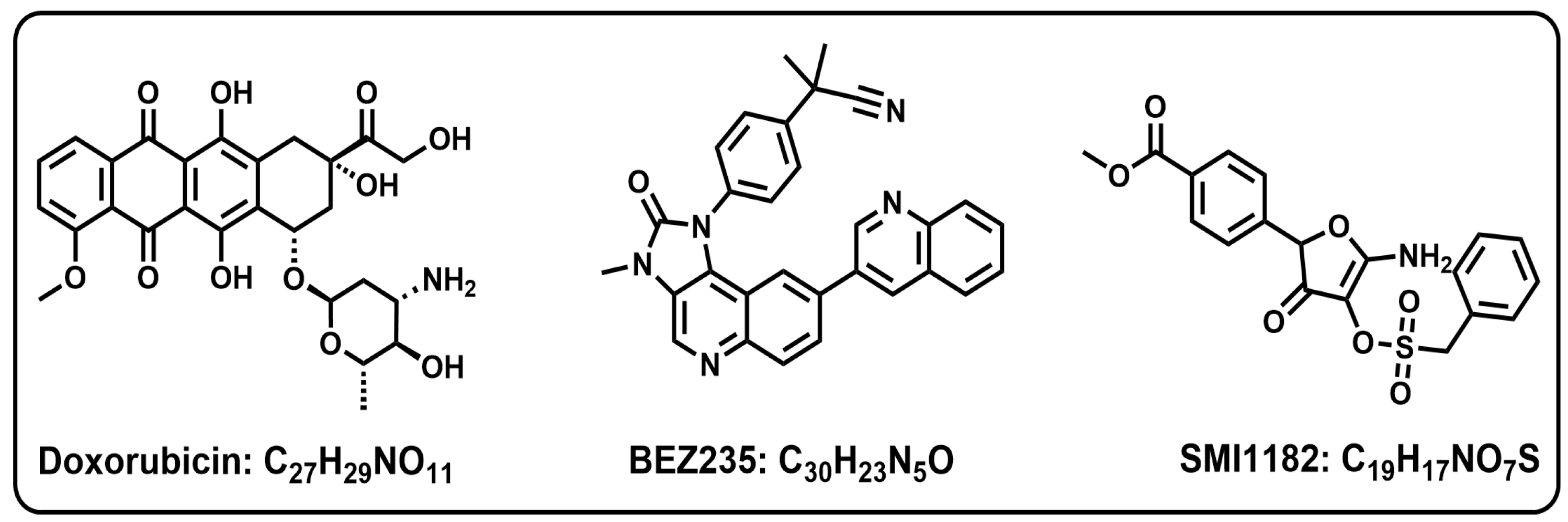
Chondrosarcoma: Multi-Targeting Therapeutic Effects of Doxorubicin, BEZ235, and the Small Molecule Aspartyl-Asparaginyl-β-hydroxylase Inhibitor SMI1182
Simple Summary
Abstract
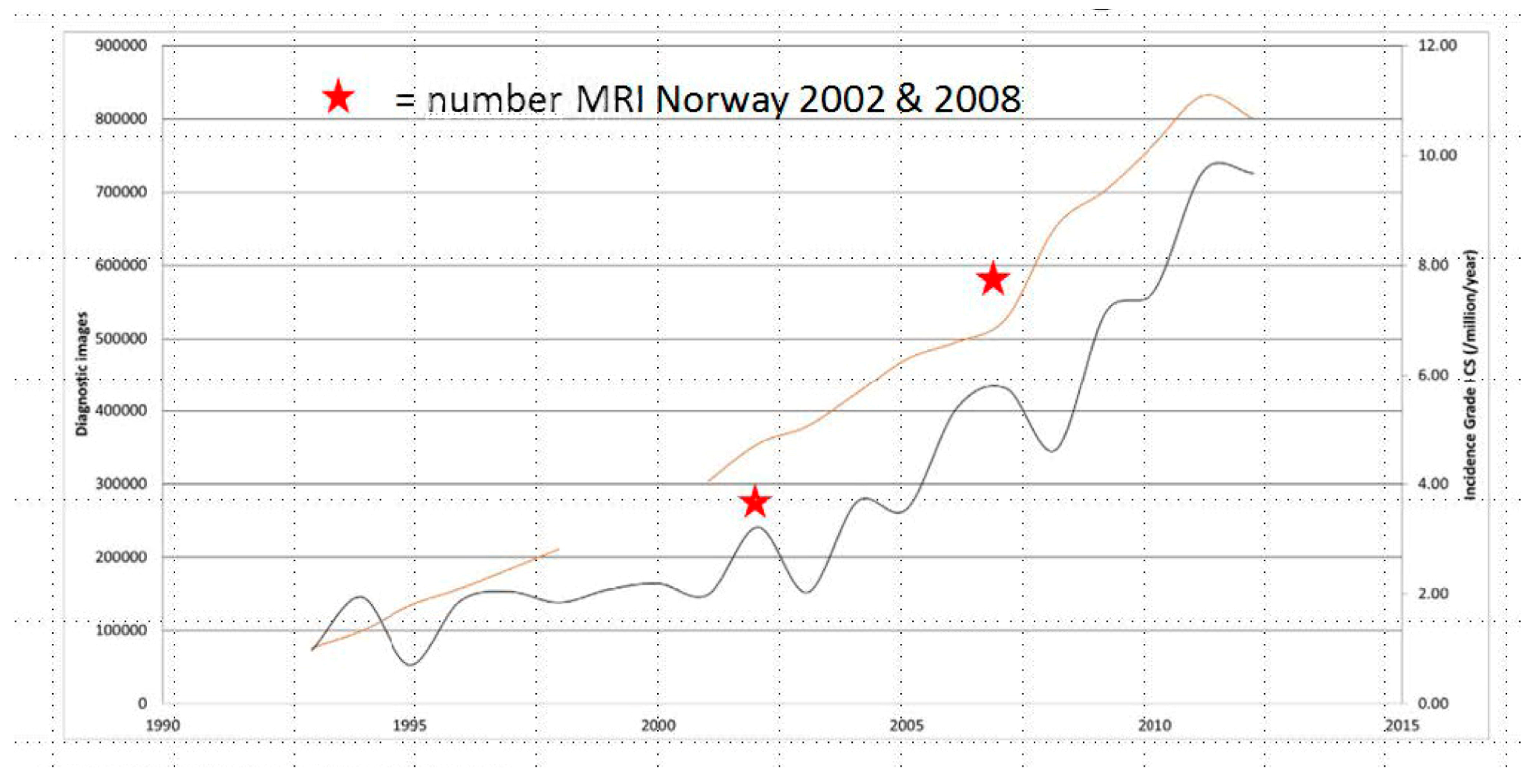
1. Introduction
2. Materials and Methods
2.1. Cell Viability and Cytotoxicity Assays
2.2. Sample Preparation for Immunoassays
2.3. Western Blot Analysis
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Immunocytochemistry
2.6. Motility Assay
2.7. Quantigene 2.0 RNA Multiplex Assay
2.8. Multiplex ELISAs (Akt-mTOR Pathway)
2.9. Data Analysis
2.10. Reagent Sources
3. Results
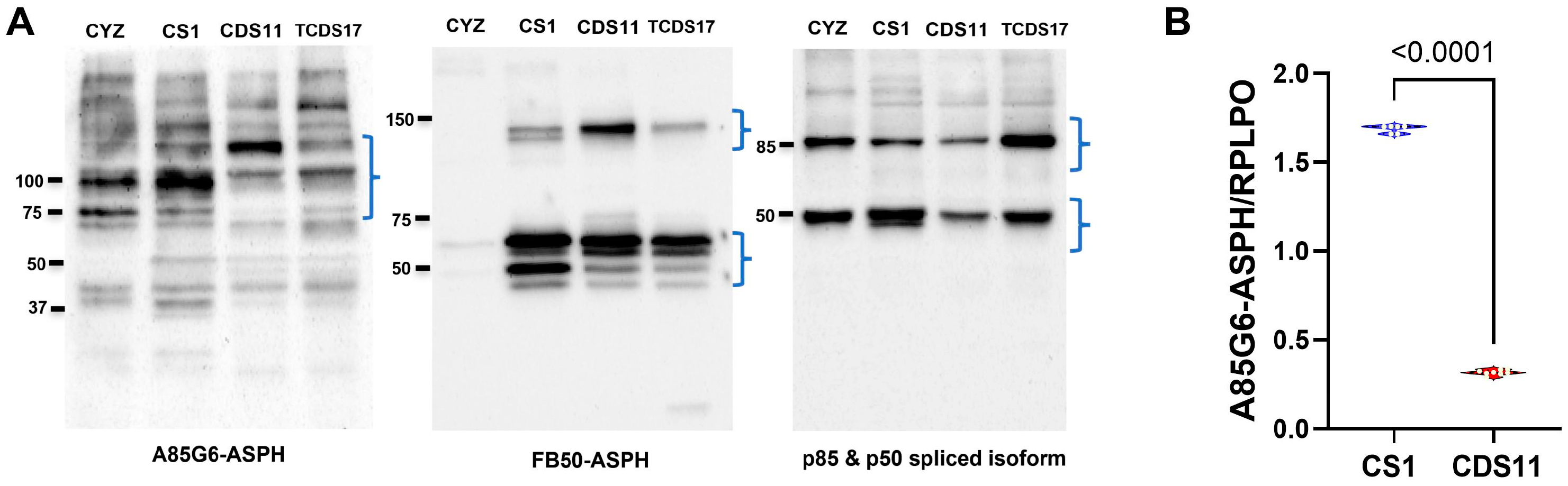
3.1. ASPH Immunoreactivity Detected in CS Cells
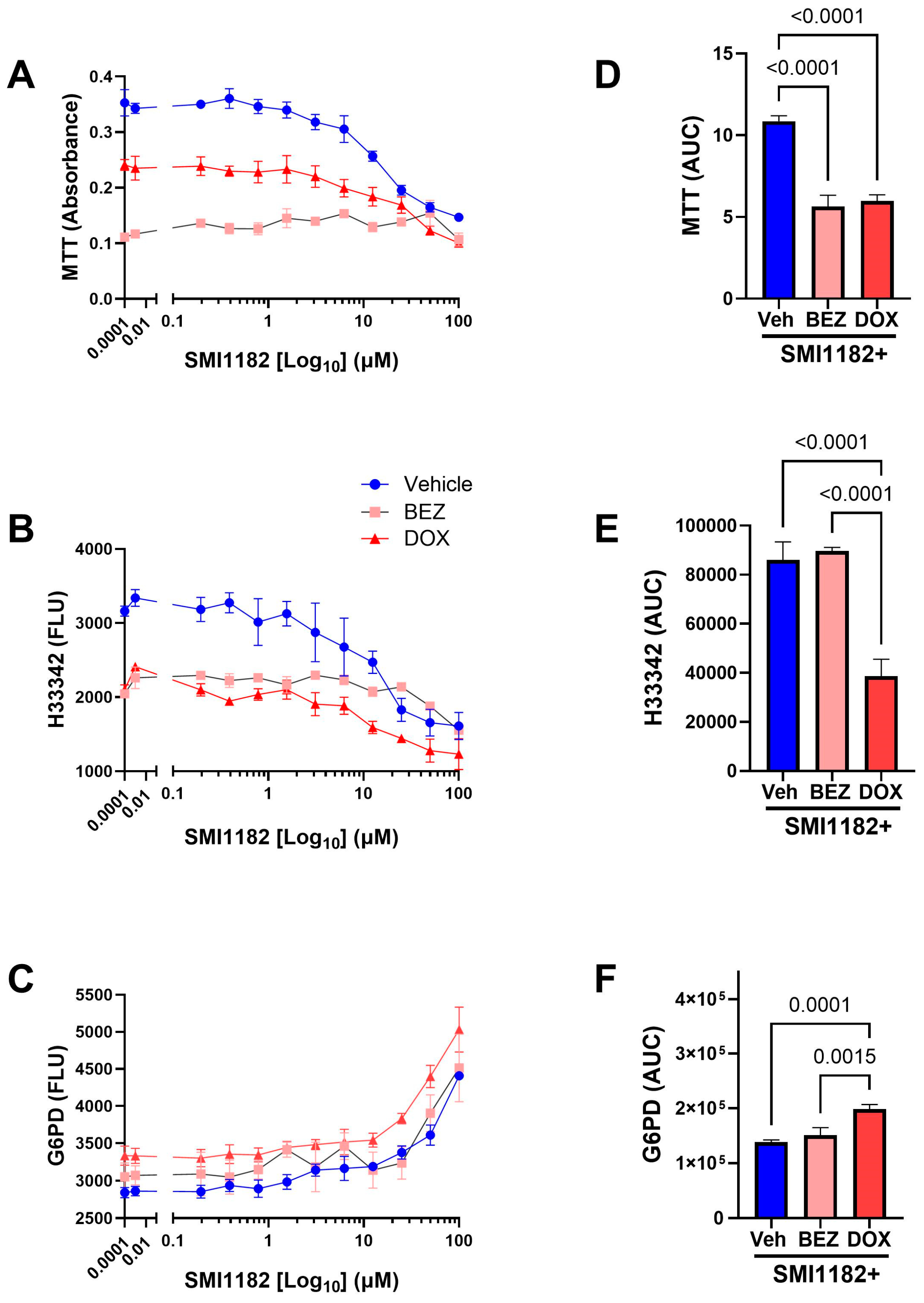
3.2. Mechanistic and Functional Differences Between CS1 and CDS11 Cells
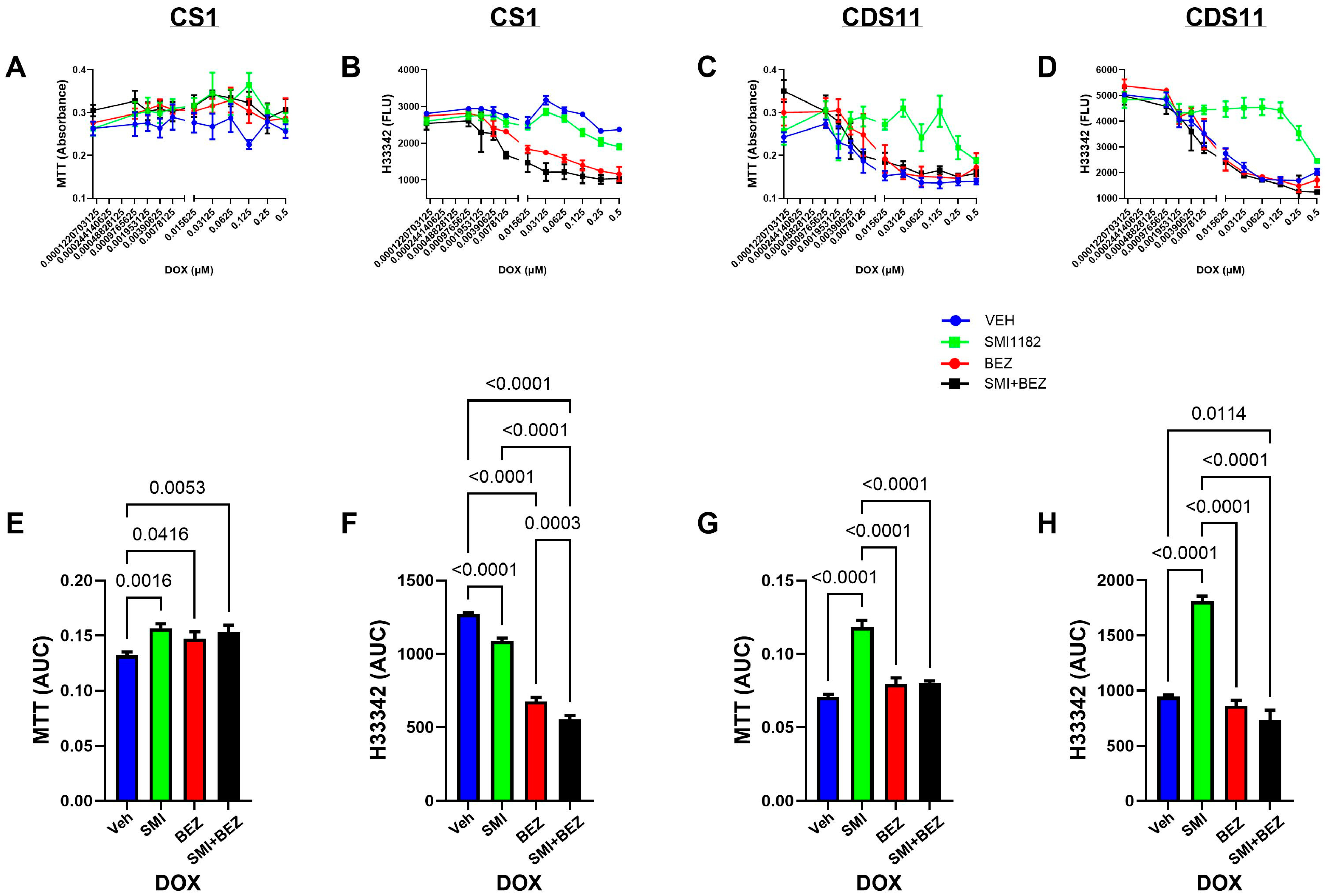
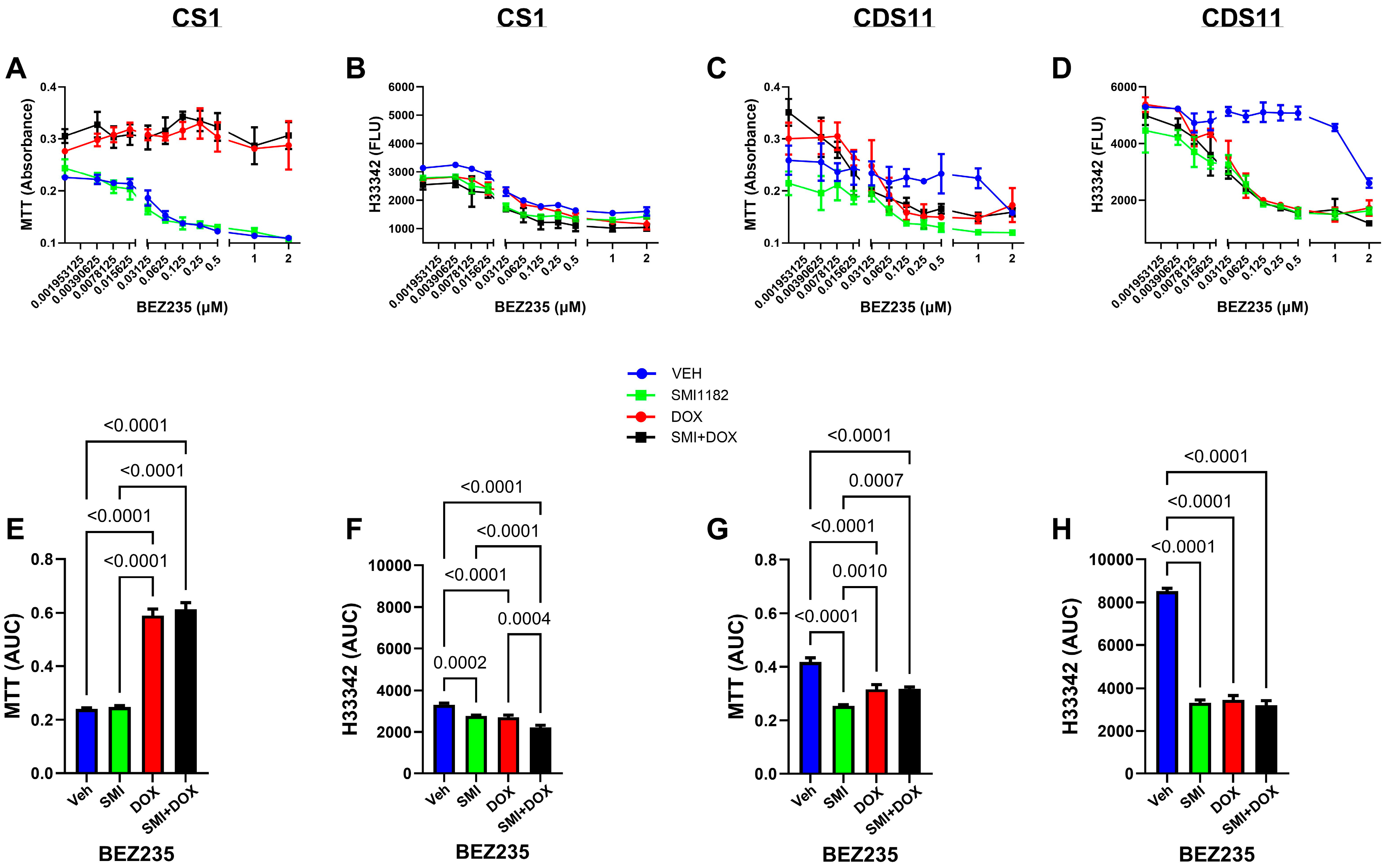
3.3. DOX, BEZ235, and SMI1182 Monotherapy and Combination Therapy Effects on Metabolic Activity, Cell Viability, and Cytotoxicity
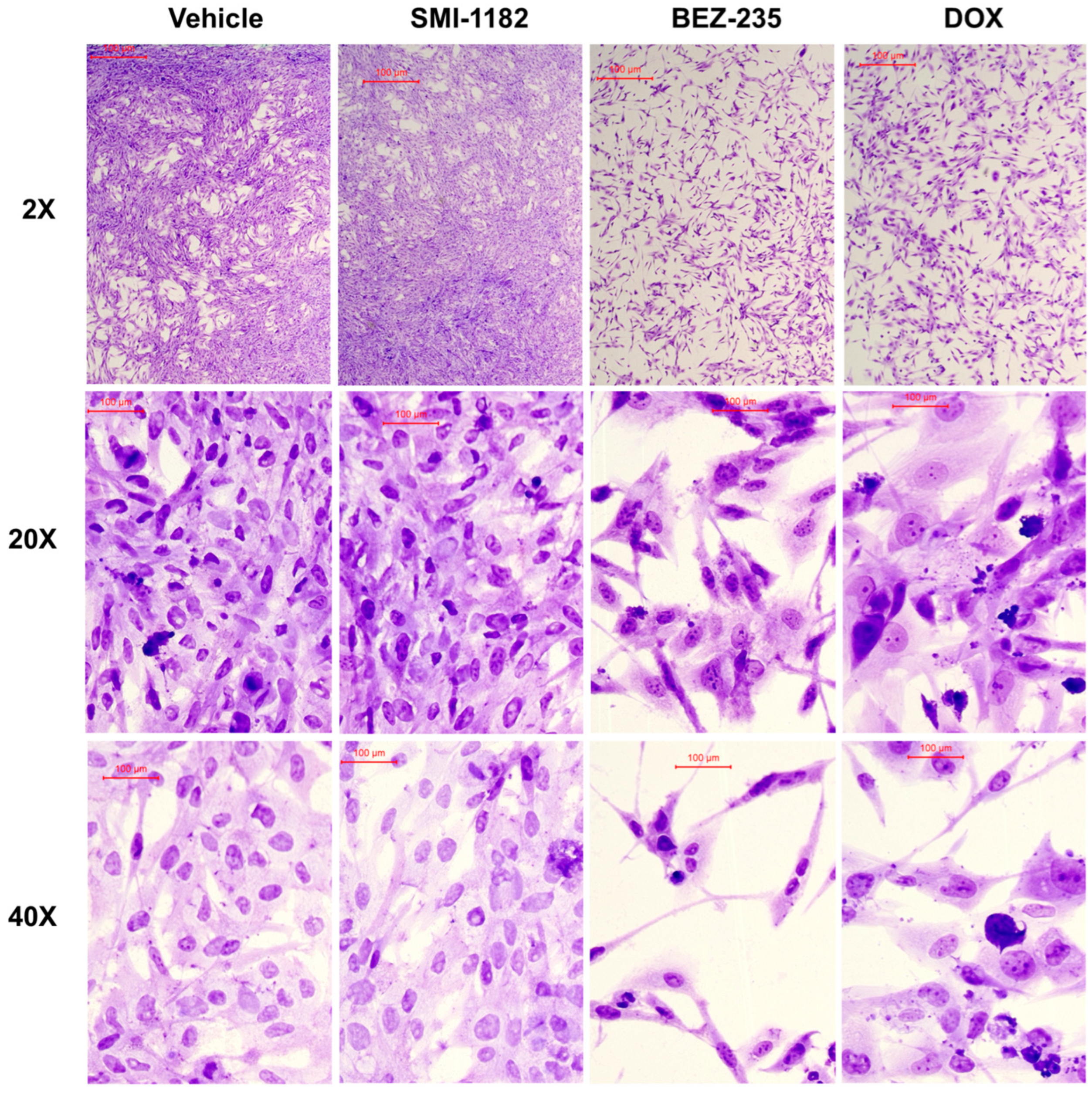
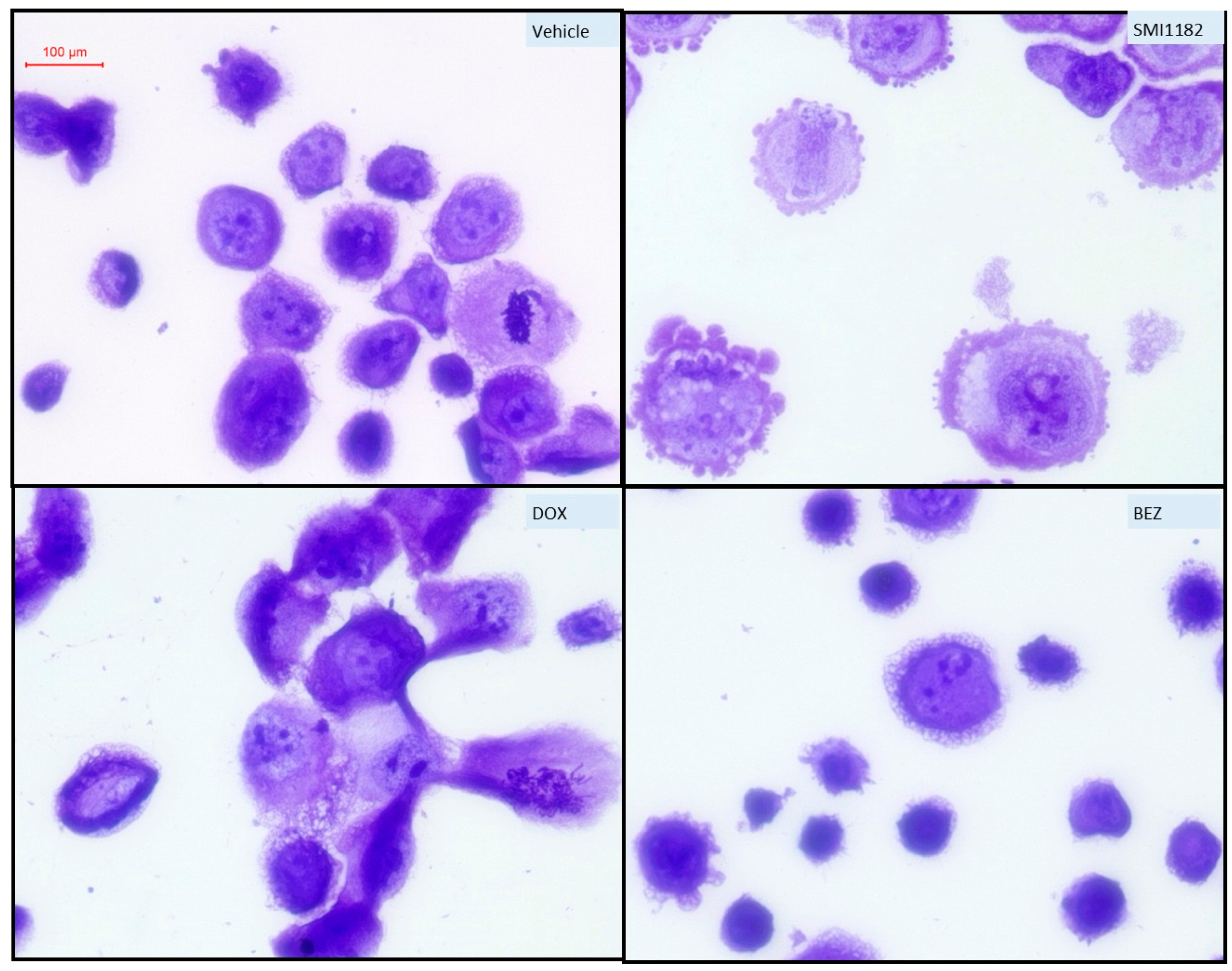
3.4. Effects of SMI1182, DOX, and BEZ on CS Morphology
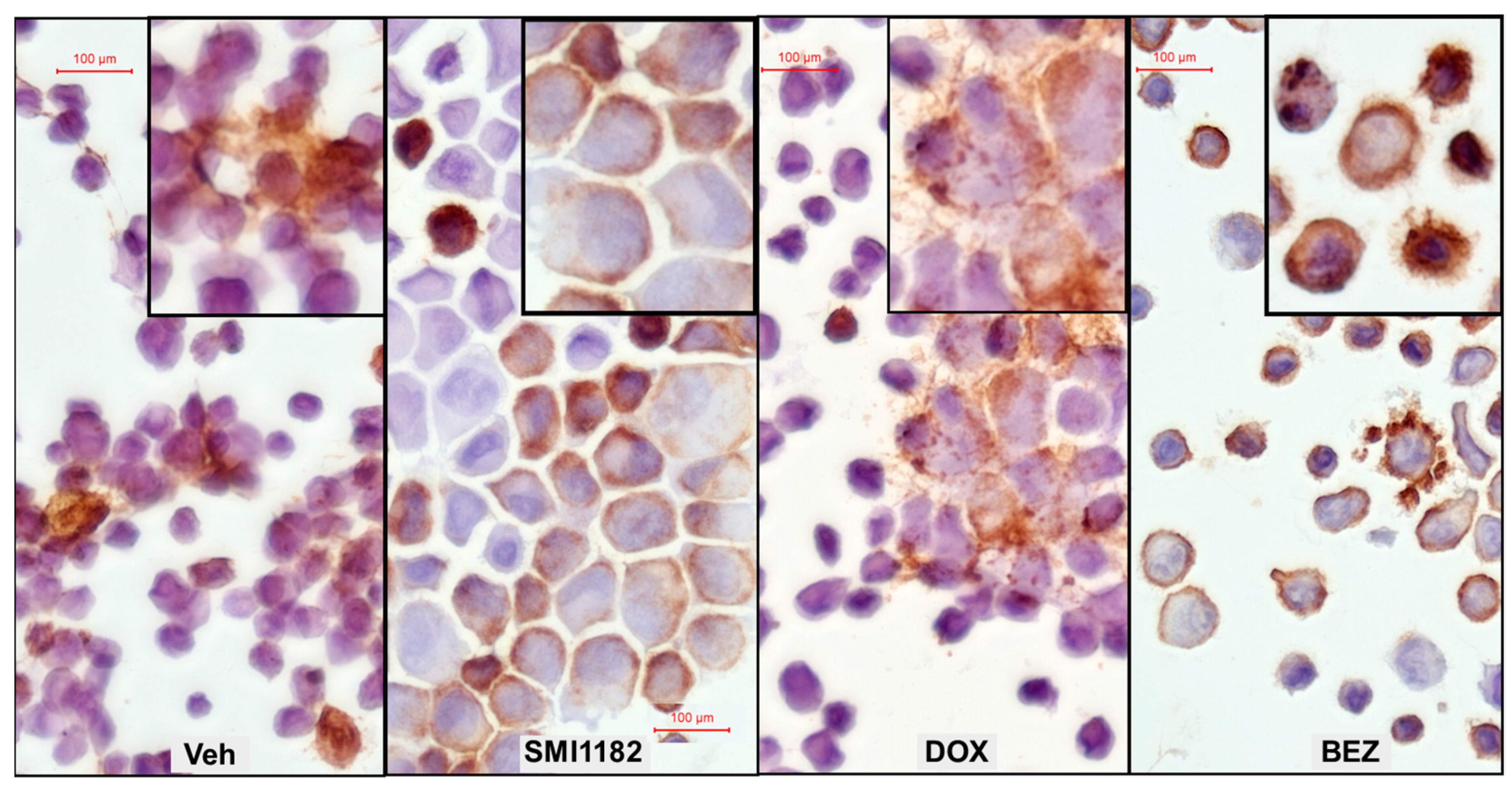
3.5. Effects of SMI1182, DOX, and BEZ on ASPH Immunoreactivity
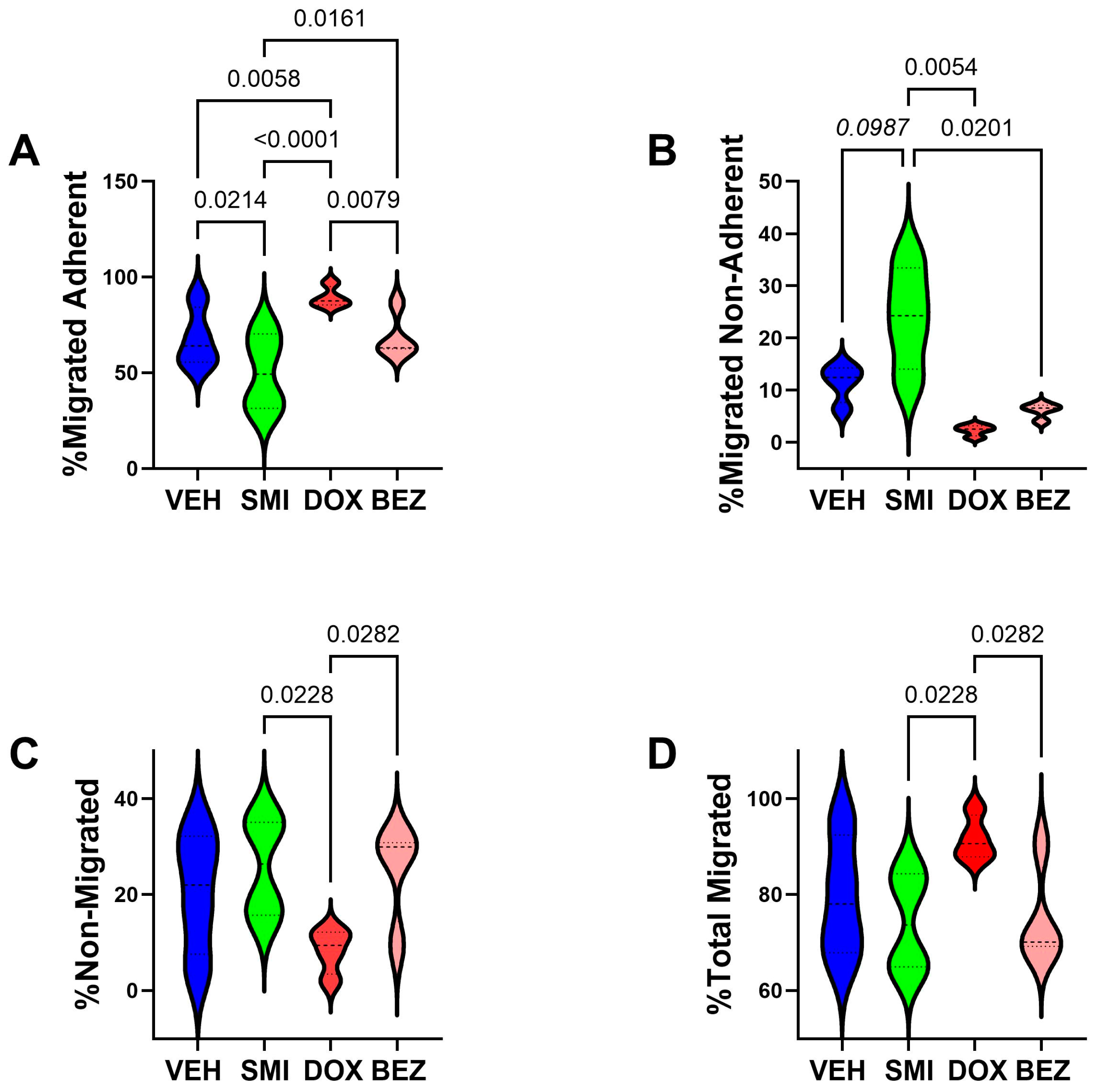
3.6. Cell Motility Studies
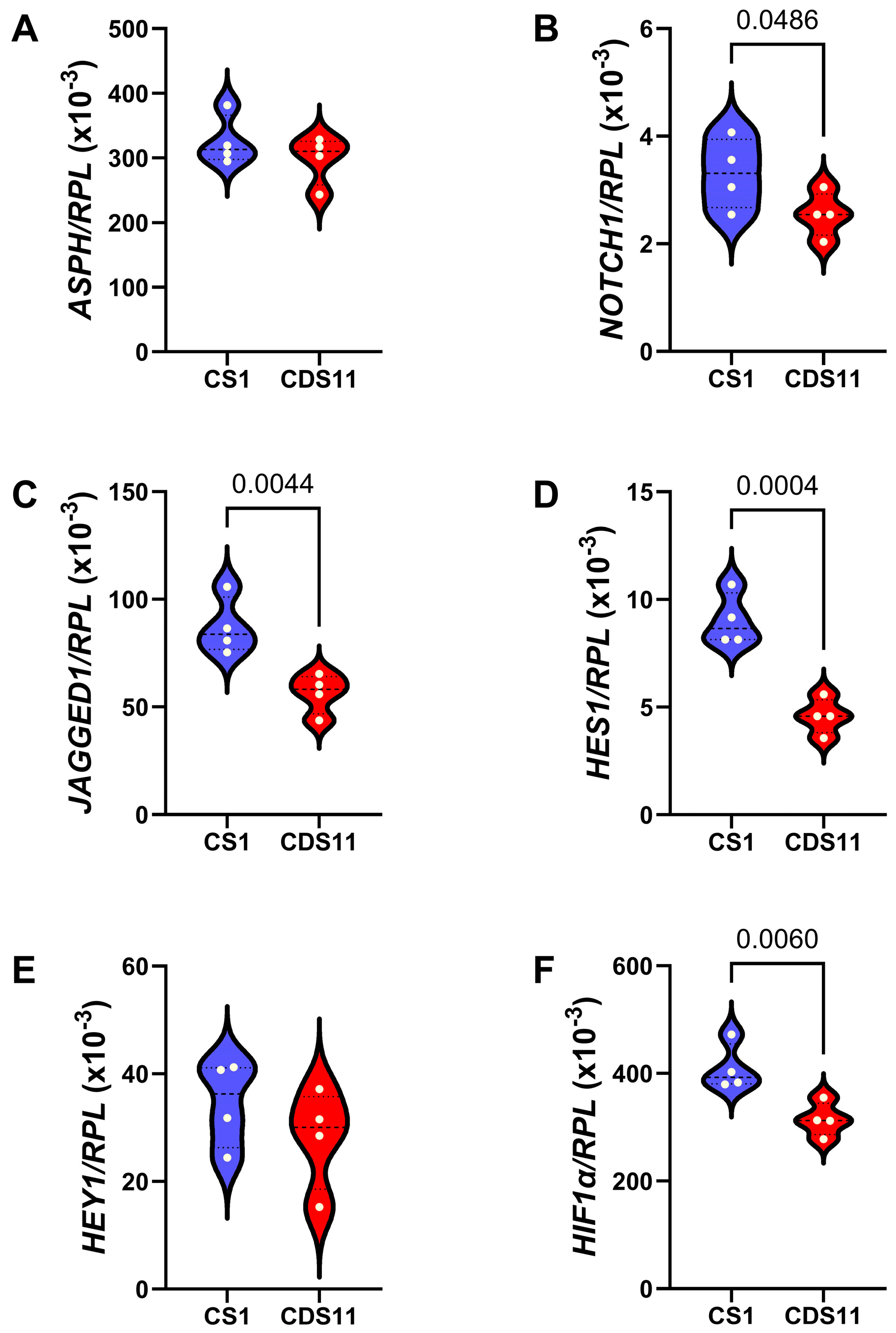
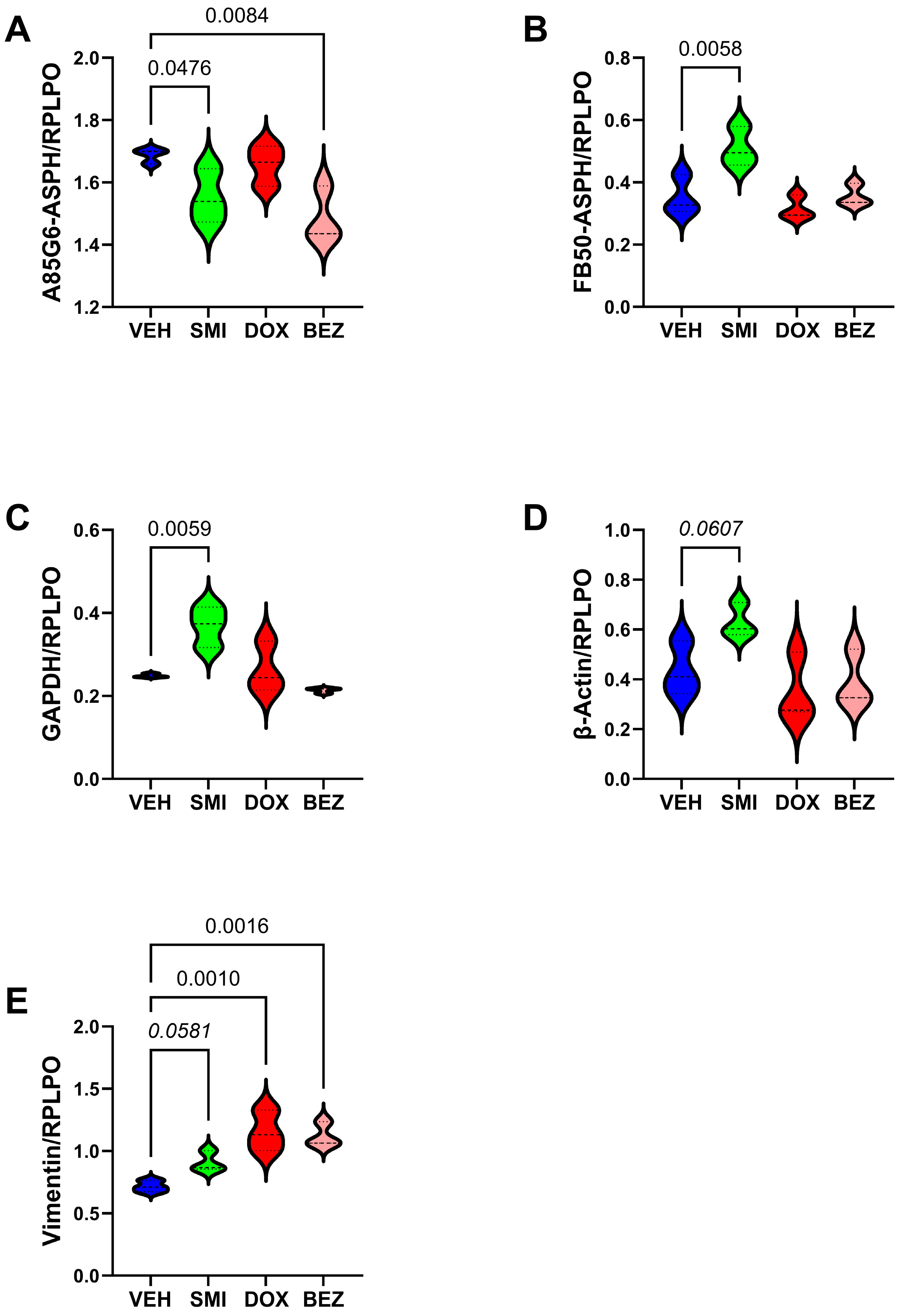
3.7. Molecular Correlates of SMI1182-, DOX-, and BEZ-Mediated Effects in CS Cells
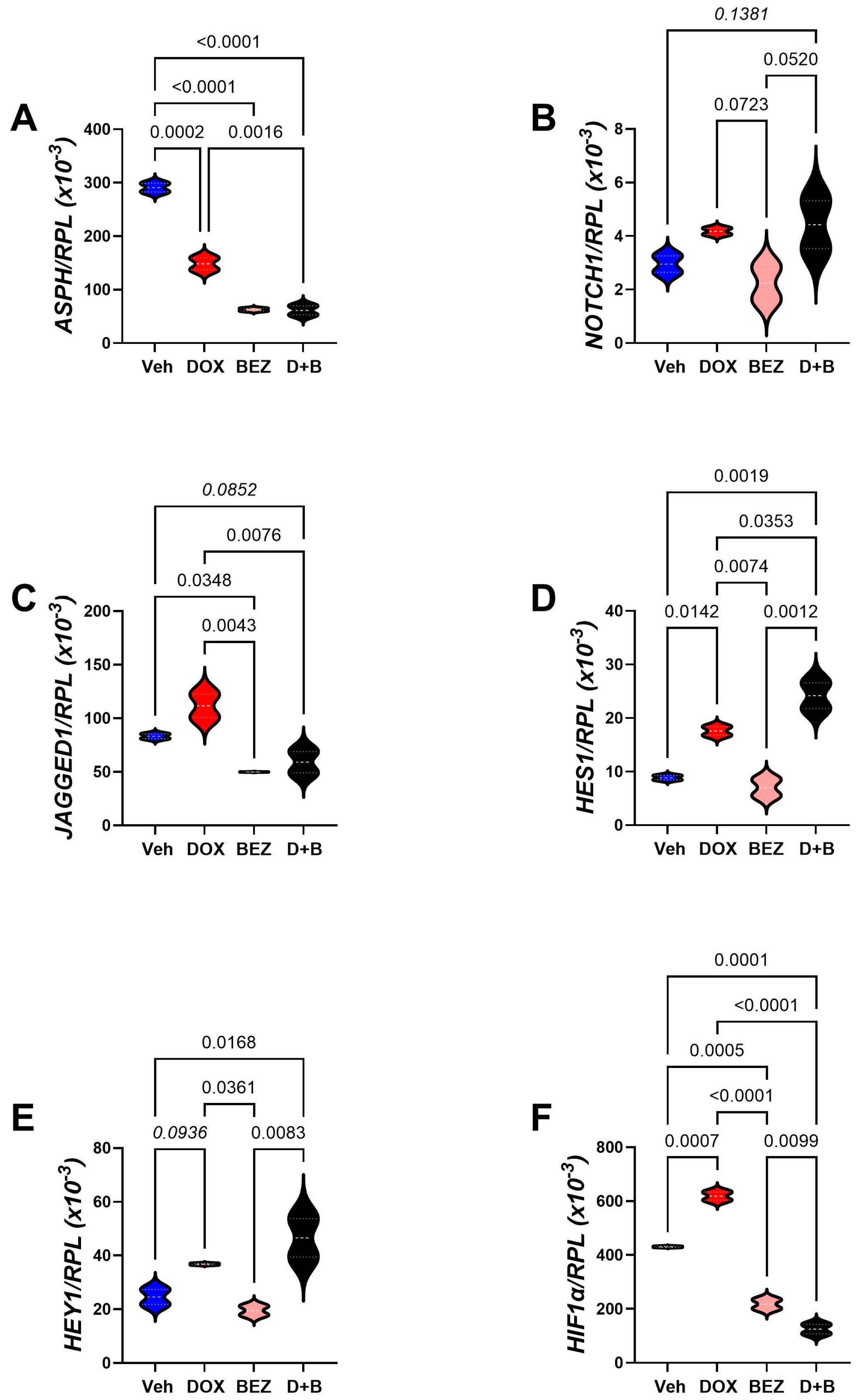
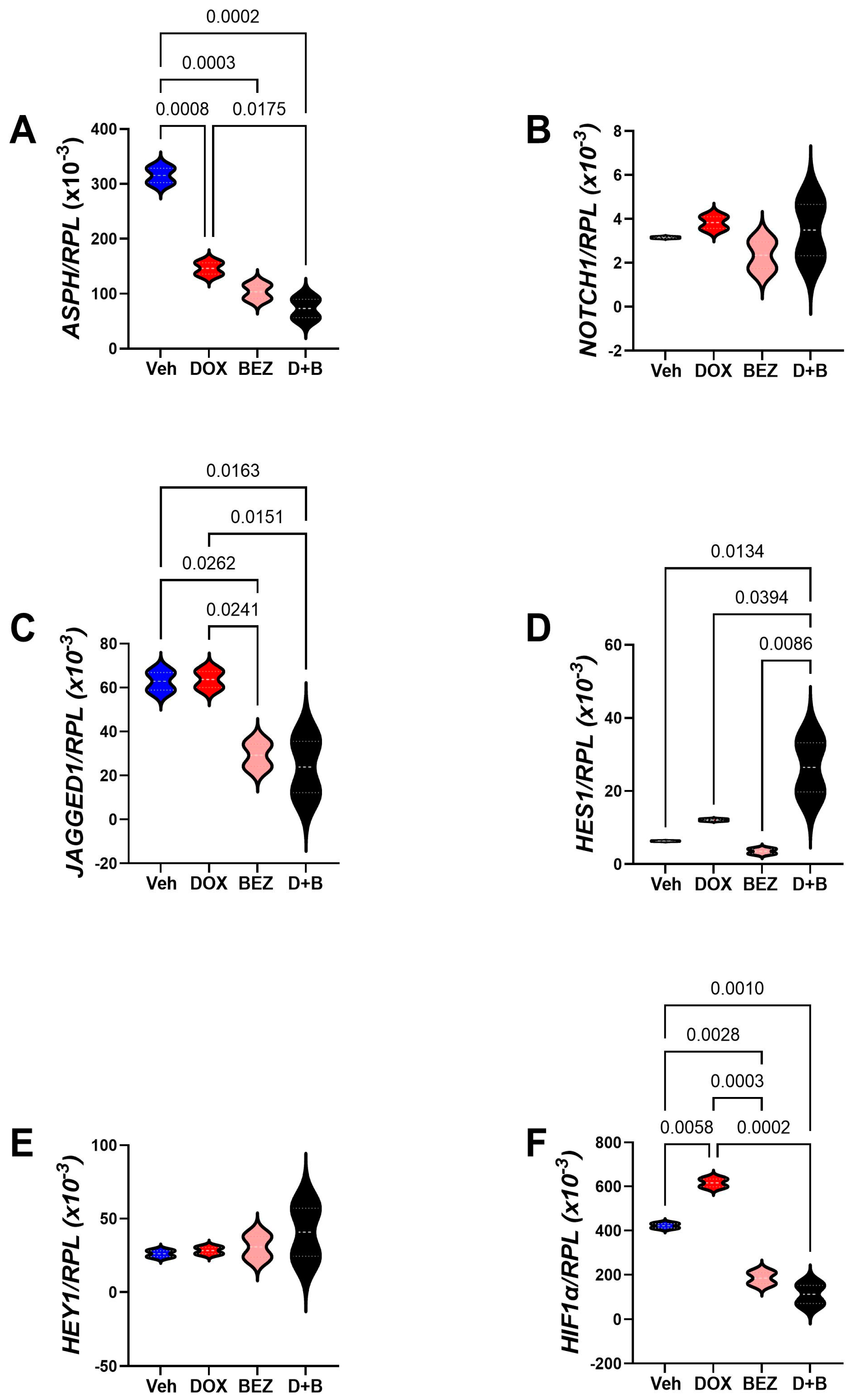
| Gene Code | Gene Name | F-Ratio | p-Value |
|---|---|---|---|
| ASPH | Aspartyl-Asparaginyl-β-Hydroxylase | 178.8 | 0.0001 |
| NOTCH1 | Notch 1 | 6.260 | 0.05 |
| JAGGED1 | Jagged 1 | 13.66 | 0.014 |
| HES1 | Hairy and enhancer of split-1 | 43.44 | 0.0016 |
| HEY1 | HES-related family bHLH transcription factor | 9.259 | 0.0268 |
| HIF1α | Hypoxia-Inducible Factor 1-alpha | 242.2 | <0.0001 |
| INS | Insulin | 1.234 | N.S. |
| IGF1 | Insulin-Like Growth Factor 1 | 1.336 | N.S. |
| IGF2 | Insulin-Like Growth Factor 2 | 2.577 | N.S. |
| INSR | Insulin Receptor | 2.637 | N.S. |
| IGF1R | Insulin-Like Growth Factor 1 Receptor | 3.164 | N.S. |
| IGF2R | Insulin-Like Growth Factor 2 Receptor | 9.891 | 0.0254 |
| IRS1 | Insulin Receptor Substrate, Type 1 | 16.01 | 0.0108 |
| IRS2 | Insulin Receptor Substrate, Type 2 | 0.667 | N.S. |
| IRS4 | Insulin Receptor Substrate, Type 4 | 0.487 | N.S. |
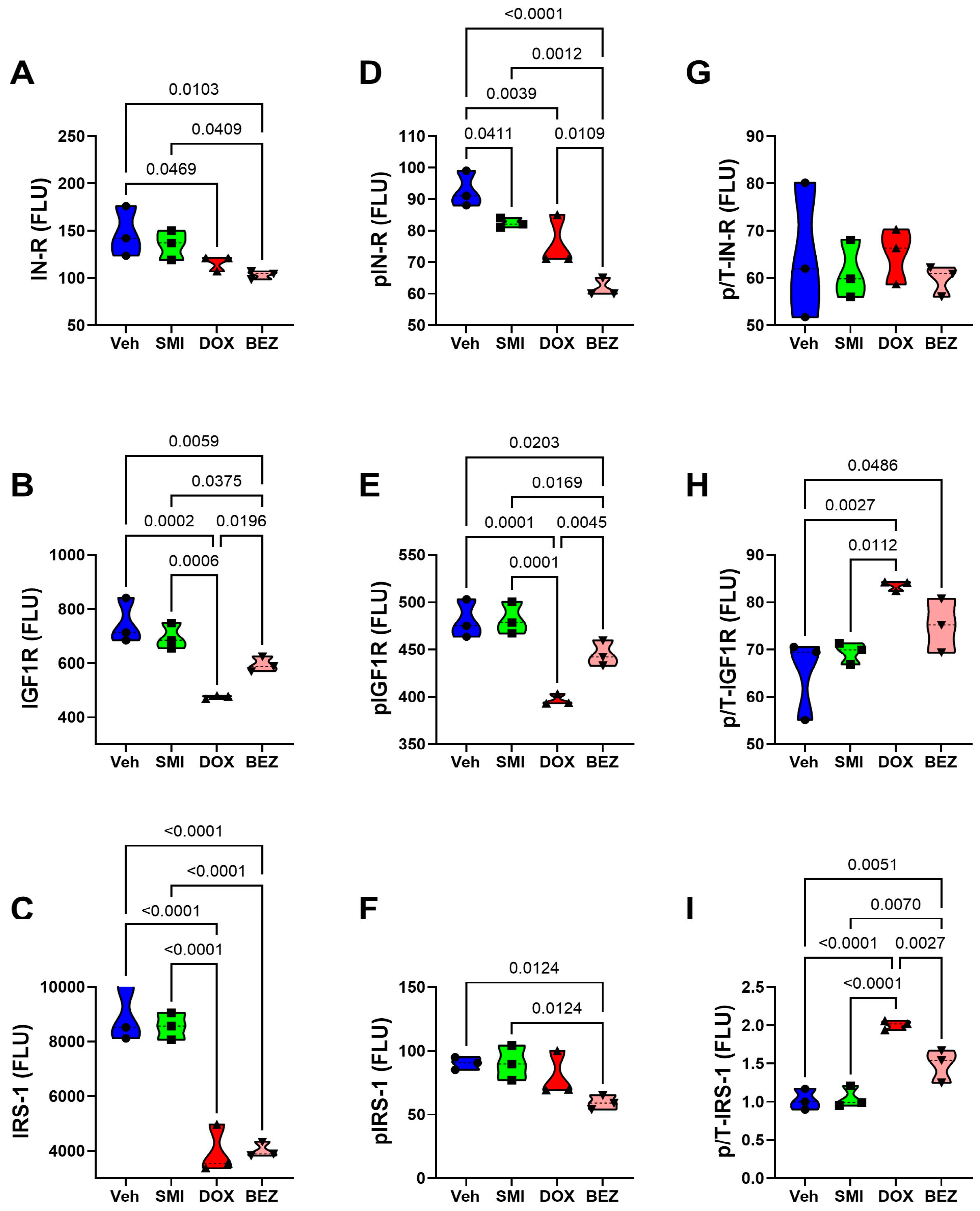
3.8. Akt-mTOR Pathway Analysis
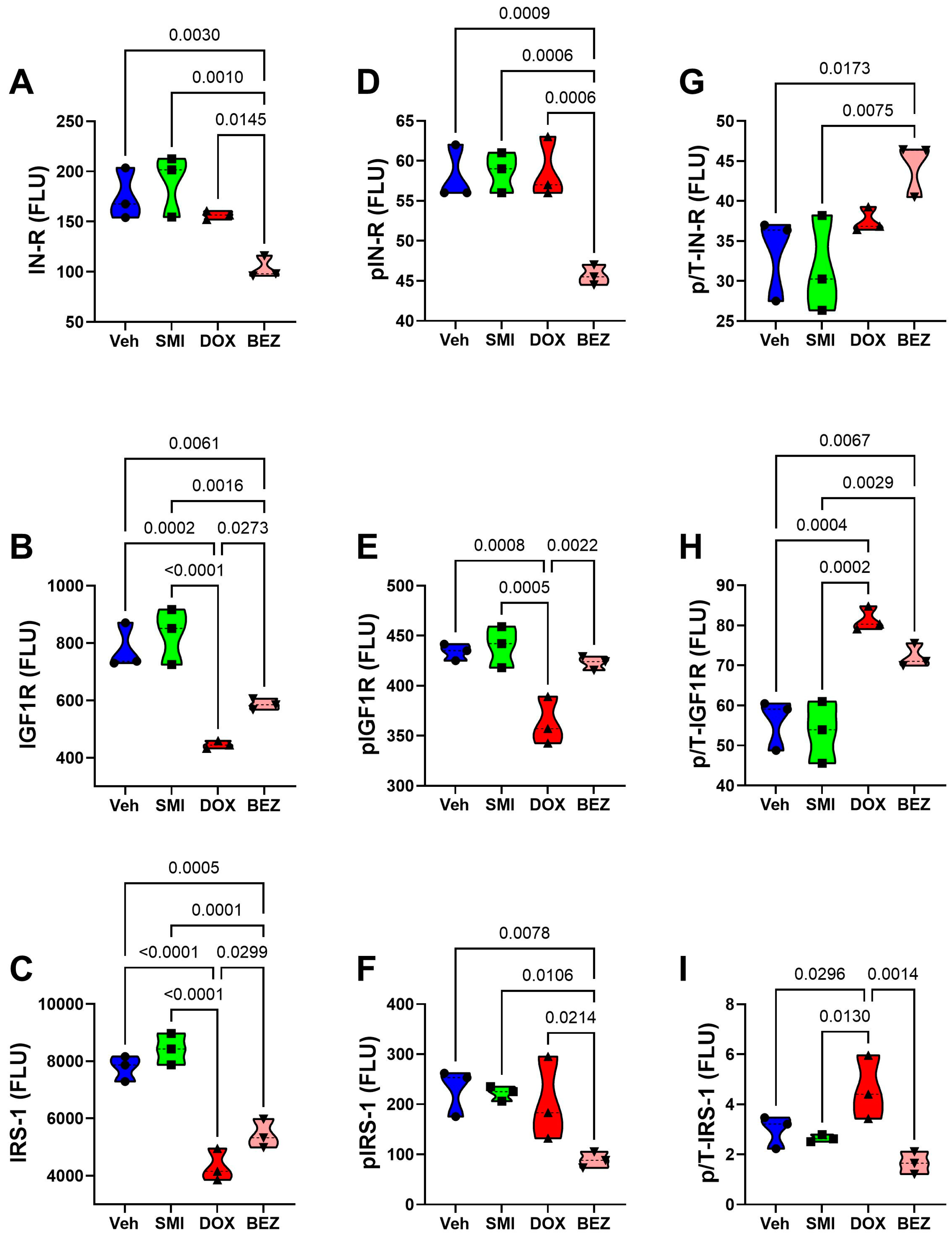
| Treatment | p-Value | CS Grade | p-Value | Treatment × CS Grade | p-Value | |
|---|---|---|---|---|---|---|
| IN-R | 13.26 | 0.0001 | 16.07 | 0.001 | 2.285 | N.S. |
| p-IN-R | 29.07 | <0.0001 | 175.5 | <0.0001 | 6.212 | 0.005 |
| IGF1R | 39.36 | <0.0001 | 1.979 | N.S. | 2.400 | N.S. |
| p-IGF1R | 33.27 | <0.0001 | 31.05 | <0.0001 | 0.703 | N.S. |
| IRS1 | 81.54 | <0.0001 | 0.25 | N.S. | 4.209 | 0.0225 |
| p-IRS1 | 7.535 | 0.0023 | 52.91 | <0.0001 | 3.132 | 0.054 |
| Akt | 28.64 | <0.0001 | 224.2 | <0.0001 | 22.0 | <0.0001 |
| p-Akt | 21.07 | <0.0001 | 11.58 | 0.0036 | 0.829 | N.S. |
| GSK-3α | 34.43 | <0.0001 | 0.863 | N.S. | 1.05 | N.S. |
| p-GSK-3α | 34.54 | <0.0001 | 25.68 | <0.0001 | 5.146 | 0.01 |
| GSK-3β | 90.35 | <0.0001 | 4.49 | 0.05 | 0.785 | N.S. |
| p-GSK-3β | 132.8 | <0.0001 | 0.284 | N.S. | 3.563 | 0.038 |
| PTEN | 91.46 | <0.0001 | 3.718 | 0.0718 | 1.89 | N.S. |
| p-PTEN | 43.21 | <0.0001 | 6.12 | 0.0249 | 0.073 | N.S. |
| TSC | 53.03 | <0.0001 | 43.21 | <0.0001 | 1.602 | N.S. |
| p-TSC | 23.81 | <0.0001 | 35.48 | <0.0001 | 0.447 | N.S. |
| mTOR | 24.57 | <0.0001 | 12.28 | 0.0029 | 1.68 | N.S. |
| p-mTOR | 49.11 | <0.0001 | 31.42 | <0.0001 | 3.459 | 0.041 |
| RPS6 | 23.97 | <0.0001 | 0.764 | N.S. | 0.909 | N.S. |
| p-RPS6 | 23.48 | <0.0001 | 47.73 | <0.0001 | 8.774 | 0.001 |
| P70S6K | 13.53 | 0.0001 | 1.191 | N.S. | 1.475 | N.S. |
| p-P70S6K | 5.049 | 0.012 | 68.16 | <0.0001 | 4.346 | 0.02 |
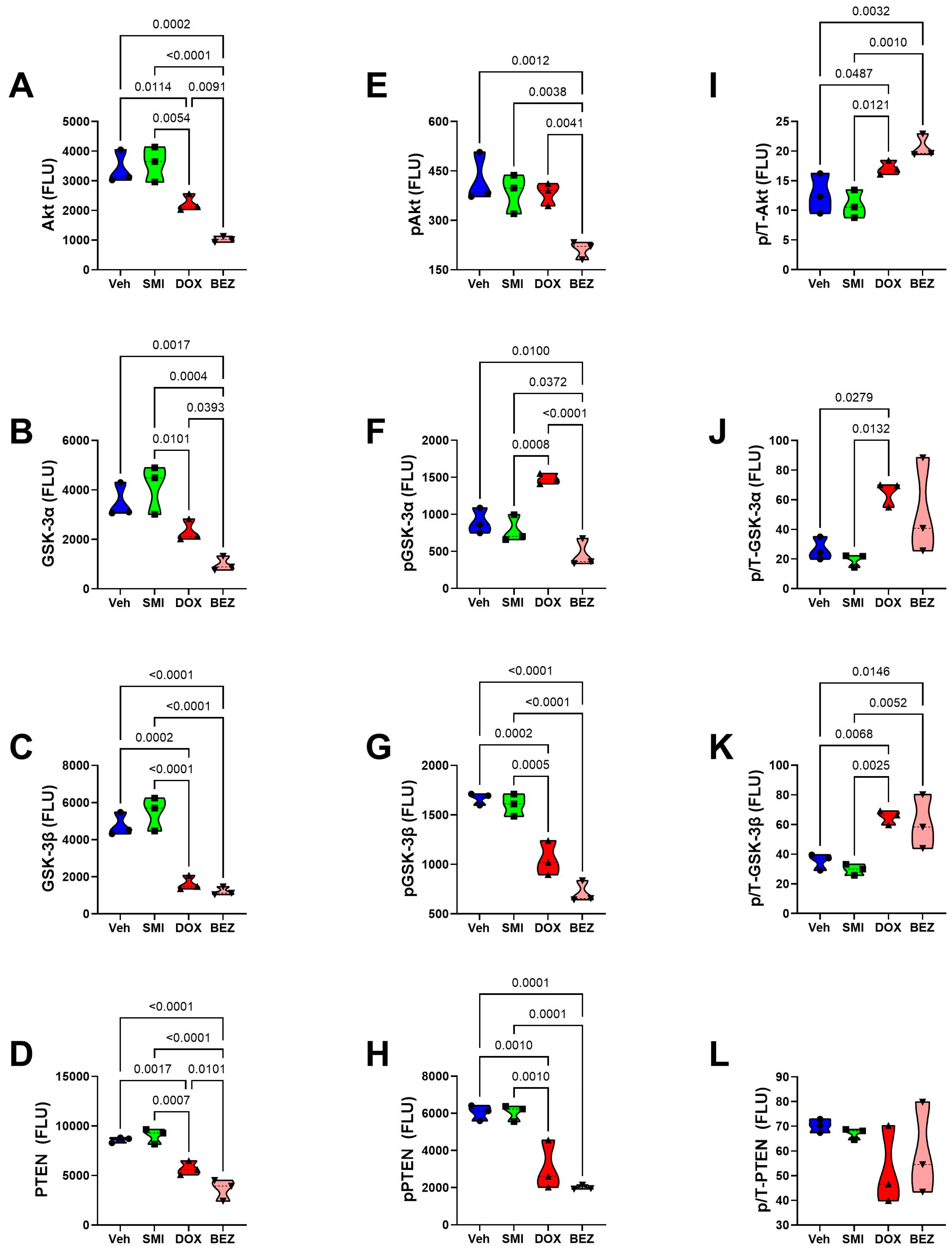
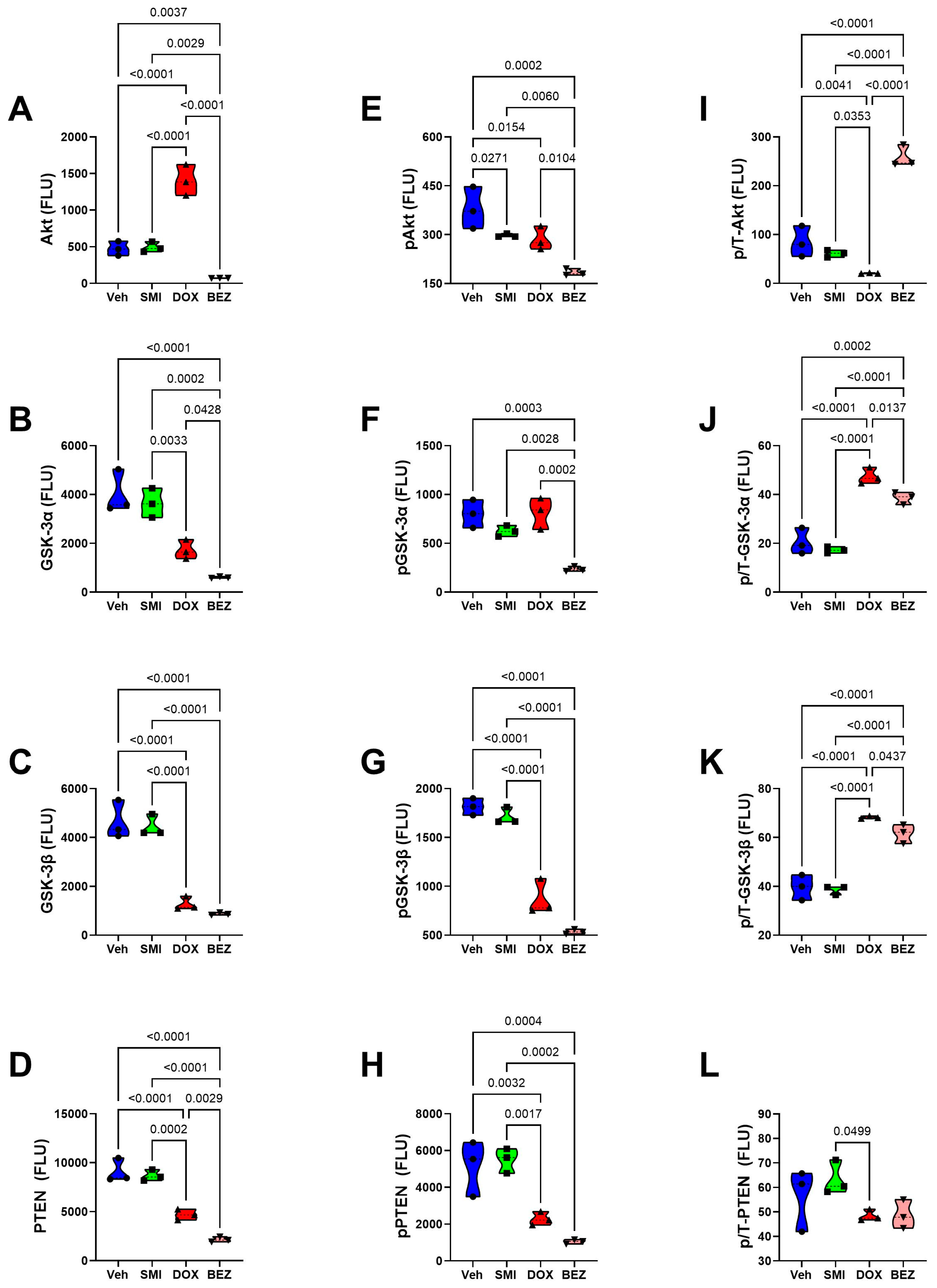
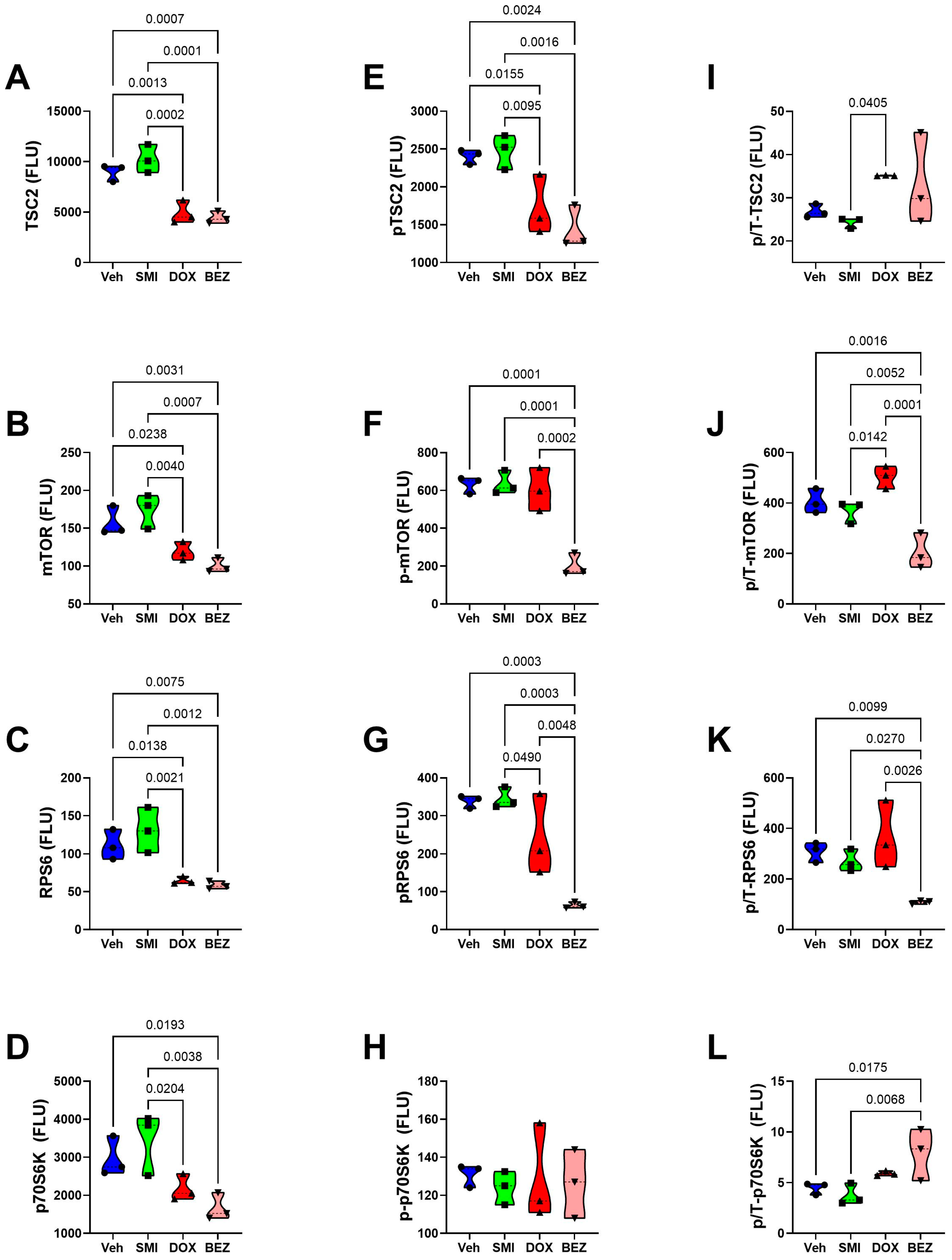
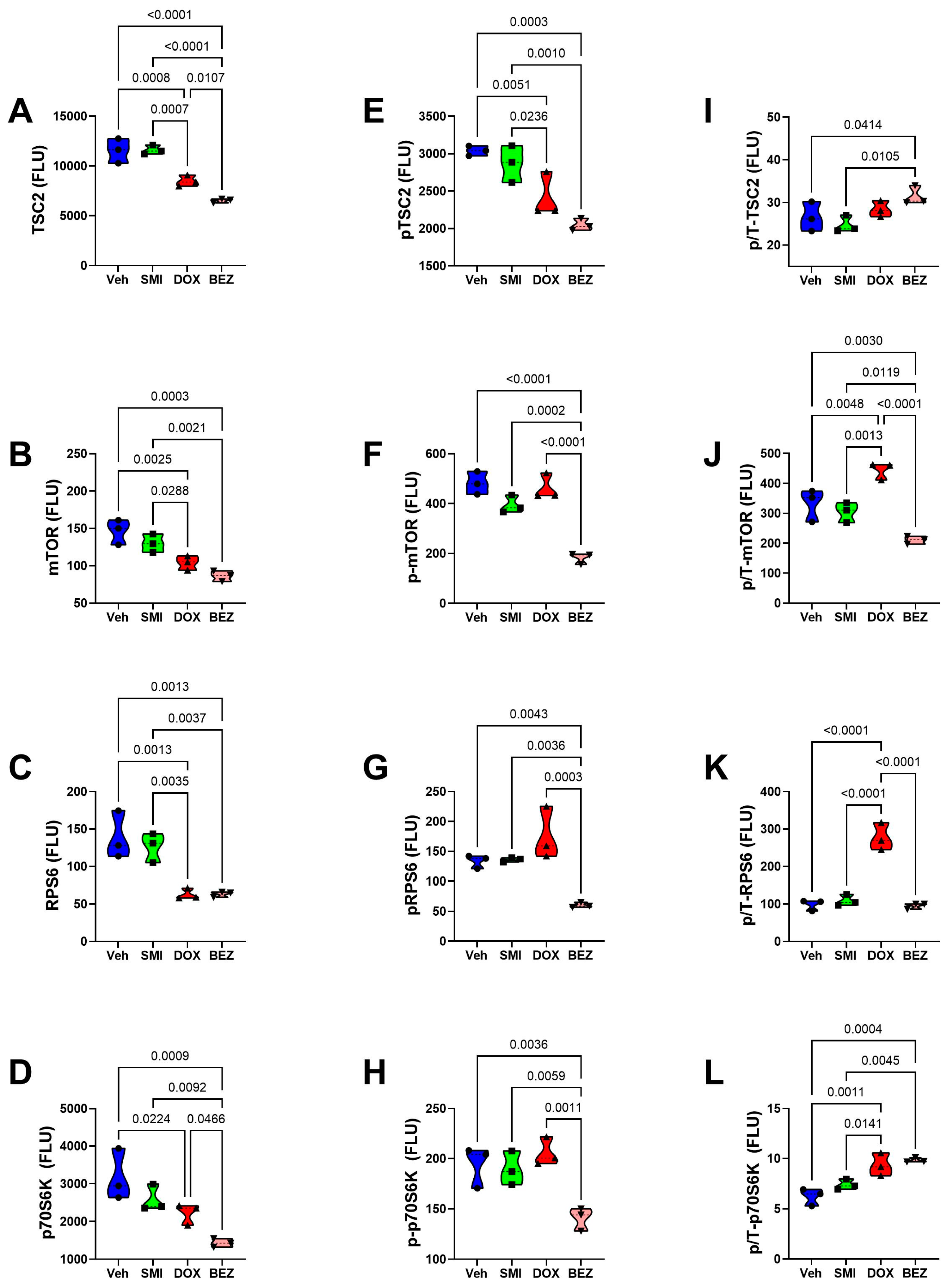
3.9. Comparative Effects of Treatment in Relation to CS Grade
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| Akt | Akt (Protein Kinase B) |
| ASPH | Aspartyl-asparaginyl-β-hydroxylase |
| AUC | Area-under-curve |
