Simple Summary
Most cancer patients are older and have concomitant diseases because the incidence of most cancer types increases with age. This leads to patients taking a variety of medications that can cause drug-related problems (DRPs). DRPs can cause harm, including increased illness, avoidable hospital stays, and even death. Common DRPs are drug–drug interactions, not taking medication as prescribed, and adverse drug reactions. In our study, we aimed to assess these medication risks in hospitalized cancer patients and to identify factors that influence their health-related quality of life (HRQOL) as a patient-relevant outcome. The results of the pharmacist-led medication reviews show that DRPs are common in hospitalized cancer patients. Therefore, patient questionnaires about therapy-related symptoms could improve the detection of DRPs. While drug-related factors had no effect on HRQOL during the hospital stay, our analysis revealed other influencing factors, such as relapse status of the cancer disease and length of hospital stay.
Abstract
Background: We aimed to assess medication risks and determine factors influencing the health-related quality of life (HRQOL) in cancer inpatients. Methods: A retrospective analysis was conducted to identify drug-related problems (DRPs) based on medication reviews, including patient-reported outcomes (PROs). Multiple linear regression analyses were performed to identify sociodemographic, disease-related, and drug therapy-related factors influencing changes from hospital admission to discharge in the scales of the EORTC QLQ-C30 questionnaire. Results: A total of 162 inpatients with various hematological and solid cancer diseases was analyzed. Patients received a mean of 11.6 drugs and 92.6% of patients exhibited polymedication resulting in a mean of 4.0 DRPs per patient. Based on PRO data, 21.5% of DRPs were identified. Multiple linear regression models described the variance of the changes in global HRQOL and physical function in a weak-to-moderate way. While drug therapy-related factors had no influence, relapse status and duration of hospital stay were identified as significant covariates for global HRQOL and physical function, respectively. Conclusion: This analysis describes underlying DRPs in a German cancer inpatient population. PROs provided valuable information for performing medication reviews. The multiple linear regression models for global HRQOL and physical function provided explanations for changes during hospital stay.
1. Introduction
Preventing medication risks in cancer patients represents a major challenge. Because the incidence of most tumor entities rises with age, most cancer patients are older and present comorbidity [1]. Compared to the general population, the comorbidity burden of cancer patients is higher [2]. With increasing comorbidity, the prevalence of polymedication also rises [3]. The common definition of polymedication is the use of five or more medications [4]. Hyperpolymedication is defined as the intake of 10 or more medicines [5]. Studies indicate that polymedication affects between 57% and 84% of older cancer patients and is associated with major risks [3,6,7,8,9,10]. Relations between polymedication and a range of health outcomes including adverse drug reactions (ADRs), falls, frailty, hospitalization, postoperative complications, and mortality were described [11,12,13,14].
One major reason for these negative outcomes upon polymedication are drug-related problems (DRPs), which are very common, especially in older cancer patients [15,16]. A DRP is defined as an event during pharmacotherapy which interferes with a desired health outcome [17]. The number of reported DRPs in cancer patients ranges between three and five per patient [6,18,19,20]. DRPs can lead to increased morbidity, unnecessary hospital admissions, and mortality [15,21]. Common DRPs are potential drug–drug interactions (DDIs), ADRs, and non-adherence [15,22]. A study on cancer patients found that 22.9% of admissions to the intensive care unit were associated with an ADR and the mortality rate of the admitted patients was 28.1% [23]. After being treated in a hospital, cancer patients are frequently readmitted to the hospital within 30 days and in almost 10% of cases, potentially because of a DRP [22].
In order to minimize the risks described for cancer patients, medication management was developed, a co-operation of all healthcare professionals involved in the medication process. The patient’s overall medication regimen is analyzed for DRPs (medication review), followed by multiprofessional care of the patient focusing on predefined treatment goals. According to the available information, three types of medication reviews can be defined: simple medication review (type 1), advanced medication review (type 2a or 2b), and complete medication review (type 3). The simple medication review (type 1) is based on medication data and basic patient data. The advanced medication review is additionally supported by a patient interview (type 2a) or clinical data, including diagnoses and laboratory parameters (type 2b). The complete medication review (type 3) is based on all the abovementioned sources of information [24].
The effect of pharmacist interventions on adult outpatients with cancer was summarized in several systematic reviews with the result that the interventions could improve outcome measures like rates of nausea, vomiting and pain control, medication adherence, patient satisfaction, quality of life, and cost savings [25,26,27,28]. A positive effect on the number of DRPs, clinical outcomes, and care processes was also shown in several studies [6,18,29].
Healthcare professionals can gain important information about their patients by assessing patient-reported outcomes (PROs) [30]. Systematic monitoring of PROs is associated with improved patient–clinician communication, clinician awareness of symptoms, symptom management, and patient satisfaction [31,32,33,34,35]. Furthermore, patient-relevant outcomes such as health-related quality of life (HRQOL) and overall survival can be improved [35,36]. Therefore, it is crucial to incorporate PROs into health care interventions, such as medication reviews, and consider HRQOL as a patient-relevant outcome for evaluation.
Most research in the field of medication safety in oncology was conducted in an outpatient setting and focuses on long-term effects of pharmaceutical care interventions. For inpatient cancer care, information about frequent DRPs and the effect of medication reviews and medication management is still limited. It is conceivable that inpatient cancer patients are exposed to distinct medication risks and that pharmaceutical interventions are associated with different challenges compared to the outpatient setting.
With this analysis, we aimed to assess medication risks in cancer inpatients and determine sociodemographic, disease-related, and drug therapy-related factors influencing HRQOL in oncology inpatients. A scheme of the study framework is shown in Figure 1.
