Reliability and Clinical Feasibility of Three Assessment Methods for Head and Neck Lymphedema in Head and Neck Cancer Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedures
2.4. Assessments
2.5. Statistical Analyses
2.5.1. Data Processing and Software
2.5.2. Descriptive Statistics
2.5.3. Absolute Agreement
2.5.4. Reliability Analyses
2.5.5. Systematic Differences
2.5.6. Measurement Error
2.5.7. Exploratory Correlation Analyses
2.5.8. Clinical Feasibility Evaluation
3. Results
3.1. Reliability
3.1.1. Inter-Rater Reliability
3.1.2. Intra-Rater Reliability
3.2. Clinical Feasibility
3.3. Correlation Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, J.; Ridner, S.H.; Dietrich, M.S.; Wells, N.; Wallston, K.A.; Sinard, R.J.; Cmelak, A.J.; Murphy, B.A. Prevalence of secondary lymphedema in patients with head and neck cancer. J. Pain Symptom Manag. 2012, 43, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Allam, O.; Park, K.E.; Chandler, L.; Mozaffari, M.A.; Ahmad, M.; Lu, X.; Alperovich, M. The impact of radiation on lymphedema: A review of the literature. Gland Surg. 2020, 9, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Pigott, A.; Brown, B.; White, N.; McPhail, S.; Porceddu, S.; Liu, H.; Jeans, C.; Panizza, B.; Nixon, J. A prospective observational cohort study examining the development of head and neck lymphedema from the time of diagnosis. Asia Pac. J. Clin. Oncol. 2023, 19, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Jeans, C.; Brown, B.; Ward, E.C.; Vertigan, A.E.; Pigott, A.E.; Nixon, J.L.; Wratten, C.; Boggess, M. A Prospective, Longitudinal and Exploratory Study of Head and Neck Lymphoedema and Dysphagia Following Chemoradiotherapy for Head and Neck Cancer. Dysphagia 2023, 38, 1059–1071. [Google Scholar] [CrossRef]
- Ridner, S.H.; Dietrich, M.S.; Niermann, K.; Cmelak, A.; Mannion, K.; Murphy, B. A Prospective Study of the Lymphedema and Fibrosis Continuum in Patients with Head and Neck Cancer. Lymphat. Res. Biol. 2016, 14, 198–205. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Feng, Y.; Zhang, J.; Swinnen, J.; Li, Y.; Ni, Y. A Review on Curability of Cancers: More Efforts for Novel Therapeutic Options Are Needed. Cancers 2019, 11, 1782. [Google Scholar] [CrossRef]
- Alfouzan, A.F. Radiation therapy in head and neck cancer. Saudi Med. J. 2021, 42, 247–254. [Google Scholar] [CrossRef]
- Kalemikerakis, I.; Evaggelakou, A.; Kavga, A.; Vastardi, M.; Konstantinidis, T.; Govina, O. Diagnosis, treatment and quality of life in patients with cancer-related lymphedema. J. BUON 2021, 26, 1735–1741. [Google Scholar]
- Tribius, S.; Pazdyka, H.; Tennstedt, P.; Busch, C.J.; Hanken, H.; Krüll, A.; Petersen, C. Prognostic factors for lymphedema in patients with locally advanced head and neck cancer after combined radio(chemo)therapy- results of a longitudinal study. Oral Oncology 2020, 109, 104856. [Google Scholar] [CrossRef]
- Deng, J.; Wulff-Burchfield, E.M.; Murphy, B.A. Late Soft Tissue Complications of Head and Neck Cancer Therapy: Lymphedema and Fibrosis. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz005. [Google Scholar] [CrossRef]
- Deng, J.; Ridner, S.H.; Dietrich, M.S.; Wells, N.; Wallston, K.A.; Sinard, R.J.; Cmelak, A.J.; Murphy, B.A. Factors associated with external and internal lymphedema in patients with head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e319–e328. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.K.; Ridner, S.H.; Deng, J.; Bartow, C.; Mannion, K.; Niermann, K.; Gilbert, J.; Dietrich, M.S.; Cmelak, A.J.; Murphy, B.A. Internal Lymphedema Correlates with Subjective and Objective Measures of Dysphagia in Head and Neck Cancer Patients. J. Palliat. Med. 2016, 19, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Jeans, C.; Brown, B.; Ward, E.C.; Vertigan, A.E.; Pigott, A.E.; Nixon, J.L.; Wratten, C. Comparing the prevalence, location, and severity of head and neck lymphedema after postoperative radiotherapy for oral cavity cancers and definitive chemoradiotherapy for oropharyngeal, laryngeal, and hypopharyngeal cancers. Head Neck 2020, 42, 3364–3374. [Google Scholar] [CrossRef] [PubMed]
