The Prevalence of Orthostatic Hypotension in Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
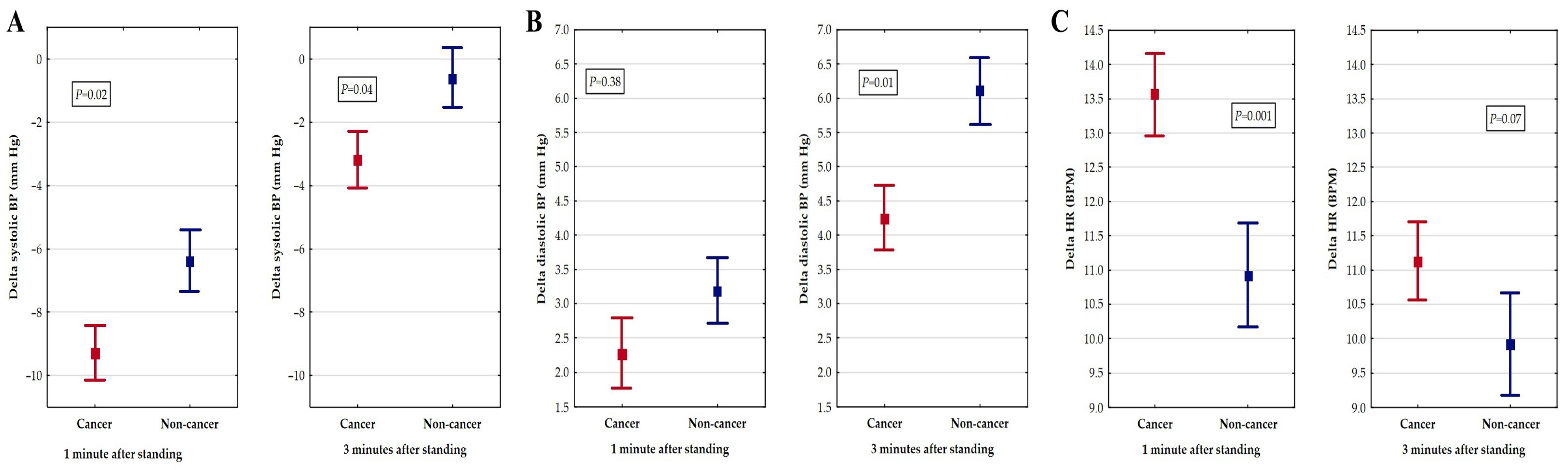
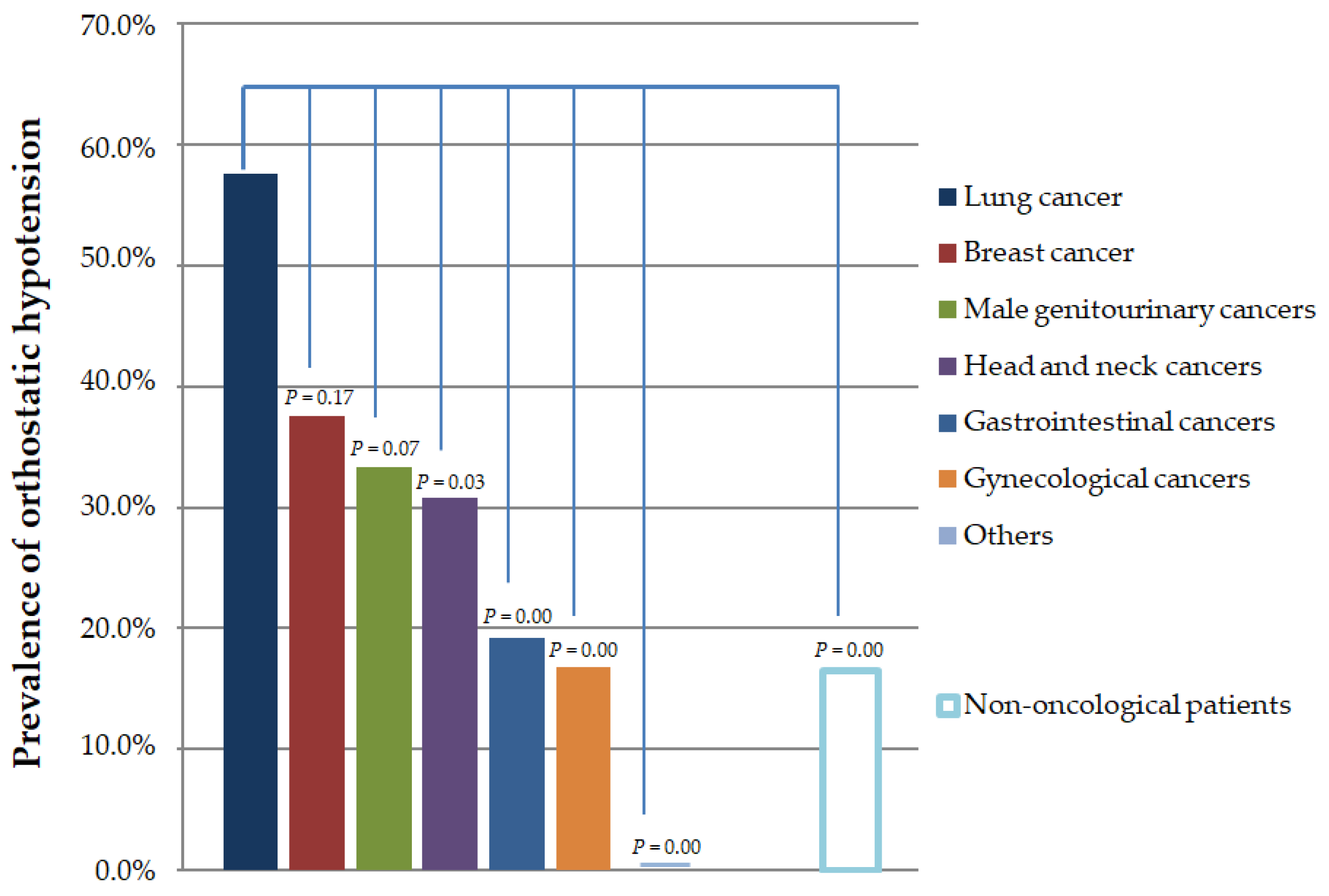
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magkas, N.; Tsioufis, C.; Thomopoulos, C.; Dilaveris, P.; Georgiopoulos, G.; Sanidas, E.; Papademetriou, V.; Tousoulis, D. Orthostatic hypotension: From pathophysiology to clinical applications and therapeutic considerations. J. Clin. Hypertens. 2019, 21, 546–554. [Google Scholar] [CrossRef]
- Freeman, R.; Abuzinadah, A.R.; Gibbons, C.; Jones, P.; Miglis, M.G.; Sinn, D.I. Orthostatic Hypotension: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 1294–1309. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Nelson, K.A. Autonomic nervous system dysfunction in advanced cancer. Support. Care Cancer 2002, 10, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Noor, B.; Akhavan, S.; Leuchter, M.; Yang, E.H.; Ajijola, O.A. Quantitative assessment of cardiovascular autonomic impairment in cancer survivors: A single center case series. Cardiooncology 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Teng, A.E.; Noor, B.; Ajijola, O.A.; Yang, E.H. Chemotherapy and Radiation-Associated Cardiac Autonomic Dysfunction. Curr. Oncol. Rep. 2021, 23, 14. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Mosnaim, A.D.; Abiola, R.; Wolf, M.E.; Perlmuter, L.C. Etiology and risk factors for developing orthostatic hypotension. Am. J. Ther. 2010, 17, 86–91. [Google Scholar] [CrossRef]
- Streeten, D.H., Jr.; Anderson, G.H. Mechanisms of orthostatic hypotension and tachycardia in patients with pheochromocytoma. Am. J. Hypertens. 1996, 9, 760–769. [Google Scholar] [CrossRef]
- Gosney, M.A.; Gosney, J.R.; Lye, M. Orthostatic hypotension and vasodilatory peptides in bronchial carcinoma. J. Clin. Pathol. 1995, 48, 1102–1105. [Google Scholar] [CrossRef][Green Version]
- Tykarski, A.; Filipiak, K.J.; Januszewicz, A.; Litwin, M.; Narkiewicz, K.; Prejbisz, A.; Ostalska-Nowicka, D.; Widecka, K.; Kostka-Jeziorny, K. Zasady postępowania w nadciśnieniu tętniczym—2019 rok. Wytyczne Polskiego Towarzystwa Nadciśnienia Tętniczego. Nadciśnienie Tętnicze Prakt. 2019, 5, 99–152. [Google Scholar]