| BEZ | BEZ235; combination of everolimus and the imidazoquinoline derivative |
| CS | Chondrosarcoma |
| DMSO | Dimethyl sulfoxide |
| DOX | Doxorubicin |
| EDTA | Ethylenediaminetetraacetic acid |
| EIA | Enzyme immunoassay |
| ELISA | Enzyme-linked immunosorbent assay |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GSK-3a | Glycogen Synthase Kinase 3a |
| GSK-3β | Glycogen Synthase Kinase 3β |
| HES1 | Hairy and enhancer of split-1 |
| HEY1 | HES-related family bHLH transcription factor |
| HIF1α | Hypoxia-Inducible Factor 1-alpha |
| HRP | Horseradish peroxidase |
| IGF1 | Insulin-Like Growth Factor 1 |
| IGF1R | Insulin-Like Growth Factor 1 Receptor |
| IGF1R | Insulin-Like Growth Factor Receptor Type 1 |
| IGF2 | Insulin-Like Growth Factor 2 |
| IGF2R | Insulin-Like Growth Factor 2 Receptor |
| INS | Insulin |
| INSR | Insulin receptor |
| Insulin-R | Insulin receptor |
| IRS1 | Insulin receptor substrate, type 1 |
| IRS1 | Insulin receptor substrate, type 1 |
| IRS2 | Insulin receptor substrate, type 2 |
| IRS4 | Insulin receptor substrate, type 4 |
| JAGGED1 | Jagged 1 |
| mTOR | Mechanistic target of rapamycin |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NOTCH1 | Notch 1 |
| P70S6K | p70 Ribosomal S6 kinase |
| PBS | Phosphate-buffered saline |
| PTEN | Phosphatase and tensin homolog |
| RPS6 | Ribosomal Protein S6 |
| SMI | Small molecule inhibitor |
| TBS | Tris-buffered saline |
| TSC2 | Tuberous sclerosis complex 2 |
References
- Limaiem, F.; Davis, D.D.; Sticco, K.L. Chondrosarcoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- van Praag Veroniek, V.M.; Rueten-Budde, A.J.; Ho, V.; Dijkstra, P.D.S.; Fiocco, M.; van de Sande, M.A.J. Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas. Surg. Oncol. 2018, 27, 402–408. [Google Scholar] [CrossRef]
- Thorkildsen, J.; Myklebust, T.A. The national incidence of chondrosarcoma of bone; a review. Acta Oncol. 2023, 62, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Tlemsani, C.; Larousserie, F.; De Percin, S.; Audard, V.; Hadjadj, D.; Chen, J.; Biau, D.; Anract, P.; Terris, B.; Goldwasser, F.; et al. Biology and Management of High-Grade Chondrosarcoma: An Update on Targets and Treatment Options. Int. J. Mol. Sci. 2023, 24, 1361. [Google Scholar] [CrossRef]
- Frezza, A.M.; Cesari, M.; Baumhoer, D.; Biau, D.; Bielack, S.; Campanacci, D.A.; Casanova, J.; Esler, C.; Ferrari, S.; Funovics, P.T.; et al. Mesenchymal chondrosarcoma: Prognostic factors and outcome in 113 patients. A European Musculoskeletal Oncology Society study. Eur. J. Cancer 2015, 51, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.P.; Papai, Z.; Mukhametshina, G.; Sankhala, K.; Vasylyev, L.; Fedenko, A.; Khamly, K.; Ganjoo, K.; Nagarkar, R.; Wieland, S.; et al. First-Line Aldoxorubicin vs Doxorubicin in Metastatic or Locally Advanced Unresectable Soft-Tissue Sarcoma: A Phase 2b Randomized Clinical Trial. JAMA Oncol. 2015, 1, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Weinschenk, R.C.; Wang, W.L.; Lewis, V.O. Chondrosarcoma. J. Am. Acad. Orthop. Surg. 2021, 29, 553–562. [Google Scholar] [CrossRef]
- Zajac, W.; Drozdz, J.; Kisielewska, W.; Karwowska, W.; Dudzisz-Sledz, M.; Zajac, A.E.; Borkowska, A.; Szumera-Cieckiewicz, A.; Szostakowski, B.; Rutkowski, P.; et al. Dedifferentiated Chondrosarcoma from Molecular Pathology to Current Treatment and Clinical Trials. Cancers 2023, 15, 3924. [Google Scholar] [CrossRef]
- Andreou, D.; Ruppin, S.; Fehlberg, S.; Pink, D.; Werner, M.; Tunn, P.U. Survival and prognostic factors in chondrosarcoma: Results in 115 patients with long-term follow-up. Acta Orthop. 2011, 82, 749–755. [Google Scholar] [CrossRef]
- Polychronidou, G.; Karavasilis, V.; Pollack, S.M.; Huang, P.H.; Lee, A.; Jones, R.L. Novel therapeutic approaches in chondrosarcoma. Future Oncol. 2017, 13, 637–648. [Google Scholar] [CrossRef]
- Boehme, K.A.; Schleicher, S.B.; Traub, F.; Rolauffs, B. Chondrosarcoma: A Rare Misfortune in Aging Human Cartilage? The Role of Stem and Progenitor Cells in Proliferation, Malignant Degeneration and Therapeutic Resistance. Int. J. Mol. Sci. 2018, 19, 311. [Google Scholar] [CrossRef]
- Rey, V.; Tornin, J.; Alba-Linares, J.J.; Robledo, C.; Murillo, D.; Rodriguez, A.; Gallego, B.; Huergo, C.; Viera, C.; Brana, A.; et al. A personalized medicine approach identifies enasidenib as an efficient treatment for IDH2 mutant chondrosarcoma. EBioMedicine 2024, 102, 105090. [Google Scholar] [CrossRef] [PubMed]
