included DICE combined with the chidamide regimen and the patient ultimately achieved a CR. One patient with a stage T2 tumor experienced central nervous system involvement after three cycles of treatment with CHOPE combined with the chidamide regimen as the first-line treatment; this patient tested positive by cerebrospinal fluid flow cytometry. Despite receiving high-dose MTX treatment and the DHAP regimen as the salvage treatment, this patient died due to disease progression. One patient with a stage T3 tumor expressing PD-1 who received tislelizumab (PD-1 inhibitor) as the second-line therapy achieved a CR again. Another patient with a stage T3 tumor received golidocitinib (JAK1 inhibitor) combined with the chidamide regimen as a second-line therapy for two cycles at the last follow-up date, and the disease remained stable. Two patients with stage T2 disease received CHOP or CHOP-like regimens alone as the first-line treatment; one of them died after four cycles of treatment with the GDP regimen as the second-line therapy, and the other patient achieved disease control after treatment with BV combined with chidamide. The detailed salvage treatments, responses, and survival data for each patient are listed in Table 3.
3.4. Survival and Prognostic Factors
With a median follow-up of 40 (range: 5–105) months, the median PFS was 21 months, and the median OS was not reached. The OS rates at 1 year, 2 years, and 3 years were 80%, 77.8%, and 77.8%, respectively, and the PFS rates were 60%, 44.4%, and 33.3%, respectively (Figure 3).
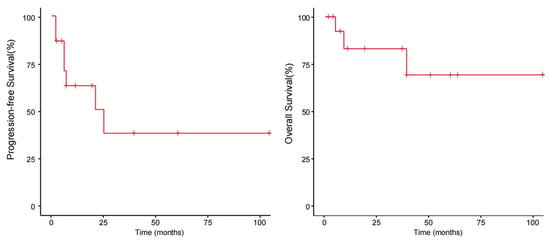
Figure 3.
Progression-free survival and overall survival of patients with pcPTCL-NOS.
An analysis of the survival data revealed that 20% (3/15) of all patients died due to lymphoma (DDL). Twenty percent (3/15) of the patients were alive with disease (AWD), and 60% (9/15) were alive without disease (AWOD) at the last follow-up. The outcomes according to the tumor stage were as follows: for T1 (solitary lesion), all five patients were alive without disease (AWOD), and for T2 (localized disease), one patient (25%, 1/4) was alive without disease (AWOD), one patient (25%, 1/4) was alive with disease (AWD), and two patients (50%, 2/4) died due to lymphoma (DDL). For T3, three patients (50%) were alive without disease (AWOD), one patient (33.33%, 2/6) was alive with disease (AWD), and one patient (16.67%, 1/6) died due to lymphoma (DDL). These group differences did not reach statistical significance.
The results of the univariate analyses indicated that patients with B symptoms and the CD4−/CD8− phenotype had inferior outcomes (p < 0.05). Age, sex, tumor stage, PIT score (calculated from 0 to 4 by age, performance status, lactic dehydrogenase level, and bone marrow involvement), Ki-67 index, elevated β2-MG levels, expression of CD20 or PD1, and selection of treatment were not associated with the prognosis (Table 4). A difference in survival trends was observed between the stage T1, T2, and T3 groups, but the difference was not significant because of the small sample size (Figure 4). We further analyzed survival according to different body regions and found a difference in survival trends between the solitary (T1) and disseminated (T2;T3) groups (Figure 5). These findings suggest that the intensity of treatment can be reduced for patients’ T1 tumors in the future.

Table 4.
Univariate logistic regression analysis of the predictive factors for progression-free survival.
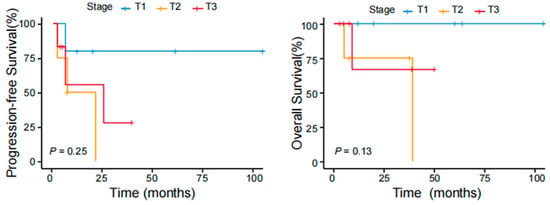
Figure 4.
PFS and OS of patients with pcPTCL-NOS stratified according to different tumor stages.
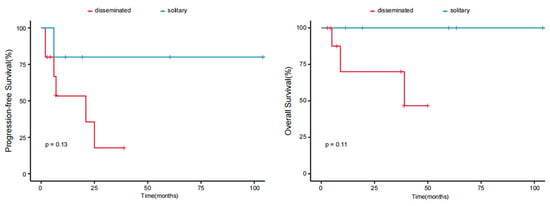
Figure 5.