- Fadhil, M.; Singh, R.; Havas, T.; Jacobson, I. Systematic review of head and neck lymphedema assessment. Head Neck 2022, 44, 2301–2315. [Google Scholar] [CrossRef]
- Jeans, C.; Ward, E.C.; Brown, B.; Vertigan, A.E.; Pigott, A.E.; Nixon, J.L.; Wratten, C.; Boggess, M. Association between external and internal lymphedema and chronic dysphagia following head and neck cancer treatment. Head Neck 2021, 43, 255–267. [Google Scholar] [CrossRef]
- Deng, J.; Ridner, S.; Rothman, R.; Murphy, B.; Sherman, K.; Moore, L.; Hall, K.; Weiner, B. Perceived Symptom Experience in Head and Neck Cancer Patients with Lymphedema. J. Palliat. Med. 2016, 19, 1267–1274. [Google Scholar] [CrossRef]
- Deng, J.; Murphy, B.A.; Dietrich, M.S.; Wells, N.; Wallston, K.A.; Sinard, R.J.; Cmelak, A.J.; Gilbert, J.; Ridner, S.H. Impact of secondary lymphedema after head and neck cancer treatment on symptoms, functional status, and quality of life. Head Neck 2013, 35, 1026–1035. [Google Scholar] [CrossRef]
- Arends, C.R.; Lindhout, J.E.; van der Molen, L.; Wilthagen, E.A.; van den Brekel, M.W.M.; Stuiver, M.M. A systematic review of validated assessments methods for head and neck lymphedema. Eur. Arch. Otorhinolaryngol. 2023, 280, 2653–2661. [Google Scholar] [CrossRef]
- Starmer, H.; Cherry, M.G.; Patterson, J.; Young, B.; Fleming, J. Assessment of Measures of Head and Neck Lymphedema Following Head and Neck Cancer Treatment: A Systematic Review. Lymphat. Res. Biol. 2023, 21, 42–51. [Google Scholar] [CrossRef]
- De Vrieze, T.; Gebruers, N.; Nevelsteen, I.; De Groef, A.; Tjalma, W.A.A.; Thomis, S.; Dams, L.; Van der Gucht, E.; Penen, F.; Devoogdt, N. Reliability of the MoistureMeterD Compact Device and the Pitting Test to Evaluate Local Tissue Water in Subjects with Breast Cancer-Related Lymphedema. Lymphat. Res. Biol. 2020, 18, 116–128. [Google Scholar] [CrossRef]
- Arends, C.R.; van der Molen, L.; van den Brekel, M.W.M.; Stuiver, M.M. Test-Retest Reliability of a Protocol for Assessment of Local Tissue Water in the Head and Neck Area. Lymphat. Res. Biol. 2024, 22, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A.; Nixon, J.; Fleming, J.; McCann, A.; Porceddu, S. Measuring head and neck lymphedema: The “ALOHA” trial. Head Neck 2016, 38, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Hidding, J.T.; Viehoff, P.B.; Beurskens, C.H.; van Laarhoven, H.W.; Nijhuis-van der Sanden, M.W.; van der Wees, P.J. Measurement Properties of Instruments for Measuring of Lymphedema: Systematic Review. Phys. Ther. 2016, 96, 1965–1981. [Google Scholar] [CrossRef] [PubMed]
- Dylke, E.S.; Benincasa Nakagawa, H.; Lin, L.; Clarke, J.L.; Kilbreath, S.L. Reliability and Diagnostic Thresholds for Ultrasound Measurements of Dermal Thickness in Breast Lymphedema. Lymphat. Res. Biol. 2018, 16, 258–262. [Google Scholar] [CrossRef]
- de Rezende, L.F.; Piloni, J.P.M.; Kempa, V.L.; Silva, J.F.R.; Vilas Boas, V.F.; Carvalho, R.L.; Marx, Â.G. Ultrasonography as an instrument to evaluate lymphedema secondary to breast cancer: Systematic review. J. Vasc. Bras. 2023, 22, e20220144. [Google Scholar] [CrossRef]
- Kilbreath, S.L.; Fearn, N.R.; Dylke, E.S. Ultrasound: Assessment of breast dermal thickness: Reliability, responsiveness to change, and relationship to patient-reported outcomes. Skin Res. Technol. 2022, 28, 111–118. [Google Scholar] [CrossRef]