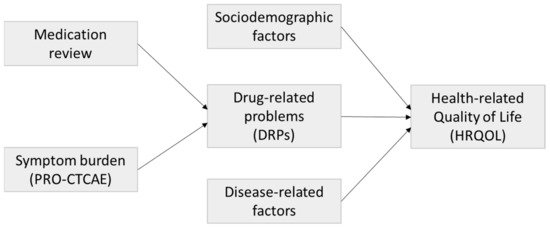
Figure 1.
Study framework.
2. Materials and Methods
2.1. Study Design
This project was a post-hoc conducted, but in advance planned, retrospective analysis including cancer inpatients of the database created during a randomized controlled trial conducted at four oncology wards of Helios hospitals in Berlin between July 2017 and February 2019 [37]. The primary aim of the underlying study was to evaluate the feasibility of an electronic patient-reported outcome (ePRO) assessment in inpatient cancer care [37]. The patients were assigned to three groups: intervention group A (ePRO assessment with presentation of patient answers to physicians and the opportunity to adapt the therapy in response), control group B (ePRO assessment without presentation of patient answers to physicians), and control group C (paper-based PRO assessment without presentation of patient answers to physicians).
In this retrospective secondary patient data analysis, medication risks were detected with the help of medication reviews, including PRO data. Subsequently, sociodemographic, disease-related, and drug therapy-related factors influencing changes in the different dimensions of HRQOL from hospital admission to discharge were determined.
The Ethics Committee of the Chamber of Physicians in Berlin, Germany, approved the study (Eth-48/16). It was carried out in accordance with the applicable German and European legal provisions as well as the Declaration of Helsinki [38].
Patients with hematological or oncological cancer entities of at least 18 years were included in the study. The planned inpatient stay had to be at least three days. Investigators recruited patients on the day of inpatient admission. Patients were informed about the study and signed a written informed consent. Patients with no or insufficient documentation of their medication within the study database were excluded from the secondary analysis. All data were anonymized before analysis.
2.2. Patient Documentation
For this secondary analysis, relevant patient data were extracted from the study database after the end of the study. The time-points of documentation are shown in Figure 2.
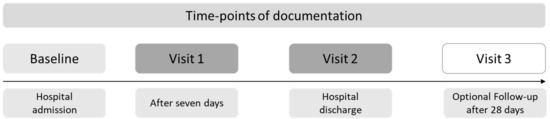
Figure 2.
Time-points of documentation of the clinical trial. Visit 1 was only performed for patients with a hospital stay of at least seven days.
Baseline documentation was undertaken at hospital admission. The following data were documented: eligibility criteria, sociodemographic parameters, and disease-anamnestic data on cancer diagnosis and concomitant diseases.
The following data were documented independent of time during the hospital stay: current therapeutic regimen and tumor therapy, concomitant medication for comorbidity, supportive drug therapy, laboratory data, and vital parameters.
2.3. Patient-Reported Outcomes
At baseline, visit 1, visit 2, and visit 3, symptom burden and health-related quality of life were assessed as ePROs via a tablet-based digital solution (group A and B) or paper-based (group C).
The symptom burden was evaluated using the PRO version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). The PRO-CTCAE item library was developed as a complementary tool to the CTCAE criteria by the US National Cancer Institute [39]. It consists of 124 items representing 78 symptomatic adverse events. The items characterize up to three symptom attributes regarding severity, frequency, and interference with daily activities [40]. Answers are requested on a verbal five-point Likert scale and refer to the last seven days. A German translation is available [41]. In this study, a validated German PRO-CTCAE core item set for patients with chemotherapy containing 31 items was used [42]. PRO-CTCAE symptom scores were calculated using the following equations. First, the raw score (RS), indicating the mean value of the symptom attributes, was calculated with Equation (1) if at least 50% of attributes were answered [43]:
- I1 = Value of item 1;
- I2 = Value of item 2;
- In = Value of item n;
- n = Number of items per scale.
Second, the raw score was linearly transformed to numerical score values ranging from 0 to 100 using Equation (2) [34]; higher values indicate a higher severity of the symptom:
Range = Difference between maximum and minimum values of the response scale (0 to 4).
HRQOL was assessed using the Quality-of-Life Questionnaire-Core 30 (QLQ-C30) Version 3.0 of the European Organization for Research and Treatment of Cancer (EORTC) [44]. The questionnaire is validated and a certified German translation is available [44,45]. It encompasses the global HRQOL scale, five functional subscales, and nine symptom scales, with a total of 30 items. Scoring was performed using the EORTC QLQ-C30 scoring manual [43]. Higher scores on the functioning subscales and the global HRQOL indicate better outcomes, whereas higher values on the symptom scales indicate a higher symptom burden.
2.4. Analysis of Medication Risks
Medication risks were recorded as DRPs. The Pharmaceutical Care Network Europe Association (PCNE) defines a DRP as follows: “A Drug-Related Problem is an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes” [17]. According to PCNE, a medication review is a “structured evaluation of a patient‘s medicines with the aim of optimising medicines use and improving health outcomes. This entails detecting drug-related problems and recommending interventions” [46]. Based on medication and clinical data, retrospective advanced medication reviews of type 2b were conducted to identify DRPs [24]. Some DRPs such as administration problems and non-adherence can only be detected in a complete medication review type 3, for which additional patient interviews would be mandatory. Instead, patients reported their symptoms under therapy using the PRO-CTCAE questionnaires. In this analysis, this source of information was used to complete the medication reviews in the absence of patient interviews to detect a broader spectrum of DRPs. The DRPs identified by PRO-CTCAE are henceforth referred to as PRO-DRPs in the following. Because therapy-related symptoms are an inherent part of tumor therapy, only a severe symptom burden with a PRO-CTCAE symptom score of at least 75 was considered as a DRP.
The DRP categories considered in the medication reviews are shown in Table 1.

Table 1.
Categories of drug-related problems (DRPs) in medication reviews of type 2b [24].
In general, a DRP was only recorded if a pharmaceutical intervention, as indicated by a therapy recommendation, would have been required [47].
