Chemopreventive and Anticancer Activity of Selected Triterpenoids in Melanoma
Simple Summary
Abstract
1. Introduction
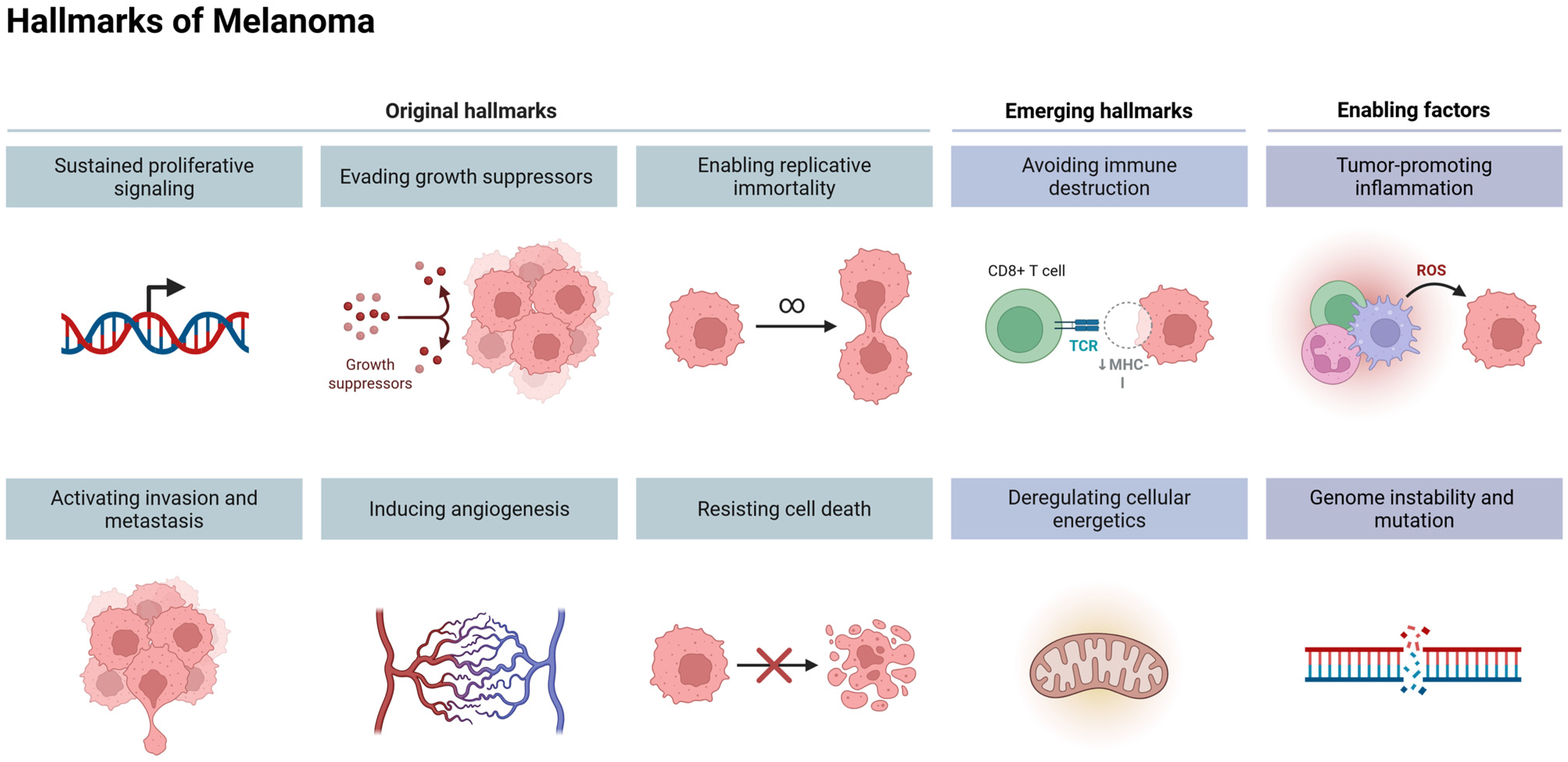
2. Genomic Changes in Melanoma
3. Terpenoids—Chemical Characteristics
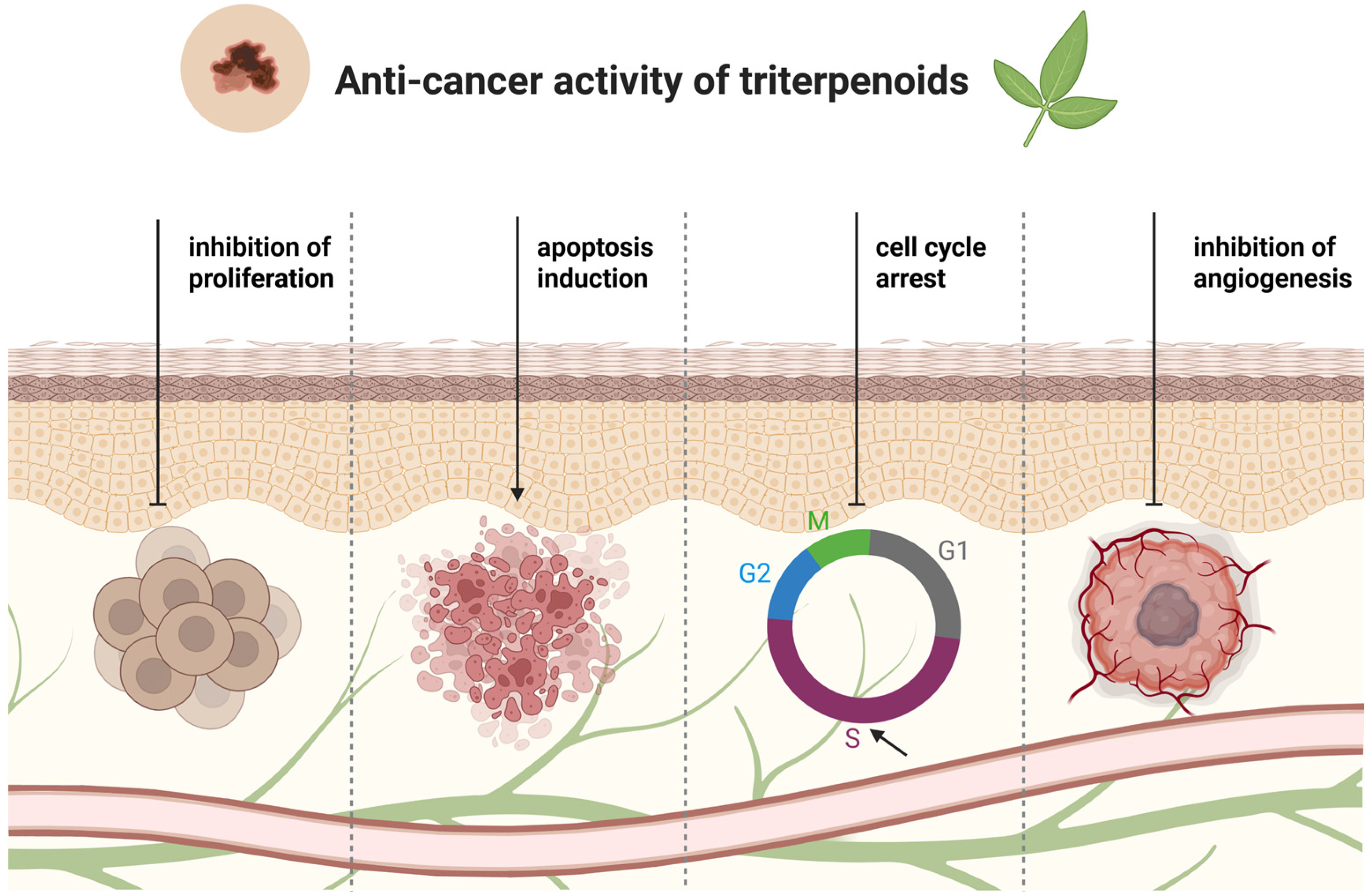
4. Lupane-Type Triterpenoids in Melanoma Treatment—In Vitro Studies
Betulinic Acid (BA)
| Type | Cell Line | Effect | Concentration/IC50 Values | Reference |
|---|---|---|---|---|
| BA | Self-generated primary equine dermal fibroblasts PriFi1 and PriFi2 and previously isolated primary equine melanoma cells (MelDuWi) | ↓ cell proliferation ↑cell cytotoxicity | MelDuWi: IC50 = 34.6 µM, PriFi1: IC50 = 20.4 µM, PriFi2: IC50 = 24.8 µM | [39] |
| BA | Human melanoma cell lines (A375, SK-MEL28, FM55P, and FM55M2), normal human keratinocytes (HaCaT) | ↓ cell viability | 1–40 µM, A375: IC50 = 15.94 µM, SK-MEL28: IC50 = 2.21 µM, FM55P: IC50 = 5.62 µM, FM55M2: IC50 = 4.08 µM | [44] |
| BA | Human melanoma cell line (IGR1), normal human keratinocytes (HaCaT) | ↑ apoptosis DNA fragmentation | IGR1: IC50 = 1.3 µg/mL (2.85 µM, respectively); normal cells: IC50 = 5 µg/mL (10.95 µM, respectively) | [45] |
| Rosemary extract containing BA | Human keratinocytes (SIK 28) cells | Inhibition of TCDD-induction of AhR-dependent reporter gene expression, inhibition of TCDD-stimulated AhR transformation and DNA binding | 10–100 µM | [40] |
| BBS and BA derivatives | MelDuW, PriFri2, and sRGO2 | ↓ cell proliferation ↑ cell cytotoxicity | 1–100 µM | [40] |
| BA and BA derivatives: N-(2,3-indolo-betulinoyl)diglycylglycine (BA1), N-(2,3-indolo-betulinoyl)glycylglycine (BA2), and N-(2,3-indolo-betulinoyl)glycine (BA3), 2,3-indolo-betulinic acid (BA4) | Murine melanoma cells (B164A5) | LDH leakage, cell membrane disruption, altered cell nuclei morphology | B164A5: 1–40 µM; BA: IC50 = 21.14 µM, BA1: IC50 = 10.34 µM, BA2: IC50 = 9.15 µM, BA3: IC50 = 8.11 µM, BA4: IC50 = 17.62 µM | [41] |
| BA and BA derivatives: N-(2,3-indolo-betulinoyl)diglycylglycine (BA1), N-(2,3-indolo-betulinoyl)glycylglycine (BA2), and N-(2,3-indolo-betulinoyl)glycine (BA3), 2,3-indolo-betulinic acid (BA4) | Human melanoma cell lines (A375) | ↓ cell viability, LDH leakage, ↓ cell migration | 1, 10, 25, 50, and 75 µM; BA: IC50 = 19.2 µM, BA1: IC50 = 5.7 µM, BA2: IC50 = 13.7 µM, BA3: IC50 = 10.0 µM, BA4: IC50 = 19.6 µM | [42] |
| BA-GNP | Human melanoma cell lines (RPMI-7951) | ↓ cell viability ↓ Bcl-2 production ↑Bax production ↓ mitochondrial respiration, cell shrinkage and deformation, nuclear condensation, shrinkage and fragmentation | 10, 25, and 50 µM | [43] |
5. Oleanane-Type Triterpenoids in Melanoma Treatment—In Vitro Studies
5.1. Oleanolic Acid (OA)
| Type | Cell Line | Effect | Concentration/IC50 Values | Reference |
|---|---|---|---|---|
| OA | Human keratinocytes (HaCaT) cells exposed to the pro-oxidative agent tBHP | ↑ cell viability, ↓ intracellular ROS levels, ↓ inducible iNOS | 1.25 μg/mL (2.74 µM, respectively) | [50] |
| OA | Human melanoma cell lines (HTB140, A375, WM793), human keratinocytes (HaCaT) | ↑cell cytotoxicity | 0.5–100 μg/mL (1.1–219 μM, respectively) | [51] |
| OA | Human melanoma cell line (A375) | ↓ cell viability, ↑ cell wall disruption, ↑ apoptosis | IC50 = 277.5 μM | [46] |
| OA | Human melanoma cell line (A375) | ↓ cell viability, ↑ apoptosis, ↑ G0/G1 proliferation arrest, ↑ inter-nucleosomal fragmentation, up-regulation of Bax, down-regulation of Bcl-2, ↑ cytochrome c release to cytosol | IC50 = 40.70 μM | [47] |
| OA | Human melanoma cell lines (A375SM, A375P) | ↓ cell viability, ↑ expression of the apoptotic proteins (PARP, Bax), ↓ expression of Bcl-2, ↓ expression of p–NF–κB and p-IκBα | 20–100 μM | [48] |
| OA; OA derivative (3-O-succinyl-28-O-benzyl oleanolate) | Mouse melanoma cell line (B16-F10) | ↓ cell proliferation, ↑ apoptosis, ↑ G0/G1 cell-cycle arrest, chromatin condensation and fragmentation, loss of membrane asymmetry, cell shrinkage | IC50 = 46.2 μg/mL (101.18 μM, respectively); IC50 = 15.3 μg/mL (22. 8 μM, respectively) | [52] |
| OA derivative (tryptamine amide of (3β)-3-(acetyloxy)olean-12-en-28-oic acid) | Human melanoma cell line (G-361) | ↑ cytotoxicity, ↑ apoptosis, ↑ accumulation of the S-phase cells, ↓ G2/M phase cells | IC50 = 9.0 µM | [53] |
| β-amyrin | Human melanoma cell line (A375) | ↓ cell proliferation | IC50 = 0.48 µM | [54] |
| Escin | Human melanoma cell line (CHL-1) | ↑ apoptosis, ↑ cell cytotoxicity, ↑ ROS generation | IC50 = 6 μg/mL (13.57 μM, respectively) | [55] |
| Escin | Human melanoma cell lines (B16F10 and SK-MEL5) | ↑ inhibitors of metalloproteinases 1 and 2 (TIMP-1 and TIMP-2) expression, ↓ phosphorylated extracellular signal-regulated kinase (p-ERK), ↓ expression of nuclear factor-kappa B (NF-κB) and its inhibitor, IκB | 20 µM | [52] |
| Hederagenin | Human melanoma cell line (SK-MEL-2) | ↓ cell viability | IC50 = 27.67 µM | [56] |
| Hederagenin; hederagenin 3-O-[α-L-rhamnopyranosyl-(1→2)-α-L-arabinopyranoside]; hederagenin 3-O-[β-D-glucopyranosyl-(1→3)-α-L-arabinopyranoside] | Human melanoma cell line (SK-MEL-2) | ↓ cell viability | IC50 = 22.0 µg/mL (17.98 µM respectively); IC50 = 2.3 µg/mL; IC50 = 7.0 µg/mL | [57] |
| δ-hederin; Glc3-O-hederagenin; hederacolchicosid A; α-hederin | Malignant melanoma cell line (M4 Beu) | ↓ cell viability | IC50 = ca. 30 µM; IC50 = ca. 35 µM; IC50 = ca. 10 µM; IC50 = ca. 25 µM | [58] |