- Devoogdt, N.; Pans, S.; De Groef, A.; Geraerts, I.; Christiaens, M.R.; Neven, P.; Vergote, I.; Van Kampen, M. Postoperative evolution of thickness and echogenicity of cutis and subcutis of patients with and without breast cancer-related lymphedema. Lymphat. Res. Biol. 2014, 12, 23–31. [Google Scholar] [CrossRef]
- Van Aperen, K.; De Groef, A.; Devoogdt, N.; De Vrieze, T.; Troosters, T.; Bollen, H.; Nuyts, S. EffEx-HN trial: Study protocol for a randomized controlled trial on the EFFectiveness and feasibility of a comprehensive supervised EXercise program during radiotherapy in Head and Neck cancer patients on health-related quality of life. Trials 2023, 24, 276. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Dias, A.C.; Jureidini, R.A.G.; Araujo-Filho, J.A.B.; Camerin, G.R.; Zattar, L.C.; Sernik, R.A.; Malhotra, A.; Cerri, L.M.O.; Cerri, G.G. Advanced US of the Skin, Nerves, and Muscles of the Neck: Pearls and Pitfalls with Use of High-Frequency Transducers. Radiographics 2024, 44, e240029. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- Lexell, J.E.; Downham, D.Y. How to assess the reliability of measurements in rehabilitation. Am. J. Phys. Med. Rehabil. 2005, 84, 719–723. [Google Scholar] [CrossRef]
- De Vrieze, T.; Gebruers, N.; Tjalma, W.A.; Nevelsteen, I.; Thomis, S.; De Groef, A.; Dams, L.; Van der Gucht, E.; Belgrado, J.P.; Vandermeeren, L.; et al. What is the best method to determine excessive arm volume in patients with breast cancer-related lymphoedema in clinical practice? Reliability, time efficiency and clinical feasibility of five different methods. Clin. Rehabil. 2019, 33, 1221–1232. [Google Scholar] [CrossRef]
- Devoogdt, N.; Van Kampen, M.; Geraerts, I.; Coremans, T.; Christiaens, M.R. Lymphoedema Functioning, Disability and Health questionnaire (Lymph-ICF): Reliability and validity. Phys. Ther. 2011, 91, 944–957. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Chotipanich, A.; Kongpit, N. Precision and reliability of tape measurements in the assessment of head and neck lymphedema. PLoS ONE 2020, 15, e0233395. [Google Scholar] [CrossRef]
- Jönsson, C.; Bjurberg, M.; Brogårdh, C.; Johansson, K. Test-Retest Reliability of Volume and Local Tissue Water Measurements in Lower Limbs of Healthy Women and Men. Lymphat. Res. Biol. 2020, 18, 261–269. [Google Scholar] [CrossRef]
- Mayrovitz, H.N.; Bernal, M.; Carson, S. Gender differences in facial skin dielectric constant measured at 300 MHz. Skin Res. Technol. 2012, 18, 504–510. [Google Scholar] [CrossRef]
- Mayrovitz, H.N.; Patel, A.; Kavadi, R.; Khan, Z.; Bartolone, S. An Approach Toward Assessing Head-and-Neck Lymphedema Using Tissue Dielectric Constant Ratios: Method and Normal Reference Values. Lymphat. Res. Biol. 2021, 19, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Feng, L.; Shan, J.; Yuan, Z.; Jin, L. Application of high-frequency ultrasound to assess facial skin thickness in association with gender, age, and BMI in healthy adults. BMC Med. Imaging 2022, 22, 113. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.M.; Seo, J.Y.; Kim, A.; Kim, Y.C.; Baek, Y.S.; Oh, C.H.; Jeon, J. Ultrasonographic analysis of facial skin thickness in relation to age, site, sex, and body mass index. Skin Res. Technol. 2023, 29, e13426. [Google Scholar] [CrossRef] [PubMed]
- Mayrovitz, H.N.; Aoki, K.; Deehan, E.; Ruppe, M. Epidermal and dermal hydration in relation to skin color parameters. Skin Res. Technol. 2024, 30, e70028. [Google Scholar] [CrossRef]
- Ferretti, R.; Pádua, C.; Passos, M.; Ferrari, G.; Fisberg, M. Elevated neck circumference and associated factors in adolescents. BMC Public Health 2015, 15, 1517. [Google Scholar] [CrossRef]