2.5. Statistical Analysis
For data entry and the processing and statistical analysis of the anonymized data, Microsoft Office® Professional Plus 2019 (Microsoft Corporation, Redmond, WA, USA), IBM SPSS® Statistics 27.0 (IBM Corporation, Armonk, NY, USA), and R 4.0.5 (The R Foundation for Statistical Computing, Vienna, Austria) were used.
Descriptive statistics were performed for patient characteristics, medication data, DRPs, and PROs. Mean values with standard deviations (SDs) or the median with interquartile range (IQR) were calculated, as applicable. Frequencies were described as absolute numbers and percentages [48,49]. In the exploratory analysis, a p-value of <0.05 was considered as statistically significant. Confidence intervals (CIs) of 95% were calculated.
Associations between changes in global HRQOL and the subscales physical function, cognitive function, and emotional function of the EORTC QLQ-C30 questionnaire from baseline to hospital discharge (visit 2) with several parameters were investigated by multiple linear regression models. The following prespecified independent variables were included in the exploratory regression analysis: study group (A, B, C), age (years), gender (male, female), educational level (high, low), hospital stay (days), cancer type (solid, hematological), time since cancer diagnosis (months), relapse status (yes, no), ECOG status (0, 1, 2, 3), concomitant diseases (number), drugs (number), DRPs (number), and PRO-DRPs (number). The goodness-of-fit of the multiple linear regression model was evaluated by using R2 and adjusted R2. It can be interpreted according to Cohen [50].
3. Results
3.1. Study Population
In total, 185 patients were included in the underlying clinical trial. Of these, 18 patients dropped out of the study. Five patients had to be excluded from the secondary data analysis because of missing documentation in the study database. Thus, the target sample for the secondary data analysis resulted in 162 patients.
The median age of the patients was 65.5 years (IQR: 18, range: 19–88) and 86 patients (53.1%) were at least 65 years old. The proportion of female patients was 56.2% (n = 91). The median hospital stay was four days (IQR: 7, range: 1–73). Most patients (n = 103, 63.6%) had a solid tumor disease. Tumor entities presented in at least 5% of patients were lymphoma (n = 31, 19.1%), sarcoma (n = 20, 12.3%), rectal cancer (n = 16, 9.9%), leukemia (n = 15, 9.3%), multiple myeloma (n = 12, 7.4%), esophageal cancer (n = 11, 6.8%), pancreatic cancer (n = 10, 6.2%), and colon cancer (n = 9, 5.6%). No relapse of their tumor disease occurred in 123 patients (75.9%). Four months represented the median time since the first diagnosis of cancer (IQR: 13.5, range: 0–208). According to their ECOG status, most patients had a rather good physical condition reflected by 87 patients (53.8%) with an ECOG status of 0 and 53 patients (32.7%) with an ECOG status of 1. The mean number of concomitant diseases listed in the Charlson Comorbidity Index (CCI) was 0.97 (SD: 1.25, range 0–6). Concomitant diseases present in at least 5% of patients were mild/severe kidney disease (18.5%), chronic pulmonary disease (13.6%), secondary tumor disease (11.1%), secondary metastatic tumor disease (10.5%), diabetes mellitus with organ damage (10.5%), diabetes mellitus without organ damage (8.0%), and heart failure (8.0%). A mean of 11.6 drugs per patient (SD: 5.15, range: 2–26, median: 11, IQR: 7), including cancer treatment and supportive/concomitant medication, was administered. Patients with solid tumor diseases (mean: 12.8, SD: 5.08, range: 3–26, median: 12, IQR: 7) received a higher number of drugs than patients with hematological diseases (mean: 9.7, SD: 4.54, range: 2–21, median: 9, IQR: 8). In total, 1884 drugs were administered to patients. Figure 3 shows the drug classes used according to their Anatomical Therapeutical Chemical (ATC) code level 1. Table S1 shows the drugs according to ATC code level 2 as well. ATC drug classes level 2 with more than 5% of drugs used were in group A (alimentary system and metabolism) remedies for acid-related diseases (A02; n = 113, 6.0%) and antiemetics/anti-nausea agents (A04; n = 168, 8.9%), in group B (blood and hematopoietic organs) antithrombotic agents (B01; n = 100, 5.3%), in group H (systemic hormone preparations excluding sexual hormones and insulin) corticosteroids for systematic use (H02; n = 136, 7.2%), in group L (antineoplastic and immunomodulatory agents) antineoplastic agents (L01; n = 372, 19.7%), and in group N (nervous system) analgesics (N02, n = 111, 5.9%). Polymedication with five or more drugs occurred in 150 patients (92.6%) during their hospital stay, and 98 patients (60.5%) exhibited hyperpolymedication with 10 or more drugs.
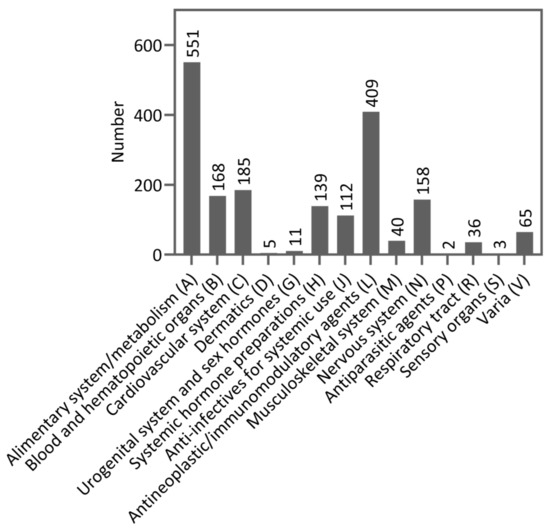
Figure 3.
Drug classes categorized by Anatomical Therapeutical Chemical (ATC) code level 1 (n = 1884).