PFS and OS of patients with pcPTCL-NOS stratified according to different body regions.
4. Discussion
To date, this is the largest series of patients with pcPTCL-NOS according to the WHO–EORTC (2018), as well as the WHO (5th edition, 2022) classification, in a single tertiary center. In our series, in addition to clinical manifestations, immunophenotypic characteristics, outcomes, and prognoses, we provided a detailed description of the selection of treatments and combination applications of new drugs for patients with pcPTCL-NOS.
Cutaneous T-cell lymphoma (CTCL) has an indolent clinical course, and 20% to 55% of CTCL patients develop late-stage disease [9,10]. Most early-stage patients who do not require systemic chemotherapy seek treatment in the Department of Dermatology. In our center, all 15 patients with pcPTCL-NOS received systemic chemotherapy as the initial treatment since the disease course was expected to be aggressive. In contrast to previous reports [11,12,13], patients in this group were predominantly female (2.75:1), and the median age at diagnosis was 54 years, with 66.67% of patients being under 60 years old. The most common clinical presentations were nodules/tumors and papules and, less often, ulcers, which was consistent with the results of previous studies [14,15,16,17,18]. Notably, lesions on the head, mainly the facial areas, were more common in this group of patients than in previous studies [11]. These lesions can also lead to early detection and attention to the disease. Patients with B symptoms had inferior outcomes. In our series, a difference in survival trends was observed among the stage T1, T2, and T3 groups. The small number of patients may, however, be one reason for the lack of a significant difference in the survival curves. In addition, in our series, the predominant phenotype was CD4+/CD8−, followed by a double-positive phenotype and, less commonly, a double-negative phenotype. Patients with the CD4−/CD8− phenotype had inferior outcomes. In Kempf’s cohort [11], aberrant expression of CD20 was observed in approximately one-fifth of the tumors. We also detected aberrant CD20 expression in one-third of the patients (5/15). The difference was that two patients who received chemotherapy plus rituximab were included in their cohort, but no such patients were included in our study. In previous studies [19], CD20 itself was reported to be a dynamic marker that may either be gained or lost during the clonal evolution of a tumor. However, whether rituximab plus chemotherapy provides a clinical benefit in this situation is not yet clear [20]. CD30 expression has been consistently reported in CD30+ lymphoproliferative disorders (LPDs) but differs in other subtypes of cutaneous T-cell lymphoma [21]. BV may be a therapeutic option, even in patients with low or even no expression of CD30, but the duration of the response can be longer in patients with high CD30 expression [22,23,24]. In our study, six patients were CD30-positive, three of whom were treated with BV (two patients with stage T3 tumors treated in the first-line setting, and another with a stage T2 tumor treated in the second-line setting), and the ORR was 100%. Additionally, among cutaneous T-cell lymphomas (CTCLs), the classification does not yet include a specific category for those with a TFH phenotype. In our group, one patient with a stage T3 tumor presenting a T follicular helper (TFH) phenotype received the CHP regimen combined with BV and chidamide as the first-line therapy for four cycles, and was in a state of disease control at the last follow-up. As targeted treatments, including histone deacetylase inhibitors [25,26] and hypomethylating agents [27,28], have been introduced for systemic TFH lymphomas, therapeutic options for these patients can be explored. In their cohort, Kempf W et al. [11] also reported a patient with the TFH phenotype of pcPTCL-NOS. A genuine TFH phenotype seems to be rare in patients with pcPTCL-NOS.
With respect to treatment modalities, all patients in our study received systemic chemotherapy. Previous studies have suggested that immediate, intense treatment with multiagent chemotherapy and hematopoietic stem cell transplantation is indicated for patients with pcPTCL-NOS [18]. In our series, all five patients with stage T1 disease also received CHOP or CHOP-like regimens for four–six cycles as the frontline treatment with complete resection of the lesion and/or radiotherapy. With a median progression-free survival (PFS) of 19 months, all of the patients with stage T1 tumors were alive without disease. Compared with previous studies [11,12,13], for patients with stage T1 or even stage T2 disease, excision, focal radiotherapy, or excision followed by focal radiotherapy were the most common upfront strategies. The question of whether an aggressive therapeutic approach with chemotherapy in patients with single lesions is effective and necessary or justified at all, which was raised by previous research [11], requires further confirmation. In 2022, Stuver R et al. [13] reported a group of patients with pcPTCL-NOS in which patients with a single lesion or regional disease experienced significantly longer survival than those with multiple regions involved. Therefore, systemic chemotherapy may not be necessary for patients with stage T1 disease, and surgery and/or radiotherapy alone are likely sufficient. Among the eight patients who relapsed after the first-line treatment, 50% (4/8) had stage T2 disease. Compared with patients with stage T2 disease, patients with stage T1 disease may also undergo surgical resection and local radiation therapy, and patients with stage T3 disease can more easily receive treatment with a new drug and even autologous stem cell transplantation. This finding may explain why recurrence is more common in patients with stage T2 disease. This finding also suggests that the treatment for patients with stage T2 disease may be insufficient.