- Lavi, S.; Aharon-Peretz, J.; Haim, N.; Vlodavsky, E.; Jacob, G. Unusual cause of partially reversible severe cardiovascular autonomic failure. Am. J. Med. Sci. 2003, 326, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.V.; Patel, K.P.; Manion, C.; Runkana, A.; Hama Amin, A.; Jain, A. Third-degree atrioventricular block followed by syncope, labile hypertension, and orthostatic hypotension in a patient with nasopharyngeal cancer: Baroreflex failure. Am. J. Cardiovasc. Dis. 2018, 8, 39–42. [Google Scholar] [PubMed]
- Ashraf, W.; Farrow, L.J. Severe orthostatic hypotension in carcinoma of the pancreas. Br. J. Clin. Pract. 1992, 46, 278–279. [Google Scholar] [CrossRef] [PubMed]
- Bortnik, M.; Occhetta, E.; Marino, P. Orthostatic hypotension as an unusual clinical manifestation of pheochromocytoma: A case report. J. Cardiovasc. Med. 2008, 9, 839–841. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chary, M.; Petersen, B.; Mascarenhas, J.O. Paraneoplastic orthostatic hypotension associated with acute myeloid leukemia. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2934–2937. [Google Scholar] [PubMed]
- Sharabi, Y.; Dendi, R.; Holmes, C.; Goldstein, D.S. Baroreflex failure as a late sequela of neck irradiation. Hypertension 2003, 42, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, H.; Norcliffe-Kaufmann, L.; Palma, J.A. Baroreflex Dysfunction. N. Engl. J. Med. 2020, 382, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Dermitzakis, E.V.; Kimiskidis, V.K.; Lazaridis, G.; Alexopoulou, Z.; Timotheadou, E.; Papanikolaou, A.; Romanidou, O.; Georgiadis, G.; Kalogeras, K.T.; Tsiptsios, I.; et al. The impact of paclitaxel and carboplatin chemotherapy on the autonomous nervous system of patients with ovarian cancer. BMC Neurol. 2016, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Vassilomanolakis, M.; Koumakis, G.; Demiri, M.; Missitzis, J.; Barbounis, V.; Efremidis, A.P. Vinorelbine and cisplatin for metastatic breast cancer: A salvage regimen in patients progressing after docetaxel and anthracycline treatment. Cancer Investig. 2003, 21, 497–504. [Google Scholar] [CrossRef]