- Ridner, S.H.; Bonner, C.M.; Doersam, J.K.; Rhoten, B.A.; Schultze, B.; Dietrich, M.S. Bioelectrical impedance self-measurement protocol development and daily variation between healthy volunteers and breast cancer survivors with lymphedema. Lymphat. Res. Biol. 2014, 12, 2–9. [Google Scholar] [CrossRef]
- De Vrieze, T.; Gebruers, N.; Nevelsteen, I.; Tjalma, W.A.A.; Thomis, S.; De Groef, A.; Dams, L.; Van der Gucht, E.; Devoogdt, N. Physical activity level and age contribute to functioning problems in patients with breast cancer-related lymphedema: A multicentre cross-sectional study. Support. Care Cancer. 2020, 28, 5717–5731. [Google Scholar] [CrossRef]
- Czerniec, S.A.; Ward, L.C.; Kilbreath, S.L. Breast Cancer-Related Arm Lymphedema: Fluctuation over Six Months and the Effect of the Weather. Lymphat. Res. Biol. 2016, 14, 148–155. [Google Scholar] [CrossRef]
- Aulino, J.M.; Wulff-Burchfield, E.M.; Dietrich, M.S.; Ridner, S.H.; Niermann, K.J.; Deng, J.; Rhoten, B.A.; Doersam, J.K.; Jarrett, L.A.; Mannion, K.; et al. Evaluation of CT Changes in the Head and Neck After Cancer Treatment: Development of a Measurement Tool. Lymphat. Res. Biol. 2018, 16, 69–74. [Google Scholar] [CrossRef]
- West, N.A.; Attia, S.K.; Kaffey, Z.; Dede, C.; Mulder, S.L.; El-Habashy, D.M.; Neuberger, R.; Naser, M.A.; Frank, S.J.; Mao, S.; et al. Evaluating observer reliability and diagnostic accuracy of CT-LEFAT criteria for post-treatment head and neck lymphedema: A prospective blinded comparative analysis. Oral Oncology 2025, 164, 107265. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Li, X.; Hao, Q.; Li, B.; Wang, R. MRI-based volume measurement methods for staging primary lower extremity lymphedema: A single-center study of asymmetric volume difference-a diagnostic study. BMC Musculoskelet. Disord. 2023, 24, 810. [Google Scholar] [CrossRef]
- Kim, G.; Smith, M.P.; Donohoe, K.J.; Johnson, A.R.; Singhal, D.; Tsai, L.L. MRI staging of upper extremity secondary lymphedema: Correlation with clinical measurements. Eur. Radiol. 2020, 30, 4686–4694. [Google Scholar] [CrossRef] [PubMed]
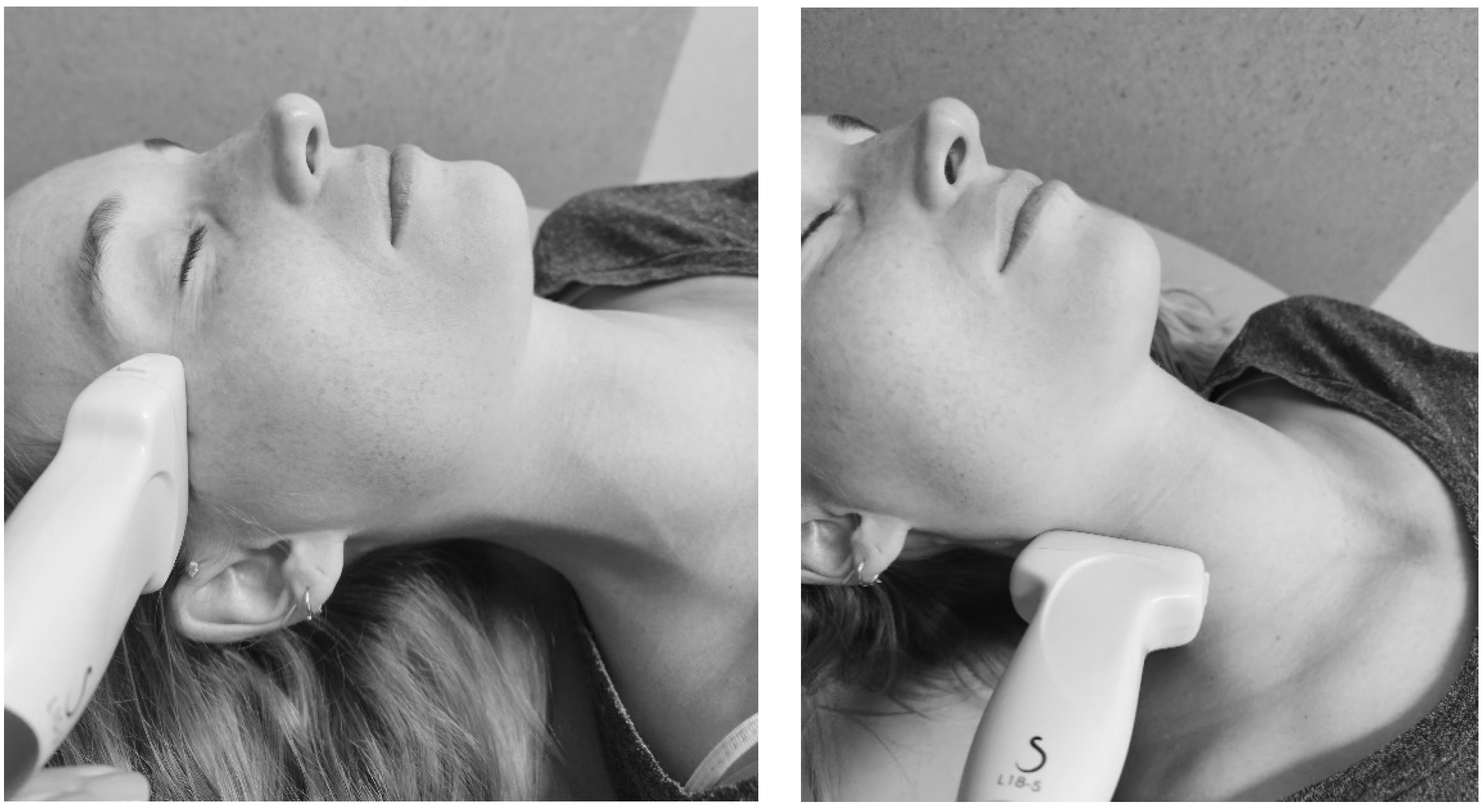
| Assessment Methods | Measurement Points | |
|---|---|---|
| % local tissue water with the MMDC Dermal thickness with B-mode ultrasound | 1—“Temporal” point left and right 2—“Mid-Tragus-Oral” point left and right 3a—“Superior Mandibular” point left and right 3b—“Inferior Mandibular” point left and right 4—“Submental” point 5a—“Superior Sternocleidomastoid” point left and right 5b—“Mid Sternocleidomastoid” point left and right 5c—“Inferior Sternocleidomastoid” point left and right | 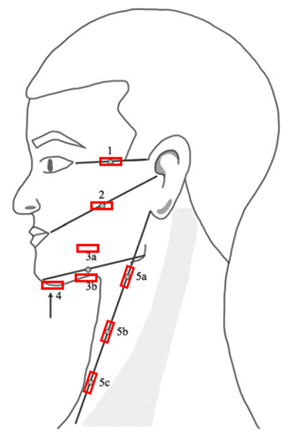 |
| Neck circumference with a tape measure | 5a—“Superior Sternocleidomastoid” point 5b—“Mid Sternocleidomastoid” point 5c—“Inferior Sternocleidomastoid” point |
 = positioning of the probes, for the measurement of dermal thickness with B-mode ultrasound. Measurement points were marked with a washable skin pencil as follows: from the lateral corner of the eye to the top of the ear (point 1); from the corner of the mouth to the tragus (point 2); and from the chin to the jaw, with the MMDC placed 1 cm above (point 3a); and 1 cm below (point 3b). The submental area was marked centrally (point 4). The tape measure was placed from the earlobe to the sternoclavicular joint, with points 5a, 5b, and 5c marked at 25%, 50%, and 75% of the total distance, respectively.
= positioning of the probes, for the measurement of dermal thickness with B-mode ultrasound. Measurement points were marked with a washable skin pencil as follows: from the lateral corner of the eye to the top of the ear (point 1); from the corner of the mouth to the tragus (point 2); and from the chin to the jaw, with the MMDC placed 1 cm above (point 3a); and 1 cm below (point 3b). The submental area was marked centrally (point 4). The tape measure was placed from the earlobe to the sternoclavicular joint, with points 5a, 5b, and 5c marked at 25%, 50%, and 75% of the total distance, respectively.| Median (IQR) [Min–Max] | ||