A clinical study initiated by our center regarding azacitidine combined with chidamide for patients with R/R peripheral T-cell lymphoma is ongoing, and two patients received this regimen as salvage treatment. One patient with stage T1 disease achieved a CR, while the other patient with stage T3 disease experienced disease progression and died due to lymphoma. The preliminary results of this study indicate that the application of the chidamide and azacitidine regimen as a salvage treatment in patients with advanced tumors may not be sufficient. However, more clinical data are needed to confirm these findings. Two patients received the CMOPE regimen as an initial treatment, and achieved CR. This was a phase I study initiated by our center, and showed promising anti-tumor activity, evidenced by an ORR of 100% and a CR rate of 66.7%, as first-line therapy in patients with untreated PTCL [29].
Two patients with stage T2 disease received CHOP or CHOP-like regimens alone as a first-line treatment, and the efficacy evaluations revealed that both regimens resulted in a PR. The difference is that one patient was switched to the GDP regimen for subsequent treatment, whereas the other patient continued to receive the CHOP-like regimen in combination with two new drugs (chidamide and brentuximab vedotin). Finally, the former died due to disease progression, whereas the latter was alive with disease at the last follow-up. From this perspective, the application of new drugs, and even combinations of drugs with multiple mechanisms, may further relieve disease in patients.
Notably, in this group of patients, one patient with stage T2 disease had central nervous system involvement. The patient experienced headaches and increased skin lesions and died due to lymphoma. Involvement of the central nervous system in patients with CTLC is rare. Further consideration is needed to determine whether it occurs simultaneously or earlier with respect to skin lesions. A physical examination and imaging examination of the central nervous system should be performed in clinical practice. Simultaneously, two patients with stage T3 disease received ASCT after the first-line treatment. One patient remained in a CR, while the other patient experienced disease recurrence. The patient with disease progression received chidamide combined with the CHOPE regimen as the first-line treatment, and immunohistochemistry showed CD30-negative and PD1-positive expression. Thus, a PD-1 inhibitor (tislelizumab) was selected for this patient as the second-line treatment. After 18 cycles of treatment, the patient achieved a CR again. PD-1, a membrane receptor expressed on activated T-cells, inhibits the immune response in peripheral tissues and promotes self-tolerance. In CTCL, skin lesion-derived tumor-infiltrating T-cells have higher expression of PD-1 (and other immune checkpoint molecules) in comparison to controls [30]. Nevertheless, ongoing studies should define more clearly the efficacy and safety of PD1 inhibitors in the T-cell lymphoma treatment.
Additionally, in China, chidamide has been approved for the treatment of relapsed/refractory peripheral T-cell lymphoma (PTCL) for 10 years, and its efficacy is recognized in PTCL patients [31,32]. In our study, most patients were treated with a combination of chidamide (seven patients in the first-line group and three patients in the salvage treatment group). Two patients died, and the remaining eight patients achieved disease control. Another patient with stage T3 disease received golidocitinib (JAK1 inhibitor) combined with the chidamide regimen as the second-line therapy for two cycles at the last follow-up date, and the disease remained stable. Next-generation sequencing (NGS) has revolutionized genomic research on many hematologic cancer types. However, genomic data on primary cutaneous lymphomas are still incomplete and sometimes contradictory [33]. Therefore, clinicians still rely on clinical and histologic information rather than genetic diagnostics for diagnosis and determining the choice of therapy. The identification of genes and pathways involved in the pathogenesis of different pcPTCL-NOS subtypes can lead to novel targeted therapies in oncology [34].
