An MRI Radiomics Approach to Predict the Hypercoagulable Status of Gliomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Information and Data Retrieval
2.2. Model Construction and Validation
2.3. Tumor Analyses and Transcriptional Signatures
2.4. Descriptive Statistical Analyses
3. Results
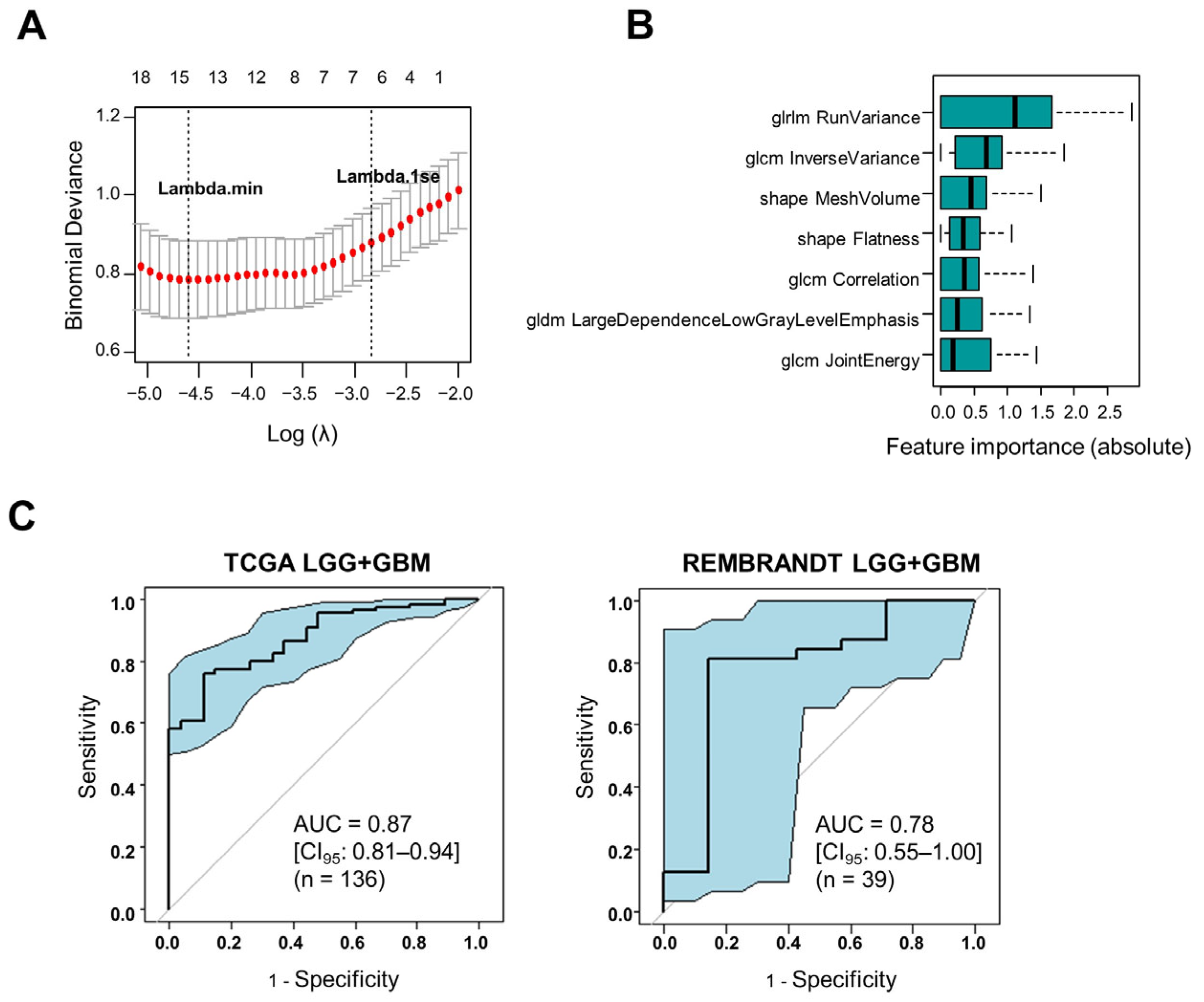
3.1. Construction and Validation of an MRI Radiomics Model That Reflects the Hypercoagulable Status of Human Gliomas
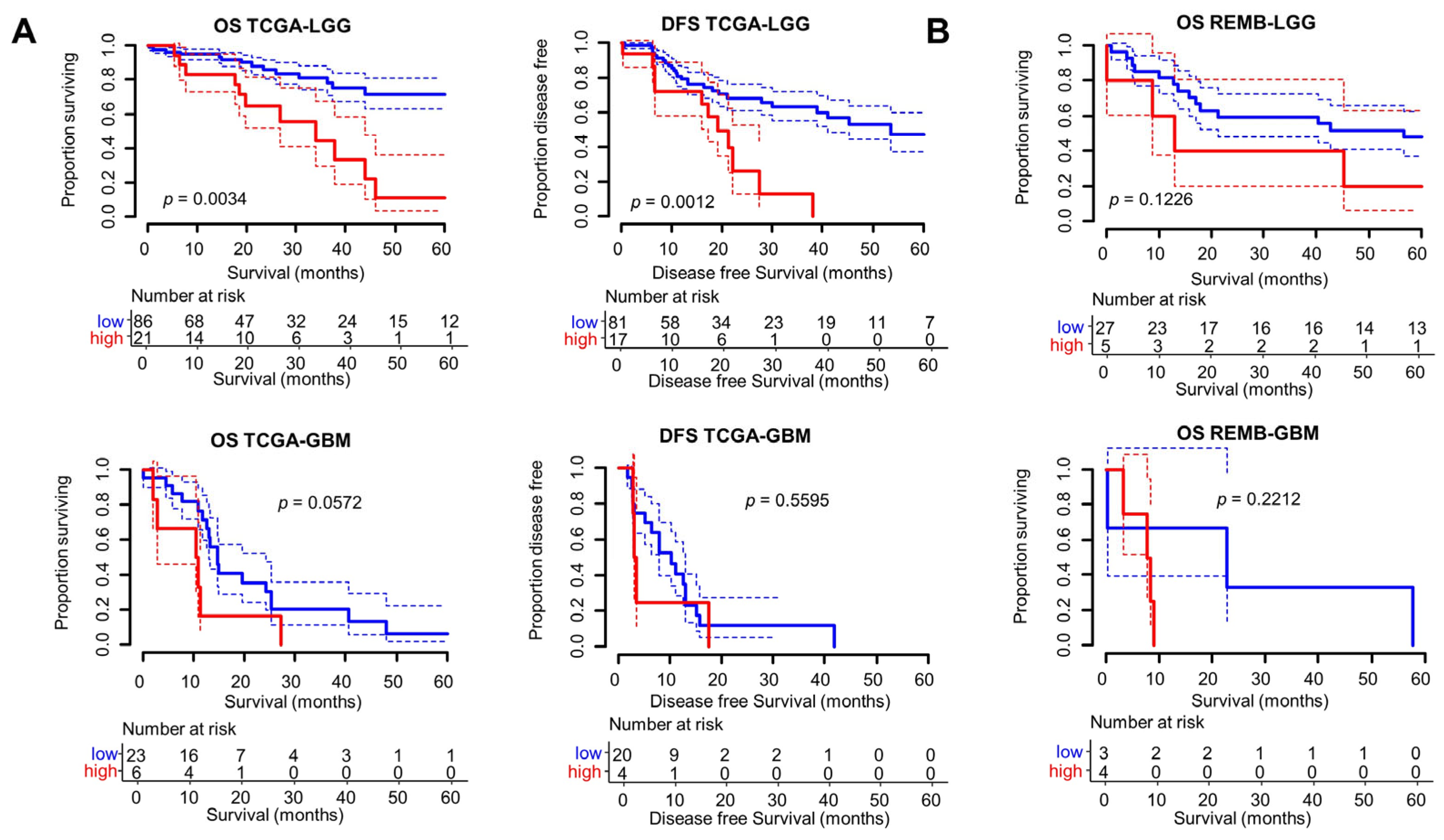
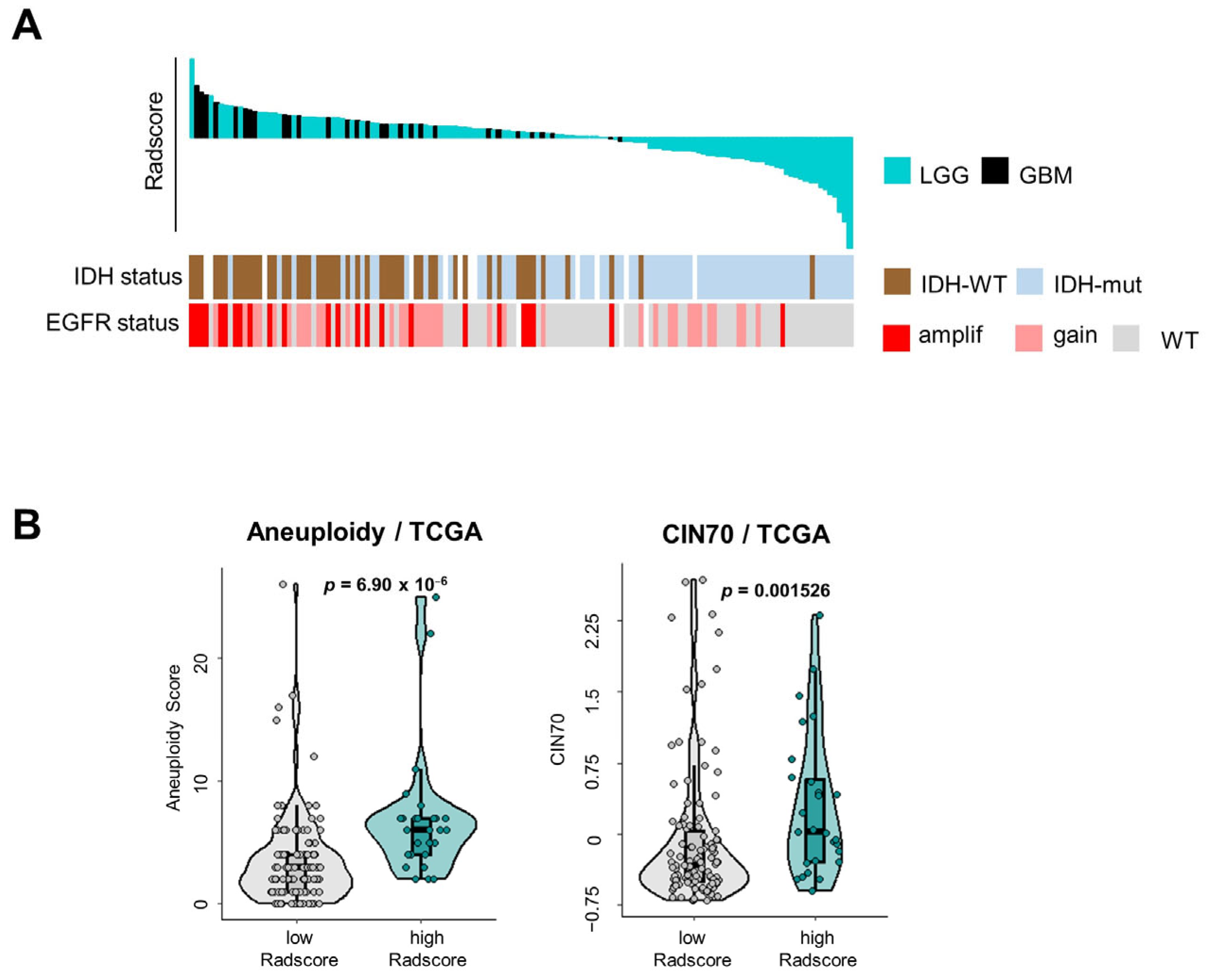
3.2. Clinical and Prognostic Significance of the Radscore
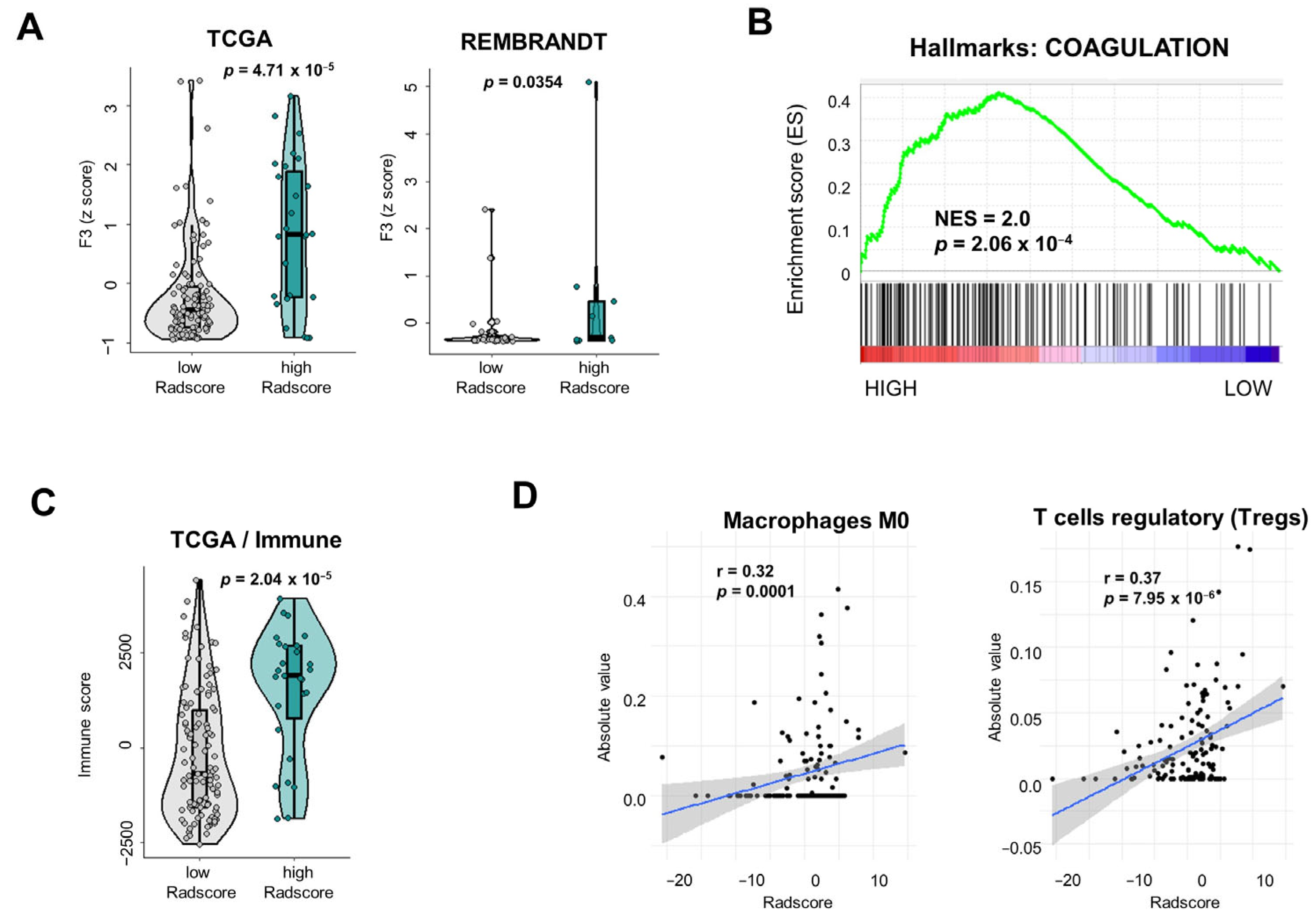
3.3. Tumor Microenvironmental Characteristics of Gliomas Stratified According to Radscore
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in Adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) Consensus Review on Current Management and Future Directions. Neuro-Oncology 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Blom, J.W.; Vanderschoot, J.P.M.; Oostindiër, M.J.; Osanto, S.; van der Meer, F.J.M.; Rosendaal, F.R. Incidence of Venous Thrombosis