3.2. Drug-Related Problems
The mean number of DRPs was 4.0 per patient (SD: 2.97, range: 0–13, median: 3.0, IQR: 4) during the hospital stay. Patients with solid tumor diseases (mean: 3.2, SD: 2.17, range: 0–10, median: 3, IQR: 4) experienced a lower number of DRPs than patients with hematological diseases (mean: 5.3, SD: 3.51, range: 0–13, median: 4, IQR: 5). Table 2 shows the number of DRPs per category. The three DRP categories indication without drug, inappropriate drug choice, and adverse drug reaction, are among the five most common DRP categories across the three patient groups (all patients, patients with solid tumor diseases, and patients with hematological tumor diseases).

Table 2.
Number of drug-related problems (DRPs) per category for all patients (n = 162) and subgroups of patients with solid (n = 103) and hematological (n = 58) tumor diseases.
Of the 641 in total detected DRPs, 138 DRPs (21.5%) could be detected by a PRO-CTCAE symptom score of at least 75 (PRO-DRP). At the time-points baseline and visit 2 (hospital discharge), the number of severe symptoms per patient was calculated, as these questionnaires were administered to every patient in the study. At baseline, 1.47 (SD: 2.03, range: 0–12, median: 1, IQR: 2) severe symptoms with a score of at least 75 occurred per patient. At visit 2, the number was 1.58 (SD: 2.32, range: 0–10, median: 1, IQR: 2). Across all visits, fatigue (26.5%) was the most often-occurring severe patient-reported symptom, followed by decreased appetite (17.4%) and insomnia (15.3%).
3.3. Determinants of Health-Related Quality of Life
The changes in the scales could not be calculated in 5.6% (n = 9) of cases for the global HRQOL and cognitive and emotional function, and in 6.2% (n = 10) of cases for the physical function due to missing values. The distribution of the changes in global HRQOL and physical function from baseline to hospital discharge is shown in the histograms of Figure 4. For histograms of cognitive function and emotional function, see Figures S1 and S2.
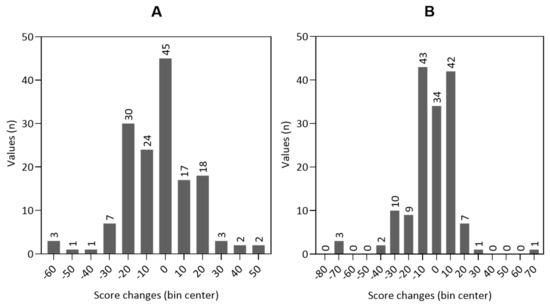
Figure 4.
Histograms of the distribution of the score changes from baseline to hospital discharge in global health-related quality of life ((A) n = 153) and physical function ((B) n = 152).
The results of the multiple linear regression models on the change in global HRQOL and the physical function subscale of the EORTC QLQ-C30 questionnaire are shown in Table 3 and Table 4.

Table 3.
Results of the multiple linear regression analysis on the change in the global HRQOL scale of the EORTC QLQ-C30 questionnaire from baseline to hospital discharge.

Table 4.
Results of the multiple linear regression analysis on the change in the physical function scale of the EORTC QLQ-C30 questionnaire from baseline to hospital discharge.
The model for global HRQOL described a significant variance of the change in the global HRQOL scale (p = 0.031), but with a weak-to-moderate variance explanation: the included independent variables explained 8.6% of the variance of the dependent variable (R2 = 0.184, adjusted R2 = 0.086). While drug therapy-related factors (“Drugs”, “DRPs”, and “PRO-DRPs”) had no influence, the variable “Relapse status” significantly influenced the change in global HRQOL (p = 0.013). Patients without a current relapse of their tumor disease showed on average an increase of 11.06 points on the global HRQOL scale of the EORTC QLQ-C30 questionnaire from baseline to hospital discharge.
The model for physical function described the variance significantly (p = 0.009) and with a weak-to-moderate variance explanation as well. The independent variables explained 11.6% of the variance of the dependent variable (R2 = 0.211, adjusted R2 = 0.116). While drug therapy-related factors (“Drugs”, “DRPs”, and “PRO-DRPs”) had no influence, “Hospital stay” had a significant influence on the change in physical function (p = 0.009). With every additional day that the patients stayed in hospital, the physical function scale decreased on average by 0.48 points.
The models for cognitive function (p = 0.122) and emotional function (p = 0.210) did not describe a significant variance of the changes in the scales.
4. Discussion
With this analysis, we aimed to assess medication risks and determine factors influencing the HRQOL in oncology inpatients. The results can contribute to the development of supportive care concepts for cancer inpatients.
The underlying clinical trial was conducted to evaluate the feasibility of a multidimensional electronic PRO (ePRO) system and to implement it in an inpatient oncology setting. The primary analysis indicates that the ePRO tool is feasible, but symptom burden and global HRQOL did not change significantly between intervention group A and control group B. These findings suggest that physicians did not respond adequately to the ePRO measures because there was no predefined supportive care concept for the study [37].
4.1. Medication Risks
The patients received a mean of 11.6 drugs per patient, including cancer treatment, supportive, and concomitant medication. This number includes drugs used only for a short duration (e.g., antiemetic prophylaxis). Because drugs were documented independent of time at discharge, not all drugs were necessarily administered concurrently and no differentiation in drugs before and after hospital admission was possible. This approach may overestimate the number of drugs. Nevertheless, every administered drug can cause a DRP. The number of drugs per patient corresponds to the findings of most comparable studies [6,18,19], which is also true for the number of patients with polymedication. In contrast, the number of patients with hyperpolymedication is slightly higher in this study [3,6,7]. Furthermore, a recent prospective study by Lavan et al. on older cancer out- and inpatients observed lower numbers of drugs than our study. A median of seven drugs was regularly prescribed to patients, resulting in 61% of patients with polymedication and 18% of patients with hyperpolymedication [10]. The difference may reflect the more frequent prescription of drugs in hospitals in addition to the regularly used drugs and emphasizes the drug-related risks of cancer inpatients.
