Updates in Cancer Cachexia: Clinical Management and Pharmacologic Interventions
Abstract
Simple Summary
Abstract
1. Introduction
“You don’t necessarily have to get on the scales, you see the bones begin to protrude and feel the end is near.” Patient [1]
2. Definition and Staging
3. Clinical Impact
“This bony thing shows up in the mirror every morning, and my eyes fall on this creature on the other side of the mirror.” Patient [1]
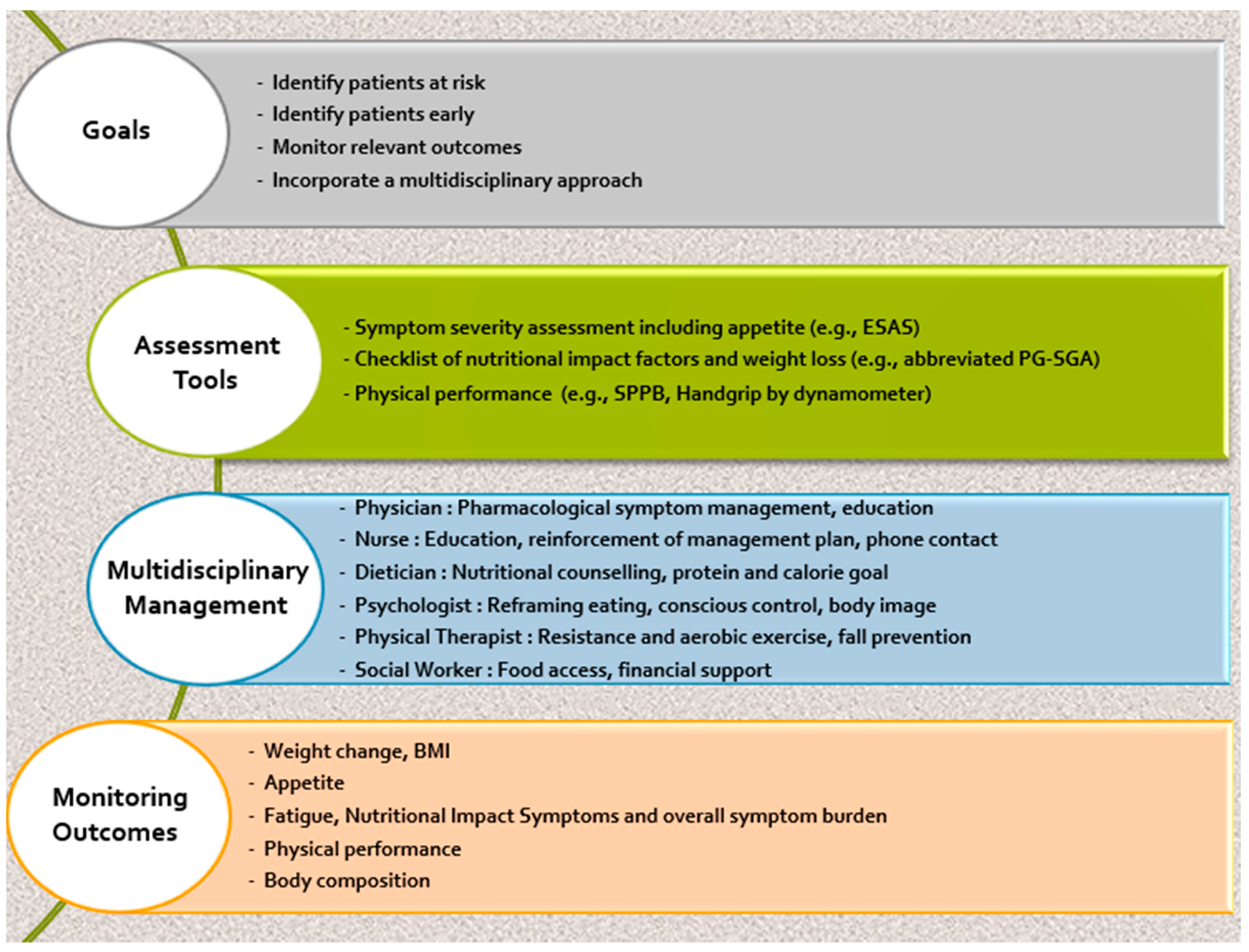
4. Assessment Tools
“I was five feet from him before he could figure out who it was. I cried, because he was a very, very good friend of mine. It seemed to confirm the fact that I was so skinny.” Patient [1]
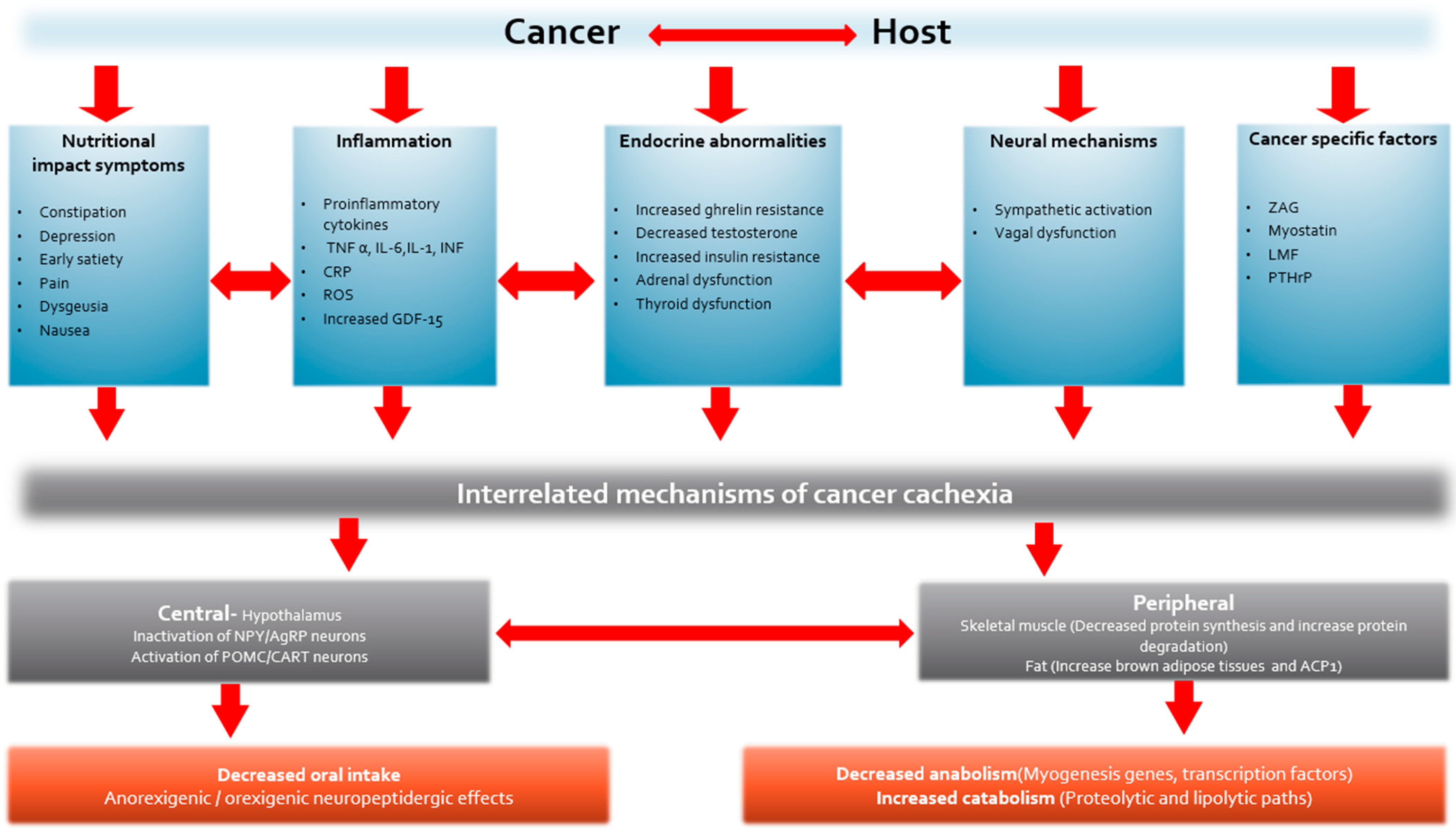
5. Mechanism of Cancer Cachexia
“I cooked a lot, I baked a lot… He ate what I made because I made what he liked… We always ate together… He wouldn’t eat if I didn’t eat, so I stopped eating when he stopped eating.” Caregiver [1]
6. Management of Cancer Cachexia
“At first I thought we were in limbo, nobody cared, that we couldn’t turn to anybody… nobody seemed to help us… we just had to cope on our own…. I thought that someone should have come and spoke to us as a family to tell us what to expect… when he wasn’t feeling well for a doctor or what or who to turn to.” Caregiver [1]
7. Non-Pharmacologic Management
- Exercise
- Nutrition
- Nutrition Impact Symptoms (NIS)
8. Pharmacologic Interventions
- Current Agents
- Corticosteroids
- Progestational agents/Progestins
- Cannabinoids
- Olanzapine
- Non-steroidal anti-inflammatory drugs (NSAIDs)
- Thalidomide
- Fish oil or Eicosapentanoic acid (EPA)
- New agents
- Androgens
- Beta Blockers
- Ghrelin and ghrelin mimetics
- Myostatin and proinflammatory cytokine inhibitors
- Anti-Growth Differentiation Factor 15 (GDF-15)
9. Multimodal Therapy for the Cancer Cachexia
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hopkinson, J.B. Psychosocial impact of cancer cachexia. J. Cachexia Sarcopenia Muscle 2014, 5, 89–94. [Google Scholar] [CrossRef]
- Fox, K.M.; Brooks, J.M.; Gandra, S.R.; Markus, R.; Chiou, C.-F. Estimation of Cachexia among Cancer Patients Based on Four Definitions. J. Oncol. 2009, 2009, e693458. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A. Weight loss in patients with advanced cancer: Effects, causes, and potential management. Curr. Opin. Support. Palliat. Care 2008, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Penet, M.-F.; Bhujwalla, Z.M. Cancer Cachexia, Recent Advances, and Future Directions. Cancer J. Sudbury Mass 2015, 21, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhang, L. Cancer Cachexia: Definition, Staging, and Emerging Treatments. Cancer Manag. Res. 2020, 12, 5597–5605. [Google Scholar] [CrossRef]
- Douglas, E.; McMillan, D.C. Towards a simple objective framework for the investigation and treatment of cancer cachexia: The Glasgow Prognostic Score. Cancer Treat. Rev. 2014, 40, 685–691. [Google Scholar] [CrossRef]
- Martin, L.; Senesse, P.; Gioulbasanis, I.; Antoun, S.; Bozzetti, F.; Deans, C.; Strasser, F.; Thoresen, L.; Jagoe, R.T.; Chasen, M.; et al. Diagnostic Criteria for the Classification of Cancer-Associated Weight Loss. J. Clin. Oncol. 2015, 33, 90–99. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, B.; Liu, H.; Yang, K.; Thapa, S.; Zhang, H.; Li, L.; Yu, S. Development and validation of a clinically applicable score to classify cachexia stages in advanced cancer patients. J. Cachexia Sarcopenia Muscle 2018, 9, 306–314. [Google Scholar] [CrossRef]
- Ueshima, J.; Maeda, K.; Shimizu, A.; Nagano, A.; Ishida, Y.; Takeuchi, T.; Nonogaki, T.; Matsuyama, R.; Yamanaka, Y.; Murotani, K.; et al. Cachexia staging score predicts survival in patients with cancer who receive palliative care. Nutrition 2023, 106, 111880. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.M.; El-Serag, H.B. The epidemiology of obesity. Gastroenterol. Clin. N. Am. 2010, 39, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, M.; Mohamadirizi, M.; Mohamadirizi, S.; Hosseini, S.A. Evaluation of body image in cancer patients and its association with clinical variables. J. Educ. Health Promot. 2017, 6, 81. [Google Scholar] [CrossRef]