5. Conclusions
In conclusion, patients with pcPTCL-NOS present tumors with rapid growth, and the disease prognosis is poor. We reported a female predominance, and the median age at diagnosis was 54 years. The most common clinical presentations were nodules/tumors and papules, and, less often, ulcers; lesions in the head were common. pcPTCL-NOS may require early and active systemic treatment. However, reducing the intensity of treatment with CHOP should be appropriately considered for patients with T1 disease to avoid overtreatment. Our study has some limitations. (1) This study has a small sample size, which limits its statistical power. (2) This study has a retrospective design. (3) This study has a referral bias to a tertiary center for patients with advanced-stage disease and treatment heterogeneity. pcPTCL-NOS is very rare; therefore, more studies on pcPTCL-NOS are needed to better characterize the tumors and clarify the best treatment modality.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers17101673/s1, Table S1. Summary of the clinicopathological characteristics of patients with primary cutaneous peripheral T-cell lymphoma, not otherwise specified.
Author Contributions
X.W., H.Z. and J.H. conceived and designed this study; G.H., Z.S., C.L., Y.S. and Y.Z. performed the research and statistical and bioinformatics analyses; X.L., X.H., L.L., L.Q., Z.Q. and S.Z. collected samples and clinical information; B.M. and W.G. reviewed the diagnosis of patients; X.W., H.Z. and J.H. provided the clinical samples and material support; G.H. wrote the manuscript and finalized the figures; X.W. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (W2412122, 82200208, and 8247012736), the State Key Laboratory of Druggability Evaluation and Systematic Translational Medicine (QZ23-6; QZ23-4), the Scientific and Technological Project of Tianjin (24ZXZSSS00050), the Haihe Yingcai (Tianjin) Project (TJSJMYXYC-D2-039), and the Tianjin Key Medical Discipline (Specialty) Construction Project grant (TJYXZDXK-009A).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Tianjin Medical University Cancer Institute and Hospital (TMUCIH) (protocol code 20240287, 15 Match 2024).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in this study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank Weiyun Z. Ai of the Division of Hematology and Oncology, Department of Medicine, University of California, San Francisco, for her valuable advice and comments. We also thank the Department of Pathology at Tianjin Medical University Cancer Institute and Hospital (TMUCIH) for providing support.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Alberti-Violetti, S.; Berti, E. Update on primary cutaneous T-cell lymphomas rare subtypes. Dermatol. Rep. 2024, 16 (Suppl. S2), 9961. [Google Scholar] [CrossRef] [PubMed]
- Dobos, G.; Pohrt, A.; Ram-Wolff, C.; Lebbé, C.; Bouaziz, J.D.; Battistella, M.; Bagot, M.; de Masson, A. Epidemiology of Cutaneous T-Cell Lymphomas: A Systematic Review and Meta-Analysis of 16,953 Patients. Cancers 2020, 12, 2921. [Google Scholar] [CrossRef] [PubMed]
- Willemze, R.; Jaffe, E.S.; Burg, G.; Cerroni, L.; Berti, E.; Swerdlow, S.H.; Ralfkiaer, E.; Chimenti, S.; Diaz-Perez, J.L.; Duncan, L.M.; et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005, 105, 3768–3785. [Google Scholar] [CrossRef]
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019, 133, 1703–1714. [Google Scholar] [CrossRef]
- Willemze, R. Primary cutaneous lymphoma: The 2018 update of the WHO-EORTC classification. Presse Med. 2022, 51, 104126. [Google Scholar] [CrossRef]
- Willemze, R.; Meijer, C.J. EORTC classification for primary cutaneous lymphomas: A comparison with the R.E.A.L. Classification and the proposed WHO Classification. Ann. Oncol. 2000, 11 (Suppl. S1), 11–15. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Willemze, R.; Pimpinelli, N.; Whittaker, S.; Olsen, E.A.; Ranki, A.; Dummer, R.; Hoppe, R.T.; ISCL and the EORTC. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007, 110, 479–484. [Google Scholar]