- Ho, M.; Moscvin, M.; Low, S.K.; Evans, B.; Close, S.; Schlossman, R.; Laubach, J.; Prada, C.P.; Glotzbecker, B.; Richardson, P.G.; et al. Risk factors for the development of orthostatic hypotension during autologous stem cell transplant in patients with multiple myeloma. Leuk. Lymphoma 2022, 63, 2403–2412. [Google Scholar] [CrossRef]
- Vecchié, A.; Thomas, G.; Bressi, E.; Bonaventura, A.; Canada, J.M.; Chuquin, D.; Kadariya, D.; Piracha, U.; Endicott, D.; Markley, R.; et al. Orthostatic intolerance syndromes after hematopoietic cell transplantation: Clinical characteristics and therapeutic interventions in a single-center experience. Cardiooncology 2021, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Fradley, M.G.; Beckie, T.M.; Brown, S.A.; Cheng, R.K.; Dent, S.F.; Nohria, A.; Patton, K.K.; Singh, J.P.; Olshansky, B. Recognition, Prevention, and Management of Arrhythmias and Autonomic Disorders in Cardio-Oncology: A Scientific Statement from the American Heart Association. Circulation 2021, 144, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, J.R.; Munk-Madsen, P.; Kehlet, H.; Gögenur, I. Orthostatic intolerance in enhanced recovery laparoscopic colorectal resection. Acta Anaesthesiol. Scand. 2019, 63, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Unaldi, H.E. Effects of mobilization within the first 4 h following anatomical lung resection with thoracotomy. Updates Surg. 2023, 75, 2027–2031. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Shirai, S.; Oya, Y.; Takahashi, Y.; Sakakura, N.; Ohtsuka, T.; Kuroda, H. Four Hours Postoperative Mobilization is Feasible After Thoracoscopic Anatomical Pulmonary Resection. World J. Surg. 2021, 45, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, C.; Wang, Y.; Feng, F.; Wang, H.; Zhang, Y.; Lin, X.; Xu, B. Lambert-Eaton Myasthenic Syndrome in Lung Cancer. Contrast Media Mol. Imaging. 2022, 2022, 3912376. [Google Scholar] [CrossRef] [PubMed]
- Worrall, A.P.; Doyle, C.P.; Ní Dhomhnall, R.; Lorton, C.; Barrett, M.; Uí Dhuibhir, P.; O’Higgins, C.; Brady, B.; Walsh, D. Blood Pressure, Orthostatic Hypotension and Falls in Patients with Advanced Cancer. Ir. Med. J. 2022, 115, 596. [Google Scholar] [PubMed]
- Ricci, F.; De Caterina, R.; Fedorowski, A. Orthostatic Hypotension: Epidemiology, Prognosis, and Treatment. J. Am. Coll. Cardiol. 2015, 66, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Fedorowski, A.; Ricci, F.; Sutton, R. Orthostatic hypotension and cardiovascular risk. Kardiol. Pol. 2019, 77, 1020–1027. [Google Scholar] [CrossRef]