| 3-O-β-D-glucopyranosyl-(1 → 2)-β-D-galactopyranosyl hederagenin 28-O-α-L-rhamnopyranosyl-(1 → 4)-β-D-glucopyranosyl-(1 → 6)-β-D-glucopyranoside | Human melanoma cell line (A375) | ↓ cell proliferation | IC50 = 21.4 µg/mL | [59] |
| GA derivative (GPD-12) | Human melanoma cell line (A375) and murine cell line (B16F10) | ↑cell cytotoxicity, ↑ nuclear fragmentation, ↑ expression of apoptosis related protein, e.g., caspase-3 and caspase-9, ↑ Bax to Bcl2 ratio | 5–100 μM | [60] |
| GA derivative (3-O-prenyl glycyrrhetinic acid) | Human melanoma cell lines (A375, SKMEL-28), murine melanoma cell line (B16F10), and normal human keratinocytes (HaCaT) | ↓ activity of MAPK signaling pathway, ↓ AKT survival signaling pathway, ↑ expression of p-mTOR, ↑ ER stress in cells, ROS generation | 16, 27, 33.5 or 50 μM | [61] |
5.2. β-Amyrin
5.3. Escin
5.4. Hederagenin
5.5. Glycyrrhetinic Acid (GA)
6. Ursane-Type Triterpenoids in Melanoma Treatment—In Vitro Studies
6.1. Ursolic Acid (UA)
| Type | Cell Line | Effect | Concentration/IC50 Values | Reference |
|---|---|---|---|---|
| UA in combination with OA (1:1, 3.5:1) | Human metastatic melanoma cell line (WM-266-4) | ↓ cell proliferation activity | 0.02, 0.2 μM, and 2 μM | [70] |
| UA | Human skin melanoma cell lines (A375 and B164A5) | ↑ cell cytotoxicity, ↑ apoptosis, ↓ bcl-2 anti-apoptotic gene expression, arresting cells in the G0/G1 phase | 25–100 μM | [72] |
| UA | Human skin melanoma cell line (SK-MEL-2) | ↓ dose-dependent effect on cell growth, cell arrest in the S phase | IC50 = 58.43 µM | [77] |
| UA | Human skin malignant melanoma (G361) | ↓ cellular growth, ↑ apoptosis via activation of caspase-3, ↓ DNA synthesis rate | 10 μM, 20 μM | [76] |
| UA | Human skin melanoma cell line (M4Beu) | ↓ cell proliferation activity, ↑ apoptosis, ↑ mitochondrial intrinsic pathway, ΔΨm collapse and accumulation of Bax proapoptotic protein, ↑ caspase activation and AIF leakage | 5–20 μM | [74] |
| UA | Human melanoma cells (M4Beu), normal human keratinocytes (HaCaT) | ↑ significant caspase-3 activation, ↑ strong ΔΨm collapse in cancer cells, change in Bax/Bcl-2-balance in favor of Bax | 12.5 μM, 15 μM | [78] |
| UA | Human melanoma cell lines (MM200, Mel-RM, Me4405, and A375) | ↓ cell proliferation after 24 and 48 h, ↑ proteolytic processing of caspase-3 | 10–40 μM | [73] |
| UA | Human melanoma cells (M4Beu) | ↓ phosphorylation of Akt and ERK-1/2 proteins, inactivation of cell growth and survival-related Akt/ERK signaling pathways, ↑ apoptosis induction | 10–17.5 μM | [75] |
| UA | Human melanoma cell line (A2058) | ↑ apoptosis | 50–75 μM | [72] |
| UA; inclusion complex of UA and 2-hydroxypropyl-β-cyclodextrin (UA: HPβCD) in the molar ratio of 1:2; inclusion complex of UA and 2- hydroxypropyl-γ-cyclodextrin (UA: HPγCD) in the molar ratio of 1:2 | Human melanoma cell line (A375 and SK-MEL-2) | ↓ cell proliferation | A375: 5–50 µM, UA: IC50 = 68.22 µM, (UA: HPβCD): IC50 = 51.73 µM, (UA: HPγCD): IC50 = 31.38 µM, SK-MEL-2: 5–50 µM, UA: IC50 = 58.44 µM, (UA: HPγCD): IC50 = 9.26 µM | [80] |
| UA; UAA | Human melanoma cell line (A375) | ↓ cell proliferation, ↑ caspases 3/7 activity, ↑ Bax levels, ↓ Bcl-2 production, cell cycle arrest at sub-G1 phase (UA) or S phase (UAA) | GI50 = 32.4 µM, GI50 = 26.7 µM (respectively) | [81] |
| Piperazine-spacered conjugate of ursolic acid and 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid | Human melanoma cell line (A375) | ↑ apoptosis, ↑ number of cells in sub-G1 phase | EC50 = 1.5 µM | [82] |
| 1H-Benzotriazole-1-yl (3β) 3-hydroxyurs-12-en-28-oate | Human melanoma cell line (A375) | ↓ cell viability, ↑ apoptotic activities (nucleus shrinkage, ↓ Bcl-2 expression, ↑ Bax production | 50 µM | [83] |
| AA | Human melanoma cell line (SK-MEL-2) | ↓ cell viability, ↑ apoptosis in a dose-dependent manner, ↑ intracellular ROS, ↑ expression of Bax, ↑ activation of caspase-3 | 40 μM | [84] |
| AA derivative | Human melanoma cell line (A375) | ↑ cell cytotoxicity | 0.0028, 0.58, 1.3, or 30 μM | [85] |
| MA; MA homopiperazinyl rhodamine B conjugate | Human melanoma cell line (A375) | no significant effect (MA), strong cytotoxic effect (conjugate) | EC50 > 30 µM, EC50 = 0.0095 µM (respectively) | [86] |
| Methyl 2-oxo-3β-(2-furoyloxy)-6,23-epoxyursa-5,12-dien-28-oate (MA derivative) | Human melanoma cell lines (SK-MEL-5, UACC-257) | ↓ cell growth | TGI = 2.6 µM, LC50 > 100 µM, TGI = 3.0 µM, LC50 = 50.1 µM (respectively) | [87] |
| α-amyrin | Human melanoma cell line (A375) | ↓ cell proliferation activity | IC50 = 1.26 µM | [54] |
6.2. Asiatic Acid (AA)
6.3. Madecassic Acid (MA)
6.4. α-Amyrin
7. Triterpenoids in Melanoma Treatment—In Vivo Studies
| Compound | Organism | Model | Duration and Dose | Effect and Mechanism | Reference |
|---|---|---|---|---|---|
| AA | 6-week-old female ICR mice | Xenografts with tumor initiator (DMBA) and tumor promoter (TPA) | 20 weeks, two doses of AA (30 and 50 μM) twice a week 1 h before TPA | ↓ average number of tumors, ↓ TPA-induced NO production, ↓ expression of NO synthase (iNOS) and cyclooxygenase-2 (COX-2) | [95] |