|---|---|---|
| Age (years) | 64.0 (52.5–69.5) [29–84] | |
| BMI (kg/m2) | 26.9 (23.6–30.5) [12.7–37.3] | |
| Time since initiation of radiotherapy (weeks) | 24 (12–52) [6–52] | |
| Number (%) | ||
| Gender | Male | 24 (73%) |
| Female | 9 (27%) | |
| Skin type | White | 32 (97%) |
| Other | 1 (3%) | |
| Primary tumor location | Nasal cavity/paranasal sinuses | 5 (15%) |
| Oropharynx | 6 (18%) | |
| Oral cavity | 11 (33%) | |
| Hypopharynx | 2 (6%) | |
| Larynx | 2 (6%) | |
| Salivary gland | 4 (12%) | |
| Thyroid | 1 (3%) | |
| Other | 2 (6%) | |
| T-Tumor classification (TNM) [32] | T1 | 7 (21%) |
| T2 | 9 (27%) | |
| T3 | 6 (18%) | |
| T4 | 10 (30%) | |
| TX | 1 (3%) | |
| N-Node classification (TNM) [32] | N0 | 16 (48%) |
| N1 | 7 (21%) | |
| N2 | 8 (27%) | |
| NX | 2 (6%) | |
| Cancer treatment | Definitive (C)RT | 19 (58%) |
| Unilateral RT | 7 (21%) | |
| Bilateral RT | 3 (9%) | |
| Unilateral CRT | 6 (18%) | |
| Bilateral CRT | 3 (9%) | |
| Post-operative (C)RT | 14 (42%) | |
| Unilateral neck dissection | 9 (27%) | |
| Bilateral neck dissection | 5 (15%) | |
| Presence of HNL based on a positive response to item 6 of the Lymphedema Symptom Intensity and Distress Survey-Head and Neck version 2.0: “Swelling in your face, head or neck?” | Subjective presence of HNL | 15 (45%) |
| Presence of HNL based on visual inspection | Subjective presence of HNL | 19 (58%) |
| Median (Q1, Q3) | p-Value Wilcoxon Signed-Rank Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R1a | N | R1b | N | R2 | N | Inter-Rater | Intra-Rater | ||
| Local tissue water (in %) | |||||||||
| 1 “Temporal” | R | 43 (40, 48) | 32 | 44 (41, 50) | 31 | 45 (41, 49) | 33 | 0.125 | 0.167 |
| L | 43 (39, 46) | 32 | 44 (38, 48) | 31 | 45 (40, 47) | 33 | 0.085 | 0.095 | |
| 2 “Mid-Tragus-Oral” | R | 46 (40, 50) | 30 | 46 (41, 51) | 29 | 46 (41, 51) | 32 | 0.914 | 0.248 |
| L | 42 (38, 46) | 30 | 42 (40, 47) | 29 | 44 (39, 48) | 31 | 0.416 | 0.046 | |
| 3a “Superior Mandibular” | R | 44 (40, 48) | 30 | 45 (39, 48) | 29 | 47 (41, 52) | 27 | 0.265 | 0.152 |
| L | 45 (40, 47) | 28 | 45 (41, 49) | 27 | 44 (40, 49) | 31 | 0.973 | 0.037 | |
| 3b “Inferior Mandibular” | R | 48 (44, 52) | 30 | 48 (41, 51) | 30 | 48 (43, 52) | 31 | 0.428 | 0.260 |
| L | 46 (44, 51) | 29 | 47 (44, 54) | 27 | 45 (42, 52) | 30 | 0.107 | 0.466 | |
| 4 “Submental” | n/a | 44 (41, 50) | 31 | 44 (40, 49) | 29 | 45 (40, 50) | 32 | 0.934 | 0.798 |
| 5a “Superior SCM” | R | 48 (44, 53) | 31 | 50 (45, 55) | 30 | 48 (43, 53) | 32 | 0.829 | 0.028 |
| L | 43 (38, 46) | 31 | 48 (43, 53) | 30 | 50 (46, 55) | 32 | 0.034 | 0.030 | |
| 5b “Mid SCM” | R | 50 (68, 56) | 31 | 52 (48, 56) | 30 | 52 (48, 57) | 32 | 0.617 | 0.284 |
| L | 50 (46, 56) | 31 | 51 (46, 58) | 30 | 52 (48, 55) | 31 | 0.496 | 0.007 | |
| 5c “Inferior SCM” | R | 49 (42, 54) | 32 | 49 (43, 53) | 30 | 52 (48, 56) | 33 | 0.277 | 0.278 |
| L | 51 (46, 53) | 31 | 50 (46, 53) | 31 | 48 (44, 52) | 33 | 0.008 | 0.329 | |
| Neck circumference (in cm) | |||||||||
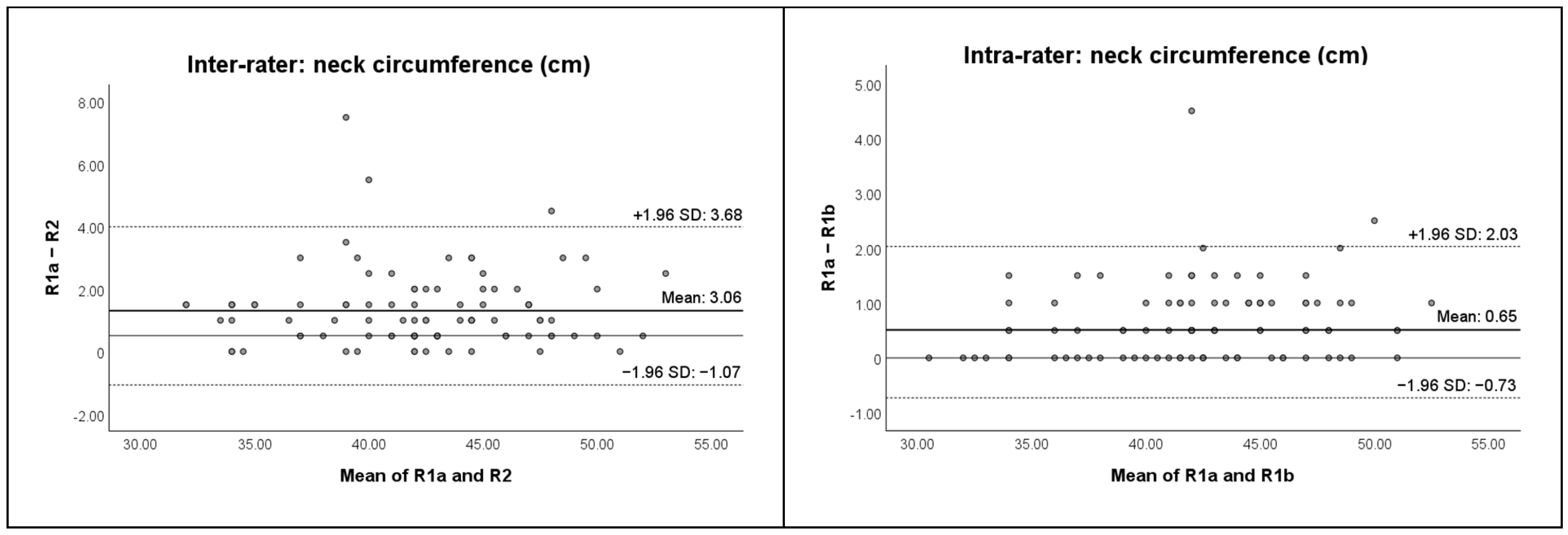
| 5a “Superior SCM” | n/a | 43.5 (42.0, 47.5) | 31 | 42.5 (41.5, 47.5) | 31 | 44.0 (40.0, 47.5) | 33 | 0.885 | 0.102 |
| 5b “Mid SCM” | n/a | 42.0 (39.5, 44.0) | 31 | 42.0 (39.0, 45.0) | 31 | 42.5 (39.0, 45.0) | 33 | 0.195 | 0.372 |
| 5c “Inferior SCM” | n/a | 42.5 (39.0, 45.5) | 31 | 42.5 (39.0, 45.0) | 31 | 42.5 (39.5, 46.0) | 33 | 0.609 | 0.562 |
| Dermal thickness (in µm) | |||||||||
| 1 “Temporal” | R | 1403 (1209, 1654) | 28 | 1418 (1220, 1784) | 25 | 1480 (1245, 1721) | 26 | 0.288 | 0.118 |
| L | 1415 (1109, 1647) | 28 | 1338 (1113, 1628) | 25 | 1413 (1068, 1721) | 26 | 0.459 | 0.581 | |
