PD-L1 Expression in High-Risk Non-Muscle-Invasive Bladder Cancer Is Influenced by Intravesical Bacillus Calmette–Guérin (BCG) Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Follow Up
2.3. Immunohistochemical Staining and Assessment
2.4. Statistical Analysis
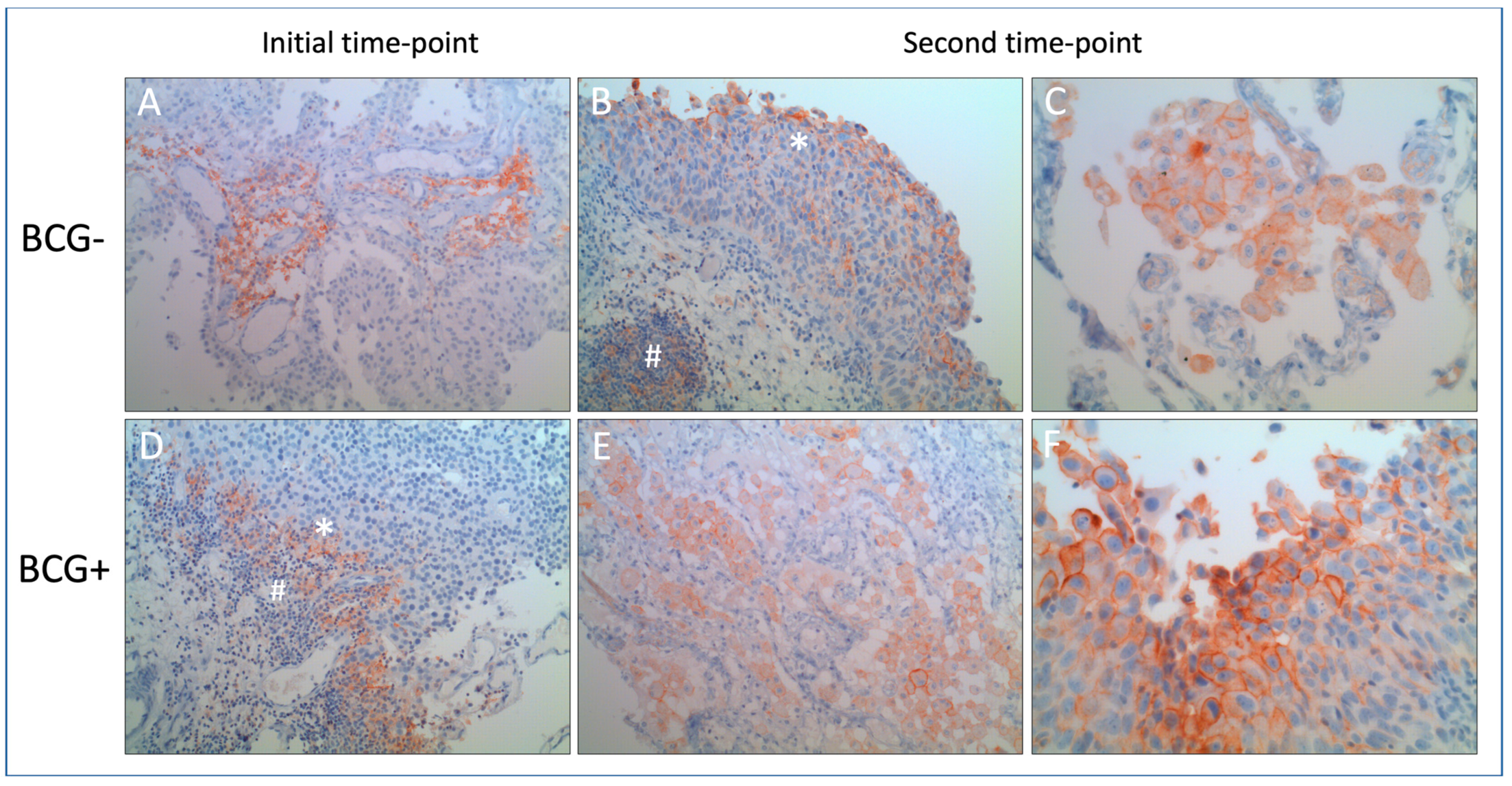
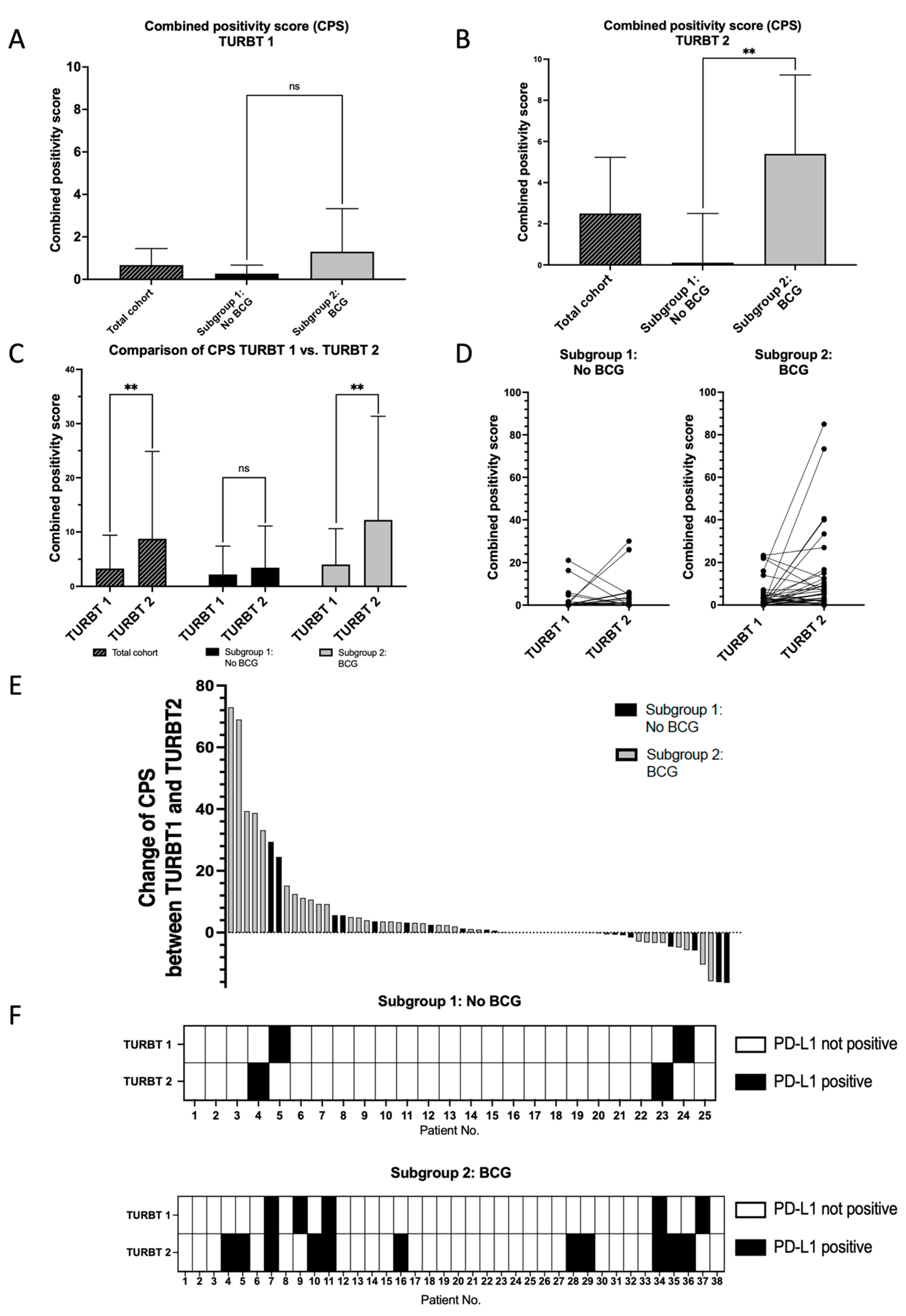
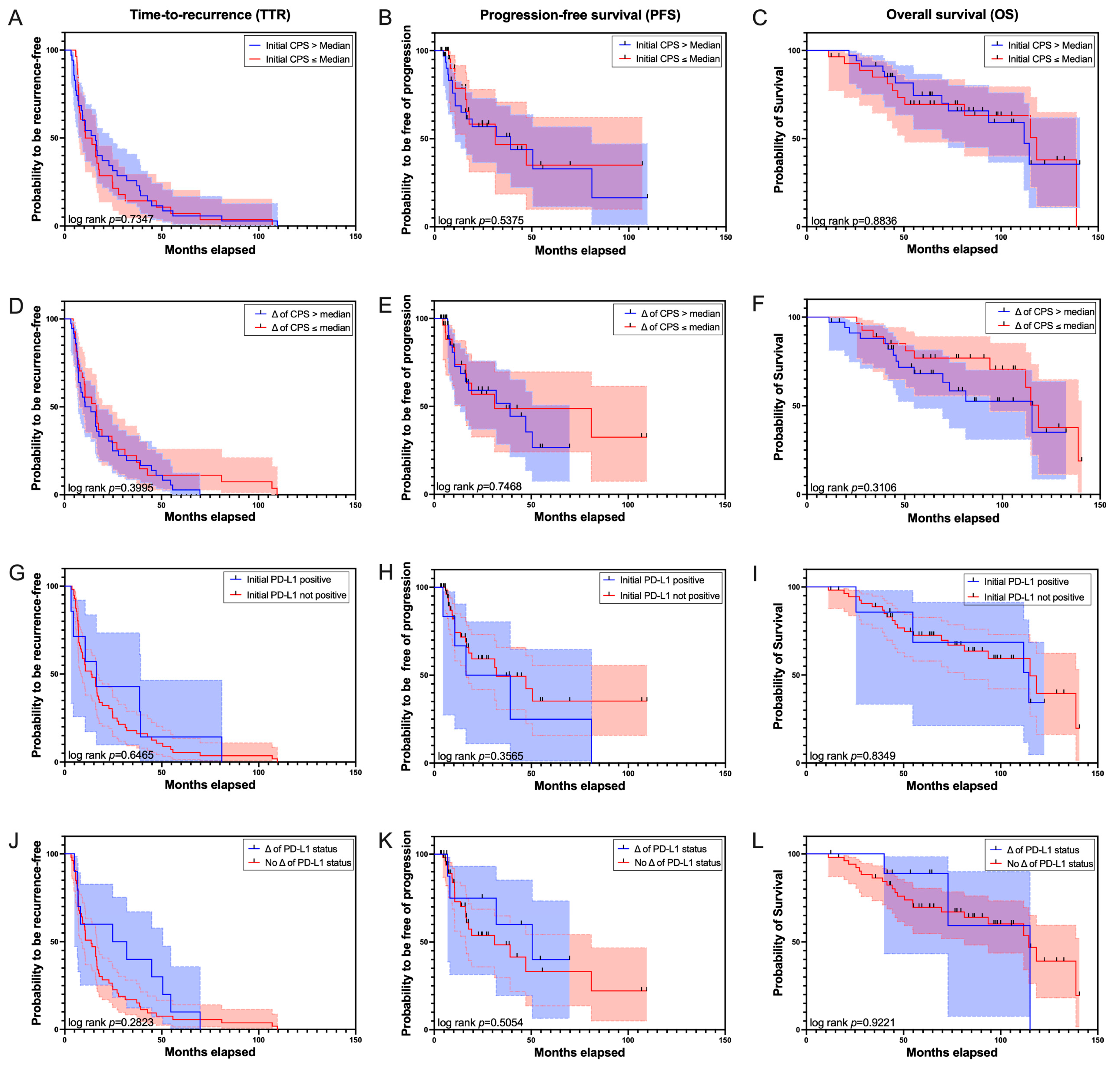
3. Results
4. Discussion
4.1. Limitations
4.2. Future Perspectives and Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1 (accessed on 15 July 2023).
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma In Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef]
- Allard, P.; Bernard, P.; Fradet, Y.; Têtu, B. The early clinical course of primary Ta and T1 bladder cancer: A proposed prognostic index. Br. J. Urol. 1998, 81, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.; Luengo-Fernandez, R.; Sullivan, R.; Witjes, J.A. Economic Burden of Bladder Cancer Across the European Union. Eur. Urol. 2016, 69, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.W. Tumour progression and survival in patients with T1G3 bladder tumours: 