| AA and naringin | 8-week-old male C51BL/6 mice | Xenografts with melanoma cells (B16F10 tumor cells) | Daily dosing, AA (10 mg/kg body weight) or NG (50 mg/kg body weight) alone or with both drugs (AA-NG, 10 mg/kg + 50 mg/kg) | ↓ volume of melanoma in the joined therapy, no cardio-, nephron or hepatotoxic effects, ↑ maturation and differentiation of NK cells, ↑ NK cells immunity against cancer cells (via Id2 and IRE2 mechanisms), ↑ Smad 7 expression, ↓ Smad 3 translation and phosphorilation | [91] |
| BA | 8-week-old C57BL/6J mice | Xenografts with melanoma cells (B164A5 tumor cells) | Daily for 21 days, 100 mg/kg i.p. BA | ↓ hyperpigmentation, ↓ erythrema, ↓ angiogenesis, ↓ proliferation, tumor size, and weight | [92] |
| BA | 9–10-week-old female C57BL/6 mice | Mouse xenografts with metastatic melanoma (B16F10 cells) | 10 mg/kg b.w. BA solution; 0.065 mg/kg b.w. vincristine; a combination of both | In the combined therapy: ↓ total number of nodules, ↓ the ratio of the total number of large to small nodules | [93] |
| OA | C57BL/6 mice | Xenografts with melanoma cells | Ten 20 μL doses of 10 mg/mL solution of oleanolic acid within 2 weeks | ↓ melanoma cell proliferation | [89] |
| OA | 4-week-old female BALB/c nude mice | Xenografts with melanoma (A375SM cells) | 75 mg/kg and 150 mg/kg OA solution (n = 5), five times a week for 13 days | ↑ apoptosis, ↑ number of TUNEL-positive cells, -> NF-ĸB-mediated anticancer effects | [48] |
| UA | 8-week-old SKH-1 hairless mice | Irradiation with UVB for 25 weeks with and without UA | UVB with 2 µM UA solution (200 µL) | ↑ IL-8 signaling pathway, ↑ Nrf2-mediated response to oxidative stress, ↓ tumor size and number | [94] |
| UA and OA | Chicken embryos (7–11 day old) inoculated with SK-MEL-2 melanoma cells | Vascular membrane assay | Five daily doses of 30 mM of each acid | ↓ number of vessels (stronger in the OA group), ↓ invasiveness of melanoma in OA-treated group, ↑ changes in the arrangement of vessels surrounding tumor cells | [77] |
| UA, OA, and loaded with UA and OA polyurethane nanoparticles | 12-week-old female SKH1 mice | Xenografts with tumor initiator (DMBA) and tumor promoter (TPA) | 29-weeks, 200 μL 10μM of UA, OA, PU-UA, or PU-OA solutions twice a week | ↓ incidence of cancer in UA and PU-OA groups, ↓ number of tumors in UA, PU-UA, PU-OA groups, ↑ increased papilloma in keratinized, ↑ squamous cell carcinoma in PU-UA | [90] |
8. Discussion and Prospects
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| ALP | alkaline phosphatase |
| APC | antigen-presenting cell |
| CAM | chicken embryo vascular membrane |
| CD | cyclodextrin |
| COX-2 | cyclooxygenase-2 |
| CTLA-4 | cytotoxic T-lymphocyte associated protein 4 |
| DCT | dopachrome tautomerase |
| DMAP | dimethylaminopyridine |
| DMAPP | dimethylallyl diphosphate |
| DMBA | 7,12-dimethylbenzanthracene |
| EGFR | epidermal growth factor receptor |
| ERK 1/2 | extracellular signal-regulated kinases 1/2 |
| GPCRs | G protein-coupled receptors |
| GTP | guanosine-5′-triphosphate |
| HO-1 | heme-oxygenase 1 |
| HPGCD | 2-hydroxypropyl-γ-cyclodextrin |
| INOS | inducible nitric oxide synthase |
| IPP | isopentenyl diphosphate |
| LDH | lactate dehydrogenase |
| LD50 | lethal dose 50 |
| MAPK | mitogen-activated protein kinase |
| MEB | 2-C-methyl-D-erythritol 4-phosphate |
| MEK 1/2 | mitogen-activated protein kinase kinase 1/2 |
| MITF | microphthalmia-associated transcription factor |
| mTOR | mechanistic target of rapamycin kinase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium |
| MPT | mitochondrial permeability transition |
| MVA | mevalonate |
| NF-κB | nuclear factor-kappa B |
| NG | naringenin |
| NMSCs | non-melanoma skin cancers |
| NO | nitric oxide |
| OA | oleanolic acid |
| PD-1 | programmed cell death protein 1 |
| PDK1 | phosphoinositide-dependent kinase-1 |
| PD-L1 | programmed cell death ligand |
| p-ERK | phosphorylated extracellular signal-regulated kinase |
| PI3K | phosphoinositide 3-kinase |
| PIP2 | phosphatidylinositol-4,5-bisphosphate |
| PIP3 | phosphatidylinositol-3,4,5-trisphosphate |
| p-IκBα | phospho-inhibitor of nuclear factor-κBα |
| p–NF–κB | phospho-nuclear factor-κB |
| PTEN | phosphatase and tensin homolog |
| PU | polyurethane |
| ROS | reactive form of oxygen |
| RTK | receptor tyrosine kinases |
| SGOT | serum glutamicoxaloacetic transaminase |
| tBHP | tert-butyl hydroperoxide |
| TGFβ | transforming growth factor-beta |
| TIMP-1 and TIMP-2 | tissue inhibitors of metalloproteinases 1 and 2 |
| TPA | 12-tetradedecumiforbol |
| TYR | tyrosinase |
| TYRP1 | tyrosinase-related protein 1 |
| UA | ursolic acid |
| UAA | 3-O-acetylursolic acid |
| UV | ultraviolet |
| VCR | vincristine |
References
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular Markers and Targets in Melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Fisher, D.E.; Garbe, C.; Gershenwald, J.E.; Grob, J.J.; Halpern, A.; Herlyn, M.; Marchetti, M.A.; McArthur, G.; Ribas, A.; et al. Melanoma. Nat. Rev. Dis. Prim. 2015, 1, 15003. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, W.; Mwamba, R.N.; Grullon, K.; Armstrong, M.; Zhao, P.; Hendren-Santiago, B.; Qin, K.H.; Li, A.J.; Hu, D.A.; Youssef, A.; et al. Melanoma: Molecular genetics, metastasis, targeted therapies, immunotherapies, and therapeutic resistance. Genes Dis. 2022, 9, 1608–1623. [Google Scholar] [CrossRef] [PubMed]