- Bernstein-Molho, R.; Kollender, Y.; Issakov, J.; Bickels, J.; Dadia, S.; Flusser, G.; Meller, I.; Sagi-Eisenberg, R.; Merimsky, O. Clinical activity of mTOR inhibition in combination with cyclophosphamide in the treatment of recurrent unresectable chondrosarcomas. Cancer Chemother. Pharmacol. 2012, 70, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Miele, L. Notch signaling. Clin. Cancer Res. 2006, 12, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.G.; Karsan, A. Recent insights into the role of Notch signaling in tumorigenesis. Blood 2006, 107, 2223–2233. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, H.; Xu, H.; Xiong, H.; Chu, Q.; Yu, S.; Wu, G.S.; Wu, K. Notch signaling: An emerging therapeutic target for cancer treatment. Cancer Lett. 2015, 369, 20–27. [Google Scholar] [CrossRef]
- Purow, B. Notch inhibition as a promising new approach to cancer therapy. Adv. Exp. Med. Biol. 2012, 727, 305–319. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Wang, Y.; Kaur, B.; Han, H.; Yu, J. The Notch signaling pathway: A potential target for cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 45. [Google Scholar] [CrossRef]
- Siar, C.H.; Ha, K.O.; Aung, L.O.; Nakano, K.; Tsujigiwa, H.; Nagatsuka, H.; Ng, K.H.; Kawakami, T. Immunolocalization of notch signaling protein molecules in a maxillary chondrosarcoma and its recurrent tumor. Eur. J. Med. Res. 2010, 15, 456–460. [Google Scholar] [CrossRef]
- Massard, C.; Azaro, A.; Soria, J.C.; Lassen, U.; Le Tourneau, C.; Sarker, D.; Smith, C.; Ohnmacht, U.; Oakley, G.; Patel, B.K.R.; et al. First-in-human study of LY3039478, an oral Notch signaling inhibitor in advanced or metastatic cancer. Ann. Oncol. 2018, 29, 1911–1917. [Google Scholar] [CrossRef]
- Gounder, M.M.; Rosenbaum, E.; Wu, N.; Dickson, M.A.; Sheikh, T.N.; D’Angelo, S.P.; Chi, P.; Keohan, M.L.; Erinjeri, J.P.; Antonescu, C.R.; et al. A Phase Ib/II Randomized Study of RO4929097, a Gamma-Secretase or Notch Inhibitor with or without Vismodegib, a Hedgehog Inhibitor, in Advanced Sarcoma. Clin. Cancer Res. 2022, 28, 1586–1594. [Google Scholar] [CrossRef]
- Collins, M.; Michot, J.M.; Bellanger, C.; Mussini, C.; Benhadji, K.; Massard, C.; Carbonnel, F. Notch inhibitors induce diarrhea, hypercrinia and secretory cell metaplasia in the human colon. EXCLI J. 2021, 20, 819–827. [Google Scholar] [CrossRef]
- Mir, O.; Azaro, A.; Merchan, J.; Chugh, R.; Trent, J.; Rodon, J.; Ohnmacht, U.; Diener, J.T.; Smith, C.; Yuen, E.; et al. Notch pathway inhibition with LY3039478 in soft tissue sarcoma and gastrointestinal stromal tumours. Eur. J. Cancer 2018, 103, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nieva, P.; Gonzalez-Sanchez, L.; Cobos-Fernandez, M.A.; Cordoba, R.; Santos, J.; Fernandez-Piqueras, J. More Insights on the Use of gamma-Secretase Inhibitors in Cancer Treatment. Oncologist 2021, 26, e298–e305. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; VanDusen, W.J.; Diehl, R.E.; Kohl, N.E.; Dixon, R.A.; Elliston, K.O.; Stern, A.M.; Friedman, P.A. cDNA cloning and expression of bovine aspartyl (asparaginyl) beta-hydroxylase. J. Biol. Chem. 1992, 267, 14322–14327. [Google Scholar] [CrossRef] [PubMed]
- Greve, J.M.; Pinkham, A.M.; Cowan, J.A. Human aspartyl (asparaginyl) hydroxylase. A multifaceted enzyme with broad intra- and extra-cellular activity. Metallomics 2021, 13, mfab044. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, X.; Meng, F.; Ogawa, K.; Li, M.; Song, R.; Zhang, S.; Zhang, Z.; Kong, X.; Xu, Q.; et al. ASPH-notch Axis guided Exosomal delivery of Prometastatic Secretome renders breast Cancer multi-organ metastasis. Mol. Cancer 2019, 18, 156. [Google Scholar] [CrossRef]
- Borgas, D.L.; Gao, J.S.; Tong, M.; Roper, N.; de la Monte, S.M. Regulation of Aspartyl-(Asparaginyl)-beta-Hydroxylase Protein Expression and Function by Phosphorylation in Hepatocellular Carcinoma Cells. J. Nat. Sci. 2015, 1, e84. [Google Scholar]
- Cantarini, M.C.; de la Monte, S.M.; Pang, M.; Tong, M.; D’Errico, A.; Trevisani, F.; Wands, J.R. Aspartyl-asparagyl beta hydroxylase over-expression in human hepatoma is linked to activation of insulin-like growth factor and notch signaling mechanisms. Hepatology 2006, 44, 446–457. [Google Scholar] [CrossRef]
- Barboro, P.; Benelli, R.; Tosetti, F.; Costa, D.; Capaia, M.; Astigiano, S.; Vene, R.; Poggi, A.; Ferrari, N. Aspartate beta-hydroxylase targeting in castration-resistant prostate cancer modulates the NOTCH/HIF1alpha/GSK3beta crosstalk. Carcinogenesis 2020, 41, 1246–1252. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, X.; Hu, J.; Bai, B.; Zhu, H. Diverse molecular functions of aspartate beta-hydroxylase in cancer (Review). Oncol. Rep. 2020, 44, 2364–2372. [Google Scholar] [CrossRef]