15-year outcome. Br. J. Urol. 1997, 80, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Redelman-Sidi, G.; Glickman, M.S.; Bochner, B.H. The mechanism of action of BCG therapy for bladder cancer—A current perspective. Nat. Rev. Urol. 2014, 11, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Meeks, J.J.; Black, P.C.; Galsky, M.; Grivas, P.; Hahn, N.M.; Hussain, S.A.; Milowsky, M.I.; Steinberg, G.D.; Svatek, R.S.; Rosenberg, J.E. Checkpoint Inhibitors in Urothelial Carcinoma-Future Directions and Biomarker Selection. Eur. Urol. 2023, 84, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, J.; Wada, Y.; Matsumoto, K.; Azuma, M.; Kikuchi, K.; Ueda, S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol. Immunother. 2007, 56, 1173–1182. [Google Scholar] [CrossRef]
- Hashizume, A.; Umemoto, S.; Yokose, T.; Nakamura, Y.; Yoshihara, M.; Shoji, K.; Wada, S.; Miyagi, Y.; Kishida, T.; Sasada, T. Enhanced expression of PD-L1 in non-muscle-invasive bladder cancer after treatment with Bacillus Calmette-Guerin. Oncotarget 2018, 9, 34066–34078. [Google Scholar] [CrossRef]