- Brederecke, J.; Heise, A.; Zimmermann, T. Body image in patients with different types of cancer. PLoS ONE 2021, 16, e0260602. [Google Scholar] [CrossRef] [PubMed]
- CClement, M.; Woodgate. Research with families in palliative care: Conceptual and methodological challenges. Eur. J. Cancer Care 1998, 7, 247–254. [Google Scholar] [CrossRef]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O., Jr.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic effect of weight loss prior tochemotherapy in cancer patients. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.J.; Ashley, S.; Norton, A.; Priest, K.; Waters, J.S.; Eisen, T.; Smith, I.E.; O’Brien, M.E. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br. J. Cancer 2004, 90, 1905–1911. [Google Scholar] [CrossRef]
- Parmar, M.P.; Swanson, T.; Jagoe, R.T. Weight changes correlate with alterations in subjective physical function in advanced cancer patients referred to a specialized nutrition and rehabilitation team. Support. Care Cancer 2013, 21, 2049–2057. [Google Scholar] [CrossRef]
- Andreyev, H.J.N.; Norman, A.R.; Oates, J.; Cunningham, D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur. J. Cancer 1998, 34, 503–509. [Google Scholar] [CrossRef]
- Ovesen, L.; Allingstrup, L.; Hannibal, J.; Mortensen, E.L.; Hansen, O.P. Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: A prospective, randomized study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1993, 11, 2043–2049. [Google Scholar] [CrossRef]
- O’Gorman, P.; McMillan, D.C.; McArdle, C.S. Impact of weight loss, appetite, and the inflammatory response on quality of life in gastrointestinal cancer patients. Nutr. Cancer 1998, 32, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Mariani, L.; Vullo, S.L.; Bozzetti, F.; Group, S.C.I.I.W. Weight loss in cancer patients: A plea for a better awareness of the issue. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2012, 20, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Wanger, T.; Foster, N.R.; Nguyen, P.L.; Jatoi, A. Patients’ rationale for declining participation in a cancer-associated weight loss study. J. Cachexia Sarcopenia Muscle 2014, 5, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Bower, P.; Brueton, V.; Gamble, C.; Treweek, S.; Tudur Smith, C.; Young, B.; Williamson, P. Interventions to improve recruitment and retention in clinical trials: A survey and workshop to assess current practice and future priorities. Trials 2014, 15, 399. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, P.H.Y.; Hamilton, S.; Tan, A.; Craig, J.C. Strategies for Increasing Recruitment to Randomised Controlled Trials: Systematic Review. PLoS Med. 2010, 7, e1000368. [Google Scholar] [CrossRef] [PubMed]
- Homsi, J.; Walsh, D.; Rivera, N.; Rybicki, L.A.; Nelson, K.A.; LeGrand, S.B.; Davis, M.; Naughton, M.; Gvozdjan, D.; Pham, H. Symptom evaluation in palliative medicine: Patient report vs systematic assessment. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2006, 14, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, E.; Jatoi, A.; Davis, M.; Fearon, K.; di Tomasso, J.; Vigano, A. Health professionals’ attitudes toward the detection and management of cancer-related anorexia-cachexia syndrome, and a proposal for standardized assessment. J. Community Support. Oncol. 2015, 13, 181–187. [Google Scholar] [CrossRef]
- Understanding the Mechanisms and Treatment Options in Cancer Cachexia|Nature Reviews Clinical Oncology. Available online: https://www.nature.com/articles/nrclinonc.2012.209 (accessed on 12 February 2024).