DRPs amounted to 4.0 DRPs per patient. This number was lower, compared to a retrospective study by Vucur et al. on head and neck cancer outpatients. DRPs per patient ranged from 4.8 (first therapy cycle) to 6.9 (fifth therapy cycle) [19]. However, our findings correspond to the studies of Nightingale et al. and Tan et al., with three DRPs per patient, and Edwards et al., showing 3.7 DRPs per patient [6,18,20]. Interestingly, patients with solid tumor diseases received more drugs per patient (12.8 vs. 9.7, respectively) but experienced fewer drug-related problems (3.2 vs. 5.3, respectively). The reason for this result is unclear and deserves further investigation.
In our study with cancer inpatients, the most frequently encountered categories of DRPs were indication without drug, inappropriate drug choice, and adverse drug reactions. Even across the subgroups of patients with solid and hematological tumor diseases, these three symptoms ranged within the five most common DRPs. In comparison, in studies with an outpatient setting, the following heterogeneous DRP categories occurred most often: indication without drug, adverse drug reactions, drug–drug interactions, inappropriate duration of use, drug without indication, inappropriate dosage, and non-adherence [6,15,18,19]. A study by Umar et al. on Turkish cancer inpatients reported adverse drug reactions, indication without drug, and drug without indication as most frequent DRP categories [29]. Non-adherence could not be detected in the present study, but it is likely that outpatients are more often affected because they are responsible for taking their own medication.
A limitation of this study is that medication reviews were only conducted retrospectively and therefore interventions could not be undertaken. The substantial proportion of DRPs that are associated with PRO symptoms (21.5%) indicates that including PRO data in medication reviews improves the detection of medication risks in cancer inpatients.
4.2. Determinants of Health-Related Quality of Life
The minor changes in scores, that can be considered clinically meaningful for patients, are called minimal important differences (MIDs). For the EORTC QLQ-C30 questionnaire, the MIDs for the global HRQOL and its subscales were evaluated in different studies [51,52,53]. As an approximation, values between five and ten can be assumed small patient-relevant changes. Values between 10 and 20 indicate a moderate difference, and values above 20 indicate a significant difference [54,55]. Therefore, it is possible to consider that all patients distributed to bin centers ±10 or higher experienced patient-relevant changes in their HRQOL (see Figure 4).
For the global HRQOL and physical function, we found multiple linear regression models describing the variance of the changes from baseline to hospital discharge. The models for cognitive function and emotional function were not significant. A possible explanation is that cognitive function and emotional function are more complex constructs of HRQOL, that require a longer time period to change than, for example, physical function. Therefore, the sample size of the study population may have been insufficient and the duration of the hospital stay too brief to manifest a significant change in these subscales of HRQOL. The models for global HRQOL and physical function may have been affected as well, but they still describe the changes in the dependent variables in a weak-to-moderate way [50].
The above circumstances may also account for the fact that there was no association with changes in HRQOL for the drug therapy-related factors “Drugs”, “DRPs”, and “PRO-DRPs”. In particular, for “PRO-DRPs”, an association with HRQOL would have been plausible because they are largely represented by symptomatic adverse events. Furthermore, the impact of pharmaceutical interventions resolving DRPs on symptom control and HRQOL has been shown [26,28]. However, because of the retrospective character of the medication reviews, no interventions to resolve DRPs and thereby influence the change in HRQOL during hospital stay could be carried out in this study.
Within the model for global HRQOL, only the variable “Relapse status” had a significant influence on the change in global HRQOL. This finding aligns with the expectation that patients with no relapse of their cancer disease benefit the most from the treatment during their hospital stay. For most cancer types, the treatment possibilities are best in the early stages of the disease and decrease upon relapses. The increase of 11.1 points on the global HRQOL scale is above the MID value and indicates a moderate patient-relevant difference [51,52].
Regarding the model for physical function, “Hospital stay” had a negatively directed influence on the change in physical function. The deterioration of physical function per day spent in hospital is not unexpected. The inpatient setting itself and therapy-related adverse events such as symptomatic and hematological toxicity like myelosuppression triggered by many chemotherapeutics affect the patients’ physical function during the hospital stay, especially if they appear closely after treatment.
4.3. Approaches to Improve Medication Safety
One approach to improve medication safety of cancer patients is the implementation of best-practice models addressing risk-adapted and interprofessional supportive care. In healthcare, best practice is defined as the optimization of a care process using evidence-based decision making for patients to ensure ongoing quality assurance [56]. ePRO assessment, pharmacist-led medication reviews, and other components like psycho-oncological care or therapeutic drug monitoring can be combined to improve patients’ health outcomes. Such complex interventions should be developed and evaluated in a phased approach. In the first phase, components of the intervention must be identified [57]. Thereafter, the following questions need to be answered: What is the trigger for an intervention? (e.g., PRO-CTCAE symptom over defined cut-off value, polymedication); how is the intervention conducted? (e.g., performing a symptom management, type of medication review performed and implementation of solutions); and which patient-relevant outcomes should be measured? (e.g., HRQOL, therapy discontinuation, adherence to treatment). Subsequently, the effectiveness of the complex intervention should be evaluated in exploratory and randomized controlled trials.
The results of this analysis can serve as a basis for designing supportive care concepts for German cancer inpatients, which are not yet established in clinical routine. In particular, pharmacist-led medication reviews, patient-reported symptom, and HRQOL monitoring are not yet routine clinical practices for German cancer inpatients. Medication reviews seem to be an important component, because most patients exhibited polymedication or even hyperpolymedication. The results show which categories of DRPs are of particular interest for the inpatient population. Moreover, PRO symptoms provided important additional information, which may increase the outcome of such medication reviews.