- Roumiguié, M.; Compérat, E.; Chaltiel, L.; Nouhaud, F.X.; Verhoest, G.; Masson-Lecomte, A.; Colin, P.; Audenet, F.; Houédé, N.; Larré, S.; et al. PD-L1 expression and pattern of immune cells in pre-treatment specimens are associated with disease-free survival for HR-NMIBC undergoing BCG treatment. World J. Urol. 2021, 39, 4055–4065. [Google Scholar] [CrossRef] [PubMed]
- Civriz, A.H.; Teke, K.; Akdas, E.M.; Dillioglugil, O.; Vural, C.; Yaprak Bayrak, B. The prognostic value of expressions of STAT3, PD-L1, and PD-L2 in Ta/T1 urothelial carcinoma before and after BCG treatment. Urol. Oncol. 2023, 41, e481–e486. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Cimadamore, A.; Hartmann, A.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M.; Montironi, R.; Gevaert, T. PD-L1 assessment in urothelial carcinoma: A practical approach. Ann. Transl. Med. 2019, 7, 690. [Google Scholar] [CrossRef] [PubMed]
- PD-L1 IHC 22C3 Pharmdx Interpretation Manual—Urothelial Carcinoma. Agilent Technologies. December 2017. Available online: https://www.agilent.com/cs/library/usermanuals/public/29276_22C3_pharmdx_uc_interpretation_manual_us.pdf (accessed on 14 September 2023).
