Disease Perception Is Correlated with Health-Related Quality of Life in Patients Suffering from Myelodysplastic Syndromes: Results of the Belgian Be-QUALMS Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. HRQOL Measurement
2.3. Illness Perception Measurement
2.4. Demographic and Clinical Parameters
2.5. Statistical Analysis
3. Results
3.1. Patient and Disease Characteristics
3.2. Patients with MDS Suffer from Impairment in HRQoL That Remains Stable during Treatment
3.3. Disease Perception Does Not Change during the Course of Treatment
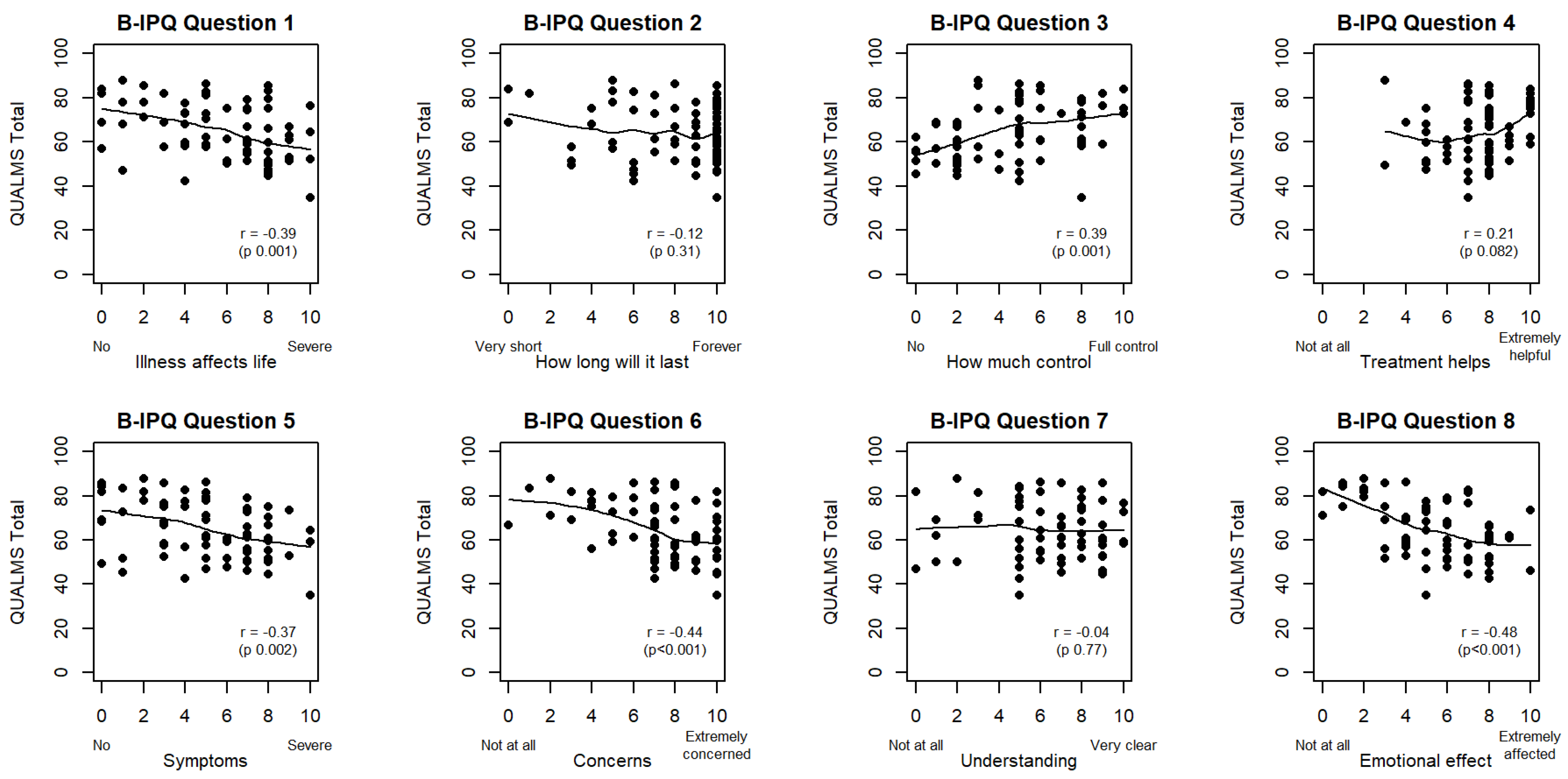
3.4. Disease Perception Is Correlated with Health-Related Quality of Life
3.5. No Significant Relation Was Found between HRQoL and Explored Interventions or Treatment Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meers, S. The myelodysplastic syndromes: The era of understanding. Eur. J. Haematol. 2015, 94, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.M. Why are myelodysplastic syndromes unrecognized and underdiagnosed? A primary care perspective. Am. J. Med. 2012, 125, S15–S17. [Google Scholar] [CrossRef] [PubMed]
- Oliva, E.N.; Platzbecker, U.; Fenaux, P.; Garcia-Manero, G.; LeBlanc, T.W.; Patel, B.J.; Kubasch, A.S.; Sekeres, M.A. Targeting health-related quality of life in patients with myelodysplastic syndromes—Current knowledge and lessons to be learned. Blood Rev. 2021, 50, 100851. [Google Scholar] [CrossRef] [PubMed]
- Efficace, F.; Gaidano, G.; Breccia, M.; Voso, M.T.; Cottone, F.; Angelucci, E.; Caocci, G.; Stauder, R.; Selleslag, D.; Sprangers, M.; et al. Prognostic value of self-reported fatigue on overall survival in patients with myelodysplastic syndromes: A multicentre, prospective, observational, cohort study. Lancet Oncol. 2015, 16, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Heyrman, B. Quality of Life in Low-Risk MDS: An Undervalued Endpoint. J. Clin. Med. 2022, 11, 5699. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, E.; Wilkes, C.; Koschwanez, H.; Weinman, J.; Norton, S.; Petrie, K.J. A systematic review and meta-analysis of the Brief Illness Perception Questionnaire. Psychol. Health 2015, 30, 1361–1385. [Google Scholar] [CrossRef] [PubMed]