- Low, P.A. Prevalence of ortostatic hypotension. Clin. Auton. Res. 2008, 18, 8–13. [Google Scholar] [CrossRef]
- Fedorowski, A.; Ricci, F.; Hamrefors, V.; Sandau, K.E.; Hwan Chung, T.; Muldowney, J.A.S.; Gopinathannair, R.; Olshansky, B. Orthostatic Hypotension: Management of a Complex, But Common, Medical Problem. Circ. Arrhythm. Electrophysiol. 2022, 15, e010573. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Radico, F.; Romanello, M. Morbidity and mortality related to orthostatic hypotension: Results of a meta-analysis of non-randomized observational studies. Eur. Heart J. 2013, 34, 4462. [Google Scholar] [CrossRef]
- Ricci, F.; Fedorowski, A.; Radico, F.; Romanello, M.; Tatasciore, A.; Di Nicola, M.; Zimarino, M.; De Caterina, R. Cardiovascular morbidity and mortality related to orthostatic hypotension: A meta-analysis of prospective observational studies. Eur. Heart J. 2015, 36, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ali, N.S.; Waqas, N.; Bhan, C.; Iftikhar, W.; Sapna, F.; Jitidhar, F.; Cheema, A.M.; Ahmad, M.Q.; Nasir, U.; et al. Management of Orthostatic Hypotension: A Literature Review. Cureus 2018, 10, 3166. [Google Scholar] [CrossRef] [PubMed]
- Rivasi, G.; Rafanelli, M.; Mossello, E.; Brignole, M.; Ungar, A. Drug-Related Orthostatic Hypotension: Beyond Anti-Hypertensive Medications. Drugs Aging 2020, 37, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Al-Ramahi, R.; Nazzal, D.; Mustafa, D.; Halabi, E.; Gnimat, S.; Gayada, S. Evaluation of types, stages and treatment of breast cancer among Palestinian women. Palest. Med. Pharm. J. 2020, 5, 35–40. [Google Scholar] [CrossRef]
- Sever, P. Severe orthostatic hypotension and weight loss associated with cancer therapy. Br. J. Cardiol. 2021, 28, 40. [Google Scholar] [CrossRef] [PubMed]
- Grześkowiak, A.; Rojek, A.; Szyndler, A.; Śmiałek, K.; Szczęch, R.; Chrostowska, M.; Narkiewicz, K. Prevalence of postural hypotension in patients with treated hypertension. Arter. Hypertens. 2005, 9, 452–457. Available online: https://journals.viamedica.pl/arterial_hypertension/article/view/12599 (accessed on 13 April 2024).
