A Review of the Paradigmatic Role of Adipose Tissue in Renal Cancer: Fat Measurement and Tumor Behavior Features
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Obesity and Cancer
1.2. Obesity and RCC
- -
- VAT (visceral adipose tissue) refers to the cross-sectional area of fat tissue measured, outlining the fat area in a single axial L3 slice, which strongly correlates with the overall amount of fat tissue in the body [15]. VAT is characterized by heightened cellularity, vascularity, and innervation and showcases an increased presence of inflammatory cells and large adipocytes [16].
- -
- SAT (subcutaneous adipose tissue) refers to the subcutaneous fat area measured, outlining the fat area in a single scan obtained at the level of the umbilicus (approximately the level of L4 and L5) [17].
- -
- PRAT (perirenal adipose tissue) refers to the perirenal adipose area obtained using a single axial slice at the level of the renal vein. The measurement involves the outlining the area of fat by manually tracing the limits of the anterior renal fascia laterally to the lateroconal ligament and including the posterior perirenal fat and retroperitoneal fat below Zuckerkandl’s fascia [18].
- -
- PFT (perirenal fat thickness) is assessed by determining the perpendicular distance from the kidney’s posterior surface to the outer border of the iliopsoas muscle at the renal hilum level, employing the ruler tool within the relevant radiological software [19].
- -
- MAP (Mayo adhesive probability) score, a validated and noninvasive tool for assessing perinephric fat “quality,” is currently associated with the presence of adherent perirenal fat encountered during partial nephrectomy (a non-tumor-related factor that can complicate PN) [20,21]. MAP score calculations are performed by measuring posterior perirenal thickness (the distance directly between the kidney capsule and the posterior abdominal wall at the level of the renal vein) and evaluating perinephric stranding. Posterior perirenal thickness lengths are assigned scores of 0, 1, or 2. Perinephric stranding at the renal vein level is assessed and assigned a score of 0 (none), 2 (type I), or 3 (type II) based on severity. The scores obtained for these two measurements are then totaled to derive the overall MAP score, ranging from 0 to 5 [22].
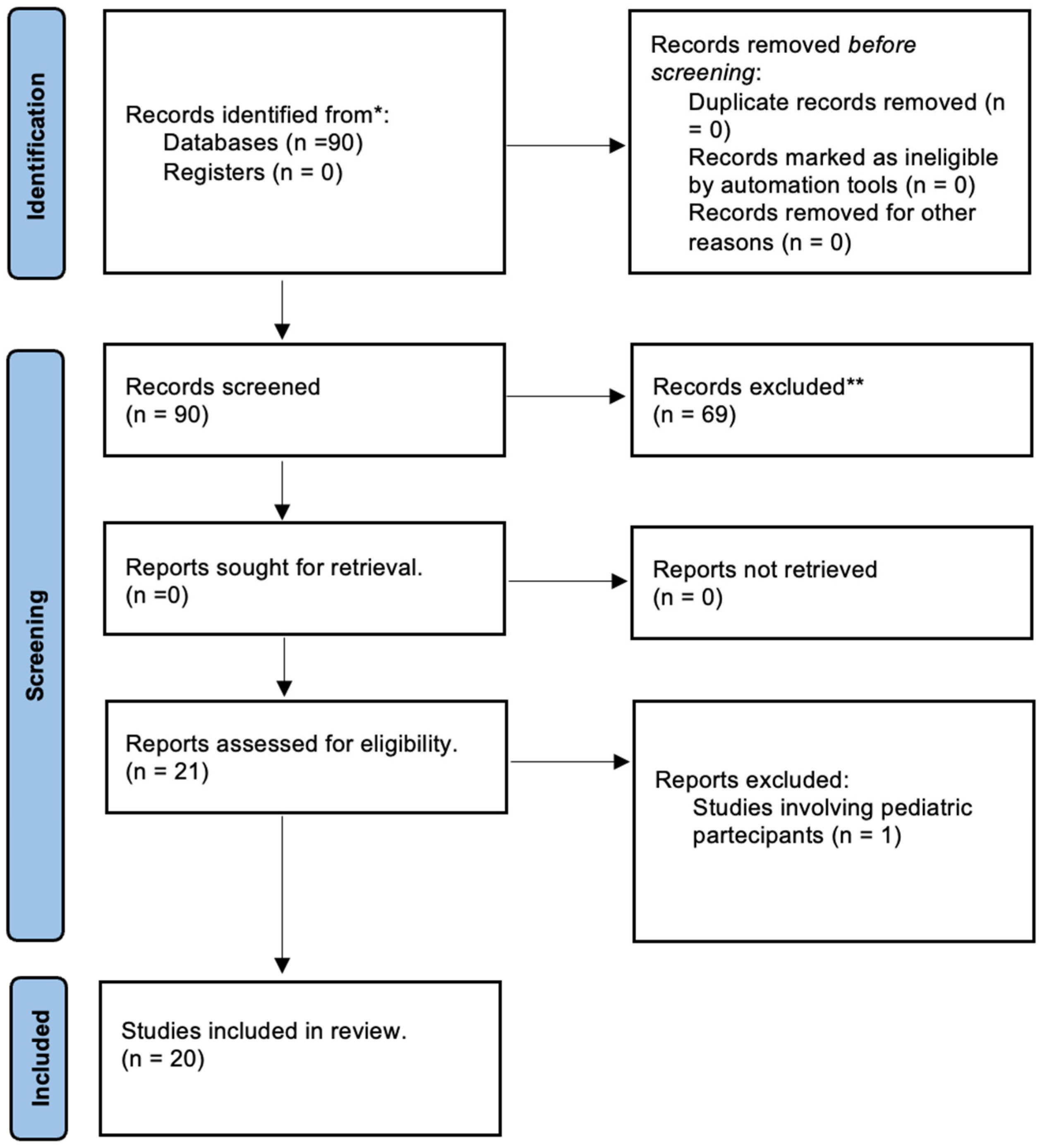
2. Materials and Methods
3. Results
3.1. VAT/SAT
3.2. MAP/PRAT
4. Discussion
- -
- Limited number of articles and retrospective design: The scarcity of articles investigating correlations between RCC and fat deposits, combined with the predominance of retrospective designs, presents limitations in terms of sample size and potential biases in data collection.
- -