- Engel, J. EGF-like domains in extracellular matrix proteins: Localized signals for growth and differentiation? FEBS Lett. 1989, 251, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Borgas, D.L.; Gao, J.S.; Tong, M.; de la Monte, S.M. Potential Role of Phosphorylation as a Regulator of Aspartyl-(asparaginyl)-beta-hydroxylase: Relevance to Infiltrative Spread of Human Hepatocellular Carcinoma. Liver Cancer 2015, 4, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Ince, N.; de la Monte, S.M.; Wands, J.R. Overexpression of human aspartyl (asparaginyl) beta-hydroxylase is associated with malignant transformation. Cancer Res. 2000, 60, 1261–1266. [Google Scholar] [PubMed]
- Dong, X.; Lin, Q.; Aihara, A.; Li, Y.; Huang, C.K.; Chung, W.; Tang, Q.; Chen, X.; Carlson, R.; Nadolny, C.; et al. Aspartate beta-Hydroxylase expression promotes a malignant pancreatic cellular phenotype. Oncotarget 2015, 6, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Sturla, L.M.; Tong, M.; Hebda, N.; Gao, J.; Thomas, J.M.; Olsen, M.; de la Monte, S.M. Aspartate-beta-hydroxylase (ASPH): A potential therapeutic target in human malignant gliomas. Heliyon 2016, 2, e00203. [Google Scholar] [CrossRef]
- Yang, H.; Song, K.; Xue, T.; Xue, X.P.; Huyan, T.; Wang, W.; Wang, H. The distribution and expression profiles of human Aspartyl/Asparaginyl beta-hydroxylase in tumor cell lines and human tissues. Oncol. Rep. 2010, 24, 1257–1264. [Google Scholar] [CrossRef]
- Aihara, A.; Huang, C.K.; Olsen, M.J.; Lin, Q.; Chung, W.; Tang, Q.; Dong, X.; Wands, J.R. A cell-surface beta-hydroxylase is a biomarker and therapeutic target for hepatocellular carcinoma. Hepatology 2014, 60, 1302–1313. [Google Scholar] [CrossRef]
- Wang, K.; Liu, J.; Yan, Z.L.; Li, J.; Shi, L.H.; Cong, W.M.; Xia, Y.; Zou, Q.F.; Xi, T.; Shen, F.; et al. Overexpression of aspartyl-(asparaginyl)-beta-hydroxylase in hepatocellular carcinoma is associated with worse surgical outcome. Hepatology 2010, 52, 164–173. [Google Scholar] [CrossRef]
- Sun, X.; Ramirez, J.; Hart, J.; Schwab, J.; Molino, J.; Dong, X.; Carlson, R.; Wands, J.R.; Terek, R. ASPH Targeted Therapy Inhibits Chondrosarcoma Progression. In Proceedings of the Orthopaedic Research Society, Tampa, FL, USA, 4–8 February 2022; p. 1217. [Google Scholar]
- Revskaya, E.; Jiang, Z.; Morgenstern, A.; Bruchertseifer, F.; Sesay, M.; Walker, S.; Fuller, S.; Lebowitz, M.S.; Gravekamp, C.; Ghanbari, H.A.; et al. A Radiolabeled Fully Human Antibody to Human Aspartyl (Asparaginyl) beta-Hydroxylase Is a Promising Agent for Imaging and Therapy of Metastatic Breast Cancer. Cancer Biother. Radiopharm. 2017, 32, 57–65. [Google Scholar] [CrossRef]
- Xue, T.; Su, J.; Li, H.; Xue, X. Evaluation of HAAH/humbug quantitative detection in the diagnosis of hepatocellular carcinoma. Oncol. Rep. 2015, 33, 329–337. [Google Scholar] [CrossRef][Green Version]
- Xue, T.; Xue, X.P.; Huang, Q.S.; Wei, L.; Sun, K.; Xue, T. Monoclonal antibodies against human aspartyl (asparaginyl) beta-hydroxylase developed by DNA immunization. Hybridoma 2009, 28, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Yeung, Y.A.; Finney, A.H.; Koyrakh, I.A.; Lebowitz, M.S.; Ghanbari, H.A.; Wands, J.R.; Wittrup, K.D. Isolation and characterization of human antibodies targeting human aspartyl (asparaginyl) beta-hydroxylase. Hum. Antibodies 2007, 16, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, V.; Adil, M.; Ahmed, N.; Rishi, A.K.; Jamal, S. Small molecule inhibitors as emerging cancer therapeutics. Integr. Cancer Sci. Ther. 2014, 1, 39–46. [Google Scholar] [CrossRef]
- McGowan, P.M.; Mullooly, M.; Caiazza, F.; Sukor, S.; Madden, S.F.; Maguire, A.A.; Pierce, A.; McDermott, E.W.; Crown, J.; O’Donovan, N.; et al. ADAM-17: A novel therapeutic target for triple negative breast cancer. Ann. Oncol. 2013, 24, 362–369. [Google Scholar] [CrossRef]
- Moss, M.L.; Minond, D. Recent Advances in ADAM17 Research: A Promising Target for Cancer and Inflammation. Mediators Inflamm. 2017, 2017, 9673537. [Google Scholar] [CrossRef]
- Huang, C.K.; Iwagami, Y.; Aihara, A.; Chung, W.; de la Monte, S.; Thomas, J.M.; Olsen, M.; Carlson, R.; Yu, T.; Dong, X.; et al. Anti-Tumor Effects of Second Generation beta-Hydroxylase Inhibitors on Cholangiocarcinoma Development and Progression. PLoS ONE 2016, 11, e0150336. [Google Scholar] [CrossRef]