- Willemze, R.; Hodak, E.; Zinzani, P.L.; Specht, L.; Ladetto, M.; ESMO Guidelines Committee. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow up. Ann. Oncol. 2018, 29 (Suppl. S4), iv30–iv40. [Google Scholar] [CrossRef]
- Dummer, R.; Vermeer, M.H.; Scarisbrick, J.J.; Kim, Y.H.; Stonesifer, C.; Tensen, C.P.; Geskin, L.J.; Quaglino, P.; Ramelyte, E. Cutaneous T cell lymphoma. Nat. Rev. Dis. Primers 2021, 7, 61. [Google Scholar] [CrossRef]
- Kempf, W.; Mitteldorf, C.; Battistella, M.; Willemze, R.; Cerroni, L.; Santucci, M.; Geissinger, E.; Jansen, P.; Vermeer, M.H.; Marschalko, M.; et al. Primary cutaneous peripheral T-cell lymphoma, not otherwise specified: Results of a multicentre European Organization for Research and Treatment of Cancer (EORTC) cutaneous lymphoma taskforce study on the clinico-pathological and prognostic features. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Tolkachjov, S.N.; Weenig, R.H.; Comfere, N.I. Cutaneous peripheral T-cell lymphoma, not otherwise specifified: A single-center prognostic analysis. J. Am. Acad. Dermatol. 2016, 75, 992–999. [Google Scholar] [CrossRef]
- Stuver, R.; Ghione, P.; Ganesan, N.; Noor, S.; Imber, B.S.; Pulitzer, M.; Horwitz, S.M. Primary Cutaneous Peripheral T-Cell Lymphoma, Not Otherwise Specified:Characterization of a Large Cohort with Long-Term Outcomes Shows Disparate Outcomes By Body Region Involvement. Blood 2022, 140 (Suppl. S1), 6556–6557. [Google Scholar] [CrossRef]
- Lee, M.H.; Choi, M.E.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Lee, W.J. Comparative Clinicopathological Analysis of Cutaneous Peripheral T-cell lymphoma, Not Otherwise Specified, According to Primary Tumor Site. J. Am. Acad. Dermatol. 2019, 80, 1771–1774. [Google Scholar] [CrossRef] [PubMed]
- Pileri, A.; Agostinelli, C.; Fuligni, F.; Broccoli, A.; Gunnella, S.; Sabattini, E.; Grandi, V.; Guglielmo, A.; Zinzani, P.L.; Patrizi, A.; et al. Primary cutaneous peripheral T-cell lymphoma not otherwise specified a rare and aggressive lymphoma. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e373–e376. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, Y.; Byun, H.J.; Oh, S.J.; Lee, J.H.; Lee, D.Y. A Retrospective Clinicopathologic Study of Korean Patients with Cutaneous Peripheral T-Cell Lymphoma Not Otherwise Specified at a Single Tertiary Center. Ann. Dermatol. 2020, 32, 337–342. [Google Scholar] [CrossRef]
- Su, C.; Nguyen, K.A.; Bai, H.X.; Cao, Y.; Tao, Y.; Karakousis, G.; Zhang, P.J.; Zhang, G.; Xiao, R. Disease site as a determinant of survival outcome in patients with primary cutaneous peripheral T-cell lymphoma, unspecified: An analysis of 4057 cases from the US National Cancer Database. Leuk. Lymphoma 2018, 59, 2105–2112. [Google Scholar] [CrossRef]
- Kempf, W.; Rozati, S.; Kerl, K.; French, L.E.; Dummer, R. Cutaneous peripheral T-cell lymphomas, unspecified/NOS and rare subtypes: A heterogeneous group of challenging cutaneous lymphomas. G. Ital. Dermatol. Venereol. 2012, 147, 553–562. [Google Scholar] [PubMed]
- Lee, A.Y.S. CD20+ T cells: An emerging T cell subset in human pathology. Inflamm. Res. 2022, 71, 1181–1189. [Google Scholar] [CrossRef]
- Mangogna, A.; Cox, M.C.; Ruco, L.; Lopez, G.; Belmonte, B.; Di Napoli, A. Rituximab Plus Chemotherapy Provides No Clinical Benefit in a Peripheral T-Cell Lymphoma Not Otherwise Specified with Aberrant Expression of CD20 and CD79a: A Case Report and Review of the Literature. Diagnostics 2020, 10, 341. [Google Scholar] [CrossRef]
- van der Weyden, C.A.; Pileri, S.A.; Feldman, A.L.; Whisstock, J.; Prince, H.M. Understanding CD30 biology and therapeutic targeting: A historical perspective providing insight into future directions. Blood Cancer J. 2017, 7, e603. [Google Scholar] [CrossRef] [PubMed]