in a Large Cohort of 66,329 Cancer Patients: Results of a Record Linkage Study. J. Thromb. Haemost. JTH 2006, 4, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.K.; Wun, T.; Harvey, D.; Zhou, H.; White, R.H. Incidence of Venous Thromboembolism and Its Effect on Survival Among Patients with Common Cancers. Arch. Intern. Med. 2006, 166, 458. [Google Scholar] [CrossRef]
- Jo, J.; Diaz, M.; Horbinski, C.; Mackman, N.; Bagley, S.; Broekman, M.; Rak, J.; Perry, J.; Pabinger, I.; Key, N.S.; et al. Epidemiology, Biology, and Management of Venous Thromboembolism in Gliomas: An Interdisciplinary Review. Neuro-Oncology 2023, 25, 1381–1394. [Google Scholar] [CrossRef]
- Yust-Katz, S.; Mandel, J.J.; Wu, J.; Yuan, Y.; Webre, C.; Pawar, T.A.; Lhadha, H.S.; Gilbert, M.R.; Armstrong, T.S. Venous Thromboembolism (VTE) and Glioblastoma. J. Neurooncol. 2015, 124, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Burdett, K.B.; Unruh, D.; Drumm, M.; Steffens, A.; Lamano, J.; Judkins, J.; Schwartz, M.; Javier, R.; Amidei, C.; Lipp, E.S.; et al. Determining Venous Thromboembolism Risk in Patients with