- Argilés, J.M.; López-Soriano, F.J.; Stemmler, B.; Busquets, S. Cancer-associated cachexia—Understanding the tumour macroenvironment and microenvironment to improve management. Nat. Rev. Clin. Oncol. 2023, 20, 250–264. [Google Scholar] [CrossRef]
- van Hateren, K.J.; Kleefstra, N.; Blanker, M.H.; Ubink-Veltmaat, L.J.; Groenier, K.H.; Houweling, S.T.; Kamper, A.M.; van der Meer, K.; Bilo, H.J. Orthostatic hypotension, diabetes, and falling in older patients: A cross-sectional study. Br. J. Gen. Pract. 2012, 62, 696–702. [Google Scholar] [CrossRef]
- Beretta, M.V.; Milan, V.B.; Hoffmeister, M.C.; Rodrigues, T.C. Orthostatic hypotension, falls and in-hospital mortality among elderly patients with and without type 2 diabetes. J. Hypertens. 2023, 41, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Tekkeşin, A.İ.; Hayıroğlu, M.İ.; Çinier, G.; Özdemir, Y.S.; İnan, D.; Yüksel, G.; Pay, L.; Parsova, K.E.; Vatanoğlu, E.G.; Şeker, M.; et al. Lifestyle intervention using mobile technology and smart devices in patients with high cardiovascular risk: A pragmatic randomised clinical trial. Atherosclerosis 2021, 319, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Asarcikli, L.D.; Hayiroglu, M.İ.; Osken, A.; Keskin, K.; Kolak, Z.; Aksu, T. Heart rate variability and cardiac autonomic functions in post-COVID period. J. Interv. Card. Electrophysiol. 2022, 63, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Hayıroğlu, M.İ.; Çınar, T.; Selçuk, M.; Çinier, G.; Alexander, B.; Doğan, S.; Çiçek, V.; Kılıç, Ş.; Atmaca, M.M.; Orhan, A.L.; et al. The significance of the morphology-voltage-P-wave duration (MVP) ECG score for prediction of in-hospital and long-term atrial fibrillation in ischemic stroke. J. Electrocardiol. 2021, 69, 44–50. [Google Scholar] [CrossRef] [PubMed]