- Ventana PD-L1 (SP142) Assay—Interpretation Guide for Urothelial Carcinoma; Ventana Medical Systems, Inc. and Roche: Oro Valley, AZ, USA, 2020.
- Udall, M.; Rizzo, M.; Kenny, J.; Doherty, J.; Dahm, S.; Robbins, P.; Faulkner, E. PD-L1 diagnostic tests: A systematic literature review of scoring algorithms and test-validation metrics. Diagn. Pathol. 2018, 13, 12. [Google Scholar] [CrossRef]
- Wang, X.; Teng, F.; Kong, L.; Yu, J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016, 9, 5023–5039. [Google Scholar] [CrossRef]
- Kluger, H.M.; Zito, C.R.; Turcu, G.; Baine, M.K.; Zhang, H.; Adeniran, A.; Sznol, M.; Rimm, D.L.; Kluger, Y.; Chen, L.; et al. PD-L1 Studies Across Tumor Types, Its Differential Expression and Predictive Value in Patients Treated with Immune Checkpoint Inhibitors. Clin. Cancer Res. 2017, 23, 4270–4279. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, S.; Alfred Witjes, J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: A systematic review. Eur. Urol. 2011, 60, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Wankowicz, S.A.M.; Werner, L.; Orsola, A.; Novak, J.; Bowden, M.; Choueiri, T.K.; de Torres, I.; Morote, J.; Freeman, G.J.; Signoretti, S.; et al. Differential Expression of PD-L1 in High Grade T1 vs Muscle Invasive Bladder Carcinoma and its Prognostic Implications. J. Urol. 2017, 198, 817–823. [Google Scholar] [CrossRef]
- Martins-Lima, C.; Chianese, U.; Benedetti, R.; Altucci, L.; Jerónimo, C.; Correia, M.P. Tumor microenvironment and epithelial-mesenchymal transition in bladder cancer: Cytokines in the game? Front. Mol. Biosci. 2022, 9, 1070383. [Google Scholar] [CrossRef]
- Inman, B.A.; Sebo, T.J.; Frigola, X.; Dong, H.; Bergstralh, E.J.; Frank, I.; Fradet, Y.; Lacombe, L.; Kwon, E.D. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: Associations with localized stage progression. Cancer 2007, 109, 1499–1505. [Google Scholar] [CrossRef]
- Aydin, A.M.; Baydar, D.E.; Hazir, B.; Babaoglu, B.; Bilen, C.Y. Prognostic significance of pre- and post-treatment PD-L1 expression in patients with primary high-grade non-muscle-invasive bladder cancer treated with BCG immunotherapy. World J. Urol. 2020, 38, 2537–2545. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Mullane, S.A.; Werner, L.; Fay, A.P.; Callea, M.; Leow, J.J.; Taplin, M.E.; Choueiri, T.K.; Hodi, F.S.; Freeman, G.J.; et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann. Oncol. 2015, 26, 812–817. [Google Scholar] [CrossRef]
- Robert, M.E.; Rüschoff, J.; Jasani, B.; Graham, R.P.; Badve, S.S.; Rodriguez-Justo, M.; Kodach, L.L.; Srivastava, A.; Wang, H.L.; Tang, L.H.; et al. High Interobserver Variability Among Pathologists Using Combined Positive Score to Evaluate PD-L1 Expression in Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma. Mod. Pathol. 2023, 36, 100154. [Google Scholar] [CrossRef]
- Meeks, J.J.; Al-Ahmadie, H.; Faltas, B.M.; Taylor, J.A., 3rd; Flaig, T.W.; DeGraff, D.J.; Christensen, E.; Woolbright, B.L.; McConkey, D.J.; Dyrskjøt, L. Genomic heterogeneity in bladder cancer: Challenges and possible solutions to improve outcomes. Nat. Rev. Urol. 2020, 17, 259–270. [Google Scholar] [CrossRef]