5. Conclusions
This secondary analysis of a clinical trial describes underlying DRPs in a German cancer inpatient population. Data on DRPs in cancer inpatients are limited because the majority of studies on pharmaceutical care are conducted on cancer outpatients. The patient population of the current trial showed a high proportion of patients with polymedication or even hyperpolymedication, indicating that these patients benefit from medication reviews. PRO symptoms provided important additional information, which may increase the outcome of such medication reviews.
The evaluation of the HRQOL suggests that a substantial proportion of patients underwent relevant changes in their HRQOL during hospital stay. While drug therapy-related factors had no influence, the multiple linear regression models for the global HRQOL and the physical function of the EORTC QLQ-C30 questionnaire partly explain the changes in HRQOL of the study population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16112110/s1, Table S1: Drug classes according to their Anatomical Therapeutical Chemical (ATC) code levels 1 and 2 (n = 1884); Figure S1: Histogram of the distribution of the score changes in cognitive function from baseline to hospital discharge (n = 153); Figure S2: Histogram of the distribution of the score changes in emotional function from baseline to hospital discharge (n = 153).
Author Contributions
M.G., M.S., L.H., H.S., M.-T.S. and U.J. contributed to the study conception and design. Data acquisition was performed by M.S. and L.H. Data analysis was performed by M.G. and M.-T.S. Data interpretation was performed by M.G., H.S. and U.J. The first draft of the manuscript was written by M.G. and all authors reviewed and commented on previous versions of the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Stiftung Oskar-Helene-Heim, Walterhöferstr. 11, 14165 Berlin, Germany.
Institutional Review Board Statement
The trial was approved by the Ethics Committee of the Chamber of Physicians in Berlin, Germany (Eth-48/16). It was carried out in accordance with the applicable German and European legal provisions and it was performed in accordance with the principles of the Declaration of Helsinki.
Informed Consent Statement
Written informed consent was obtained from all individual participants included in the study. Consent for publication was obtained within the written informed consent. Only anonymized data are published.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We express our gratitude to all patients who participated in our project despite their challenging situation.
Conflicts of Interest
M.G., M.S., L.H., H.S., M.-T.S. and U.J. declare that they have no conflicts of interest.
References
- Williams, G.R.; Mackenzie, A.; Magnuson, A.; Olin, R.; Chapman, A.; Mohile, S.; Allore, H.; Somerfield, M.R.; Targia, V.; Extermann, M.; et al. Comorbidity in older adults with cancer. J. Geriatr. Oncol. 2016, 7, 249–257. [Google Scholar] [CrossRef]
- Jørgensen, T.L.; Hallas, J.; Friis, S.; Herrstedt, J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br. J. Cancer 2012, 106, 1353–1360. [Google Scholar] [CrossRef]
- Turner, J.P.; Shakib, S.; Singhal, N.; Hogan-Doran, J.; Prowse, R.; Johns, S.; Bell, J.S. Prevalence and factors associated with polypharmacy in older people with cancer. Support. Care Cancer 2014, 22, 1727–1734. [Google Scholar] [CrossRef]
- Turner, J.P.; Jamsen, K.M.; Shakib, S.; Singhal, N.; Prowse, R.; Bell, J.S. Polypharmacy cut-points in older people with cancer: How many medications are too many? Support. Care Cancer 2016, 24, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Gnjidic, D.; Hilmer, S.N.; Blyth, F.M.; Naganathan, V.; Cumming, R.G.; Handelsman, D.J.; McLachlan, A.J.; Abernethy, D.R.; Banks, E.; Le Couteur, D.G. High-Risk Prescribing and Incidence of Frailty Among Older Community-Dwelling Men. Clin. Pharmacol. Ther. 2012, 91, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, G.; Hajjar, E.; Pizzi, L.T.; Wang, M.; Pigott, E.; Doherty, S.; Prioli, K.M.; Swartz, K.; Chapman, A.E. Implementing a pharmacist-led, individualized medication assessment and planning (iMAP) intervention to reduce medication related problems among older adults with cancer. J. Geriatr. Oncol. 2017, 8, 296–302. [Google Scholar] [CrossRef]
- Prithviraj, G.K.; Koroukian, S.; Margevicius, S.; Berger, N.A.; Bagai, R.; Owusu, C. Patient characteristics associated with polypharmacy and inappropriate prescribing of medications among older adults with cancer. J. Geriatr. Oncol. 2012, 3, 228–237. [Google Scholar] [CrossRef]
- Sharma, M.; Loh, K.P.; Nightingale, G.; Mohile, S.G.; Holmes, H.M. Polypharmacy and potentially inappropriate medication use in geriatric oncology. J. Geriatr. Oncol. 2016, 7, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Ortland, I.; Ott, M.M.; Kowar, M.; Sippel, C.; Ko, Y.-D.; Jacobs, A.H.; Jaehde, U. Medication risks in older patients (70+) with cancer and their association with therapy-related toxicity. BMC Geriatr. 2022, 22, 716. [Google Scholar] [CrossRef]
- Lavan, A.H.; O’Mahony, D.; O’Mahony, D.; Gallagher, P. Potentially inappropriate medication (PIM) use and severe drug interactions (SDIs) in older adults with cancer. J. Geriatr. Oncol. 2021, 12, 872–880. [Google Scholar] [CrossRef]
- Nightingale, G.; Skonecki, E.; Boparai, M.K. The impact of polypharmacy on patient outcomes in older adults with cancer. Cancer J. 2017, 23, 211–218. [Google Scholar] [PubMed]
- Hersh, L.R.; Beldowski, K.; Hajjar, E.R. Polypharmacy in the Geriatric Oncology Population. Curr. Oncol. Rep. 2017, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Balducci, L.; Goetz-Parten, D.; Steinman, M.A. Polypharmacy and the management of the older cancer patient. Ann. Oncol. 2013, 24 (Suppl S7), vii36–vii40. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Trares, K.; Laetsch, D.C.; Nguyen, T.N.M.; Brenner, H.; Schöttker, B. Systematic Review and Meta-Analysis on the Associations of Polypharmacy and Potentially Inappropriate Medication with Adverse Outcomes in Older Cancer Patients. J. Gerontol. Ser. A 2021, 76, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, T.T.; Tay, X.Y.; Si, P.; Chew, L. Drug-related problems in elderly patients with cancer receiving outpatient chemotherapy. J. Geriatr. Oncol. 2015, 6, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Jayalakshmi, V.; Shalini, S.; Rajasekaran, A.; Deepika, K. Drug-related problems in cancer patients: A systematic review. J. Oncol. Pharm. Pract. 2024, 30, 562–571. [Google Scholar] [CrossRef] [PubMed]
- PCNE Classification for Drug-Related Problems; Version 9.1; Pharmaceutical Care Network Europe (PCNE): Zuidlaren, The Netherlands, 2020; Available online: https://www.pcne.org (accessed on 20 May 2024).