- Barbera, L.; Seow, H.; Howell, D.; Sutradhar, R.; Earle, C.; Liu, Y.; Stitt, A.; Husain, A.; Sussman, J.; Dudgeon, D. Symptom burden and performance status in a population-based cohort of ambulatory cancer patients. Cancer 2010, 116, 5767–5776. [Google Scholar] [CrossRef]
- ESPEN Guidelines on Nutrition in Cancer Patients—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27637832/ (accessed on 12 February 2024).
- Vigano, A.L.; di Tomasso, J.; Kilgour, R.D.; Trutschnigg, B.; Lucar, E.; Morais, J.A.; Borod, M. The abridged patient-generated subjective global assessment is a useful tool for early detection and characterization of cancer cachexia. J. Acad. Nutr. Diet. 2014, 114, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Verweij, N.M.; Schiphorst, A.H.W.; Pronk, A.; van den Bos, F.; Hamaker, M.E. Physical performance measures for predicting outcome in cancer patients: A systematic review. Acta Oncol. 2016, 55, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Amasene, M.; Besga, A.; Medrano, M.; Urquiza, M.; Rodriguez-Larrad, A.; Tobalina, I.; Barroso, J.; Irazusta, J.; Labayen, I. Nutritional status and physical performance using handgrip and SPPB tests in hospitalized older adults. Clin. Nutr. Edinb. Scotl. 2021, 40, 5547–5555. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobäus, N.; Gonzalez, M.C.; Schulzke, J.-D.; Pirlich, M. Hand grip strength: Outcome predictor and marker of nutritional status. Clin. Nutr. Edinb. Scotl. 2011, 30, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Grip Strength: An Indispensable Biomarker For Older Adults. Clin. Interv. Aging 2019, 14, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Rolland, Y.; Cruz-Jentoft, A.J.; Bauer, J.M.; Sieber, C.; Cooper, C.; Al-Daghri, N.; Araujo de Carvalho, I.; Bautmans, I.; Bernabei, R.; et al. Assessment of Muscle Function and Physical Performance in Daily Clinical Practice: A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif. Tissue Int. 2019, 105, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.A.; DuMontier, C.; Murillo, A.; Hshieh, T.T.; Bean, J.F.; Soiffer, R.J.; Stone, R.M.; Abel, G.A.; Driver, J.A. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood 2019, 134, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short Physical Performance Battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016, 14, 215. [Google Scholar] [CrossRef]
- Klepin, H.D.; Geiger, A.M.; Tooze, J.A.; Kritchevsky, S.B.; Williamson, J.D.; Pardee, T.S.; Ellis, L.R.; Powell, B.L. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 2013, 121, 4287–4294. [Google Scholar] [CrossRef]
- Hanada, M.; Yamauchi, K.; Miyazaki, S.; Oyama, Y.; Yanagita, Y.; Sato, S.; Miyazaki, T.; Nagayasu, T.; Kozu, R. Short-Physical Performance Battery (SPPB) score is associated with postoperative pulmonary complications in elderly patients undergoing lung resection surgery: A prospective multicenter cohort study. Chron. Respir. Dis. 2020, 17, 1479973120961846. [Google Scholar] [CrossRef]
- Del Fabbro, E. Current and future care of patients with the cancer anorexia-cachexia syndrome. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2015, 35, e229–e237. [Google Scholar] [CrossRef]
- Del Fabbro, E.; Orr, T.A.; Stella, S.M. Practical approaches to managing cancer patients with weight loss. Curr. Opin. Support. Palliat. Care 2017, 11, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, E.; Parsons, H.; Warneke, C.L.; Pulivarthi, K.; Litton, J.K.; Dev, R.; Palla, S.L.; Brewster, A.; Bruera, E. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist 2012, 17, 1240–1245. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Dalal, S.; Hui, D.; Bidaut, L.; Lem, K.; Del Fabbro, E.; Crane, C.; Reyes-Gibby, C.C.; Bedi, D.; Bruera, E. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: A pilot study. J. Pain Symptom Manag. 2012, 44, 181–191. [Google Scholar] [CrossRef]
- Cooper, A.B.; Slack, R.; Fogelman, D.; Holmes, H.M.; Petzel, M.; Parker, N.; Balachandran, A.; Garg, N.; Ngo-Huang, A.; Varadhachary, G.; et al. Characterization of Anthropometric Changes that Occur During Neoadjuvant Therapy for Potentially Resectable Pancreatic Cancer. Ann. Surg. Oncol. 2015, 22, 2416–2423. [Google Scholar] [CrossRef]
- Di Sebastiano, K.M.; Mourtzakis, M. A critical evaluation of body composition modalities used to assess adipose and skeletal muscle tissue in cancer. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2012, 37, 811–821. [Google Scholar] [CrossRef]
- Prado, C.M.; Batsis, J.A.; Donini, L.M.; Gonzalez, M.C.; Siervo, M. Sarcopenic obesity in older adults: A clinical overview. Nat. Rev. Endocrinol. 2024, 20, 261–277. [Google Scholar] [CrossRef]
- Wang, J.-C.; Wu, W.-T.; Chang, K.-V.; Chen, L.-R.; Chi, S.-Y.; Kara, M.; Özçakar, L. Ultrasound Imaging for the Diagnosis and Evaluation of Sarcopenia: An Umbrella Review. Life 2021, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Wang, L.; Zhang, W.; Lu, J.; Yang, M. Diagnostic test accuracy of ultrasound for sarcopenia diagnosis: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 57–70. [Google Scholar] [CrossRef]
- Estimating Survival in Patients with Cancer Receiving Palliative Care: Is Analysis of Body Composition Using Bioimpedance Helpful?—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/19663711/ (accessed on 12 February 2024).