- Alqathama, A.; Prieto, J.M. Natural products with therapeutic potential in melanoma metastasis. Nat. Prod. Rep. 2015, 32, 1170–1182. [Google Scholar] [CrossRef]
- Tímár, J.; Ladányi, A. Molecular Pathology of Skin Melanoma: Epidemiology, Differential Diagnostics, Prognosis and Therapy Prediction. Int. J. Mol. Sci. 2022, 23, 5384. [Google Scholar] [CrossRef]
- Guo, W.; Wang, H.; Li, C. Signal pathways of melanoma and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 424. [Google Scholar] [CrossRef]
- Inamdar, G.S.; Madhunapantula, S.R.V.; Robertson, G.P. Targeting the MAPK pathway in melanoma: Why some approaches succeed and other fail. Biochem. Pharmacol. 2010, 80, 624–637. [Google Scholar] [CrossRef]
- Smalley, K.S.M. Understanding Melanoma Signaling Networks as the Basis for Molecular Targeted Therapy. J. Investig. Dermatol. 2010, 130, 28–37. [Google Scholar] [CrossRef]
- Liu, Y.; Sheikh, M.S. Melanoma: Molecular Pathogenesis and Therapeutic Management. Mol. Cell. Pharmacol. 2014, 6, 228. [Google Scholar]
- Busse, A.; Keilholz, U. Role of TGF-β in melanoma. Curr. Pharm. Biotechnol. 2011, 12, 2165–2175. [Google Scholar] [CrossRef]
- Humbert, L.; Lebrun, J.J. TGF-beta inhibits human cutaneous melanoma cell migration and invasion through regulation of the plasminogen activator system. Cell. Signal. 2013, 25, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Vlašić, I.; Horvat, A.; Tadijan, A.; Slade, N. p53 Family in Resistance to Targeted Therapy of Melanoma. Int. J. Mol. Sci. 2022, 24, 65. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Chen, Y.; Jian, Y.; Zhang, F.; Wu, R. Chemotaxonomic investigation of plant terpenoids with an established database (TeroMOL). New Phytol. 2022, 235, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar]
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Moss, G.P.; Smith, P.A.S.; Tavernier, D. Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995). Pure Appl. Chem. 1995, 67, 1307–1375. [Google Scholar] [CrossRef]
- Mathur, A.; Meena, A.; Luqman, S. Monoterpenoids: An upcoming class of therapeutic agents for modulating cancer metastasis. Phytother. Res. 2024, 38, 939–969. [Google Scholar] [CrossRef]
- Ahmad, B.; Tian, C.; Tang, J.X.; Dumbuya, J.S.; Li, W.; Lu, J. Anticancer activities of natural abietic acid. Front. Pharmacol. 2024, 15, 1392203. [Google Scholar] [CrossRef]
- Roshni, P.T.; Rekha, P.D. Biotechnological interventions for the production of forskolin, an active compound from the medicinal plant, Coleus forskohlii. Physiol. Mol. Biol. Plants 2024, 30, 213–226. [Google Scholar] [CrossRef]
- Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and oleanolic acids: Plant metabolites with neuroprotective potential. Int. J. Mol. Sci. 2021, 22, 4599. [Google Scholar] [CrossRef]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.R.L.; Calado, L.L.; Duarte, A.B.S.; de Sousa, D.P. Centella asiatica and Its Metabolite Asiatic Acid: Wound Healing Effects and Therapeutic Potential. Metabolites 2023, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Tang, Y.; Xie, Y.; He, B.B.; Ding, G.; Zhou, X. Research progress on differentiation and regulation of plant chromoplasts. Mol. Biol. Rep. 2024, 51, 810. [Google Scholar] [CrossRef] [PubMed]
- Ziętal, K.; Mirowska-Guzel, D.; Nowaczyk, A.; Blecharz-Klin, K. Cnicus benedictus: Folk Medicinal Uses, Biological Activities, and In Silico Screening of Main Phytochemical Constituents. Planta Med. 2024, 90, 976–991. [Google Scholar] [CrossRef]
- Trendafilova, A.; Moujir, L.M.; Sousa, P.M.C.; Seca, A.M.L. Research Advances on Health Effects of Edible Artemisia Species and Some Sesquiterpene Lactones Constituents. Foods 2020, 10, 65. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Gomulski, J. Biologically Active Diterpenoids in the Clerodendrum Genus—A Review. Int. J. Mol. Sci. 2022, 23, 11001. [Google Scholar] [CrossRef]
- Otsuki, K.; Li, W. Tigliane and daphnane diterpenoids from Thymelaeaceae family: Chemistry, biological activity, and potential in drug discovery. J. Nat. Med. 2023, 77, 625–643. [Google Scholar] [CrossRef]
- Sochacki, M.; Vogt, O. Triterpenoid Saponins from Washnut (Sapindus mukorossi Gaertn.)-A Source of Natural Surfactants and Other Active Components. Plants 2022, 11, 2355. [Google Scholar] [CrossRef]
- Lu, Z.; Mao, T.; Chen, K.; Chai, L.; Dai, Y.; Liu, K. Ginsenoside Rc: A potential intervention agent for metabolic syndrome. J. Pharm. Anal. 2023, 13, 1375–1387. [Google Scholar] [CrossRef]
- Riyadi, S.A.; Naini, A.A.; Supratman, U. Sesquiterpenoids from Meliaceae Family and Their Biological Activities. Molecules 2023, 28, 4874. [Google Scholar] [CrossRef]
- Chen, D.L.; Wang, B.W.; Sun, Z.C.; Yang, J.S.; Xu, X.D.; Ma, G.X. Natural Nitrogenous Sesquiterpenoids and Their Bioactivity: A Review. Molecules 2020, 25, 2485. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shang, J.; Tao, C.; Huang, M.; Wei, D.; Yang, L.; Yang, J.; Fan, Q.; Ding, Q.; Zhou, M. Advancements in Betulinic Acid-Loaded Nanoformulations for Enhanced Anti-Tumor Therapy. Int. J. Nanomed. 2024, 19, 14075–14103. [Google Scholar] [CrossRef] [PubMed]
- Nemli, E.; Saricaoglu, B.; Kirkin, C.; Ozkan, G.; Capanoglu, E.; Habtemariam, S.; Sharifi-Rad, J.; Calina, D. Chemopreventive and Chemotherapeutic Potential of Betulin and Betulinic Acid: Mechanistic Insights From In Vitro, In Vivo and Clinical Studies. Food Sci. Nutr. 2024, 12, 10059–10069. [Google Scholar] [CrossRef]