- de Jong, J.J.; Stoop, H.; Boormans, J.L.; van Leenders, G. PD-L1 expression in urothelial bladder cancer varies more among specimen types than between companion assays. Virchows Arch. Int. J. Pathol. 2021, 479, 705–713. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, A.S.; Alva, A.; Zhan, T.; Xiao, H.; Cao, X.; Gursky, A.; Siddiqui, J.; Chinnaiyan, A.M.; Jiang, H.; Lee, C.T.; et al. Expression of PDL1 (B7-H1) Before and After Neoadjuvant Chemotherapy in Urothelial Carcinoma. Eur. Urol. Focus. 2016, 1, 265–268. [Google Scholar] [CrossRef]
- Wang, B.; Pan, W.; Yang, M.; Yang, W.; He, W.; Chen, X.; Bi, J.; Jiang, N.; Huang, J.; Lin, T. Programmed death ligand-1 is associated with tumor infiltrating lymphocytes and poorer survival in urothelial cell carcinoma of the bladder. Cancer Sci. 2019, 110, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Breyer, J.; Wirtz, R.M.; Otto, W.; Erben, P.; Worst, T.S.; Stoehr, R.; Eckstein, M.; Denzinger, S.; Burger, M.; Hartmann, A. High PDL1 mRNA expression predicts better survival of stage pT1 non-muscle-invasive bladder cancer (NMIBC) patients. Cancer Immunol. Immunother. 2018, 67, 403–412. [Google Scholar] [CrossRef]
- Le Goux, C.; Damotte, D.; Vacher, S.; Sibony, M.; Delongchamps, N.B.; Schnitzler, A.; Terris, B.; Zerbib, M.; Bieche, I.; Pignot, G. Correlation between messenger RNA expression and protein expression of immune checkpoint-associated molecules in bladder urothelial carcinoma: A retrospective study. Urol. Oncol. 2017, 35, 257–263. [Google Scholar] [CrossRef]
- Martínez, R.; Tapia, G.; De Muga, S.; Hernández, A.; Cao, M.G.; Teixidó, C.; Urrea, V.; García, E.; Pedreño-López, S.; Ibarz, L.; et al. Combined assessment of peritumoral Th1/Th2 polarization and peripheral immunity as a new biomarker in the prediction of BCG response in patients with high-risk NMIBC. Oncoimmunology 2019, 8, 1602460. [Google Scholar] [CrossRef] [PubMed]
- Faraj, S.F.; Munari, E.; Guner, G.; Taube, J.; Anders, R.; Hicks, J.; Meeker, A.; Schoenberg, M.; Bivalacqua, T.; Drake, C.; et al. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology 2015, 85, e701–e706. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Hori, S.; Ohnishi, S.; Owari, T.; Iida, K.; Ohnishi, K.; Morizawa, Y.; Gotoh, D.; Itami, Y.; Nakai, Y.; et al. Clinical Impact of the Increase in Immunosuppressive Cell-Related Gene Expression in Urine Sediment during Intravesical Bacillus Calmette-Guérin. Diseases 2019, 7, 44. [Google Scholar] [CrossRef] [PubMed]

| Initial TURBT | ||
|---|---|---|
| n | ||
| Patients | 63 | 100% |
| Male | 56 | 88.89% |
| Female | 7 | 11.11% |
| Age in years | ||
| Median | 73.9 | 95% CI: 69.70–78.50 |
| Range | 38.00–96.80 | |
| Pathological stage | ||
| pTis | 18 | 28.57% |
| pTa | 15 | 23.81% |
| pT1 | 30 | 47.62% |
| Grading | ||
| WHO 1973 | ||
| G1 | 1 | 1.59% |
| G2 | 20 | 31.75% |
| G3 | 39 | 61.90% |
| X (unknown) | 3 | 4.76% |
| WHO 2004/2016 | ||
| LG | 7 | 11.11% |