- Edwards, S.J.; Abbott, R.; Edwards, J.; LeBlanc, M.; Dranitsaris, G.; Donnan, J.; Laing, K.; Whelan, M.A.; MacKinnon, N.J. Outcomes Assessment of a Pharmacist-Directed Seamless Care Program in an Ambulatory Oncology Clinic. J. Pharm. Pract. 2014, 27, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Vucur, C.; Wirtz, D.A.; Weinhold, L.; Zipfel, M.; Schmid, M.; Schmidt-Wolf, I.G.; Jaehde, U. Drug-related problems in head and neck cancer patients identified by repeated medication reviews on consecutive therapy cycles. J. Oncol. Pharm. Pract. 2021, 27, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Chua, S.S.; Chen, L.-C.; Chang, K.M.; Balashanker, S.; Bee, P.C. Acceptability of pharmacist-led interventions to resolve drug-related problems in patients with chronic myeloid leukaemia. J. Oncol. Pharm. Pract. 2021, 27, 1644–1656. [Google Scholar] [CrossRef]
- Chan, A.; Soh, D.; Ko, Y.; Huang, Y.-C.; Chiang, J. Characteristics of unplanned hospital admissions due to drug-related problems in cancer patients. Support. Care Cancer 2014, 22, 1875–1881. [Google Scholar] [CrossRef]
- Koubaity, M.; Lechon, A.-S.; Amighi, K.; Van Nuffelen, M.; Moreau, M.; Meert, A.-P.; De Vriese, C. Drug-related problems and risk factors related to unplanned hospital readmission among cancer patients in Belgium. Support. Care Cancer 2021, 29, 3911–3919. [Google Scholar] [CrossRef]
- Nazer, L.H.; Eljaber, R.; Rimawi, D.; Hawari, F.I. Adverse drug events resulting in admission to the intensive care unit in oncology patients: Incidence, characteristics and associated cost. J. Oncol. Pharm. Pract. 2013, 19, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Grundsatzpapier zur Medikationsanalyse und zum Medikationsmanagement; Version 24/06/2014; Bundesvereinigung Deutscher Apothekerverbände e. V. (ABDA): Berlin, Germany, 2014; Available online: https://www.abda.de (accessed on 20 May 2024).
- Colombo, L.R.P.; Aguiar, P.M.; Lima, T.M.; Storpirtis, S. The effects of pharmacist interventions on adult outpatients with cancer: A systematic review. J. Clin. Pharm. Ther. 2017, 42, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Alexander, M.; Fua, T.; Liu, C.; Rischin, D.; Lingaratnam, S. A systematic review of the impact of outpatient clinical pharmacy services on medication-related outcomes in patients receiving anticancer therapies. J. Oncol. Pharm. Pract. 2019, 25, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Fentie, A.M.; Huluka, S.A.; Gebremariam, G.T.; Gebretekle, G.B.; Abebe, E.; Fenta, T.G. Impact of pharmacist-led interventions on medication-related problems among patients treated for cancer: A systematic review and meta-analysis of randomized control trials. Res. Soc. Adm. Pharm. 2024, 20, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Lattard, C.; Baudouin, A.; Larbre, V.; Herledan, C.; Cerutti, A.; Cerfon, M.-A.; Kimbidima, R.; Caffin, A.-G.; Vantard, N.; Schwiertz, V.; et al. Clinical and economic impact of clinical oncology pharmacy in cancer patients receiving injectable anticancer treatments: A systematic review. J. Cancer Res. Clin. Oncol. 2023, 149, 7905–7924. [Google Scholar] [CrossRef] [PubMed]
- Umar, R.M.; Apikoglu-Rabus, S.; Yumuk, P.F. Significance of a clinical pharmacist-led comprehensive medication management program for hospitalized oncology patients. Int. J. Clin. Pharm. 2020, 42, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, A. Quality of life data as prognostic indicators of survival in cancer patients: An overview of the literature from 1982 to 2008. Health Qual. Life Outcomes 2009, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Y.; Manhas, D.S.; Howard, A.F.; Olson, R.A. Patient-reported outcome use in oncology: A systematic review of the impact on patient-clinician communication. Support. Care Cancer 2018, 26, 41–60. [Google Scholar] [CrossRef]
- Kotronoulas, G.; Kearney, N.; Maguire, R.; Harrow, A.; Di Domenico, D.; Croy, S.; MacGillivray, S. What Is the Value of the Routine Use of Patient-Reported Outcome Measures toward Improvement of Patient Outcomes, Processes of Care, and Health Service Outcomes in Cancer Care? A Systematic Review of Controlled Trials. J. Clin. Oncol. 2014, 32, 1480–1501. [Google Scholar] [CrossRef]
- Velikova, G.; Booth, L.; Smith, A.B.; Brown, P.M.; Lynch, P.; Brown, J.M.; Selby, P.J. Measuring Quality of Life in Routine Oncology Practice Improves Communication and Patient Well-Being: A Randomized Controlled Trial. J. Clin. Oncol. 2004, 22, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Barbera, L.; Kerrigan, C.L.; Velikova, G. Implementation of Patient-Reported Outcomes in Routine Medical Care. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Deal, A.M.; Kris, M.G.; Scher, H.I.; Hudis, C.A.; Sabbatini, P.; Rogak, L.; Bennett, A.V.; Dueck, A.C.; Atkinson, T.M.; et al. Symptom Monitoring with Patient-Reported Outcomes during Routine Cancer Treatment: A Randomized Controlled Trial. J. Clin. Oncol. 2016, 34, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA-J. Am. Med. Assoc. 2017, 318, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Salm, H.; Hentschel, L.; Eichler, M.; Pink, D.; Fuhrmann, S.; Kramer, M.; Reichardt, P.; Schuler, M.K. Evaluation of electronic patient–reported outcome assessment in inpatient cancer care: A feasibility study. Support. Care Cancer 2023, 31, 575. [Google Scholar] [CrossRef] [PubMed]