Adult-Type Diffuse Glioma. Blood 2023, 141, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Schieppati, F.; Russo, L. Pathophysiology 1. Mechanisms of Thrombosis in Cancer Patients. In Thrombosis and Hemostasis in Cancer; Soff, G., Ed.; Cancer Treatment and Research; Springer International Publishing: Cham, Switerzland, 2019; Volume 179, pp. 11–36. ISBN 978-3-030-20314-6. [Google Scholar]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Rondon, A.M.R.; Kroone, C.; Kapteijn, M.Y.; Versteeg, H.H.; Buijs, J.T. Role of Tissue Factor in Tumor Progression and Cancer-Associated Thrombosis. Semin. Thromb. Hemost. 2019, 45, 396–412. [Google Scholar] [CrossRef]
- Saidak, Z.; Soudet, S.; Lottin, M.; Salle, V.; Sevestre, M.-A.; Clatot, F.; Galmiche, A. A Pan-Cancer Analysis of the Human Tumor Coagulome and Its Link to the Tumor Immune Microenvironment. Cancer Immunol. Immunother. CII 2021, 70, 923–933. [Google Scholar] [CrossRef]
- Galmiche, A.; Rak, J.; Roumenina, L.T.; Saidak, Z. Coagulome and the Tumor Microenvironment: An Actionable Interplay. Trends Cancer 2022, 8, 369–383. [Google Scholar] [CrossRef]