- Nagaoka, K.; Ogawa, K.; Ji, C.; Cao, K.Y.; Bai, X.; Mulla, J.; Cheng, Z.; Wands, J.R.; Huang, C.-K. Targeting Aspartate Beta-Hydroxylase with the Small Molecule Inhibitor MO-I-1182 Suppresses Cholangiocarcinoma Metastasis. Dig. Dis. Sci. 2021, 66, 1080–1089. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, X.; Meng, F.; Ogawa, K.; Li, M.; Song, R.; Zhang, S.; Zhang, Z.; Kong, X.; Xu, Q.; et al. Multi-organ metastasis as destination for breast cancer cells guided by biomechanical architecture. Am. J. Cancer Res. 2021, 11, 2537–2567. [Google Scholar]
- Susa, M.; Morii, T.; Yabe, H.; Horiuchi, K.; Toyama, Y.; Weissbach, L.; Hornicek, F.J.; Morioka, H. Alendronate inhibits growth of high-grade chondrosarcoma cells. Anticancer Res. 2009, 29, 1879–1888. [Google Scholar]
- Rey, V.; Menendez, S.T.; Estupinan, O.; Rodriguez, A.; Santos, L.; Tornin, J.; Martinez-Cruzado, L.; Castillo, D.; Ordonez, G.R.; Costilla, S.; et al. New Chondrosarcoma Cell Lines with Preserved Stem Cell Properties to Study the Genomic Drift During In Vitro/In Vivo Growth. J. Clin. Med. 2019, 8, 455. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Tamaki, S.; Cantarini, M.C.; Ince, N.; Wiedmann, M.; Carter, J.J.; Lahousse, S.A.; Califano, S.; Maeda, T.; Ueno, T.; et al. Aspartyl-(asparaginyl)-beta-hydroxylase regulates hepatocellular carcinoma invasiveness. J. Hepatol. 2006, 44, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Eliasz, S.; Liang, S.; Chen, Y.; De Marco, M.A.; Machek, O.; Skucha, S.; Miele, L.; Bocchetta, M. Notch-1 stimulates survival of lung adenocarcinoma cells during hypoxia by activating the IGF-1R pathway. Oncogene 2010, 29, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Chen, Y.; Zheng, X.; Li, Y.; Zhang, Q.; Mo, D.; Yang, G. MicroRNA-139-5p Suppresses 3T3-L1 Preadipocyte Differentiation Through Notch and IRS1/PI3K/Akt Insulin Signaling Pathways. J. Cell Biochem. 2015, 116, 1195–1204. [Google Scholar] [CrossRef]
- Patravale, V.; Dandekar, P.; Jain, R. Nanotoxicology: Evaluating toxicity potential of drug-nanoparticles. In Nanoparticulate Drug Delivery; Patravale, V., Dandekar, P., Jain, R., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 123–155. [Google Scholar] [CrossRef]
- Jain, A.K.; Singh, D.; Dubey, K.; Maurya, R.; Mittal, S.; Pandey, A.K. Models and Methods for In Vitro Toxicity. In In Vitro Toxicology; Dhawan, A., Kwon, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 45–65. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. In Cancer Cell Culture: Methods and Protocols; Cree, I.A., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 237–245. [Google Scholar] [CrossRef]
- Gundogan, F.; Elwood, G.; Longato, L.; Tong, M.; Feijoo, A.; Carlson, R.I.; Wands, J.R.; de la Monte, S.M. Impaired placentation in fetal alcohol syndrome. Placenta 2008, 29, 148–157. [Google Scholar] [CrossRef]
- Tong, M.; Ziplow, J.L.; Mark, P.; de la Monte, S.M. Dietary Soy Prevents Alcohol-Mediated Neurocognitive Dysfunction and Associated Impairments in Brain Insulin Pathway Signaling in an Adolescent Rat Model. Biomolecules 2022, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, E.B.; Tong, M.; Delikkaya, B.; Pelit, W.; Yang, Y.; de la Monte, S.M. Differential effects of moderate chronic ethanol consumption on neurobehavior, white matter glial protein expression, and mTOR pathway signaling with adolescent brain maturation. Am. J. Drug Alcohol Abuse 2024, 50, 492–516. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tong, M.; de la Monte, S.M. Early-Stage Moderate Alcohol Feeding Dysregulates Insulin-Related Metabolic Hormone Expression in the Brain: Potential Links to Neurodegeneration Including Alzheimer’s Disease. J. Alzheimers Dis. Rep. 2024, 8, 1211–1228. [Google Scholar] [CrossRef]
- Alexander, M.C.; Lomanto, M.; Nasrin, N.; Ramaika, C. Insulin stimulates glyceraldehyde-3-phosphate dehydrogenase gene expression through cis-acting DNA sequences. Proc. Natl. Acad. Sci. USA 1988, 85, 5092–5096. [Google Scholar] [CrossRef]
- Nicholls, C.; Li, H.; Liu, J.P. GAPDH: A common enzyme with uncommon functions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 674–679. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Lahousse, S.A.; Carter, J.; Wands, J.R. ATP luminescence-based motility-invasion assay. Biotechniques 2002, 33, 98–100, 102, 104 passim. [Google Scholar] [CrossRef]