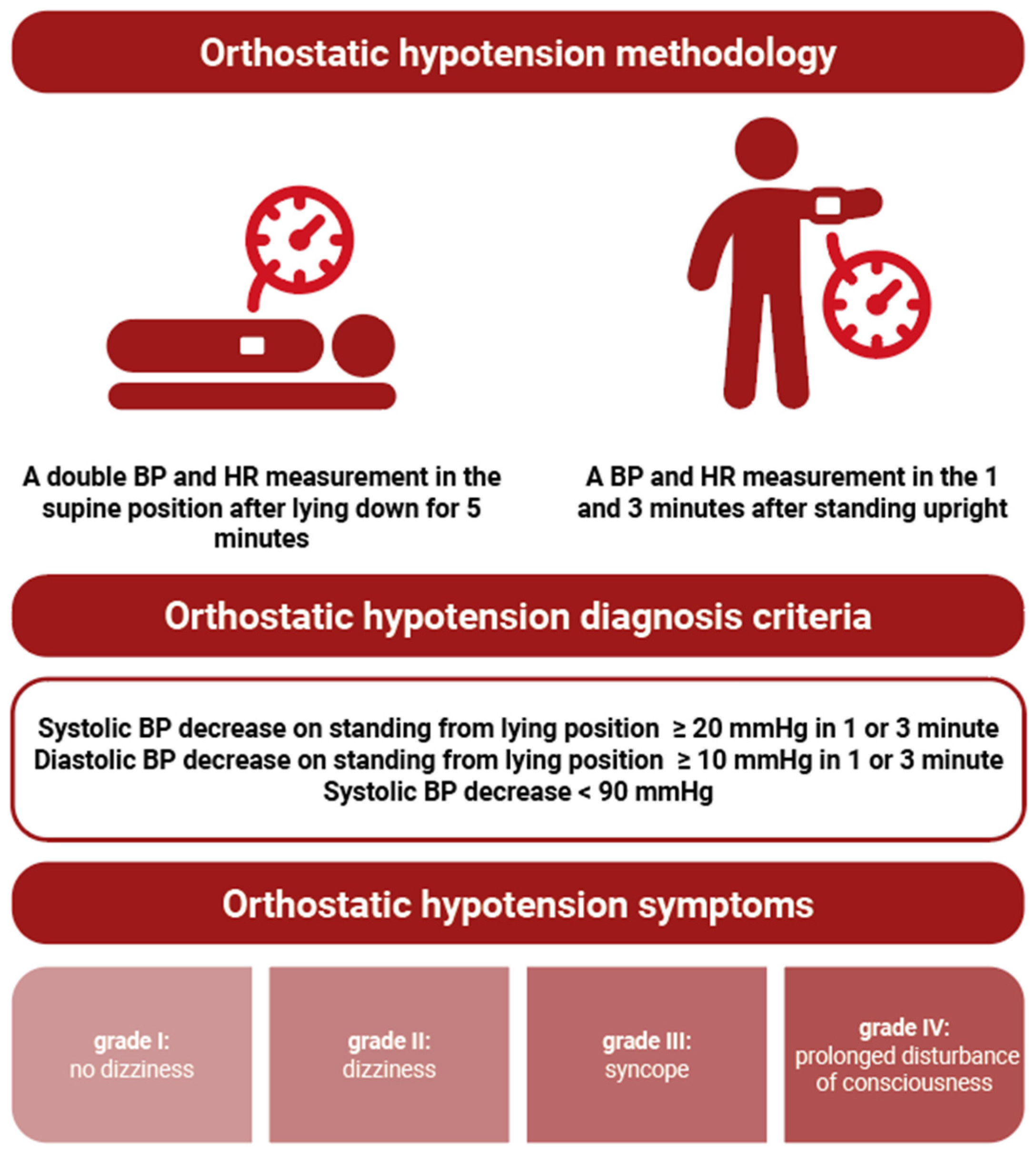

| Hospital Department | Number of Cases | % |
|---|---|---|
| Clinical Oncology | 106 | 25.8 |
| Oncological Gynecology | 58 | 14.1 |
| Radiotherapy | 56 | 13.6 |
| Neurology | 108 | 26.3 |
| Internal diseases | 49 | 11.9 |
| Dermatology | 20 | 4.9 |
| Rehabilitation | 10 | 2.4 |
| Urology | 4 | 1.0 |
| Total | 411 | 100 |
| Type of Cancer | Number of Cases | % |
|---|---|---|
| Lung cancer | 40 | 17.9 |
| Breast cancer | 16 | 7.2 |
| Male genitourinary cancers | 21 | 9.4 |
| Head and neck cancers | 26 | 11.7 |
| Gastrointestinal cancers | 52 | 23.3 |
| Gynecological cancers | 60 | 26.9 |
| Others | 8 | 3.6 |
| Total | 223 | 100 |
| Oncological (n = 223) | Non-Oncological (n = 188) | p-Value | |
|---|---|---|---|
| Age in years, mean (SD) | 65.0 (8.9) | 61.5 (12.2) | 0.01 |
| Female sex, n (%) | 116 (52.0) | 113 (60.1) | 0.09 |
| BMI (kg/m2), mean (SD) | 26.2 (5.7) | 28.1 (5.0) | 0.01 |
| SBP (SD) | 127.7 (16.6) | 129.2 (18.4) | 0.35 |
| DBP (SD) | 80.0 (10.4) | 79.3 (9.7) | 0.42 |
| HR (SD) | 69.4 (12.4) | 78.4 (14.0) | 0.001 |
| Orthostatic hypotension, n (%) | 64 (28.7) | 31 (16.5) | 0.003 |
| Orthostatic hypotension symptoms: | 0.00001 | ||
| grade I, n (%) | 198 (88.8) | 187 (99.5) | |
| grade II, n (%) | 25 (11.2) | 1 (0.5) | |
| grade III, n (%) | 0 | 0 | |
| grade IV, n (%) | 0 | 0 | |
| Hypertension, n (%) | 114 (51.1) | 98 (52.1) | 0.83 |
| CAD, n (%) | 22 (9.9) | 21 (11.2) | 0.66 |
| Stroke, n (%) | 5 (2.2) | 9 (4.8) | 0.15 |
| Dyslipidaemia, n (%) | 25 (11.2) | 43 (22.9) | 0.001 |
| Diabetes, n (%) | 38 (17.0) | 48 (25.5) | 0.03 |
| CKD, n (%) | 5 (2.2) | 7 (3.7) | 0.37 |
| Thyroid diseases, n (%) | 19 (8.5) | 20 (10.6) | 0.46 |
| VTE, n (%) | 15 (6.7) | 7 (3.7) | 0.17 |
| PD, n (%) | 1 (0.5) | 3 (1.6) | 0.23 |