- Donohoe, C.L.; Ryan, A.M.; Reynolds, J.V. Cancer Cachexia: Mechanisms and Clinical Implications. Gastroenterol. Res. Pract. 2011, 2011, 601434. [Google Scholar] [CrossRef]
- Arends, J. Struggling with nutrition in patients with advanced cancer: Nutrition and nourishment-focusing on metabolism and supportive care. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29 (Suppl. S2), ii27–ii34. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.T.; Noble, S.; Chester, J.; Davies, H.E.; Evans, W.D.; Farewell, D.; Lester, J.F.; Parry, D.; Pettit, R.; Byrne, A. The value of physical performance measurements alongside assessment of sarcopenia in predicting receipt and completion of planned treatment in non-small cell lung cancer: An observational exploratory study. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2018, 26, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Hardee, J.P.; Counts, B.R.; Carson, J.A. Understanding the Role of Exercise in Cancer Cachexia Therapy. Am. J. Lifestyle Med. 2017, 13, 46–60. [Google Scholar] [CrossRef]
- Segal, R.; Zwaal, C.; Green, E.; Tomasone, J.R.; Loblaw, A.; Petrella, T. Exercise for people with cancer: A systematic review. Curr. Oncol. 2017, 24, e290–e315. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.C.; Cook, J.; Maddocks, M.; Skipworth, R.J.E.; Fallon, M.; Laird, B.J. Combined exercise and nutritional rehabilitation in outpatients with incurable cancer: A systematic review. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2019, 27, 2371–2384. [Google Scholar] [CrossRef]
- Oldervoll, L.M.; Loge, J.H.; Lydersen, S.; Paltiel, H.; Asp, M.B.; Nygaard, U.V.; Oredalen, E.; Frantzen, T.L.; Lesteberg, I.; Amundsen, L.; et al. Physical exercise for cancer patients with advanced disease: A randomized controlled trial. Oncologist 2011, 16, 1649–1657. [Google Scholar] [CrossRef]
- Malcolm, L.; Mein, G.; Jones, A.; Talbot-Rice, H.; Maddocks, M.; Bristowe, K. Strength in numbers: Patient experiences of group exercise within hospice palliative care. BMC Palliat. Care 2016, 15, 97. [Google Scholar] [CrossRef]
- Solheim, T.S.; Laird, B.J.A.; Balstad, T.R.; Stene, G.B.; Bye, A.; Johns, N.; Pettersen, C.H.; Fallon, M.; Fayers, P.; Fearon, K.; et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, E. Combination therapy in cachexia. Ann. Palliat. Med. 2019, 8, 59–66. [Google Scholar] [CrossRef]
- Koeppel, M.; Mathis, K.; Schmitz, K.H.; Wiskemann, J. Muscle hypertrophy in cancer patients and survivors via strength training.A meta-analysis and meta-regression. Crit. Rev. Oncol. Hematol. 2021, 163, 103371. [Google Scholar] [CrossRef]
- Garg, S.; Yoo, J.; Winquist, E. Nutritional support for head and neck cancer patients receiving radiotherapy: A systematic review. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2010, 18, 667–677. [Google Scholar] [CrossRef]
- Langius, J.A.; Zandbergen, M.C.; Eerenstein, S.E.; van Tulder, M.W.; Leemans, C.R.; Kramer, M.H.; Weijs, P.J. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: A systematic review. Clin. Nutr. Edinb. Scotl. 2013, 32, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.C.; Leong, L.P.; Lim, S.L. Nutrition intervention approaches to reduce malnutrition in oncology patients: A systematic review. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2016, 24, 469–480. [Google Scholar] [CrossRef] [PubMed]
- de van der Schueren, M.A.E.; Laviano, A.; Blanchard, H.; Jourdan, M.; Arends, J.; Baracos, V.E. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: Current evidence and guidance for design of future trials. Ann. Oncol. 2018, 29, 1141–1153. [Google Scholar] [CrossRef]
- Bouleuc, C.; Anota, A.; Cornet, C.; Grodard, G.; Thiery-Vuillemin, A.; Dubroeucq, O.; Crétineau, N.; Frasie, V.; Gamblin, V.; Chvetzoff, G.; et al. Impact on Health-Related Quality of Life of Parenteral Nutrition for Patients with Advanced Cancer Cachexia: Results from a Randomized Controlled Trial. Oncologist 2020, 25, e843–e851. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, E.; Hui, D.; Dalal, S.; Dev, R.; Nooruddin, Z.I.; Bruera, E. Clinical outcomes and contributors to weight loss in a cancer cachexia clinic. J. Palliat. Med. 2011, 14, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Shivshanker, K.; Bennett, R.W.; Haynie, T.P. Tumor-associated gastroparesis: Correction with metoclopramide. Am. J. Surg. 1983, 145, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Silverman, W. Diagnostic and therapeutic approach to pancreatic cancer-associated gastroparesis: Literature review and our experience. Dig. Dis. Sci. 2009, 54, 401–405. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. Edinb. Scotl. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Navari, R.M.; Pywell, C.M.; Le-Rademacher, J.G.; White, P.; Dodge, A.B.; Albany, C.; Loprinzi, C.L. Olanzapine for the Treatment of Advanced Cancer-Related Chronic Nausea and/or Vomiting: A Randomized Pilot Trial. JAMA Oncol. 2020, 6, 895–899. [Google Scholar] [CrossRef]
- Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting|NEJM. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa1515725 (accessed on 12 February 2024).