- Isah, M.B.; Tajuddeen, N.; Umar, M.I.; Alhafiz, Z.A.; Mohammed, A.; Ibrahim, M.A. Terpenoids as Emerging Therapeutic Agents: Cellular Targets and Mechanisms of Action against Protozoan Parasites. Stud. Nat. Prod. Chem. 2018, 59, 227–250. [Google Scholar]
- Huang, L.N.; Liu, Y.Y.; Fang, H.B.; Jiao, Y.B.; Cheng, Y.X. Six new diterpenoids from the resins of Populus euphratica and their anti-inflammatory activities. Fitoterapia 2023, 168, 105545. [Google Scholar] [CrossRef]
- Li, H.Y.; Yang, W.Q.; Zhou, X.Z.; Shao, F.; Shen, T.; Guan, H.Y.; Zheng, J.; Zhang, L.M. Antibacterial and Antifungal Sesquiterpenoids: Chemistry, Resource, and Activity. Biomolecules 2022, 12, 1271. [Google Scholar] [CrossRef]
- Mu, H.; Sun, Y.; Yuan, B.; Wang, Y. Betulinic acid in the treatment of breast cancer: Application and mechanism progress. Fitoterapia 2023, 169, 105617. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Leśków, A.; Szczuka, I.; Zaprutko, L.; Diakowska, D. The Effect of Oleanolic Acid and Its Four New Semisynthetic Derivatives on Human MeWo and A375 Melanoma Cell Lines. Pharmaceuticals 2023, 16, 746. [Google Scholar] [CrossRef]
- Kallimanis, P.; Chinou, I.; Panagiotopoulou, A.; Soshilov, A.A.; He, G.; Denison, M.S.; Magiatis, P. Rosmarinus officinalis L. Leaf Extracts and Their Metabolites Inhibit the Aryl Hydrocarbon Receptor (AhR) Activation In Vitro and in Human Keratinocytes: Potential Impact on Inflammatory Skin Diseases and Skin Cancer. Molecules 2022, 27, 2499. [Google Scholar] [CrossRef]
- Lombrea, A.; Watz, C.G.; Bora, L.; Dehelean, C.A.; Diaconeasa, Z.; Dinu, S.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Danciu, C. Enhanced Cytotoxicity and Antimelanoma Activity of Novel Semisynthetic Derivatives of Betulinic Acid with Indole Conjugation. Plants 2023, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Lombrea, A.; Semenescu, A.D.; Magyari-Pavel, I.Z.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Muntean, D.; Dehelean, C.A.; Dinu, S.; Danciu, C. Comparison of In Vitro Antimelanoma and Antimicrobial Activity of 2,3-Indolo-betulinic Acid and Its Glycine Conjugates. Plants 2023, 12, 1253. [Google Scholar] [CrossRef] [PubMed]
- Ghiulai, R.; Mioc, A.; Racoviceanu, R.; Mioc, M.; Milan, A.; Prodea, A.; Semenescu, A.; Dehelean, C.; Barbu Tudoran, L.; Avram, Ș.; et al. The Anti-Melanoma Effect of Betulinic Acid Functionalized Gold Nanoparticles: A Mechanistic In Vitro Approach. Pharmaceuticals 2022, 15, 1362. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska-Łuczka, P.; Cabaj, J.; Bąk, W.; Bargieł, J.; Grabarska, A.; Góralczyk, A.; Łuszczki, J.J. Additive Interactions between Betulinic Acid and Two Taxanes in In Vitro Tests against Four Human Malignant Melanoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 9641. [Google Scholar] [CrossRef]
- Galgon, T.; Wohlrab, W.; Dräger, B. Betulinic acid induces apoptosis in skin cancer cells and differentiation in normal human keratinocytes. Exp. Dermatol. 2005, 14, 736–743. [Google Scholar] [CrossRef]
- Cijo George, V.; Naveen Kumar, D.R.; Suresh, P.K.; Kumar, R.A. Oleanolic acid inhibits cell growth and induces apoptosis in A375 melanoma cells. Biomed. Prev. Nutr. 2014, 4, 95–99. [Google Scholar] [CrossRef]
- Ghosh, S.; Bishayee, K.; Khuda-Bukhsh, A.R. Oleanolic acid isolated from ethanolic extract of Phytolacca decandra induces apoptosis in A375 skin melanoma cells: Drug-DNA interaction and signaling cascade. J. Integr. Med. 2014, 12, 102–114. [Google Scholar] [CrossRef]
- Woo, J.S.; Yoo, E.S.; Kim, S.H.; Lee, J.H.; Han, S.H.; Jung, S.H.; Jung, G.H.; Jung, J.Y. Anticancer effects of oleanolic acid on human melanoma cells. Chem. Biol. Interact. 2021, 347, 109619. [Google Scholar] [CrossRef]
- Bernabé-García, Á.; Armero-Barranco, D.; Liarte, S.; Ruzafa-Martínez, M.; Ramos-Morcillo, A.J.; Nicolás, F.J. Oleanolic acid induces migration in Mv1Lu and MDA-MB-231 epithelial cells involving EGF receptor and MAP kinases activation. PLoS ONE 2017, 12, e0172574. [Google Scholar] [CrossRef]
- Vasarri, M.; Bergonzi, M.C.; Leri, M.; Castellacci, R.; Bucciantini, M.; De Marchi, L.; Degl’Innocenti, D. Protective Effects of Oleanolic Acid on Human Keratinocytes: A Defense Against Exogenous Damage. Pharmaceuticals 2025, 18, 238. [Google Scholar] [CrossRef]
- Grabowska, K.; Galanty, A.; Pecio, Ł.; Stojakowska, A.; Malarz, J.; Żmudzki, P.; Zagrodzki, P.; Podolak, I. Selectivity Screening and Structure-Cytotoxic Activity Observations of Selected Oleanolic Acid (OA)-Type Saponins from the Amaranthaceae Family on a Wiade Panel of Human Cancer Cell Lines. Molecules 2024, 29, 3794. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Zurita, F.J.; Medina-O’Donnell, M.; Ferrer-Martin, R.M.; Rufino-Palomares, E.E.; Martin-Fonseca, S.; Rivas, F.; Martínez, A.; García-Granados, A.; Pérez-Jiménez, A.; García-Salguero, L.; et al. The oleanolic acid derivative, 3-O-succinyl-28-O-benzyl oleanolate, induces apoptosis in B16–F10 melanoma cells via the mitochondrial apoptotic pathway. RSC Adv. 2016, 6, 93590–93601. [Google Scholar] [CrossRef]