- Richtlinie (EU) 2016/680 des Europäischen Parlaments und des Rates vom 27 April 2016 zum Schutz natürlicher Personen bei der Verarbeitung Personenbezogener Daten durch die zuständigen Behörden zum Zwecke der Verhütung, Ermittlung, Aufdeckung oder Verfolgung von Straftaten oder der Strafvollstreckung sowie zum freien Datenverkehr und zur Aufhebung des Rahmenbeschlusses 2008/977/JI des Rates; Version 27/04/2016; Europäisches Parlament und Rat der Europäischen Union: Strasbourg, France, 2016; Available online: https://eur-lex.europa.eu/homepage.html (accessed on 20 May 2024).
- Basch, E.; Reeve, B.B.; Mitchell, S.A.; Clauser, S.B.; Minasian, L.M.; Dueck, A.C.; Mendoza, T.R.; Hay, J.; Atkinson, T.M.; Abernethy, A.P.; et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J. Natl. Cancer Inst. 2014, 106, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE); Version 11/29/2023; National Cancer Institute: Rockville, MD, USA, 2023. Available online: https://healthcaredelivery.cancer.gov/pro-ctcae (accessed on 20 May 2024).
- Kirsch, M.; Mitchell, S.A.; Dobbels, F.; Stussi, G.; Basch, E.; Halter, J.P.; De Geest, S. Linguistic and content validation of a German-language PRO-CTCAE-based patient-reported outcomes instrument to evaluate the late effect symptom experience after allogeneic hematopoietic stem cell transplantation. Eur. J. Oncol. Nurs. 2015, 19, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hagelstein, V.; Ortland, I.; Wilmer, A.; Mitchell, S.A.; Jaehde, U. Validation of the German patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE™). Ann. Oncol. 2016, 27, 2294–2299. [Google Scholar] [CrossRef] [PubMed]
- Fayers, P.M.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. The EORTC QLQ-C30 Scoring Manual, 3rd ed.; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 2001. [Google Scholar]
- European Organisation for Research and Treatment of Cancer. EORTC Quality of Life Questionnaires. Available online: https://qol.eortc.org/questionnaires (accessed on 20 May 2024).
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. JNCI J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Position Paper on PCNE Medication Review; Pharmaceutical Care Network Europe (PCNE): Zuidlaren, The Netherlands, 2016.
- Maes, K.A.; Bruch, S.; Hersberger, K.E.; Lampert, M.L. Documentation of pharmaceutical care: Development of an intervention oriented classification system. Int. J. Clin. Pharm. 2017, 39, 354–363. [Google Scholar] [CrossRef]
- Bortz, J.; Schuster, C. Statistik für Human- und Sozialwissenschaftler, 7th ed.; Springer: Heidelberg, Germany, 2010. [Google Scholar]
- Weiß, C. Basiswissen Medizinische Statistik, 7th ed.; Springer: Heidelberg, Germany, 2019. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Maringwa, J.T.; Quinten, C.; King, M.; Ringash, J.; Osoba, D.; Coens, C.; Martinelli, F.; Vercauteren, J.; Cleeland, C.S.; Flechtner, H.; et al. Minimal important differences for interpreting health-related quality of life scores from the EORTC QLQ-C30 in lung cancer patients participating in randomized controlled trials. Support. Care Cancer 2011, 19, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Bedard, G.; Zeng, L.; Zhang, L.; Lauzon, N.; Holden, L.; Tsao, M.; Danjoux, C.; Barnes, E.; Sahgal, A.; Poon, M.; et al. Minimal important differences in the EORTC QLQ-C30 in patients with advanced cancer. Asia-Pacific J. Clin. Oncol. 2014, 10, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Musoro, J.Z.; Sodergren, S.C.; Coens, C.; Pochesci, A.; Terada, M.; King, M.T.; Sprangers, M.A.; Groenvold, M.; Cocks, K.; Velikova, G.; et al. Minimally important differences for interpreting the EORTC QLQ-C30 in patients with advanced colorectal cancer treated with chemotherapy. Color. Dis. 2020, 22, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef] [PubMed]
- King, M.T. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual. Life Res. 1996, 5, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Perleth, M.; Jakubowski, E.; Busse, R. What is ‘best practice’ in health care? State of the art and perspectives in improving the effectiveness and efficiency of the European health care systems. Health Policy 2001, 56, 235–250. [Google Scholar] [CrossRef]
- Campbell, M.; Fitzpatrick, R.; Haines, A.; Kinmonth, A.L.; Sandercock, P.; Spiegelhalter, D.; Tyrer, P. Framework for design and evaluation of complex interventions to improve health. BMJ 2000, 321, 694–696. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).