- Ashley, L.; Marti, J.; Jones, H.; Velikova, G.; Wright, P. Illness perceptions within 6 months of cancer diagnosis are an independent prospective predictor of health-related quality of life 15 months post-diagnosis. Psycho-Oncology 2015, 24, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Thong, M.S.Y.; Kaptein, A.A.; Vissers, P.A.J.; Vreugdenhil, G.; van de Poll-Franse, L.V. Illness perceptions are associated with mortality among 1552 colorectal cancer survivors: A study from the population-based PROFILES registry. J. Cancer Surviv. 2016, 10, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Abel, G.A.; Efficace, F.; Buckstein, R.J.; Tinsley, S.; Jurcic, J.G.; Martins, Y.; Steensma, D.P.; Watts, C.D.; Raza, A.; Lee, S.J.; et al. Prospective international validation of the Quality of Life in Myelodysplasia Scale (QUALMS). Haematologica 2016, 101, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Efficace, F.; Koinig, K.A.; Cottone, F.; Bowen, D.; Mittelman, M.; Langemeijer, S.M.C.; Culligan, D.; Filanovsky, K.; Abel, G.A.; Storck, M.; et al. Validation of the Qualms Questionnaire to Assess Health-Related Quality of Life in European and Israeli Patients with Myelodysplastic Syndromes: Results from the MDS-Right Project. Blood 2021, 138, 1982. [Google Scholar] [CrossRef]
- Broadbent, E.; Petrie, K.J.; Main, J.; Weinman, J. The brief illness perception questionnaire. J. Psychosom. Res. 2006, 60, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Karataş, T.; Özen, Ş.; Kutlutürkan, S. Factor Structure and Psychometric Properties of the Brief Illness Perception Questionnaire in Turkish Cancer Patients. Asia Pac. J. Oncol. Nurs. 2017, 4, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.M.; Xiao, H.; LeBlanc, M.; Hershman, D.L.; Blanke, C.D. Cancer Clinical Trial Participation at the 1-Year Anniversary of the Outbreak of the COVID-19 Pandemic. JAMA Netw. Open. 2021, 4, e2118433. [Google Scholar] [CrossRef] [PubMed]
- Stauder, R.; Yu, G.; Koinig, K.A.; Bagguley, T.; Fenaux, P.; Symeonidis, A.; Sanz, G.; Cermak, J.; Mittelman, M.; Hellström-Lindberg, E.; et al. Health-related quality of life in lower-risk MDS patients compared with age- and sex-matched reference populations: A European LeukemiaNet study. Leukemia 2018, 32, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Stanworth, S.J.; Killick, S.; McQuilten, Z.K.; Karakantza, M.; Weinkove, R.; Smethurst, H.; Pankhurst, L.A.; Hodge, R.L.; Hopkins, V.; Thomas, H.L.; et al. Red cell transfusion in outpatients with myelodysplastic syndromes: A feasibility and exploratory randomised trial. Br. J. Haematol. 