- Kanwal, M.; Smahel, M.; Olsen, M.; Smahelova, J.; Tachezy, R. Aspartate beta-hydroxylase as a target for cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 163. [Google Scholar] [CrossRef] [PubMed]
- Lavaissiere, L.; Jia, S.; Nishiyama, M.; de la Monte, S.; Stern, A.M.; Wands, J.R.; Friedman, P.A. Overexpression of human aspartyl(asparaginyl)beta-hydroxylase in hepatocellular carcinoma and cholangiocarcinoma. J. Clin. Investig. 1996, 98, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M.; Tong, M.; Carlson, R.I.; Carter, J.J.; Longato, L.; Silbermann, E.; Wands, J.R. Ethanol inhibition of aspartyl-asparaginyl-beta-hydroxylase in fetal alcohol spectrum disorder: Potential link to the impairments in central nervous system neuronal migration. Alcohol 2009, 43, 225–240. [Google Scholar] [CrossRef]
- Dinchuk, J.E.; Focht, R.J.; Kelley, J.A.; Henderson, N.L.; Zolotarjova, N.I.; Wynn, R.; Neff, N.T.; Link, J.; Huber, R.M.; Burn, T.C.; et al. Absence of post-translational aspartyl beta-hydroxylation of epidermal growth factor domains in mice leads to developmental defects and an increased incidence of intestinal neoplasia. J. Biol. Chem. 2002, 277, 12970–12977. [Google Scholar] [CrossRef]
- Feriotto, G.; Finotti, A.; Breveglieri, G.; Treves, S.; Zorzato, F.; Gambari, R. Transcriptional activity and Sp 1/3 transcription factor binding to the P1 promoter sequences of the human AbetaH-J-J locus. FEBS J. 2007, 274, 4476–4490. [Google Scholar] [CrossRef]
- Finotti, A.; Treves, S.; Zorzato, F.; Gambari, R.; Feriotto, G. Upstream stimulatory factors are involved in the P1 promoter directed transcription of the A beta H-J-J locus. BMC Mol. Biol. 2008, 9, 110. [Google Scholar] [CrossRef]
- Bandyopadhyay, G.K.; Yu, J.G.; Ofrecio, J.; Olefsky, J.M. Increased p85/55/50 expression and decreased phosphotidylinositol 3-kinase activity in insulin-resistant human skeletal muscle. Diabetes 2005, 54, 2351–2359. [Google Scholar] [CrossRef]
- Lee, J.H. Overexpression of humbug promotes malignant progression in human gastric cancer cells. Oncol. Rep. 2008, 19, 795–800. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luu, M.; Sabo, E.; de la Monte, S.M.; Greaves, W.; Wang, J.; Tavares, R.; Simao, L.; Wands, J.R.; Resnick, M.B.; Wang, L. Prognostic value of aspartyl (asparaginyl)-beta-hydroxylase/humbug expression in non-small cell lung carcinoma. Hum. Pathol. 2009, 40, 639–644. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; de la Monte, S.M.; Sabo, E.; Kethu, S.; Tavares, R.; Branda, M.; Simao, L.; Wands, J.R.; Resnick, M.B. Prognostic value of humbug gene overexpression in stage II colon cancer. Hum. Pathol. 2007, 38, 17–25. [Google Scholar] [CrossRef]
- Qu, Y.; Qi, L.; Hao, L.; Zhu, J. Upregulation of circ-ASPH contributes to glioma cell proliferation and aggressiveness by targeting the miR-599/AR/SOCS2-AS1 signaling pathway. Oncol. Lett. 2021, 21, 388. [Google Scholar] [CrossRef] [PubMed]
- Benelli, R.; Costa, D.; Mastracci, L.; Grillo, F.; Olsen, M.J.; Barboro, P.; Poggi, A.; Ferrari, N. Aspartate-beta-Hydroxylase: A Promising Target to Limit the Local Invasiveness of Colorectal Cancer. Cancers 2020, 12, 971. [Google Scholar] [CrossRef]
- Kanwal, M.; Smahelova, J.; Ciharova, B.; Johari, S.D.; Nunvar, J.; Olsen, M.; Smahel, M. Aspartate beta-hydroxylase Regulates Expression of Ly6 Genes. J. Cancer 2024, 15, 1138–1152. [Google Scholar] [CrossRef]
- Radaelli, S.; Stacchiotti, S.; Casali, P.G.; Gronchi, A. Emerging therapies for adult soft tissue sarcoma. Expert Rev. Anticancer Ther. 2014, 14, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Maira, S.M.; Stauffer, F.; Brueggen, J.; Furet, P.; Schnell, C.; Fritsch, C.; Brachmann, S.; Chene, P.; De Pover, A.; Schoemaker, K.; et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008, 7, 1851–1863. [Google Scholar] [CrossRef]
- Schnell, C.R.; Stauffer, F.; Allegrini, P.R.; O’Reilly, T.; McSheehy, P.M.; Dartois, C.; Stumm, M.; Cozens, R.; Littlewood-Evans, A.; Garcia-Echeverria, C.; et al. Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: Implications for clinical imaging. Cancer Res. 2008, 68, 6598–6607. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.E.; Tang, C.H.; Wu, S.H.; Chen, H.T.; Fong, Y.C.; Lu, Y.C.; Chen, W.C.; Huang, H.D.; Lin, C.Y.; Wang, S.W. CCN6-mediated MMP-9 activation enhances metastatic potential of human chondrosarcoma. Cell Death Dis. 2018, 9, 955. [Google Scholar] [CrossRef] [PubMed]