- Variability and lack of standardization: There is a lack of standardized approaches across various facets, including diverse types of fat measurements, different RCC aspects, and variability in cohort populations. This variability impedes comparability and reproducibility of results. However, this wide variability also underscores the numerous aspects open to assessment and the potential for further advancement.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, K.D.; Goding Sauer, A.; Ortiz, A.P.; Fedewa, S.A.; Pinheiro, P.S.; Tortolero-Luna, G.; Martinez-Tyson, D.; Jemal, A.; Siegel, R.L. Cancer statistics for hispanics/latinos. Cancer J. Clin. 2018, 68, 425–445. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.H.; Klatte, T.; Usher-Smith, J.; Stewart, G.D. Epidemiology and screening for renal cancer. World J. Urol. 2018, 36, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Agnello, F.; Albano, D.; Micci, G.; Di Buono, G.; Agrusa, A.; Salvaggio, G.; Pardo, S.; Sparacia, G.; Bartolotta, T.V.; Midiri, M.; et al. CT and MR imaging of cystic renal lesions. Insights Imaging 2020, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Benjaminov, O.; Atri, M.; O’Malley, M.; Lobo, K.; Tomlinson, G. Enhancing Component on CT to Predict Malignancy in Cystic Renal Masses and Interobserver Agreement of Different CT Features. Am. J. Roentgenol. 2006, 186, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Sun, H.Y.; Hwang, J.; Hong, S.S.; Cho, Y.J.; Doo, S.W.; Yang, W.J.; Song, Y.S. Diagnostic accuracy of contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging of small renal masses in real practice: Sensitivity and specificity according to subjective radiologic interpretation. World J. Surg. Oncol. 2016, 14, 260. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Thompson, R.H.; Yee, D.S.; Kaag, M.; Donat, S.M.; Russo, P. Obesity is associated with a higher risk of clear-cell renal cell carcinoma than with other histologies. BJU Int. 2010, 105, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, A.A.; Furberg, H.; Zabor, E.C.; Jacobsen, A.; Schultz, N.; Ciriello, G.; Mikklineni, N.; Fiegoli, B.; Kim, P.H.; Voss, M.H.; et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J. Natl. Cancer Inst. 2013, 105, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Lennon, H.; Sperrin, M.; Badrick, E.; Renehan, A.G. The Obesity Paradox in Cancer: A Review. Curr. Oncol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef]
- Berger, N.A. Obesity and cancer pathogenesis. Ann. N. Y. Acad. Sci. 2014, 1311, 57–76. [Google Scholar] [CrossRef]
- Vertulli, D.; Santucci, D.; Esperto, F.; Beomonte Zobel, B.; Grasso, R.F.; Faiella, E. Impact of adipose tissue distribution on prostate cancer recurrence after radical prostatectomy. Actas. Urol. Esp. 2023, 47, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Rosenblatt, D.N.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer—Systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Jousilahti, P.; Stehouwer, C.D.; Söderberg, S.; Onat, A.; Laatikainen, T.; Yudkin, J.S.; Dankner, R.; Morris, R.; Tuomilehto, J.; et al. Comparison of various surrogate obesity indicators as predictors of cardiovascular mortality in four European populations. Eur. J. Clin. Nutr. 2013, 67, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. BMI-related errors in the measurement of obesity. Int. J. Obes. 2008, 32 (Suppl. S3), 56–59. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2009, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, T.; Nakamura, T.; Yamane, M.; Islam, A.H.; Menju, M.; Yamasaki, K.; Arai, T.; Kotani, K.; Funahashi, T.; Yamashita, S.; et al. Abdominal Fat: Standardized Technique for Measurement at CT. Radiology 1999, 211, 283–286. [Google Scholar] [CrossRef]
- Garcia-Rojo, D.; Capdevila, M.; Preciado, D.; Prera, A.; Abad, C.; Vicente, E.; Hannaoui, N.; Gonzalez-Sala, J.; Muñoz, J.; Dominguez, A.; et al. Visceral adipose tissue and perirenal fat surface area as a predictor of renal cortical neoplasm histopathology. Eur. Urol. Suppl. 2016, 15, e1381–e1382. [Google Scholar] [CrossRef]
- Okhunov, Z.; Mues, A.C.; Kline, M.; Haramis, G.; Xu, B.; Mirabile, G.; Vira, M.; Landman, J. Evaluation of Perirenal Fat as a Predictor of cT1a Renal Cortical Neoplasm Histopathology and Surgical Outcomes. J. Endourol. 2012, 26, 911–916. [Google Scholar] [CrossRef]
- Davidiuk, A.J.; Parker, A.S.; Thomas, C.S.; Leibovich, B.C.; Castle, E.P.; Heckman, M.G.; Custer, K.; Thiel, D.D. Mayo Adhesive Probability Score: An Accurate Image-based Scoring System to Predict Adherent Perinephric Fat in Partial Nephrectomy. Eur. Urol. 2014, 66, 1165–1171. [Google Scholar] [CrossRef]
- Bier, S.; Aufderklamm, S.; Todenhöfer, T.; Kruck, S.; Schuster, K.; Rausch, S.; Othman, A.; Notohamiprodjo, M.; Nikolaou, K.; Schwentner, C.; et al. Prediction of postoperative risks in laparascoping partial nephrectomy using RENAL, Mayo adhesive probability and renal pelvic score. Anticancer Res. 2017, 37, 1369–1373. [Google Scholar] [PubMed]
- Bernstein, A.P.; Fram, E.B.; Sankin, A.; Kovac, E.; Srivastava, A.; DiVito, J.; Stern, J.M. A comparison of perinephric fat surface area and Mayo Adhesive Probability score in predicting malignancy in T1 renal masses. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 499.e17–499.e22. [Google Scholar] [CrossRef] [PubMed]
- Maurits, J.S.F.; Sedelaar, J.P.M.; Aben, K.K.H.; Kiemeney, L.A.L.M.; Vrieling, A. Association of visceral and subcutaneous adiposity with tumor stage and Fuhrman grade in renal cell carcinoma. Sci. Rep. 2022, 12, 16718. [Google Scholar] [CrossRef] [PubMed]
- Naya, Y.; Zenbutsu, S.; Araki, K.; Nakamura, K.; Kobayashi, M.; Kamijima, S.; Imamoto, T.; Nihei, N.; Suzuki, H.; Ichikawa, T.; et al. Influence of Visceral Obesity on Oncologic Outcome in Patients with Renal Cell Carcinoma. Urol. Int. 2010, 85, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Choi, H.Y.; Jeon, H.G. Prognostic significance of visceral obesity in patients with advanced renal cell carcinoma undergoing nephrectomy. Int. J. Urol. 2015, 22, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, G.; Miyajima, A.; Yuge, K.; Yazawa, S.; Mizuno, R.; Kikuchi, E.; Jinzaki, M.; Oya, M. Visceral obesity is associated with better recurrence-free survival after curative surgery for Japanese patients with localized clear cell renal cell carcinoma. Ultrasound Med. Biol. 2015, 45, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Jang, W.S.; Kim, J.; Lee, S.H.; Rha, K.H.; Ham, W.S. Association between visceral adiposity and DDX11 as a predictor of aggressiveness of small clear-cell renal-cell carcinoma: A prospective clinical trial. Cancer Metab. 