| Diuretics, n (%) | 42 (18.8) | 27 (14.4) | 0.22 |
| ACEi, n (%) | 59 (26.5) | 56 (29.8) | 0.45 |
| ARBs, n (%) | 20 (9.0) | 22 (11.7) | 0.36 |
| Beta-blockers, n (%) | 82 (36.8) | 60 (31.9) | 0.30 |
| Nitrates, n (%) | 10 (4.5) | 6 (3.2) | 0.49 |
| Alpha-blockers, n (%) | 5 (2.2) | 6 (3.2) | 0.55 |
| CCB, n (%) | 34 (15.3) | 38 (20.2) | 0.18 |
| Antiparkinsonian agents, n (%) | 1 (0.5) | 3 (1.6) | 0.23 |
| Variable | Single-Variable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Cancer | 2.04 | 1.26–3.31 | 0.003 | 2.06 | 1.24–3.43 | 0.005 |
| Age ≥ 65 | 2.45 | 1.44–4.18 | 0.0002 | - | - | - |
| Male sex | 1.31 | 0.83–2.08 | 0.24 | - | - | - |
| BMI ≥ 30 kg/m2 | 0.47 | 0.26–0.82 | 0.007 | 0.40 | 0.22–0.72 | 0.002 |
| Hypertension | 1.47 | 0.92–2.35 | 0.10 | - | - | - |
| Diabetes | 1.61 | 0.94–2.74 | 0.07 | 1.90 | 1.06–3.40 | 0.03 |
| Stroke | 2.60 | 0.87–7.70 | 0.08 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwański, M.A.; Sokołowska, A.; Sokołowski, A.; Wojdyła, R.; Styczkiewicz, K. The Prevalence of Orthostatic Hypotension in Cancer Patients. Cancers 2024, 16, 1541. https://doi.org/10.3390/cancers16081541
Iwański MA, Sokołowska A, Sokołowski A, Wojdyła R, Styczkiewicz K. The Prevalence of Orthostatic Hypotension in Cancer Patients. Cancers. 2024; 16(8):1541. https://doi.org/10.3390/cancers16081541
Chicago/Turabian StyleIwański, Mateusz A., Aldona Sokołowska, Andrzej Sokołowski, Roman Wojdyła, and Katarzyna Styczkiewicz. 2024. "The Prevalence of Orthostatic Hypotension in Cancer Patients" Cancers 16, no. 8: 1541. https://doi.org/10.3390/cancers16081541
APA StyleIwański, M. A., Sokołowska, A., Sokołowski, A., Wojdyła, R., & Styczkiewicz, K. (2024). The Prevalence of Orthostatic Hypotension in Cancer Patients. Cancers, 16(8), 1541. https://doi.org/10.3390/cancers16081541