- Mantha, S.; Rak, J. Cancer Genetic Alterations and Risk of Venous Thromboembolism. Thromb. Res. 2022, 213, S29–S34. [Google Scholar] [CrossRef]
- Magnus, N.; Gerges, N.; Jabado, N.; Rak, J. Coagulation-Related Gene Expression Profile in Glioblastoma Is Defined by Molecular Disease Subtype. J. Thromb. Haemost. 2013, 11, 1197–1200. [Google Scholar] [CrossRef]
- Furuta, T.; Negoto, T.; Miyoshi, H.; Moritsubo, M.; Nakamura, H.; Morioka, M.; Akiba, J.; Ohshima, K.; Sugita, Y. Intratumoral Thrombosis as a Histological Biomarker for Predicting Epidermal Growth Factor Receptor Alteration and Poor Prognosis in Patients with Glioblastomas. J. Neurooncol. 2023, 164, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Kaye, B.; Ali, A.; Correa Bastianon Santiago, R.A.; Ibrahim, B.; Isidor, J.; Awad, H.; Sabahi, M.; Obrzut, M.; Adada, B.; Ranjan, S.; et al. The Role of EGFR Amplification in Deep Venous Thrombosis Occurrence in IDH Wild-Type Glioblastoma. Curr. Oncol. 2023, 30, 4946–4956. [Google Scholar] [CrossRef]