2021, 9, 15. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, J.; Lai, S.; Xu, Y.; Zhan, J.; Li, R.; Liu, X.; Wang, N.; Wei, X.; Jiang, X.; et al. Clear cell renal cell carcinoma: The value of sex-specific abdominal visceral fat measured on CT for prediction of Fuhrman nuclear grade. Eur. Radiol. 2020, 30, 3977–3986. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.-K.; Zhang, H.-L.; Yao, X.-D.; Zhang, S.-L.; Dai, B.; Shen, Y.-J.; Liu, X.-H.; Zhou, L.-P.; Ye, D.-W. Visceral Obesity and Risk of High Grade Disease in Clinical T1a Renal Cell Carcinoma. J. Urol. 2013, 189, 447–453. [Google Scholar] [CrossRef]
- Keehn, A.; Srivastava, A.; Maiman, R.; Taylor, J.; DiVito, J.; Ghavamian, R.; Stern, J.M. The Relationship between Visceral Obesity and the Clinicopathologic Features of Patients with Small Renal Masses. J. Endourol. 2015, 29, 372–376. [Google Scholar] [CrossRef]
- Nguyen, G.K.; Mellnick, V.M.; Yim, A.K.-Y.; Salter, A.; Ippolito, J.E. Synergy of Sex Differences in Visceral Fat Measured with CT and Tumor Metabolism Helps Predict Overall Survival in Patients with Renal Cell Carcinoma. Radiology 2018, 287, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Zhang, B.; Qu, Z.; Chen, C. Elevated visceral obesity quantified by CT is associated with adverse postoperative outcome of laparoscopic radical nephrectomy for renal clear cell carcinoma patients. Int. Urol. Nephrol. 2018, 50, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Lee, J.K.; Kim, K.M.; Kook, H.R.; Lee, H.; Kim, K.B.; Lee, S.; Byun, S.S.; Lee, S.E. Visceral obesity in predicting oncologic outcomes of localized renal cell carcinoma. J. Urol. 2014, 192, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Mano, R.; Hakimi, A.A.; Zabor, E.C.; A Bury, M.; Donati, O.F.; A Karlo, C.; Bazzi, W.M.; Furberg, H.; Russo, P. Association between visceral and subcutaneous adiposity and clinicopathological outcomes in non-metastatic clear cell renal cell carcinoma. Can. Urol. Assoc. J. 2014, 8, E675–E680. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, X.; Cheng, Y.; Qu, Y.; Zhang, S.; Dai, B.; Zhang, H.; Shen, Y.; Zhu, Y.; Shi, G.; et al. Visceral fat accumulation is associated with different pathological subtypes of renal cell carcinoma (RCC): A multicentre study in China. BJU Int. 2014, 114, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Preza-Fernandes, J.; Passos, P.; Mendes-Ferreira, M.; Rodrigues, A.R.; Gouveia, A.; Fraga, A.; Medeiros, R.; Ribeiro, R. A hint for the obesity paradox and the link between obesity, perirenal adipose tissue and Renal Cell Carcinoma progression. Sci. Rep. 2022, 12, 19956. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, S.; Li, W.; Wu, X.; Xing, J. High perirenal fat thickness predicts a poor progression-free survival in patients with localized clear cell renal cell carcinoma. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 157.e1–157.e6. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-P.; Lin, D.-C.; Huang, W.-M.; Chen, M.; Chen, Y.-H. Comparison of perinephric fat measurements between malignant and benign renal tumours. J. Int. Med. Res. 2022, 50. [Google Scholar] [CrossRef] [PubMed]
- Thiel, D.D.; Davidiuk, A.J.; Meschia, C.; Serie, D.; Custer, K.; Petrou, S.P.; Parker, A.S. Mayo Adhesive Probability Score Is Associated with Localized Renal Cell Carcinoma Progression-free Survival. Urology 2015, 89, 54–62. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO classification of tumours of the urinary system and male genital organs—Part A: Renal, penile, and testicular tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Turajlic, S.; Swanton, C.; Boshoff, C. Kidney cancer: The next decade. J. Exp. Med. 2018, 215, 2477–2479. [Google Scholar] [CrossRef] [PubMed]
- Wagener, N.; Edelmann, D.; Benner, A.; Zigeuner, R.; Borgmann, H.; Wolff, I.; Krabbe, L.M.; Musquera, M.; Dell’oglio, P.; Capitanio, U.; et al. Outcome of papillary versus clear cell renal cell carcinoma varies significantly in non-metastatic disease. PLoS ONE 2017, 12, e0184173. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.W.; Kapur, P.; Serie, D.J.; Parasramka, M.; Ho, T.H.; Cheville, J.C.; Frenkel, E.; Parker, A.S.; Brugarolas, J. Clear Cell Renal Cell Carcinoma Subtypes Identified by BAP1 and PBRM1 Expression. J. Urol. 2016, 195, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Escudier, B.; Martini, J.-F.; Magheli, A.; Svedman, C.; Lopatin, M.; Knezevic, D.; Goddard, A.D.; Febbo, P.G.; Li, R.; et al. Validation of the 16-Gene Recurrence Score in Patients with Locoregional, High-Risk Renal Cell Carcinoma from a Phase III Trial of Adjuvant Sunitinib. Clin. Cancer Res. 2018, 24, 4407–4415. [Google Scholar] [CrossRef] [PubMed]
- Köhn, L.; Svenson, U.; Ljungberg, B.; Roos, G. Specific Genomic Aberrations Predict Survival, But Low Mutation Rate in Cancer Hot Spots, in Clear Cell Renal Cell Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Beltran, A.; Henriques, V.; Cimadamore, A.; Santoni, M.; Cheng, L.; Gevaert, T.; Blanca, A.; Massari, F.; Scarpelli, M.; Montironi, R. The Identification of Immunological Biomarkers in Kidney Cancers. Front. Oncol. 2018, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Bektas, S.; Bahadir, B.; Kandemir, N.O.; Barut, F.; Gul, A.E.; Ozdamar, S.O. Intraobserver and Interobserver Variability of Fuhrman and Modified Fuhrman Grading Systems for Conventional Renal Cell Carcinoma. Kaohsiung J. Med. Sci. 2009, 25, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Rizkalla, C.; Tretiakova, M. PathologyOutlines.com. Available online: https://www.pathologyoutlines.com/topic/kidneytumormalignantnucleargrading.html (accessed on 23 January 2024).