- Mery, B.; Espenel, S.; Guy, J.B.; Rancoule, C.; Vallard, A.; Aloy, M.T.; Rodriguez-Lafrasse, C.; Magne, N. Biological aspects of chondrosarcoma: Leaps and hurdles. Crit. Rev. Oncol. Hematol. 2018, 126, 32–36. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Wu, H.C.; Wu, J.E.; Huang, K.Y.; Yang, S.C.; Chen, S.X.; Tsao, C.J.; Hsu, K.F.; Chen, Y.L.; Hong, T.M. The dual PI3K/mTOR inhibitor BEZ235 restricts the growth of lung cancer tumors regardless of EGFR status, as a potent accompanist in combined therapeutic regimens. J. Exp. Clin. Cancer Res. 2019, 38, 282. [Google Scholar] [CrossRef]
- Huyan, T.; Tang, R.; Li, J.; Li, Q.; Xue, X.; Yang, H. Optimized Expression and Purification of Humbug in Pichia pastoris and Its Monoclonal Antibody Preparation. Iran. J. Public Health 2015, 44, 1632–1642. [Google Scholar]
- Durrant, D.E.; Das, A.; Dyer, S.; Kukreja, R.C. A dual PI3 kinase/mTOR inhibitor BEZ235 reverses doxorubicin resistance in ABCB1 overexpressing ovarian and pancreatic cancer cell lines. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129556. [Google Scholar] [CrossRef] [PubMed]
- Durrant, D.E.; Das, A.; Dyer, S.; Tavallai, S.; Dent, P.; Kukreja, R.C. Targeted Inhibition of Phosphoinositide 3-Kinase/Mammalian Target of Rapamycin Sensitizes Pancreatic Cancer Cells to Doxorubicin without Exacerbating Cardiac Toxicity. Mol. Pharmacol. 2015, 88, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Durrant, D.E.; Dyer, S.; Das, A.; Kukreja, R.C. Abstract 16201: Combined Treatment of the PI3k/mTOR Inhibitor BEZ235 With Doxorubicin Does Not Increase Cardiotoxicity While Improving Efficacy in Breast Cancer Cells. Circulation 2014, 130 (Suppl. 2). [Google Scholar] [CrossRef]
- Ikenouchi, J.; Aoki, K. Membrane bleb: A seesaw game of two small GTPases. Small GTPases 2017, 8, 85–89. [Google Scholar] [CrossRef]
- Aoki, K.; Satoi, S. Coordinated changes in cell membrane and cytoplasm during maturation of apoptotic bleb. Mol. Biol. Cell 2020, 31, 833–844. [Google Scholar] [CrossRef]
- Tacar, O.; Dass, C.R. Doxorubicin-induced death in tumour cells and cardiomyocytes: Is autophagy the key to improving future clinical outcomes? J. Pharm. Pharmacol. 2013, 65, 1577–1589. [Google Scholar] [CrossRef]
- Brachmann, S.M.; Hofmann, I.; Schnell, C.; Fritsch, C.; Wee, S.; Lane, H.; Wang, S.; Garcia-Echeverria, C.; Maira, S.M. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 22299–22304. [Google Scholar] [CrossRef]
- Mo, J.S.; Yoon, J.H.; Ann, E.J.; Ahn, J.S.; Baek, H.J.; Lee, H.J.; Kim, S.H.; Kim, Y.D.; Kim, M.Y.; Park, H.S. Notch1 modulates oxidative stress induced cell death through suppression of apoptosis signal-regulating kinase 1. Proc. Natl. Acad. Sci. USA 2013, 110, 6865–6870. [Google Scholar] [CrossRef]
- Tasca, A.; Helmstadter, M.; Brislinger, M.M.; Haas, M.; Mitchell, B.; Walentek, P. Notch signaling induces either apoptosis or cell fate change in multiciliated cells during mucociliary tissue remodeling. Dev. Cell 2021, 56, 525–539.e526. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Li, Y.; Banerjee, S.; Liao, J.; Sarkar, F.H. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol. Cancer Ther. 2006, 5, 483–493. [Google Scholar] [CrossRef]
- Sahlgren, C.; Gustafsson, M.V.; Jin, S.; Poellinger, L.; Lendahl, U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc. Natl. Acad. Sci. USA 2008, 105, 6392–6397. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, L.; Zhao, S.; Zhao, X.Y.; Min, P.X.; Ma, Y.D.; Wang, Y.Y.; Chen, Y.; Tang, S.J.; Zhang, Y.J.; et al. Non-canonical Notch Signaling Regulates Actin Remodeling in Cell Migration by Activating PI3K/AKT/Cdc42 Pathway. Front. Pharmacol. 2019, 10, 370. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, X.; Ma, J.; Tong, Y.; Yao, J. Effects of NVP-BEZ235 on the proliferation, migration, apoptosis and autophagy in HT-29 human colorectal adenocarcinoma cells. Int. J. Oncol. 2016, 49, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, N.; Lopez, A.; Cuello, V.; Persans, M.; Schuenzel, E.; Innis-Whitehouse, W.; Keniry, M. NVP-BEZ235 or JAKi Treatment leads to decreased survival of examined GBM and BBC cells. Cancer Treat. Res. Commun. 2021, 27, 100340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; van Oosterwijk, J.G.; Sicinska, E.; Moss, S.; Remillard, S.P.; van Wezel, T.; Buhnemann, C.; Hassan, A.B.; Demetri, G.D.; Bovee, J.V.; et al. Functional profiling of receptor tyrosine kinases and downstream signaling in human chondrosarcomas identifies pathways for rational targeted therapy. Clin. Cancer Res. 2013, 19, 3796–3807. [Google Scholar] [CrossRef]