- Librach, S.L.; Bouvette, M.; De Angelis, C.; Farley, J.; Oneschuk, D.; Pereira, J.L.; Syme, A. Consensus recommendations for the management of constipation in patients with advanced, progressive illness. J. Pain Symptom Manag. 2010, 40, 761–773. [Google Scholar] [CrossRef]
- Sánchez-Torralvo, F.J.; Contreras-Bolívar, V.; Ruiz-Vico, M.; Abuín-Fernández, J.; González-Almendros, I.; Barrios, M.; Olveira, G. Relationship between malnutrition and the presence of symptoms of anxiety and depression in hospitalized cancer patients. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2022, 30, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E.; Foley, K.F. Cancer chemotherapy and cachexia: Mirtazapine and olanzapine are 5-HT3 antagonists with good antinausea effects. Eur. J. Cancer Care 2007, 16, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Riechelmann, R.P.; Burman, D.; Tannock, I.F.; Rodin, G.; Zimmermann, C. Phase II trial of mirtazapine for cancer-related cachexia and anorexia. Am. J. Hosp. Palliat. Care 2010, 27, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shin, I.; Kim, J.; Kang, H.; Mun, J.; Yang, S.; Yoon, J. Mirtazapine for severe gastroparesis unresponsive to conventional prokinetic treatment. Psychosomatics 2006, 47, 440–442. [Google Scholar] [CrossRef]
- Hunter, C.N.; Abdel-Aal, H.H.; Elsherief, W.A.; Farag, D.E.; Riad, N.M.; Alsirafy, S.A. Mirtazapine in Cancer-Associated Anorexia and Cachexia: A Double-Blind Placebo-Controlled Randomized Trial. J. Pain Symptom Manag. 2021, 62, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Dev, R.; Del Fabbro, E.; Schwartz, G.G.; Hui, D.; Palla, S.L.; Gutierrez, N.; Bruera, E. Preliminary report: Vitamin D deficiency in advanced cancer patients with symptoms of fatigue or anorexia. Oncologist 2011, 16, 1637–1641. [Google Scholar] [CrossRef]
- Khorasanchi, A.; Nemani, S.; Pandey, S.; Del Fabbro, E. Managing Nutrition Impact Symptoms in Cancer Cachexia: A Case Series and Mini Review. Front. Nutr. 2022, 9, 831934. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.P. Cannabinoids for Symptom Management and Cancer Therapy: The Evidence. J. Natl. Compr. Cancer Netw. JNCCN 2016, 14, 915–922. [Google Scholar] [CrossRef]
- Brisbois, T.D.; de Kock, I.H.; Watanabe, S.M.; Mirhosseini, M.; Lamoureux, D.C.; Chasen, M.; MacDonald, N.; Baracos, V.E.; Wismer, W.V. Delta-9-tetrahydrocannabinol may palliate altered chemosensory perception in cancer patients: Results of a randomized, double-blind, placebo-controlled pilot trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 2086–2093. [Google Scholar] [CrossRef]
- Ripamonti, C.; Zecca, E.; Brunelli, C.; Fulfaro, F.; Villa, S.; Balzarini, A.; Bombardieri, E.; De Conno, F. A randomized, controlled clinical trial to evaluate the effects of zinc sulfate on cancer patients with taste alterations caused by head and neck irradiation. Cancer 1998, 82, 1938–1945. [Google Scholar] [CrossRef]
- Halyard, M.Y.; Jatoi, A.; Sloan, J.A.; Bearden, J.D., III; Vora, S.A.; Atherton, P.J.; Perez, E.A.; Soori, G.; Zalduendo, A.C.; Zhu, A.; et al. Does zinc sulfate prevent therapy-induced taste alterations in head and neck cancer patients? Results of phase III double-blind, placebo-controlled trial from the North Central Cancer Treatment Group (N01C4). Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Yennurajalingam, S.; Frisbee-Hume, S.; Palmer, J.L.; Delgado-Guay, M.O.; Bull, J.; Phan, A.T.; Tannir, N.M.; Litton, J.K.; Reddy, A.; Hui, D.; et al. Reduction of cancer-related fatigue with dexamethasone: A double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 3076–3082. [Google Scholar] [CrossRef] [PubMed]
- Bruera, E.; Roca, E.; Cedaro, L.; Carraro, S.; Chacon, R. Action of oral methylprednisolone in terminal cancer patients: A prospective randomized double-blind study. Cancer Treat. Rep. 1985, 69, 751–754. [Google Scholar] [PubMed]
- Moertel, C.G.; Schutt, A.J.; Reitemeier, R.J.; Hahn, R.G. Corticosteroid therapy of preterminal gastrointestinal cancer. Cancer 1974, 33, 1607–1609. [Google Scholar] [CrossRef]
- Paulsen, Ø.; Klepstad, P.; Rosland, J.H.; Aass, N.; Albert, E.; Fayers, P.; Kaasa, S. Efficacy of Methylprednisolone on Pain, Fatigue, and Appetite Loss in Patients with Advanced Cancer Using Opioids: A Randomized, Placebo-Controlled, Double-Blind Trial. J. Clin. Oncol. 2014, 32, 3221–3228. [Google Scholar] [CrossRef]
- Sturdza, A.; Millar, B.A.; Bana, N.; Laperriere, N.; Pond, G.; Wong, R.K.; Bezjak, A. The use and toxicity of steroids in the management of patients with brain metastases. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2008, 16, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-García, V.; Juan, O.; Pérez Hoyos, S.; Peiró, R.; Ramón, N.; Rosero, M.A.; García, M.A. Megestrol acetate: A systematic review usefulness about the weight gain in neoplastic patients with cachexia. Med. Clin. 2002, 119, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Pascual López, A.; Roqué i Figuls, M.; Urrútia Cuchi, G.; Berenstein, E.G.; Almenar Pasies, B.; Balcells Alegre, M.; Herdman, M. Systematic review of megestrol acetate in the treatment of anorexia-cachexia syndrome. J. Pain Symptom Manag. 2004, 27, 360–369. [Google Scholar] [CrossRef]
- Leśniak, W.; Bała, M.; Jaeschke, R.; Krzakowski, M. Effects of megestrol acetate in patients with cancer anorexia-cachexia syndrome--a systematic review and meta-analysis. Pol. Arch. Med. Wewn. 2008, 118, 636–644. [Google Scholar] [CrossRef]
- Lambert, C.P.; Sullivan, D.H.; Freeling, S.A.; Lindquist, D.M.; Evans, W.J. Effects of testosterone replacement and/or resistance exercise on the composition of megestrol acetate stimulated weight gain in elderly men: A randomized controlled trial. J. Clin. Endocrinol. Metab. 2002, 87, 2100–2106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bruera, E.; Macmillan, K.; Kuehn, N.; Hanson, J.; MacDonald, R.N. A controlled trial of megestrol acetate on appetite, caloric intake, nutritional status, and other symptoms in patients with advanced cancer. Cancer 1990, 66, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.P.; Aaronson, N.K.; Vansteenkiste, J.F.; ten Velde, G.P.; Muller, M.J.; Drenth, B.M.; Erdkamp, F.L.; Cobben, E.G.; Schoon, E.J.; Smeets, J.B.; et al. Effects of medroxyprogesterone acetate on appetite, weight, and quality of life in advanced-stage non-hormone-sensitive cancer: A placebo-controlled multicenter study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1996, 14, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Munshi, L.B.; Tsushima, Y.; Cheng, K.; Brito, M. Megestrol Acetate-Induced Symptomatic Hypogonadism in a Male Patient. Case Rep. Endocrinol. 2018, 2018, 7048610. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J. Megestrol Acetate Use for Weight Gain Should Be Carefully Considered. J. Clin. Endocrinol. Metab. 2007, 92, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Poster, D.S.; Penta, J.S.; Bruno, S.; Macdonald, J.S. delta 9-tetrahydrocannabinol in clinical oncology. JAMA 1981, 245, 2047–2051. [Google Scholar] [CrossRef] [PubMed]