| HG | 53 | 84.13% |
| X (unknown) | 3 | 4.76% |
| HR according to | ||
| EAU 2022 | 61 | 96.83% |
| EAU 2020/AUA 2020 | 2 | 3.17% |
| BCG therapy | ||
| yes | 38 | 60.32% |
| no | 25 | 39.68% |
| Variant histology | ||
| Yes | 0 | 0.00% |
| No | 63 | 100.00% |
| Tumor size in cm | ||
| Median | 2.2 | 95% CI: 1.7–2.7 |
| Range | 0.5–10.0 | |
| Multilocular lesions | ||
| yes | 49 | 77.78% |
| no | 14 | 22.22% |
| Parameter | Initial CPS > Median | Initial PD-L1 Status Positive | ||||
|---|---|---|---|---|---|---|
| p-Value | Odds Ratio | 95% CI | p-Value | Odds Ratio | 95% CI | |
| T1 | 0.453 | 0.6373 | 0.2529–1.764 | 0.429 | 0.4 | 0.07568–2.193 |
| CIS | 0.094 | 2.737 | 0.8954–9.234 | 0.017 | 8.269 | 1.399–43.50 |
| >LG/G1 | 0.238 | 0.0013 | 0.0010–2.072 | 0.212 | 0.1091 | 0.005684–2.399 |
| Tumor size > 3 cm | 0.439 | 1.589 | 0.5827–4.395 | 0.041 | 0.394 | 0.021–0.9339 |
| Multilocular lesions | 0.365 | 2.035 | 0.6326–6.323 | >0.9999 | 0.5513 | 0.04490–4.215 |
| Age > 70 years | 0.124 | 0.4174 | 0.1424–1.242 | 0.699 | 1.618 | 0.2935–8.633 |
| Sex (male) | 0.257 | 0.3467 | 0.06573–1.904 | 0.17 | 0.2451 | 0.04702–1.529 |
| Parameter | Initial CPS | ||
|---|---|---|---|
| Spearman’s r | 95% CI | p-Value | |
| CIS | 0.268 | 0.0139–0.4892 | 0.0338 |
| Age | −0.1105 | −0.3553–0.1484 | 0.3884 |
| Tumor size | 0.0406 | −0.2164–0.2924 | 0.7520 |
| Grading G1–G3 | 0.2727 | 0.0125–0.4983 | 0.0351 |
| Grading LG–HG | 0.0716 | −0.1931–0.3266 | 0.5867 |
| Multilocular lesions | 0.902 | −0.1667–0.3388 | 0.4733 |
| Sex (male vs. female) | 0.1896 | −0.0684–0.4239 | 0.1366 |
| T1 | 0.041 | −0.2163–0.2925 | 0.7513 |
| (A) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TTR | PFS | OS | |||||||
| p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | |
| BCG therapy (yes/no) | 0.8344 | 0.9464 | 0.5675–1.602 | 0.9203 | 1.042 | 0.4700–2.410 | 0.1068 | 0.5221 | 0.2337–1.157 |
| Initial T-stage T1 | 0.1478 | 0.6878 | 0.4135–1.145 | 0.2045 | 0.5991 | 0.2678–1.330 | 0.3953 | 0.709 | 0.3189–1.588 |
| CIS (yes/no) | 0.1168 | 0.6279 | 0.3407–1.099 | 0.2263 | 0.563 | 0.2027–1.349 | 0.5704 | 0.779 | 0.3112–1.790 |
| Grading > LG/G1 (yes/no) | 0.2158 | 0.4067 | 0.06605–1.328 | 0.4967 | 0.494 | 0.02717–2.442 | 0.923 | 1.104 | 0.06141–5.337 |
| Tumor size ≥ 3 cm | 0.5749 | 0.8603 | 0.5121–1.474 | 0.7874 | 1.124 | 0.4937–2.776 | 0.4554 | 1.398 | 0.6046–3.617 |
| Multilocular lesions | 0.7691 | 1.094 | 0.6151–2.074 | 0.5642 | 1.338 | 0.5350–4.050 | 0.937 | 1.041 | 0.4152–3.168 |
| Age > 70 years | 0.8682 | 0.9573 | 0.5646–1.591 | 0.1223 | 0.4818 | 0.1742–1.149 | 0.1665 | 0.5185 | 0.1870–1.246 |
| Sex (male) | 0.5698 | 0.7943 | 0.3277–1.644 | 0.3381 | 0.4922 | 0.07875–1.677 | 0.3367 | 1.695 | 0.4920–4.491 |
| Initial PD-L1 status positive | 0.645 | 0.8297 | 0.3422–1.716 | 0.3712 | 1.573 | 0.5179–3.934 | 0.835 | 1.124 | 0.3204–3.049 |
| Initial CPS > median | 0.5698 | 0.7943 | 0.3277–1.644 | 0.3381 | 0.4922 | 0.07875–1.677 | 0.3367 | 1.695 | 0.4920–4.491 |