| 2 “Mid-Tragus-Oral” | R | 1342 (1159, 1670) | 28 | 1412 (1174, 1693) | 25 | 1419 (1186, 1685) | 26 | 0.534 | 0.701 |
| L | 1418 (1067, 1657) | 28 | 1418 (1121, 1567) | 25 | 1459 (1094, 1625) | 26 | 0.280 | 0.532 | |
| 3a “Superior Mandibular” | R | 1250 (1189, 1474) | 28 | 1256 (1174, 1487) | 25 | 1288 (1203, 1432) | 26 | 0.638 | 0.809 |
| L | 1296 (1153, 1412) | 28 | 1279 (1141, 1358) | 25 | 1276 (1156, 1358) | 26 | 0.048 | 0.269 | |
| 3b “Inferior Mandibular” | R | 1456 (1281, 1631) | 28 | 1494 (1252, 1630) | 25 | 1469 (1345, 1593) | 26 | 0.265 | 0.889 |
| L | 1417 (1253, 1641) | 28 | 1379 (1231, 1612) | 25 | 1469 (1237, 1621) | 26 | 0.694 | 0.597 | |
| 4 “Submental” | n/a | 1357 (1220, 1494) | 28 | 1433 (1189, 1528) | 25 | 1404 (1250, 1566) | 26 | 0.638 | 0.895 |
| 5a “Superior SCM” | R | 1436 (1241, 1569) | 28 | 1482 (1243, 1582) | 25 | 1428 (1227, 1578) | 26 | 0.968 | 0.882 |
| L | 1408 (1243, 1552) | 28 | 1389 (1236, 1515) | 25 | 1503 (1250, 1584) | 26 | 0.932 | 0.220 | |
| 5b “Mid SCM” | R | 1278 (1032, 1525) | 28 | 1316 (1125, 1539) | 25 | 1358 (1194, 1491) | 26 | 0.737 | 0.220 |
| L | 1301 (1202, 1483) | 28 | 1284 (1174, 1416) | 25 | 1351 (1139, 1570) | 26 | 0.182 | 0.115 | |
| 5c “Inferior SCM” | R | 1242 (1016, 1401) | 28 | 1247 (1031, 1391) | 25 | 1282 (1086, 1385) | 26 | 0.770 | 0.829 |
| L | 1281 (1067, 1397) | 28 | 1249 (1117, 1377) | 25 | 1286 (1104, 1425) | 26 | 0.454 | 0.166 | |
| Inter-Rater | Intra-Rater | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ICC2,1 (95% CI) | SEM | %SEM | SRD | ICC2,1 (95% CI) | SEM | %SEM | SRD | ||
| Local tissue water (in %) | |||||||||
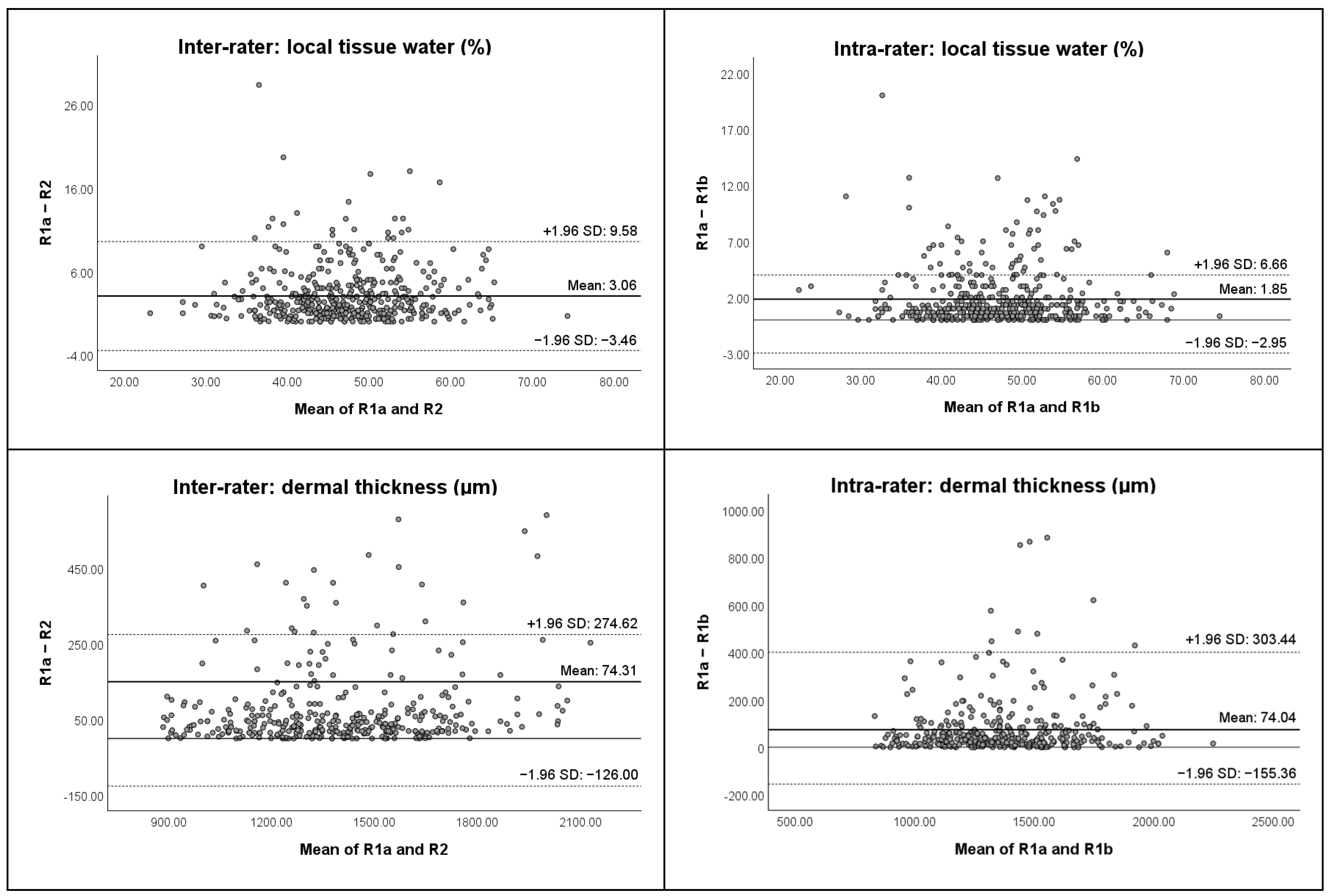
| 1 “Temporal” | R | 0.727 (0.448–0.865) | 3 | 7 | 8 | 0.919 (0.836–0.960) | 2 | 4 | 5 |
| L | 0.813 (0.625–0.907) | 2 | 6 | 8 | 0.963 (0.923–0.982) | 1 | 3 | 4 | |
| 2 “Mid-Tragus-Oral” | R | 0.882 (0.754–0.944) | 2 | 5 | 6 | 0.922 (0.839–0.962) | 2 | 4 | 5 |
| L | 0.774 (0.514–0.894) | 4 | 9 | 11 | 0.851 (0.691–0.929) | 3 | 7 | 8 | |
| 3a “Superior Mandibular” | R | 0.644 (0.254–0.833) | 4 | 9 | 12 | 0.914 (0.819–0.960) | 2 | 5 | 6 |
| L | 0.745 (0.454–0.880) | 3 | 8 | 9 | 0.908 (0.798–0.957) | 2 | 4 | 5 | |
| 3b “Inferior Mandibular” | R | 0.951 (0.900–0.976) | 2 | 4 | 6 | 0.990 (0.979–0.995) | 1 | 2 | 3 |
| L | 0.938 (0.872–0.971) | 2 | 4 | 6 | 0.970 (0.937–0.986) | 2 | 3 | 4 | |
| 4 “Submental” | n/a | 0.964 (0.924–0.982) | 1 | 3 | 4 | 0.939 (0.875–0.971) | 2 | 4 | 6 |
| 5a “Superior SCM” | R | 0.906 (0.808–0.954) | 2 | 4 | 6 | 0.974 (0.947–0.987) | 1 | 2 | 3 |
| L | 0.939 (0.874–0.970) | 2 | 4 | 5 | 0.970 (0.939–0.986) | 1 | 3 | 4 | |
| 5b “Mid SCM” | R | 0.938 (0.873–0.970) | 2 | 4 | 5 | 0.988 (0.974–0.995) | 1 | 2 | 2 |
| L | 0.949 (0.885–0.976) | 2 | 3 | 4 | 0.961 (0.908–0.982) | 1 | 3 | 4 | |
| 5c “Inferior SCM” | R | 0.924 (0.845–0.962) | 2 | 4 | 6 | 0.974 (0.948–0.987) | 1 | 3 | 4 |
| L | 0.888 (0.768–0.945) | 2 | 4 | 5 | 0.972 (0.943–0.986) | 1 | 2 | 3 | |
| Neck circumference (in cm) | |||||||||
| 5a “Superior SCM” | n/a | 0.958 (0.915–0.980) | 1.0 | 2.5 | 3.0 | 0.982 (0.963–0.992) | 0.5 | 1.5 | 2.0 |
| 5b “Mid SCM” | n/a | 0.973 (0.945–0.987) | 1.0 | 2.0 | 2.0 | 0.993 (0.986–0.997) | 0.5 | 1.0 | 1.0 |
| 5c “Inferior SCM” | n/a | 0.959 (0.916–0.980) | 1.0 | 2.5 | 2.5 | 0.994 (0.988–0.997) | 0.5 | 1.0 | 1.0 |
| Dermal thickness (in µm) | |||||||||
| 1 “Temporal” | R | 0.956 (0.902–0.981) | 67 | 5 | 186 | 0.354 (−0.392–0.703) | 253 | 16 | 703 |
| L | 0.945 (0.877–0.975) | 82 | 6 | 227 | 0.969 (0.932–0.986) | 58 | 4 | 161 | |
| 2 “Mid-Tragus-Oral” | R | 0.964 (0.921–0.984) | 60 | 4 | 167 | 0.781 (0.518–0.900) | 159 | 11 | 442 |
| L | 0.958 (0.907–0.981) | 70 | 5 | 195 | 0.968 (0.931–0.986) | 57 | 4 | 160 | |