- Bildziukevich, U.; Kvasnicová, M.; Šaman, D.; Rárová, L.; Wimmer, Z. Novel oleanolic acid-tryptamine and-fluorotryptamine amides: From adaptogens to agents targeting in vitro cell apoptosis. Plants 2021, 10, 2082. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Peron, G.; Ferrari, S.; Gandin, V.; Bramucci, M.; Quassinti, L.; Mártonfi, P.; Maggi, F. Phytochemical investigations and antiproliferative secondary metabolites from Thymus alternans growing in Slovakia. Pharm. Biol. 2017, 55, 1162–1170. [Google Scholar] [CrossRef]
- Vejselova Sezer, C. Escin induces cell death in human skin melanoma cells through apoptotic mechanisms. Toxicol. Res. 2024, 13, 7. [Google Scholar] [CrossRef]
- Woo, K.W.; Cha, J.M.; Choi, S.U.; Lee, K.R. A new triterpene glycoside from the stems of Lagerstroemia indica. Arch. Pharm. Res. 2016, 39, 631–635. [Google Scholar] [CrossRef]
- Jung, H.J.; Lee, C.O.; Lee, K.T.; Choi, J.; Park, H.J. Structure-activity relationship of oleanane disaccharides isolated from Akebia quinata versus cytotoxicity against cancer cells and NO inhibition. Biol. Pharm. Bull. 2004, 27, 744–747. [Google Scholar] [CrossRef]
- Barthomeuf, C.; Debiton, E.; Mshvildadze, V.; Kemertelidze, E.; Balansard, G. In vitro activity of hederacolchisid A1 compared with other saponins from Hedera colchica against proliferation of human carcinoma and melanoma cells. Planta Med. 2002, 68, 672–675. [Google Scholar] [CrossRef]
- Ye, W.; Ji, N.N.; Zhao, S.; Che, C.T. A New Cytotoxic Saponin from Pulsatilla patens var. multifida. Pharm. Biol. 2001, 39, 7–10. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Gupta, R.; Rashid, H.; Bhat, A.M.; Sharma, R.R.; Naikoo, S.H.; Kaur, S.; Tasduq, S.A. Synthesis, molecular docking, and biological evaluation of [3,2-b]indole fused 18β-glycyrrhetinic acid derivatives against skin melanoma. RSC Adv. 2023, 13, 11130–11141. [Google Scholar] [CrossRef]
- Nazir, L.A.; Shahid, N.H.; Amit, K.; Umar, S.A.; Rajni, S.; Bharate, S.; Sangwan, P.L.; Tasduq, S.A. Synthesis and anti-melanoma effect of 3-O-prenyl glycyrrhetinic acid against B16F10 cells via induction of endoplasmic reticulum stress-mediated autophagy through ERK/AKT signaling pathway. Front. Oncol. 2022, 12, 890299. [Google Scholar] [CrossRef] [PubMed]
- Fazliev, S.; Tursunov, K.; Razzokov, J.; Sharipov, A. Escin’s Multifaceted Therapeutic Profile in Treatment and Post-Treatment of1. Fazliev, S.; Tursunov, K.; Razzokov, J.; Sharipov, A. Escin’s Multifaceted Therapeutic Profile in Treatment and Post-Treatment of Various Cancers: A Comprehensive Review. Biomol. Biomolecules 2023, 13, 315. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.S.; An, H.; Alam, M.B.; Choi, W.S.; Lee, S.Y.; Lee, S.H. Inhibition of Migration and Invasion in Melanoma Cells by β-Escin via the ERK/NF-κB Signaling Pathway. Biol. Pharm. Bull. 2018, 41, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Fang, X.; Li, H.; Lu, X.; Yang, D.; Han, S.; Bi, Y. Advances in the anti-tumor potential of hederagenin and its analogs. Eur. J. Pharmacol. 2023, 959, 176073. [Google Scholar] [CrossRef]
- Moustafa, G.O.; Shalaby, A.; Naglah, A.M.; Mounier, M.M.; El-Sayed, H.; Anwar, M.M.; Nossier, E.S. Synthesis, Characterization, In Vitro Anticancer Potentiality, and Antimicrobial Activities of Novel Peptide–Glycyrrhetinic-Acid-Based Derivatives. Molecules 2021, 26, 4573. [Google Scholar] [CrossRef]
- Csuk, R.; Schwarz, S.; Kluge, R.; Ströhl, D. Improvement of the Cytotoxicity and Tumor Selectivity of Glycyrrhetinic Acid by Derivatization with Bifunctional Aminoacids. Arch. Pharm. 2011, 344, 505–513. [Google Scholar] [CrossRef]
- Alho, D.P.S.; Salvador, J.A.R.; Cascante, M.; Marin, S. Synthesis and Antiproliferative Activity of Novel Heterocyclic Glycyrrhetinic Acid Derivatives. Molecules 2019, 24, 766. [Google Scholar] [CrossRef]
- Serbian, I.; Wolfram, R.K.; Fischer, L.; Al-Harrasi, A.; Csuk, R. Hydroxylated boswellic and glycyrrhetinic acid derivatives: Synthesis and cytotoxicity. Mediterr. J. Chem. 2018, 7, 286–293. [Google Scholar] [CrossRef]
- Csuk, R.; Schwarz, S.; Siewert, B.; Kluge, R.; Ströhl, D. Synthesis and antitumor activity of ring A modified glycyrrhetinic acid derivatives. Eur. J. Med. Chem. 2011, 46, 5356–5369. [Google Scholar] [CrossRef]
- Isaković-Vidović, S.; Dariš, B.; Knez, Ž.; Vidović, K.; Oprić, D.; Ferk, P. Antiproliferative Activity of Selected Triterpene Acids from Rosemary on Metastatic Melanoma Cell Line WM-266-4. Open Access Maced. J. Med. Sci. 2021, 9, 515–521. [Google Scholar] [CrossRef]
- Soica, C.; Oprean, C.; Borcan, F.; Danciu, C.; Trandafirescu, C.; Coricovac, D.; Crainiceanu, Z.; Dehelean, C.A.; Dehelean, M.M. The synergistic biologic activity of oleanolic and ursolic acids in complex with hydroxypropyl-γ-cyclodextrin. Molecules 2014, 19, 4924–4940. [Google Scholar] [CrossRef] [PubMed]
- Oprean, C.; Ivan, A.; Bojin, F.; Cristea, M.; Soica, C.; Drăghia, L.; Caunii, A.; Paunescu, V.; Tatu, C. Selective in vitro anti-melanoma activity of ursolic and oleanolic acids. Toxicol. Mech. Methods 2018, 28, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Rabe, S.Z.T.; Balali-Mood, M.; Karimi, G.; Tabasi, N.; Riahi-Zanjani, B. Ursolic acid induced apoptotic cell death following activation of caspases in isolated human melanoma cells. Cell Biol. Int. 2015, 39, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Harmand, P.O.; Duval, R.; Delage, C.; Simon, A. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. Int. J. Cancer 2005, 114, 1–11. [Google Scholar] [CrossRef]