| Change PD-L1 status (not positive to positive) | 0.2838 | 0.6857 | 0.3247–1.309 | 0.5066 | 0.6906 | 0.1979–1.865 | 0.9222 | 0.9411 | 0.2212–2.757 |
| Change CPS > median | 0.3519 | 0.7562 | 0.4060–1.329 | 0.7839 | 1.127 | 0.4536–2.564 | 0.5782 | 0.7672 | 0.2758–1.849 |
| (B) | |||||||||
| TTR | PFS | OS | |||||||
| p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | |
| BCG therapy (yes/no) | 0.9043 | 0.954 | 0.4391–2.050 | 0.2262 | 2.076 | 0.6302–6.884 | 0.1233 | 0.3373 | 0.07754–1.287 |
| Initial T-stage T1 | 0.5632 | 0.8108 | 0.3895–1.627 | 0.848 | 0.8961 | 0.2720–2.646 | 0.1722 | 0.4354 | 0.1215–1.378 |
| CIS (yes/no) | 0.3408 | 0.6077 | 0.2191–1.720 | 0.1228 | 0.2637 | 0.04661–1.455 | 0.4708 | 1.972 | 0.3160–13.29 |
| Grading > LG/G1 (yes/no) | 0.0721 | 0.1897 | 0.02328–0.9600 | 0.3099 | 0.2633 | 0.01021–2.339 | 0.8214 | 0.7396 | 0.02844–7.214 |
| Tumor size ≥ 3 cm | 0.9431 | 0.977 | 0.5148–1.857 | 0.8426 | 0.8972 | 0.3065–2.674 | 0.1817 | 2.037 | 0.7268–6.058 |
| Multilocular lesions | 0.5697 | 1.21 | 0.6410–2.411 | 0.9131 | 1.062 | 0.3764–3.470 | 0.7069 | 1.245 | 0.4279–4.343 |
| Age > 70 years | 0.81 | 1.079 | 0.5770–1.992 | 0.1235 | 0.4089 | 0.1211–1.214 | 0.2767 | 0.5645 | 0.1852–1.500 |
| Sex (male) | 0.3855 | 0.6851 | 0.2689–1.521 | 0.313 | 0.4554 | 0.06937–1.728 | 0.3879 | 1.746 | 0.4424–5.927 |
| Initial PD-L1 status positive | 0.3848 | 1.532 | 0.5392–3.795 | 0.0583 | 3.392 | 0.8965–11.92 | 0.945 | 0.9514 | 0.2053–3.705 |
| BCG therapy (yes/no) | 0.8551 | 0.931 | 0.4288–2.002 | 0.3977 | 1.644 | 0.5143–5.285 | 0.1217 | 0.3365 | 0.07747–1.277 |
| Initial T-stage T1 | 0.4804 | 0.7761 | 0.3753–1.547 | 0.5842 | 0.7349 | 0.2271–2.126 | 0.1728 | 0.436 | 0.1217–1.379 |
| CIS (yes/no) | 0.4273 | 0.6693 | 0.2512–1.841 | 0.2865 | 0.434 | 0.09389–2.115 | 0.4693 | 1.943 | 0.3323–12.64 |
| Grading > LG/G1 (yes/no) | 0.1054 | 0.2661 | 0.03828–1.080 | 0.6212 | 0.5628 | 0.02685–3.828 | 0.7716 | 0.7086 | 0.03312–5.113 |
| Tumor size ≥ 3 cm | 0.9512 | 1.02 | 0.5453–1.919 | 0.8697 | 1.089 | 0.3953–3.119 | 0.1809 | 2.026 | 0.7312–5.987 |
| Multilocular lesions | 0.6147 | 1.185 | 0.6260–2.369 | 0.9315 | 1.049 | 0.3739–3.438 | 0.7002 | 1.249 | 0.4347–4.344 |
| Age > 70 years | 0.8817 | 1.047 | 0.5654–1.908 | 0.1075 | 0.4124 | 0.1299–1.156 | 0.278 | 0.5652 | 0.1851–1.500 |
| Sex (male) | 0.3792 | 0.7284 | 0.3509–1.452 | 0.5791 | 0.528 | 0.02555–3.502 | 0.8762 | 1.05 | 0.5643–1.931 |
| Initial CPS > median | 0.4184 | 0.6997 | 0.2719–1.568 | 0.378 | 0.4951 | 0.07337–1.940 | 0.3766 | 1.723 | 0.4539–5.364 |
| BCG therapy (yes/no) | 0.6609 | 1.188 | 0.5433–2.555 | 0.1508 | 2.328 | 0.7177–7.426 | 0.123 | 0.3438 | 0.08057–1.277 |
| Initial T-stage T1 | 0.36 | 0.7148 | 0.3405–1.444 | 0.4148 | 0.625 | 0.1876–1.847 | 0.1309 | 0.3871 | 0.1047–1.277 |
| CIS (yes/no) | 0.2257 | 0.5292 | 0.1908–1.508 | 0.163 | 0.326 | 0.06758–1.646 | 0.4671 | 1.954 | 0.3319–12.79 |
| Grading > LG/G1 (yes/no) | 0.0735 | 0.233 | 0.03370–0.9394 | 0.5587 | 0.5086 | 0.02450–3.412 | 0.7468 | 0.6828 | 0.03212–4.882 |
| Tumor size ≥ 3 cm | 0.9854 | 0.994 | 0.5203–1.909 | 0.9264 | 1.051 | 0.3659– 3.132 | 0.1828 | 2.02 | 0.7290–5.964 |