- Beal, J.E.; Olson, R.; Laubenstein, L.; Morales, J.O.; Bellman, P.; Yangco, B.; Lefkowitz, L.; Plasse, T.F.; Shepard, K.V. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J. Pain Symptom Manag. 1995, 10, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Strasser, F.; Luftner, D.; Possinger, K.; Ernst, G.; Ruhstaller, T.; Meissner, W.; Ko, Y.D.; Schnelle, M.; Reif, M.; Cerny, T. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 3394–3400. [Google Scholar] [CrossRef] [PubMed]
- Plasse, T.F.; Gorter, R.W.; Krasnow, S.H.; Lane, M.; Shepard, K.V.; Wadleigh, R.G. Recent clinical experience with dronabinol. Pharmacol. Biochem. Behav. 1991, 40, 695–700. [Google Scholar] [CrossRef]
- Jatoi, A.; Windschitl, H.; Loprinzi, C.; Sloan, J.; Dakhil, S.; Mailliard, J.; Pundaleeka, S.; Kardinal, C.; Fitch, T.; Krook, J.; et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: A North Central Cancer Treatment Group study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 567–573. [Google Scholar] [CrossRef]
- Turcott, J.G.; Del Rocío Guillen Núñez, M.; Flores-Estrada, D.; Oñate-Ocaña, L.F.; Zatarain-Barrón, Z.L.; Barrón, F.; Arrieta, O. The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: A randomized, double-blind clinical trial. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2018, 26, 3029–3038. [Google Scholar] [CrossRef]
- Parsons, B.; Allison, D.B.; Loebel, A.; Williams, K.; Giller, E.; Romano, S.; Siu, C. Weight effects associated with antipsychotics: A comprehensive database analysis. Schizophr. Res. 2009, 110, 103–110. [Google Scholar] [CrossRef]
- Davis, M.; Hui, D.; Davies, A.; Ripamonti, C.; Capela, A.; DeFeo, G.; Del Fabbro, E.; Bruera, E. MASCC antiemetics in advanced cancer updated guideline. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2021, 29, 8097–8107. [Google Scholar] [CrossRef]
- Navari, R.M.; Einhorn, L.H.; Passik, S.D.; Loehrer, P.J., Sr.; Johnson, C.; Mayer, M.L.; McClean, J.; Vinson, J.; Pletcher, W. A phase II trial of olanzapine for the prevention of chemotherapy-induced nausea and vomiting: A Hoosier Oncology Group study. Support. Care Cancer 2005, 13, 529–534. [Google Scholar] [CrossRef]
- Navari, R.M.; Einhorn, L.H.; Loehrer, P.J., Sr.; Passik, S.D.; Vinson, J.; McClean, J.; Chowhan, N.; Hanna, N.H.; Johnson, C.S. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: A Hoosier oncology group study. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2007, 15, 1285. [Google Scholar] [CrossRef]
- Passik, S.D.; Lundberg, J.; Kirsh, K.L.; Theobald, D.; Donaghy, K.; Holtsclaw, E.; Cooper, M.; Dugan, W. A pilot exploration of the antiemetic activity of olanzapine for the relief of nausea in patients with advanced cancer and pain. J. Pain Symptom Manag. 2002, 23, 526–532. [Google Scholar] [CrossRef]
- Naing, A.; Dalal, S.; Abdelrahim, M.; Wheler, J.; Hess, K.; Fu, S.; Hong, D.S.; Janku, F.; Falchook, G.S.; Ilustre, A.; et al. Olanzapine for cachexia in patients with advanced cancer: An exploratory study of effects on weight and metabolic cytokines. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2015, 23, 2649–2654. [Google Scholar] [CrossRef]
- Navari, R.M.; Brenner, M.C. Treatment of cancer-related anorexia with olanzapine and megestrol acetate: A randomized trial. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2010, 18, 951–956. [Google Scholar] [CrossRef]
- Sandhya, L.; Devi Sreenivasan, N.; Goenka, L.; Dubashi, B.; Kayal, S.; Solaiappan, M.; Govindarajalou, R.; Kt, H.; Ganesan, P. Randomized Double-Blind Placebo-Controlled Study of Olanzapine for Chemotherapy-Related Anorexia in Patients with Locally Advanced or Metastatic Gastric, Hepatopancreaticobiliary, and Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 2617–2627. [Google Scholar] [CrossRef]
- Solheim, T.S.; Fearon, K.C.H.; Blum, D.; Kaasa, S. Non-steroidal anti-inflammatory treatment in cancer cachexia: A systematic literature review. Acta Oncol. Stockh. Swed. 2013, 52, 6–17. [Google Scholar] [CrossRef]
- Reid, J.; Mills, M.; Cantwell, M.M.; Cardwell, C.R.; Murray, L.J.; Donnelly, M. Thalidomide for managing cancer cachexia. Cochrane Database Syst. Rev. 2012, 2012, CD008664. [Google Scholar] [CrossRef]
- Wilkes, E.A.; Selby, A.L.; Cole, A.T.; Freeman, J.G.; Rennie, M.J.; Khan, Z.H. Poor tolerability of thalidomide in end-stage oesophageal cancer. Eur. J. Cancer Care 2011, 20, 593–600. [Google Scholar] [CrossRef]
- Gordon, J.N.; Trebble, T.M.; Ellis, R.D.; Duncan, H.D.; Johns, T.; Goggin, P.M. Thalidomide in the treatment of cancer cachexia: A randomised placebo controlled trial. Gut 2005, 54, 540–545. [Google Scholar] [CrossRef]
- Khan, Z.H.; Simpson, E.J.; Cole, A.T.; Holt, M.; MacDonald, I.; Pye, D.; Austin, A.; Freeman, J.G. Oesophageal cancer and cachexia: The effect of short-term treatment with thalidomide on weight loss and lean body mass. Aliment. Pharmacol. Ther. 2003, 17, 677–682. [Google Scholar] [CrossRef]
- Davis, M.; Lasheen, W.; Walsh, D.; Mahmoud, F.; Bicanovsky, L.; Lagman, R. A Phase II dose titration study of thalidomide for cancer-associated anorexia. J. Pain Symptom Manag. 2011, 43, 78–86. [Google Scholar] [CrossRef]
- Yennurajalingam, S.; Willey, J.S.; Palmer, J.L.; Allo, J.; Del Fabbro, E.; Cohen, E.N.; Tin, S.; Reuben, J.M.; Bruera, E. The role of thalidomide and placebo for the treatment of cancer-related anorexia-cachexia symptoms: Results of a double-blind placebo-controlled randomized study. J. Palliat. Med. 2012, 15, 1059–1064. [Google Scholar] [CrossRef]
- Anticachectic and Antitumor Effect of Eicosapentaenoic Acid and Its Effect on Protein Turnover|Semantic Scholar. Available online: https://www.semanticscholar.org/paper/Anticachectic-and-antitumor-effect-of-acid-and-its-Beck-Smith/c37c2ec76348086be6ee49be857052234d15a0d0 (accessed on 12 February 2024).