2020, 189, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Harnan, S.; Ren, S.; Gomersall, T.; Everson-Hock, E.S.; Sutton, A.; Dhanasiri, S.; Kulasekararaj, A. Association between Transfusion Status and Overall Survival in Patients with Myelodysplastic Syndromes: A Systematic Literature Review and Meta-Analysis. Acta Haematol. 2016, 136, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.L. The impact of myelodysplastic syndromes on quality of life: Lessons learned from 70 voices. J. Support. Oncol. 2012, 10, 37–44. [Google Scholar] [CrossRef] [PubMed]


| B-IPQ Item | Baseline (n = 67) | Week 4 (n = 59) | Week 12 (n = 45) | Week 24 (n = 35) | |
|---|---|---|---|---|---|
| How much does your illness affect your life? (“0” no effects at all—severely affects my life “10”) | Mean (sd) | 5.8 (2.8) | 5.7 (2.9) | 5.9 (2.8) | 6.1 (3.1) |
| How long do you think your illness will continue? (“0” a very short time—forever “10”) | Mean (sd) | 7.8 (2.7) | 8.0 (2.3) | 7.4 (3.3) | 8.6 (2.4) |
| How much control do you feel you have over your illness? (“0” absolutely no control—extreme amount of control “10”) | Mean (sd) | 4.5 (2.9) | 4.8 (2.8) | 5.4 (2.8) | 4.5 (3.1) |
| How much do you think your treatment can help your illness? (“0” not at all—extremely helpful “10”) | Mean (sd) | 7.5 (1.7) | 7.8 (1.6) | 8.0 (1.5) | 7.5 (2.8) |
| How much do you experience symptoms from your illness? (“0” no symptoms at all—many severe symptoms “10”) | Mean (sd) | 5.0 (2.8) | 4.1 (2.8) | 4.2 (2.7) | 3.9 (2.6) |
| How concerned are you about your illness? (“0” not at all concerned—extremely concerned “10”) | Mean (sd) | 7.1 (2.4) | 6.6 (2.8) | 6.7 (2.6) | 6.9 (2.9) |
| How well do you feel you understand your illness? (“0” don’t understand at all—understand very clearly “10”) | Mean (sd) | 6.4 (2.5) | 6.6 (2.9) | 6.2 (2.6) | 5.7 (3.2) |
| How much does your illness affect you emotionally (“0” not at all affected emotionally—extremely affected emotionally “10”) | Mean (sd) | 5.4 (2.4) | 5.3 (3.1) | 5.4 (3.1) | 5.3 (3.0) |
| QUALMS Final | QUALMS Emotional | QUALMS Benefit | QUALMS Burden | |||||
|---|---|---|---|---|---|---|---|---|
| B-IPQ1 (life-affecting) | r = −0.39 (p = 0.001) | ** | r = −0.27 (p = 0.026) | * | r = 0.28 (p = 0.022) | * | r = −0.48 (p < 0.001) | *** |
| B-IPQ2 (duration) | r = −0.12 (p = 0.31) | r = −0.08 (p = 0.50) | r = 0.29 (p = 0.018) | * | r = −0.18 (p = 0.14) | |||