| 3a “Superior Mandibular” | R | 0.849 (0.662–0.933) | 69 | 5 | 191 | 0.852 (0.680–0.932) | 71 | 5 | 198 |
| L | 0.860 (0.690–0.937) | 63 | 5 | 174 | 0.969 (0.932–0.986) | 31 | 2 | 86 | |
| 3b “Inferior Mandibular” | R | 0.900 (0.772–0.956) | 69 | 5 | 191 | 0.915 (0.814–0.961) | 70 | 5 | 194 |
| L | 0.982 (0.960–0.992) | 28 | 2 | 79 | 0.941 (0.870–0.973) | 56 | 4 | 156 | |
| 4 “Submental” | n/a | 0.950 (0.890–0.977) | 48 | 3 | 133 | 0.879 (0.735–0.945) | 73 | 5 | 202 |
| 5a “Superior SCM” | R | 0.903 (0.782–0.956) | 76 | 5 | 124 | 0.840 (0.652–0.927) | 100 | 7 | 277 |
| L | 0.968 (0.928–0.986) | 45 | 3 | 186 | 0.964 (0.922–0.984) | 45 | 3 | 124 | |
| 5b “Mid SCM” | R | 0.921 (0.825–0.965) | 70 | 5 | 194 | 0.896 (0.775–0.953) | 77 | 6 | 212 |
| L | 0.916 (0.813–0.962) | 67 | 5 | 997 | 0.913 (0.802–0.961) | 65 | 5 | 180 | |
| 5c “Inferior SCM” | R | 0.946 (0.878–0.976) | 55 | 4 | 152 | 0.948 (0.887–0.977) | 56 | 5 | 154 |
| L | 0.136 (−0.906–0.611) | 360 | 28 | 1906 | 0.254 (−0.617–0.658) | 339 | 26 | 939 | |
| Local Tissue Water | Neck Circumference | Dermal Thickness | |
|---|---|---|---|
| Time efficiency (in seconds) | |||
| Median preparation time (SD) | 153 (15) | 21 (6) | 153 (15) |
| Median execution time (SD) | 405 (70) | 39 (8) | 557 (81) |
| Median process time (SD) | n/a | n/a | 1778 (124) |
| Median total time (SD) | 579 (71) | 57 (11) | 2476 (257) |
| Clinical limitations | |||
| Training of raters is needed. “The quality and accuracy of the measurements can vary significantly based on the raters’ skill and experience.” “The head and neck area is anatomically complex with many curves and contours. Achieving consistent contact with the device on curved surfaces like the jawline or chin can be challenging, potentially leading to variations in readings.” [21] “A discrepancy between evaluators could arise if the tape measure is pulled at different degrees of tension.” [38] “Variability in how the MMDC and ultrasound probes are placed, the angle of contact and the pressure applied to the skin during the measurement can affect the results.” [21] | X | X | X |
| No direct assessment of HNL, measures only an aspect of potential HNL. “In areas where the skin is thicker or where subcutaneous tissues are deeper, such as certain parts of the neck, the device may not provide an accurate reflection of lymphedema throughout the entire subcutaneous layer.” “It does not provide information on underlying tissue changes, such as fluid composition, and cannot differentiate between fat accumulation, muscle hypertrophy, and true lymphedema.” | X | X | X |
| Effect of movement in the head and neck area. “Any movement can affect the precision and repeatability of the measurements. Repeated measurements can show variability due to slight changes in device positioning or the patients’ movements (for example breathing, swallowing). This can make it difficult to obtain highly precise or reproducible measurements, especially in the head and neck area where movement is common.” | X | X | X |
| “Very sensitive device. The measurement is highly sensitive to surface conditions, such as the presence of sweat on the skin, open wounds and beard growth. This might lead to missing data.” | X | X | |
| “Changes in room temperature or skin temperature during measurement can introduce variability, especially since the skin on the face and neck is sensitive to temperature changes.” [21] | X | X | |
| “The device can be sensitive to the presence of veins and other blood vessels beneath the skin. The results are also affected by other factors like blood flow and vessel density.” | X | ||
| “Expensive device because high-frequency ultrasound is needed to achieve detailed images of thin structures like the dermis.” | X | ||