- Fearon, K.C.; Von Meyenfeldt, M.F.; Moses, A.G.; Van Geenen, R.; Roy, A.; Gouma, D.J.; Giacosa, A.; Van Gossum, A.; Bauer, J.; Barber, M.D.; et al. Effect of a protein and energy dense n-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: A randomised double blind trial. Gut 2003, 52, 1479–1486. [Google Scholar] [CrossRef]
- Fearon, K.; Barber, M.; Moses, A.; Ahmedzai, S.; Taylor, G.; Tisdale, M.; Murray, G. Double-blind, placebo-controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 3401–3407. [Google Scholar] [CrossRef]
- Murphy, R.A.; Mourtzakis, M.; Chu, Q.S.C.; Baracos, V.E.; Reiman, T.; Mazurak, V.C. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer 2011, 117, 1775–1782. [Google Scholar] [CrossRef]
- van der Meij, B.S.; Langius, J.A.; Smit, E.F.; Spreeuwenberg, M.D.; von Blomberg, B.M.; Heijboer, A.C.; Paul, M.A.; van Leeuwen, P.A. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. J. Nutr. 2010, 140, 1774–1780. [Google Scholar] [CrossRef]
- Dewey, A.; Baughan, C.; Dean, T.; Higgins, B.; Johnson, I. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst. Rev. 2007, 2017, CD004597. [Google Scholar] [CrossRef] [PubMed]
- Ries, A.; Trottenberg, P.; Elsner, F.; Stiel, S.; Haugen, D.; Kaasa, S.; Radbruch, L. A systematic review on the role of fish oil for the treatment of cachexia in advanced cancer: An EPCRC cachexia guidelines project. Palliat. Med. 2012, 26, 294–304. [Google Scholar] [CrossRef]
- Mazzotta, P.; Jeney, C.M. Anorexia-cachexia syndrome: A systematic review of the role of dietary polyunsaturated Fatty acids in the management of symptoms, survival, and quality of life. J. Pain Symptom Manag. 2009, 37, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- de Castro, G.S.; Andrade, M.F.; Pinto, F.C.S.; Faiad, J.Z.; Seelaender, M. Omega-3 Fatty Acid Supplementation and Its Impact on Systemic Inflammation and Body Weight in Patients with Cancer Cachexia-A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 797513. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.R.; López-Briz, E.; Sanchis, R.C.; Perales, J.L.G.; Bort-Marti, S. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst. Rev. 2013, 2013, CD004310. [Google Scholar] [CrossRef] [PubMed]
- Grunfeld, C.; Kotler, D.P.; Dobs, A.; Glesby, M.; Bhasin, S. Oxandrolone in the treatment of HIV-associated weight loss in men: A randomized, double-blind, placebo-controlled study. J. Acquir. Immune Defic. Syndr. 1999 2006, 41, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.; Kugler, J.; Sloan, J.; Mailliard, J.; Krook, J.; Wilwerding, M.; Rowland Jr, K.; Camoriano, J.; Novotny, P.; Christensen, B. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 3299–3306. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Herrold, J.; Ali, I.; Oktay, E.; Chlebowski, J.S.; Ponce, A.T.; Heber, D.; Block, J.B. Influence of nandrolone decanoate on weight loss in advanced non-small cell lung cancer. Cancer 1986, 58, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Dobs, A.S.; Boccia, R.V.; Croot, C.C.; Gabrail, N.Y.; Dalton, J.T.; Hancock, M.L.; Johnston, M.A.; Steiner, M.S. Effects of enobosarm on muscle wasting and physical function in patients with cancer: A double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013, 14, 335–345. [Google Scholar] [CrossRef]
- Crawford, J.; Prado, C.M.; Johnston, M.A.; Gralla, R.J.; Taylor, R.P.; Hancock, M.L.; Dalton, J.T. Study Design and Rationale for the Phase 3 Clinical Development Program of Enobosarm, a Selective Androgen Receptor Modulator, for the Prevention and Treatment of Muscle Wasting in Cancer Patients (POWER Trials). Curr. Oncol. Rep. 2016, 18, 37. [Google Scholar] [CrossRef]
- Crawford, J.; Johnston, M.A.; Taylor, R.P.; Dalton, J.T.; Steiner, M.S. Enobosarm and lean body mass in patients with non-small cell lung cancer. J. Clin. Oncol. 2014, 32, 9618. [Google Scholar] [CrossRef]
- Del Fabbro, E.; Garcia, J.M.; Dev, R.; Hui, D.; Williams, J.; Engineer, D.; Palmer, J.L.; Schover, L.; Bruera, E. Testosterone replacement for fatigue in hypogonadal ambulatory males with advanced cancer: A preliminary double-blind placebo-controlled trial. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2013, 21, 2599–2607. [Google Scholar] [CrossRef]
- Garcia, J.M. Improving Cancer-related Fatigue, Sexual Dysfunction and Quality of Life in Older Men with Cancer and Androgen Deficiency. clinicaltrials.gov, Clinical Trial Registration NCT04301765. February 2024. Available online: https://clinicaltrials.gov/study/NCT04301765 (accessed on 31 December 2023).
- Herndon, D.N.; Hart, D.W.; Wolf, S.E.; Chinkes, D.L.; Wolfe, R.R. Reversal of catabolism by beta-blockade after severe burns. N. Engl. J. Med. 2001, 345, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Hyltander, A.; Daneryd, P.; Sandström, R.; Körner, U.; Lundholm, K. Beta-adrenoceptor activity and resting energy metabolism in weight losing cancer patients. Eur. J. Cancer 2000, 36, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Vulić, A.; Perši, N. β-adrenergic agonists: Substances with anabolic effect in animals for meat production. MESO 2012, 14, 80–87. [Google Scholar]
- Espindolol for the Treatment and Prevention of Cachexia in Patients with Stage III/IV Non-Small Cell Lung Cancer or Colorectal Cancer: A Randomized, Double-Blind, Placebo-Controlled, International Multicentre Phase II Study (the ACT-ONE Trial)—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27386169/ (accessed on 13 February 2023).
- Ghrelin Is a Growth-Hormone-Releasing Acylated Peptide from Stomach|Nature. Available online: https://www.nature.com/articles/45230 (accessed on 13 February 2023).
- Hanada, T.; Toshinai, K.; Kajimura, N.; Nara-Ashizawa, N.; Tsukada, T.; Hayashi, Y.; Osuye, K.; Kangawa, K.; Matsukura, S.; Nakazato, M. Anti-cachectic effect of ghrelin in nude mice bearing human melanoma cells. Biochem. Biophys. Res. Commun. 2003, 301, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Andersson, M.; Iresjö, B.-M.; Lönnroth, C.; Lundholm, K. Effects of ghrelin on anorexia in tumor-bearing mice with eicosanoid-related cachexia. Int. J. Oncol. 2006, 28, 1393–1400. [Google Scholar] [CrossRef]
- DeBoer, M.; Zhu, X.; Levasseur, P.; Meguid, M.; Suzuki, S.; Inui, A.; Taylor, J.; Halem, H.; Dong, J.; Datta, R.; et al. Ghrelin Treatment Causes Increased Food Intake and Retention of Lean Body Mass in a Rat Model of Cancer Cachexia. Endocrinology 2007, 148, 3004–3012. [Google Scholar] [CrossRef]
- Date, Y.; Murakami, N.; Toshinai, K.; Matsukura, S.; Niijima, A.; Matsuo, H.; Kangawa, K.; Nakazato, M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002, 123, 1120–1128. [Google Scholar] [CrossRef]
- Ghrelin Inhibits Leptin- and Activation-Induced Proinflammatory Cytokine Expression by Human Monocytes and T Cells—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/15232612/ (accessed on 13 February 2023).