- International Agency for Research on Cancer. WHO Classification of Tumours of the Urinary System and Male Genital Organs (IARC WHO Classification of Tumours), 4th ed.; WHO/IARC Press: Lyon, France, 2016. [Google Scholar]
- Delahunt, B.; Cheville, J.C.; Martignoni, G.; Humphrey, P.A.; Magi-Galluzzi, C.; McKenney, J.; Egevad, L.; Algaba, F.; Moch, H.; Grignon, D.J.; et al. The International Society of Urological Pathology (ISUP) Grading System for Renal Cell Carcinoma and Other Prognostic Parameters. Am. J. Surg. Pathol. 2013, 37, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Warren, A.Y.; Harrison, D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: Standards and controversies. World J. Urol. 2018, 36, 1913–1926. [Google Scholar] [CrossRef]
- Tsui, K.-H.; Shvarts, O.; Smith, R.B.; Figlin, R.; de Kernion, J.B.; Belldegrun, A. Renal cell Carcinoma: Prognostic significance of incidentally detected tumors. J. Urol. 2000, 163, 426–430. [Google Scholar] [CrossRef]
- Bahadoram, S.; Davoodi, M.; Hassanzadeh, S.; Bahadoram, M.; Barahman, M.; Mafakher, L. Renal cell carcinoma: An overview of the epidemiology, diagnosis, and treatment. G. Ital. Nefrol. 2022, 39, 2022-vol3. [Google Scholar] [PubMed]
- Israel, G.M.; Hindman, N.; Bosniak, M.A. Evaluation of cystic renal masses: Comparison of CT and MR imaging by using the Bosniak classification system. Radiology 2004, 231, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Delahunt, B.; Eble, J.N.; Samaratunga, H.; Thunders, M.; Yaxley, J.W.; Egevad, L. Staging of renal cell carcinoma: Current progress and potential advances. Pathology 2021, 53, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Kutikov, A.; Uzzo, R.G. The R.E.N.A.L. Nephrometry Score: A Comprehensive Standardized System for Quantitating Renal Tumor Size, Location and Depth. J. Urol. 2009, 182, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Mori, K.; Kawada, T.; Motlagh, R.S.; Mostafaei, H.; Quhal, F.; Laukhtina, E.; Rajwa, P.; Aydh, A.; König, F.; et al. Differential efficacy of ablation therapy versus partial nephrectomy between clinical T1a and T1b renal tumors: A systematic review and meta-analysis. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 315–330. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faiella, E.; Vergantino, E.; Vaccarino, F.; Bruno, A.; Perillo, G.; Grasso, R.F.; Zobel, B.B.; Santucci, D. A Review of the Paradigmatic Role of Adipose Tissue in Renal Cancer: Fat Measurement and Tumor Behavior Features. Cancers 2024, 16, 1697. https://doi.org/10.3390/cancers16091697
Faiella E, Vergantino E, Vaccarino F, Bruno A, Perillo G, Grasso RF, Zobel BB, Santucci D. A Review of the Paradigmatic Role of Adipose Tissue in Renal Cancer: Fat Measurement and Tumor Behavior Features. Cancers. 2024; 16(9):1697. https://doi.org/10.3390/cancers16091697
Chicago/Turabian StyleFaiella, Eliodoro, Elva Vergantino, Federica Vaccarino, Amalia Bruno, Gloria Perillo, Rosario Francesco Grasso, Bruno Beomonte Zobel, and Domiziana Santucci. 2024. "A Review of the Paradigmatic Role of Adipose Tissue in Renal Cancer: Fat Measurement and Tumor Behavior Features" Cancers 16, no. 9: 1697. https://doi.org/10.3390/cancers16091697
APA StyleFaiella, E., Vergantino, E., Vaccarino, F., Bruno, A., Perillo, G., Grasso, R. F., Zobel, B. B., & Santucci, D. (2024). A Review of the Paradigmatic Role of Adipose Tissue in Renal Cancer: Fat Measurement and Tumor Behavior Features. Cancers, 16(9), 1697. https://doi.org/10.3390/cancers16091697