| “The device is not portable or easily moved.” | X | ||
| “In cases of severe HNL, it can be difficult to distinguish between the dermis and subcutis on ultrasound.” | X | ||
| Number of limitations | 6 | 3 | 8 |
| Expected Correlations | R | L |
|---|---|---|
| 1. Positive correlation between local tissue water and dermal thickness of the “Temporal” (1) point | 0.076 (N = 29) | 0.310 (N = 29) |
| 2. Positive correlation between local tissue water and dermal thickness of the “Mid-Tragus-Oral” (2) point | 0.143 (N = 28) | 0.246 (N = 27) |
| 3. Positive correlation between local tissue water and dermal thickness of the “Superior Mandibular” (3a) point | 0.095 (N = 26) | 0.267 (N = 25) |
| 4. Positive correlation between local tissue water and dermal thickness of the “Inferior Mandibular” (3b) point | 0.143 (N = 27) | 0.377 (N = 27) |
| 5. Positive correlation between local tissue water and dermal thickness of the “Submental” (4) point | 0.232 (N = 28) | |
| 6. Positive correlation between local tissue water and neck circumference of the “Superior Sternocleidomastoid” (5a) point | 0.067 (N = 33) | 0.229 (N = 33) |
| 7. Positive correlation between local tissue water and dermal thickness of the “Superior Sternocleidomastoid” (5a) point | 0.266 (N = 28) | 0.133 (N = 28) |
| 8. Positive correlation between the neck circumference and dermal thickness of the “Superior Sternocleidomastoid” (5a) point | 0.240 (N = 29) | −0.008 (N = 29) |
| 9.Positive correlation between local tissue water and neck circumference of the “Mid Sternocleidomastoid” (5b) point | 0.192 (N = 33) | 0.155 (N = 33) |
| 10.Positive correlation between local tissue water and dermal thickness of the “Mid Sternocleidomastoid” (5b) point | 0.390 * (N = 28) | 0.352 (N = 28) |
| 11. Positive correlation between the neck circumference and dermal thickness of the “Mid Sternocleidomastoid” (5b) point | 0.165 (N = 29) | 0.208 (N 29) |
| 12. Positive correlation between local tissue water and neck circumference of the “Inferior Sternocleidomastoid” (5c) point | 0.092 (N = 34) | 0.140 (N = 34) |
| 13. Positive correlation between local tissue water and dermal thickness of the “Inferior Sternocleidomastoid” (5c) point | 0.500 **(N = 29) | 0.268 (N = 29) |
| 14. Positive correlation between the neck circumference and dermal thickness of the “Inferior Sternocleidomastoid” (5c) point | 0.033 (N = 29) | −0.270 (N = 29) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Aperen, K.; Nuyts, S.; Troosters, T.; Devoogdt, N.; De Vrieze, T.; Gürsen, C.; Verbeelen, K.; Devos, J.; De Groef, A. Reliability and Clinical Feasibility of Three Assessment Methods for Head and Neck Lymphedema in Head and Neck Cancer Patients. Cancers 2025, 17, 1672. https://doi.org/10.3390/cancers17101672
Van Aperen K, Nuyts S, Troosters T, Devoogdt N, De Vrieze T, Gürsen C, Verbeelen K, Devos J, De Groef A. Reliability and Clinical Feasibility of Three Assessment Methods for Head and Neck Lymphedema in Head and Neck Cancer Patients. Cancers. 2025; 17(10):1672. https://doi.org/10.3390/cancers17101672
Chicago/Turabian StyleVan Aperen, Kaat, Sandra Nuyts, Thierry Troosters, Nele Devoogdt, Tessa De Vrieze, Ceren Gürsen, Kaat Verbeelen, Johannes Devos, and An De Groef. 2025. "Reliability and Clinical Feasibility of Three Assessment Methods for Head and Neck Lymphedema in Head and Neck Cancer Patients" Cancers 17, no. 10: 1672. https://doi.org/10.3390/cancers17101672
APA StyleVan Aperen, K., Nuyts, S., Troosters, T., Devoogdt, N., De Vrieze, T., Gürsen, C., Verbeelen, K., Devos, J., & De Groef, A. (2025). Reliability and Clinical Feasibility of Three Assessment Methods for Head and Neck Lymphedema in Head and Neck Cancer Patients. Cancers, 17(10), 1672. https://doi.org/10.3390/cancers17101672