| B-IPQ3 (control) | r = 0.39 (p = 0.001) | ** | r = 0.36 (p = 0.003) | ** | r = −0.04 (p = 0.74) | r = 0.35 (p = 0.004) | ** | |
| B-IPQ4 (treatment) | r = 0.21 (p = 0.082) | ˙ | r = 0.16 (p = 0.202) | r = 0.16 (p = 0.19) | r = 0.14 (p = 0.25) | |||
| B-IPQ5 (symptoms) | r = −0.37 (p = 0.002) | ** | r = −0.27 (p = 0.028) | * | r = 0.14 (p = 0.25) | r = −0.43 (p < 0.001) | *** | |
| B-IPQ6 (concerns) | r = −0.44 (p < 0.001) | *** | r = −0.50 (p < 0.001) | *** | r = 0.17 (p = 0.17) | r = −0.30 (p = 0.014) | * | |
| B-IPQ7 (understanding) | r = −0.04 (p = 0.77) | r = −0.15 (p = 0.21) | r = 0.38 (p = 0.002) | ** | r = −0.07 (p = 0.55) | |||
| B-IPQ8 (emotional) | r = −0.48 (p < 0.001) | *** | r = −0.39 (p = 0.001) | ** | r = 0.14 (p = 0.27) | r = −0.42 (p < 0.001) | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heyrman, B.; Meers, S.; De Becker, A.; Wouters, K.; Van Hoof, A.; Van De Velde, A.; Graux, C.; Mazure, D.; Selleslag, D.; Maes, H.; et al. Disease Perception Is Correlated with Health-Related Quality of Life in Patients Suffering from Myelodysplastic Syndromes: Results of the Belgian Be-QUALMS Study. Cancers 2023, 15, 3296. https://doi.org/10.3390/cancers15133296
Heyrman B, Meers S, De Becker A, Wouters K, Van Hoof A, Van De Velde A, Graux C, Mazure D, Selleslag D, Maes H, et al. Disease Perception Is Correlated with Health-Related Quality of Life in Patients Suffering from Myelodysplastic Syndromes: Results of the Belgian Be-QUALMS Study. Cancers. 2023; 15(13):3296. https://doi.org/10.3390/cancers15133296
Chicago/Turabian StyleHeyrman, Bert, Stef Meers, Ann De Becker, Kristien Wouters, Achiel Van Hoof, Ann Van De Velde, Carlos Graux, Dominiek Mazure, Dominik Selleslag, Helena Maes, and et al. 2023. "Disease Perception Is Correlated with Health-Related Quality of Life in Patients Suffering from Myelodysplastic Syndromes: Results of the Belgian Be-QUALMS Study" Cancers 15, no. 13: 3296. https://doi.org/10.3390/cancers15133296
APA StyleHeyrman, B., Meers, S., De Becker, A., Wouters, K., Van Hoof, A., Van De Velde, A., Graux, C., Mazure, D., Selleslag, D., Maes, H., Lemmens, J., Beckers, M., Breems, D., Sid, S., Berneman, Z., & Anguille, S. (2023). Disease Perception Is Correlated with Health-Related Quality of Life in Patients Suffering from Myelodysplastic Syndromes: Results of the Belgian Be-QUALMS Study. Cancers, 15(13), 3296. https://doi.org/10.3390/cancers15133296