| Multilocular lesions | 0.2855 | 1.446 | 0.7514–2.934 | 0.6051 | 1.339 | 0.4677–4.467 | 0.5785 | 1.39 | 0.4659–4.933 |
| Age > 70 years | 0.8172 | 0.9295 | 0.4937–1.714 | 0.0643 | 0.3409 | 0.09937–1.000 | 0.2211 | 0.5111 | 0.1592–1.410 |
| Sex (male) | 0.7865 | 0.8871 | 0.3442–1.994 | 0.6909 | 0.7276 | 0.1076–2.892 | 0.2588 | 2.168 | 0.5067–7.974 |
| Change PD-L1 status (not positive to positive) | 0.032 | 0.4202 | 0.1819–0.9001 | 0.0834 | 0.3173 | 0.07624–1.080 | 0.4779 | 0.5773 | 0.1012–2.257 |
| BCG therapy (yes/no) | 0.8608 | 0.9335 | 0.4294–2.012 | 0.32 | 1.824 | 0.5555–6.082 | 0.1195 | 0.3363 | 0.07788–1.268 |
| Initial T-stage T1 | 0.4755 | 0.7713 | 0.3694–1.550 | 0.5307 | 0.6989 | 0.2116–2.048 | 0.1631 | 0.4264 | 0.1184–1.354 |
| CIS (yes/no) | 0.4237 | 0.6649 | 0.2466–1.840 | 0.2189 | 0.3656 | 0.07289–1.883 | 0.4068 | 2.191 | 0.3539–14.98 |
| Grading > LG/G1 (yes/no) | 0.1225 | 0.2568 | 0.03382–1.207 | 0.447 | 0.3928 | 0.01731–3.119 | 0.9837 | 0.9734 | 0.03829–9.712 |
| Tumor size ≥ 3 cm | 0.9385 | 1.025 | 0.5450–1.952 | 0.751 | 1.188 | 0.4174–3.597 | 0.272 | 1.838 | 0.6358–5.764 |
| Multilocular lesions | 0.6114 | 1.188 | 0.6265–2.382 | 0.9354 | 1.047 | 0.3634–3.507 | 0.7443 | 1.206 | 0.4238–4.186 |
| Age > 70 years | 0.8674 | 1.054 | 0.5632–1.948 | 0.1451 | 0.4426 | 0.1378–1.272 | 0.2296 | 0.5196 | 0.1663–1.457 |
| Sex (male) | 0.4241 | 0.7021 | 0.2723–1.578 | 0.4793 | 0.5637 | 0.08222–2.297 | 0.4601 | 1.592 | 0.4112–5.160 |
| Change CPS > median | 0.9127 | 1.039 | 0.5089–2.008 | 0.3303 | 1.672 | 0.5686–4.642 | 0.6064 | 0.734 | 0.2083–2.279 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maas, M.; Hilsendecker, A.; Pertoll, A.; Stühler, V.; Walz, S.; Rausch, S.; Stenzl, A.; Tsaur, I.; Hennenlotter, J.; Aufderklamm, S. PD-L1 Expression in High-Risk Non-Muscle-Invasive Bladder Cancer Is Influenced by Intravesical Bacillus Calmette–Guérin (BCG) Therapy. Cancers 2024, 16, 1356. https://doi.org/10.3390/cancers16071356
Maas M, Hilsendecker A, Pertoll A, Stühler V, Walz S, Rausch S, Stenzl A, Tsaur I, Hennenlotter J, Aufderklamm S. PD-L1 Expression in High-Risk Non-Muscle-Invasive Bladder Cancer Is Influenced by Intravesical Bacillus Calmette–Guérin (BCG) Therapy. Cancers. 2024; 16(7):1356. https://doi.org/10.3390/cancers16071356
Chicago/Turabian StyleMaas, Moritz, Andreas Hilsendecker, Alexandra Pertoll, Viktoria Stühler, Simon Walz, Steffen Rausch, Arnulf Stenzl, Igor Tsaur, Jörg Hennenlotter, and Stefan Aufderklamm. 2024. "PD-L1 Expression in High-Risk Non-Muscle-Invasive Bladder Cancer Is Influenced by Intravesical Bacillus Calmette–Guérin (BCG) Therapy" Cancers 16, no. 7: 1356. https://doi.org/10.3390/cancers16071356
APA StyleMaas, M., Hilsendecker, A., Pertoll, A., Stühler, V., Walz, S., Rausch, S., Stenzl, A., Tsaur, I., Hennenlotter, J., & Aufderklamm, S. (2024). PD-L1 Expression in High-Risk Non-Muscle-Invasive Bladder Cancer Is Influenced by Intravesical Bacillus Calmette–Guérin (BCG) Therapy. Cancers, 16(7), 1356. https://doi.org/10.3390/cancers16071356