- Prince, H.M.; Kim, Y.H.; Horwitz, S.M.; Dummer, R.; Scarisbrick, J.; Quaglino, P. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): An international, open-label, randomized, phase 3, multicentre trial. Lancet 2017, 390, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Papadavid, E.; Kapniari, E.; Pappa, V.; Nikolaou, V.; Iliakis, T.; Dalamaga, M.; Jonak, C.; Porkert, S.; Engelina, S.; Quaglino, P.; et al. Multicentric EORTC retrospective study shows efficacy of brentuximab vedotin in patients who have mycosis fungoides and Sézary syndrome with variable CD30 positivity. Br. J. Dermatol. 2021, 185, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Gosmann, J.; Stadler, R. Long term survival, time to next treatment and CD30 expression in patients with advanced CD30+ cutaneous T-cell lymphoma treated with Brentuximab vedotin—A monocentric retrospective analysis of twelve patients. J. Dtsch. Dermatol. Ges. 2022, 20, 514–517. [Google Scholar] [CrossRef]
- Ghione, P.; Faruque, P.; Mehta-Shah, N.; Seshan, V.; Ozkaya, N.; Bhaskar, S.; Yeung, J.; Spinner, M.A.; Lunning, M.; Inghirami, G.; et al. T follicular helper phenotype predicts response to histone deacetylase inhibitors in relapsed/refractory peripheral T-cell lymphoma. Blood Adv. 2020, 4, 4640–4647. [Google Scholar] [CrossRef]
- Bachy, E.; Camus, V.; Thieblemont, C.; Sibon, D.; Casasnovas, R.O.; Ysebaert, L.; Damaj, G.; Guidez, S.; Pica, G.M.; Kim, W.S.; et al. Romidepsin plus CHOP versus CHOP in patients with previously untreated peripheral T cell lymphoma: Results of the Ro-CHOP phase III study(conducted by LYSA). J. Clin. Oncol. 2022, 40, 242–251. [Google Scholar] [CrossRef]
- Lemonnier, F.; Dupuis, J.; Sujobert, P.; Tournillhac, O.; Cheminant, M.; Sarkozy, C.; Pelletier, L.; Marçais, A.; Robe, C.; Fataccioli, V.; et al. Treatment with 5-azacytidine induces a sustained response in patients with angioimmunoblastic T-cell lymphoma. Blood 2018, 132, 2305–2309. [Google Scholar] [CrossRef]
- Falchi, L.; Ma, H.; Klein, S.; Lue, J.K.; Montanari, F.; Marchi, E.; Deng, C.; Kim, H.A.; Rada, A.; Jacob, A.T.; et al. Combined oral 5-azacytidine and romidepsin are highly effective in patients with PTCL: A multicenter phase 2 study. Blood 2021, 137, 2161–2170. [Google Scholar] [CrossRef]
- Yu, J.; Sun, X.; Gao, G.; Yu, L.; Wang, J.; Qiu, L.; Qian, Z.; Li, W.; Zhang, H. CMOEP regimen in the treatment of untreated peripheral T-cell lymphoma: A multicenter, single-arm, phase I study. Front. Immunol. 2025, 16, 1551723. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, A.; Al-Juhaishi, T.; Davila, E.; Haverkos, B. Enhancing antitumor immunity through checkpoint blockade as a therapeutic strategy in T-cell lymphomas. Blood Adv. 2020, 4, 4256–4266. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, N.; Fang, Y.; Ma, S.; Zhang, Y.; Cai, J.; Zou, Q.; Tian, X.; Xia, Y.; Liu, P.; et al. Comparison of Chemotherapy Combined With Chidamide Versus Chemotherapy in the Frontline Treatment for Peripheral T-Cell Lymphoma. Front. Immunol. 2022, 13, 835103. [Google Scholar] [CrossRef]
- Wang, J.; Fang, Y.; Ma, S.; Su, N.; Zhang, Y.; Huang, H.; Li, Z.; Huang, H.; Tian, X.; Cai, J.; et al. Comparison of chidamide-contained treatment modalities versus chemotherapy in the second-line treatment for relapsed or refractory peripheral T-cell lymphoma. Leuk. Res. 2021, 111, 106705. [Google Scholar] [CrossRef]
- Tensen, C.P.; Quint, K.D.; Vermeer, M.H. Genetic and epigenetic insights into cutaneous T-cell lymphoma. Blood 2022, 139, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Yumeen, S.; Girardi, M. Insights Into the Molecular and Cellular Underpinnings of Cutaneous T Cell Lymphoma. Yale J. Biol. Med. 2020, 93, 111–121. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).