- Hassan, L.; Pinon, A.; Limami, Y.; Seeman, J.; Fidanzi-Dugas, C.; Martin, F.; Badran, B.; Simon, A.; Liagre, B. Resistance to ursolic acid-induced apoptosis through involvement of melanogenesis and COX-2/PGE2 pathways in human M4Beu melanoma cancer cells. Exp. Cell Res. 2016, 345, 60–69. [Google Scholar] [CrossRef]
- Paduszyński, P.; Chodurek, E.; Jaworska-Kik, M.; Orchel, A.; Kaps, A.; Kasperczyk, J. Antiproliferative and proapoptotic activity of ursolic acid in human skin malignant melanoma cells. Postepy Hig. Med. Dosw. 2018, 72, 1148–1155. [Google Scholar] [CrossRef]
- Caunii, A.; Oprean, C.; Cristea, M.; Ivan, A.; Danciu, C.; Tatu, C.; Paunescu, V.; Marti, D.; Tzanakakis, G.; Spandidos, D.A.; et al. Effects of ursolic and oleanolic on SK-MEL-2 melanoma cells: In vitro and in vivo assays. Int. J. Oncol. 2017, 51, 1651–1660. [Google Scholar] [CrossRef]
- Duval, R.E.; Harmand, P.O.; Jayat-Vignoles, C.; Cook-Moreau, J.; Pinon, A.; Delage, C.; Simon, A. Differential involvement of mitochondria during ursolic acid-induced apoptotic process in HaCaT and M4Beu cells. Oncol. Rep. 2008, 19, 145–149. [Google Scholar] [CrossRef][Green Version]
- Manu, K.A.; Kuttan, G. Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-kappaB mediated activation of bcl-2 in B16F-10 melanoma cells. Int. Immunopharmacol. 2008, 8, 974–981. [Google Scholar] [CrossRef]
- Oprean, C.; Mioc, M.; Csányi, E.; Ambrus, R.; Bojin, F.; Tatu, C.; Cristea, M.; Ivan, A.; Danciu, C.; Dehelean, C.; et al. Improvement of ursolic and oleanolic acids’ antitumor activity by complexation with hydrophilic cyclodextrins. Biomed. Pharmacother. 2016, 83, 1095–1104. [Google Scholar] [CrossRef]
- Alqathama, A.; Shao, L.; Bader, A.; Khondkar, P.; Gibbons, S.; Prieto, J.M. Differential Anti-Proliferative and Anti-Migratory Activities of Ursolic Acid, 3-O-Acetylursolic Acid and Their Combination Treatments with Quercetin on Melanoma Cells. Biomolecules 2020, 10, 894. [Google Scholar] [CrossRef] [PubMed]
- Kahnt, M.; Hoenke, S.; Fischer, L.; Al-Harrasi, A.; Csuk, R. Synthesis and Cytotoxicity Evaluation of DOTA-Conjugates of Ursolic Acid. Molecules 2019, 24, 2254. [Google Scholar] [CrossRef] [PubMed]
- Mioc, M.; Mioc, A.; Prodea, A.; Milan, A.; Balan-Porcarasu, M.; Racoviceanu, R.; Ghiulai, R.; Iovanescu, G.; Macasoi, I.; Draghici, G.; et al. Novel Triterpenic Acid-Benzotriazole Esters Act as Pro-Apoptotic Antimelanoma Agents. Int. J. Mol. Sci. 2022, 23, 9992. [Google Scholar] [CrossRef] [PubMed]
- Park, B.C.; Bosire, K.O.; Lee, E.S.; Lee, Y.S.; Kim, J.A. Asiatic acid induces apoptosis in SK-MEL-2 human melanoma cells. Cancer Lett. 2005, 218, 81–90. [Google Scholar] [CrossRef]
- Kraft, O.; Hartmann, A.K.; Brandt, S.; Hoenke, S.; Heise, N.V.; Csuk, R.; Mueller, T. Asiatic acid as a leading structure for derivatives combining sub-nanomolar cytotoxicity, high selectivity, and the ability to overcome drug resistance in human preclinical tumor models. Eur. J. Med. Chem. 2023, 250, 115189. [Google Scholar] [CrossRef]
- Kraft, O.; Hartmann, A.K.; Hoenke, S.; Serbian, I.; Csuk, R. Madecassic Acid-A New Scaffold for Highly Cytotoxic Agents. Int. J. Mol. Sci. 2022, 23, 4362. [Google Scholar] [CrossRef]
- Valdeira, A.S.C.; Darvishi, E.; Woldemichael, G.M.; Beutler, J.A.; Gustafson, K.R.; Salvador, J.A.R. Madecassic Acid Derivatives as Potential Anticancer Agents: Synthesis and Cytotoxic Evaluation. J. Nat. Prod. 2019, 82, 2094–2105. [Google Scholar] [CrossRef]
- Gómez-Pulido, L.D.M.; González-Cano, R.C.; Benítez, J.J.; Domínguez, E.; Heredia, A. Structural analysis of mixed α- and β-amyrin samples. R. Soc. Open Sci. 2022, 9, 211787. [Google Scholar] [CrossRef]
- Lúcio, K.A.; da Rocha, G.G.; Monção-Ribeiro, L.C.; Fernandes, J.; Takiya, C.M.; Gattass, C.R. Oleanolic Acid Initiates Apoptosis in Non-Small Cell Lung Cancer Cell Lines and Reduces Metastasis of a B16F10 Melanoma Model In Vivo. PLoS ONE 2011, 6, e28596. [Google Scholar] [CrossRef]
- Oprean, C.; Borcan, F.; Pavel, I.; Dema, A.; Danciu, C.; Soica, C.; Dehelean, C.; Nicu, A.; Ardelean, A.; Cristea, M.; et al. In Vivo Biological Evaluation of Polyurethane Nanostructures with Ursolic and Oleanolic Acids on Chemically-induced Skin Carcinogenesis. In Vivo 2016, 30, 633–638. [Google Scholar]
- Lian, G.Y.; Wang, Q.M.; Tang, P.M.K.; Zhou, S.; Huang, X.R.; Lan, H.Y. Combination of Asiatic Acid and Naringenin Modulates NK Cell Anti-cancer Immunity by Rebalancing Smad3/Smad7 Signaling. Mol. Ther. 2018, 26, 2255–2266. [Google Scholar] [CrossRef] [PubMed]
- Soica, C.; Danciu, C.; Savoiu-Balint, G.; Borcan, F.; Ambrus, R.; Zupko, I.; Bojin, F.; Coricovac, D.; Ciurlea, S.; Avram, S.; et al. Betulinic acid in complex with a gamma-cyclodextrin derivative decreases proliferation and in vivo tumor development of non-metastatic and metastatic B164A5 cells. Int. J. Mol. Sci. 2014, 15, 8235–8255. [Google Scholar] [CrossRef] [PubMed]
- Sawada, N.; Kataoka, K.; Kondo, K.; Arimochi, H.; Fujino, H.; Takahashi, Y.; Miyoshi, T.; Kuwahara, T.; Monden, Y.; Ohnishi, Y. Betulinic acid augments the inhibitory effects of vincristine on growth and lung metastasis of B16F10 melanoma cells in mice. Br. J. Cancer 2004, 90, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, R.; Wu, R.; Ramirez, C.N.; Sargsyan, D.; Li, S.; Wang, L.; Cheng, D.; Wang, C.; Hudlikar, R.; et al. DNA methylome and transcriptome alterations and cancer prevention by triterpenoid ursolic acid in UVB-induced skin tumor in mice. Mol. Carcinog. 2019, 58, 1738–1753. [Google Scholar] [CrossRef] [PubMed]