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 5992. [Google Scholar] [CrossRef]
- Neary, N.M.; Small, C.J.; Wren, A.M.; Lee, J.L.; Druce, M.R.; Palmieri, C.; Frost, G.S.; Ghatei, M.A.; Coombes, R.C.; Bloom, S.R. Ghrelin increases energy intake in cancer patients with impaired appetite: Acute, randomized, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2004, 89, 2832–2836. [Google Scholar] [CrossRef] [PubMed]
- Hiura, Y.; Takiguchi, S.; Yamamoto, K.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Miyata, H.; Fujiwara, Y.; Mori, M.; et al. Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients. Cancer 2012, 118, 4785–4794. [Google Scholar] [CrossRef] [PubMed]
- Inui, A. Ghrelin: An orexigenic and somatotrophic signal from the stomach. Nat. Rev. Neurosci. 2001, 2, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Boccia, R.V.; Graham, C.D.; Yan, Y.; Duus, E.M.; Allen, S.; Friend, J. Anamorelin for patients with cancer cachexia: An integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol. 2015, 16, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Temel, J.S.; Abernethy, A.P.; Currow, D.C.; Friend, J.; Duus, E.M.; Yan, Y.; Fearon, K.C. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): Results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016, 17, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Carnac, G.; Vernus, B.; Bonnieu, A. Myostatin in the pathophysiology of skeletal muscle. Curr. Genomics 2007, 8, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-M.; Zhao, Y.-P.; Zhao, Y.; Deng, S.-L.; Yu, K. Regulation of Myostatin on the Growth and Development of Skeletal Muscle. Front. Cell Dev. Biol. 2021, 9, 785712. [Google Scholar] [CrossRef] [PubMed]
- Han, H.Q.; Mitch, W.E. Targeting the myostatin signaling pathway to treat muscle wasting diseases. Curr. Opin. Support. Palliat. Care 2011, 5, 334–341. [Google Scholar] [CrossRef]
- Wu, C.; Fernandez, S.A.; Criswell, T.; Chidiac, T.A.; Guttridge, D.; Villalona-Calero, M.; Bekaii-Saab, T.S. Disrupting cytokine signaling in pancreatic cancer: A phase I/II study of Etanercept in combination with Gemcitabine in patients with advanced disease. Pancreas 2013, 42, 813–818. [Google Scholar] [CrossRef]
- Wiedenmann, B.; Malfertheiner, P.; Friess, H.; Ritch, P.; Arseneau, J.; Mantovani, G.; Caprioni, F.; Van Cutsem, E.; Richel, D.; DeWitte, M.; et al. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J. Support. Oncol. 2008, 6, 18–25. [Google Scholar]
- Hong, D.S.; Hui, D.; Bruera, E.; Janku, F.; Naing, A.; Falchook, G.S.; Piha-Paul, S.; Wheler, J.J.; Fu, S.; Tsimberidou, A.M.; et al. MABp1, a first-in-class true human antibody targeting interleukin-1α in refractory cancers: An open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014, 15, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Johnen, H.; Lin, S.; Kuffner, T.; Brown, D.A.; Tsai, V.W.; Bauskin, A.R.; Wu, L.; Pankhurst, G.; Jiang, L.; Junankar, S.; et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat. Med. 2007, 13, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Lerner, L.; Hayes, T.G.; Tao, N.; Krieger, B.; Feng, B.; Wu, Z.; Nicoletti, R.; Chiu, M.I.; Gyuris, J.; Garcia, J.M. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J. Cachexia Sarcopenia Muscle 2015, 6, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.C.; Bock, A.J.; Becker, C.; Kempf, T.; Wollert, K.C.; Davidson, B. Growth differentiation factor-15 as a prognostic biomarker in ovarian cancer. Gynecol. Oncol. 2010, 118, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Casal, I.; Wang, J.; Li, P.; Zhang, W.; Xu, E.; Lai, M.; Zhang, H. Growth differentiation factor 15 is a promising diagnostic and prognostic biomarker in colorectal cancer. J. Cell. Mol. Med. 2016, 20, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Non-Homeostatic Body Weight Regulation through a Brainstem-Restricted Receptor for GDF15|Nature. Available online: https://www.nature.com/articles/nature24042 (accessed on 13 February 2023).
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- GFRAL Is the Receptor for GDF15 and Is Required for the Anti-Obesity Effects of the Ligand|Nature Medicine. Available online: https://www.nature.com/articles/nm.4394 (accessed on 13 February 2023).
- Emmerson, P.J.; Wang, F.; Du, Y.; Liu, Q.; Pickard, R.T.; Gonciarz, M.D.; Coskun, T.; Hamang, M.J.; Sindelar, D.K.; Ballman, K.K.; et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 2017, 23, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Suriben, R.; Chen, M.; Higbee, J.; Oeffinger, J.; Ventura, R.; Li, B.; Mondal, K.; Gao, Z.; Ayupova, D.; Taskar, P.; et al. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat. Med. 2020, 26, 1264–1270. [Google Scholar] [CrossRef]
- Kim-Muller, J.Y.; Song, L.; LaCarubba Paulhus, B.; Pashos, E.; Li, X.; Rinaldi, A.; Joaquim, S.; Stansfield, J.C.; Zhang, J.; Robertson, A.; et al. GDF15 neutralization restores muscle function and physical performance in a mouse model of cancer cachexia. Cell Rep. 2023, 42, 111947. [Google Scholar] [CrossRef]
- More Is Better: A Multimodality Approach to Cancer Cachexia—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3227946/ (accessed on 13 February 2023).
- Randomized Phase III Clinical Trial of Five Different Arms of Treatment in 332 Patients with Cancer Cachexia—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/20156909/ (accessed on 13 February 2023).
- Insulin Treatment in Cancer Cachexia: Effects on Survival, Metabolism, and Physical Functioning—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/17473202/ (accessed on 13 February 2023).
- Effects of Nutrition and Physical Exercise Intervention in Palliative Cancer Patients: A Randomized Controlled Trial—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/28651827/ (accessed on 13 February 2023).
- Early Interdisciplinary Supportive Care in Patients with Previously Untreated Metastatic Esophagogastric Cancer: A Phase III Randomized Controlled Trial—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/33417481/ (accessed on 13 February 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, S.; Bradley, L.; Del Fabbro, E. Updates in Cancer Cachexia: Clinical Management and Pharmacologic Interventions. Cancers 2024, 16, 1696. https://doi.org/10.3390/cancers16091696
Pandey S, Bradley L, Del Fabbro E. Updates in Cancer Cachexia: Clinical Management and Pharmacologic Interventions. Cancers. 2024; 16(9):1696. https://doi.org/10.3390/cancers16091696
Chicago/Turabian StylePandey, Sudeep, Lauren Bradley, and Egidio Del Fabbro. 2024. "Updates in Cancer Cachexia: Clinical Management and Pharmacologic Interventions" Cancers 16, no. 9: 1696. https://doi.org/10.3390/cancers16091696
APA StylePandey, S., Bradley, L., & Del Fabbro, E. (2024). Updates in Cancer Cachexia: Clinical Management and Pharmacologic Interventions. Cancers, 16(9), 1696. https://doi.org/10.3390/cancers16091696