- Bhola, N.E.; Jansen, V.M.; Koch, J.P.; Li, H.; Formisano, L.; Williams, J.A.; Grandis, J.R.; Arteaga, C.L. Treatment of Triple-Negative Breast Cancer with TORC1/2 Inhibitors Sustains a Drug-Resistant and Notch-Dependent Cancer Stem Cell Population. Cancer Res. 2016, 76, 440–452. [Google Scholar] [CrossRef]
- Zhdanovskaya, N.; Firrincieli, M.; Lazzari, S.; Pace, E.; Scribani Rossi, P.; Felli, M.P.; Talora, C.; Screpanti, I.; Palermo, R. Targeting Notch to Maximize Chemotherapeutic Benefits: Rationale, Advanced Strategies, and Future Perspectives. Cancers 2021, 13, 5106. [Google Scholar] [CrossRef]
| Antibody Name * | Antibody Type | Source | Catalog # | Concentration |
|---|---|---|---|---|
| A85G6-ASPH) | Mouse Monoclonal | Liver Research Center, Brown University Health, RI USA | A85G6 | 1.3 µg/mL |
| FB50-ASPH) | Mouse Monoclonal | Liver Research Center, Brown University Health, RI USA | FB50 | 0.845 µg/mL |
| GAPDH | Mouse Monoclonal | Santa Cruz Biotechnology | sc-365062 | 0.067 µg/ml |
| β-Actin | Mouse Monoclonal | Santa Cruz Biotechnology | sc-47778 | 0.2 µg/mL |
| Vimentin | Mouse Monoclonal | RV-202; Abcam | ab8978 | 2.5 µg/mL |
| RPLPO | Mouse Monoclonal | Santa Cruz Biotechnology | sc-293260 | 0.1 µg/mL |
| HRP-conjugated secondary | Goat Polyclonal | Thermo Fisher Scientific; | Anti-mouse-31430Anti-rabbit-31461 | 0.04 µg/mL |
| Akt/mTOR Pathway Molecules | Protein Abbreviation | Phosphoprotein |
|---|---|---|
| Insulin Receptor | Insulin-R | pYpY1162/1163-Insulin R |
| Insulin-Like Growth Factor Receptor Type 1 | IGF-1R | pYpY1135/1136-IGF-1R |
| Insulin Receptor Substrate, Type 1 | IRS1 | pS636-IRS-1 |
| Akt (Protein Kinase B) | Akt | pS473-Akt |
| Phosphatase and tensin homolog | PTEN | pS380-PTEN |
| Glycogen Synthase Kinase 3α | GSK-3α | pS21-GSK3α |
| Glycogen Synthase Kinase 3β | GSK-3β | pS9-GSK3β |
| Tuberous Sclerosis Complex 2 | TSC2 | pS939-TSC2 |
| Mechanistic Target of Rapamycin | mTOR | pS2448-mTOR |
| p70 Ribosomal S6 kinase | P70S6K | pT412-p70S6K |
| Ribosomal Protein S6 | RPS6 | pS235/S236-RPS6 |
| Gene Code | Gene Name | F-Ratio | p-Value |
|---|---|---|---|
| ASPH | Aspartyl-Asparaginyl-β-Hydroxylase | 66.64 | 0.0007 |
| NOTCH1 | Notch 1 | 0.8998 | N.S. |
| JAG1 | Jagged 1 | 9.533 | 0.0270 |
| HES1 | Hairy and enhancer of split-1 | 9.187 | 0.0288 |
| HEY1 | HES-related family bHLH transcription factor | 0.5119 | N.S. |
| HIF1α | Hypoxia-Inducible Factor 1-alpha | 80.51 | 0.0005 |
| INS | Insulin | 4.845 | 0.0808 |
| IGF1 | Insulin-Like Growth Factor 1 | 0.5891 | N.S. |
| IGF2 | Insulin-Like Growth Factor 2 | 0.8381 | N.S. |
| INSR | Insulin Receptor | 0.4804 | N.S. |
| IGF1R | Insulin-Like Growth Factor 1 Receptor | 1.100 | N.S. |
| IGF2R | Insulin-Like Growth Factor 2 Receptor | 2.613 | N.S. |
| IRS1 | Insulin Receptor Substrate, Type 1 | 13.75 | 0.0142 |
| IRS2 | Insulin Receptor Substrate, Type 2 | 1.245 | N.S. |
| IRS4 | Insulin Receptor Substrate, Type 4 | 0.4039 | N.S. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fife, M.; Tong, M.; Das, B.; Rodriguez, R.; Chokkalingam, P.; Carlson, R.I.; de la Monte, S.M. Chondrosarcoma: Multi-Targeting Therapeutic Effects of Doxorubicin, BEZ235, and the Small Molecule Aspartyl-Asparaginyl-β-hydroxylase Inhibitor SMI1182. Cancers 2025, 17, 1671. https://doi.org/10.3390/cancers17101671
Fife M, Tong M, Das B, Rodriguez R, Chokkalingam P, Carlson RI, de la Monte SM. Chondrosarcoma: Multi-Targeting Therapeutic Effects of Doxorubicin, BEZ235, and the Small Molecule Aspartyl-Asparaginyl-β-hydroxylase Inhibitor SMI1182. Cancers. 2025; 17(10):1671. https://doi.org/10.3390/cancers17101671
Chicago/Turabian StyleFife, Megan, Ming Tong, Bhaskar Das, Rene Rodriguez, Parthiban Chokkalingam, Rolf I. Carlson, and Suzanne M. de la Monte. 2025. "Chondrosarcoma: Multi-Targeting Therapeutic Effects of Doxorubicin, BEZ235, and the Small Molecule Aspartyl-Asparaginyl-β-hydroxylase Inhibitor SMI1182" Cancers 17, no. 10: 1671. https://doi.org/10.3390/cancers17101671
APA StyleFife, M., Tong, M., Das, B., Rodriguez, R., Chokkalingam, P., Carlson, R. I., & de la Monte, S. M. (2025). Chondrosarcoma: Multi-Targeting Therapeutic Effects of Doxorubicin, BEZ235, and the Small Molecule Aspartyl-Asparaginyl-β-hydroxylase Inhibitor SMI1182. Cancers, 17(10), 1671. https://doi.org/10.3390/cancers17101671