- Park, B.C.; Paek, S.H.; Lee, Y.S.; Kim, S.J.; Lee, E.S.; Choi, H.G.; Yong, C.S.; Kim, J.A. Inhibitory effects of asiatic acid on 7,12-dimethylbenz[a]anthracene and 12-O-tetradecanoylphorbol 13-acetate-induced tumor promotion in mice. Biol. Pharm. Bull. 2007, 30, 176–179. [Google Scholar] [CrossRef]
- Saini, V.; Mata Espinosa, D.; Pandey, A.; Dighe, V.; Barrios Payán, J.; Prasad Myneedu, V.; Valdez Zarate, I.; Rajani, D.P.; Anande, L.D.; Hernandez Pando, R.; et al. Antimycobacterial Activity of Solid Lipid Microparticles Loaded with Ursolic Acid and Oleanolic Acid: In Vitro, In Vivo, and Toxicity Assessments. Microorganisms 2024, 12, 2140. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, J.; Liu, L.; Sun, Y.; Lai, Y.; Ji, H.; Knaus, E.E.; Tian, J.; Zhang, Y. Glycosylated diazeniumdiolate-based oleanolic acid derivatives: Synthesis, in vitro and in vivo biological evaluation as anti-human hepatocellular carcinoma agents. Org. Biomol. Chem. 2012, 10, 3882–3891. [Google Scholar] [CrossRef]
- Mishra, V.; Soren, A.D.; Yadav, A.K. Toxicological evaluations of betulinic acid and ursolic acid; common constituents of Houttuynia cordata used as an anthelmintic by the Naga tribes in North-east India. Future J. Pharm. Sci. 2021, 7, 39. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. Toxicity of escin-triterpene saponins from Aesculus. Toxicol. Environ. Chem. 2022, 104, 141–148. [Google Scholar] [CrossRef]
- Tatia, R.; Zalaru, C.; Tarcomnicu, I.; Moldovan, L.; Craciunescu, O.; Calinescu, I. Isolation and characterization of hederagenin from hedera helix L. Extract with antitumor activity. Rev. Chim. 2019, 70, 1157–1161. [Google Scholar] [CrossRef]
- Aragão, G.F.; Nogueira, A.O.; Xavier Júnior, F.A.F.; Evangelista, J.S.A.M.; Bandeira, P.N.; Fernandes, C.; de Moraes, M.E.A.; Assreuy, A.M.S. Acute toxicity study of the isomeric mixture of alpha and beta amyrin from Protium heptaphyllum (Aubl.) Marchand. Acta Sci. Biol. Sci. 2023, 45, e66144. [Google Scholar] [CrossRef]
- Selzer, E.; Pimentel, E.; Wacheck, V.; Schlegel, W.; Pehamberger, H.; Jansen, B.; Kodym, R. Effects of Betulinic Acid Alone and in Combination with Irradiation in Human Melanoma Cells. J. Investig. Dermatol. 2000, 114, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, Y. Terpenoids and membrane dynamics evolution. Front. Ecol. Evol. 2024, 12, 1345733. [Google Scholar] [CrossRef]
- Serafim, T.L.; Carvalho, F.S.; Bernardo, T.C.; Pereira, G.C.; Perkins, E.; Holy, J.; Krasutsky, D.A.; Kolomitsyna, O.N.; Krasutsky, P.A.; Oliveira, P.J. New derivatives of lupane triterpenoids disturb breast cancer mitochondria and induce cell death. Bioorg. Med. Chem. 2014, 22, 6270–6287. [Google Scholar] [CrossRef]
- Spivak, A.Y.; Nedopekina, D.A.; Gubaidullin, R.R.; Dubinin, M.V.; Belosludtsev, K.N. Conjugation of Natural Triterpenic Acids with Delocalized Lipophilic Cations: Selective Targeting Cancer Cell Mitochondria. J. Pers. Med. 2021, 11, 470. [Google Scholar] [CrossRef]




| Group of Terpenoids | Example of Compound | Plant Sources | Reference |
|---|---|---|---|
| Monoterpenoids | Linalol | Lavandula angustifolia, Citrus bergamia | [17] |
| Thymol | Thymus vulgaris, Origanum vulgare | [17] | |
| Thujone | Artemisia vulgaris, Salvia officinalis | [17] | |
| Diterpenoids | Abietic acid | Pinus palustris | [18] |
| Forskolin | Coleus forskohlii | [19] | |
| Triterpenoids | Oleanolic acid | Olea europea | [20] |
| Betulinic acid | Betula pendula | [21] | |
| Ursolic acid | Sambucus nigra | [20] | |
| Asiatic acid | Centella asiatica | [22] | |
| Tetraterpenoids | Β-carotene | Solanum lycopersicum | [23] |
| Lutein | Citrus sinensis | [23] | |
| Sesquiterpenoids | Cnicin | Cnicus benedictus | [24] |
| Santonin | Artemisia annua | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dycha, N.; Michalak-Tomczyk, M.; Jachuła, J.; Okoń, E.; Jarząb, A.; Tokarczyk, J.; Koch, W.; Gaweł-Bęben, K.; Kukula-Koch, W.; Wawruszak, A. Chemopreventive and Anticancer Activity of Selected Triterpenoids in Melanoma. Cancers 2025, 17, 1625. https://doi.org/10.3390/cancers17101625
Dycha N, Michalak-Tomczyk M, Jachuła J, Okoń E, Jarząb A, Tokarczyk J, Koch W, Gaweł-Bęben K, Kukula-Koch W, Wawruszak A. Chemopreventive and Anticancer Activity of Selected Triterpenoids in Melanoma. Cancers. 2025; 17(10):1625. https://doi.org/10.3390/cancers17101625
Chicago/Turabian StyleDycha, Natalia, Magdalena Michalak-Tomczyk, Jacek Jachuła, Estera Okoń, Agata Jarząb, Joanna Tokarczyk, Wojciech Koch, Katarzyna Gaweł-Bęben, Wirginia Kukula-Koch, and Anna Wawruszak. 2025. "Chemopreventive and Anticancer Activity of Selected Triterpenoids in Melanoma" Cancers 17, no. 10: 1625. https://doi.org/10.3390/cancers17101625
APA StyleDycha, N., Michalak-Tomczyk, M., Jachuła, J., Okoń, E., Jarząb, A., Tokarczyk, J., Koch, W., Gaweł-Bęben, K., Kukula-Koch, W., & Wawruszak, A. (2025). Chemopreventive and Anticancer Activity of Selected Triterpenoids in Melanoma. Cancers, 17(10), 1625. https://doi.org/10.3390/cancers17101625