- Kapteijn, M.Y.; Kaptein, F.H.J.; Stals, M.A.M.; Klaase, E.E.; García-Ortiz, I.; Van Eijk, R.; Ruano, D.; Van Duinen, S.G.; Cannegieter, S.C.; Taphoorn, M.J.B.; et al. Targeted DNA Sequencing to Identify Genetic Aberrations in Glioblastoma That Underlie Venous Thromboembolism; a Cohort Study. Thromb. Res. 2023, 221, 10–18. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Ngo, B.; Laughney, A.M.; Cavallo, J.-A.; Murphy, C.J.; Ly, P.; Shah, P.; Sriram, R.K.; Watkins, T.B.K.; Taunk, N.K.; et al. Chromosomal Instability Drives Metastasis through a Cytosolic DNA Response. Nature 2018, 553, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Magnus, N.; Garnier, D.; Meehan, B.; McGraw, S.; Lee, T.H.; Caron, M.; Bourque, G.; Milsom, C.; Jabado, N.; Trasler, J.; et al. Tissue Factor Expression Provokes Escape from Tumor Dormancy and Leads to Genomic Alterations. Proc. Natl. Acad. Sci. USA 2014, 111, 3544–3549. [Google Scholar] [CrossRef] [PubMed]
- Auvergne, R.; Wu, C.; Connell, A.; Au, S.; Cornwell, A.; Osipovitch, M.; Benraiss, A.; Dangelmajer, S.; Guerrero-Cazares, H.; Quinones-Hinojosa, A.; et al. PAR1 Inhibition Suppresses the Self-Renewal and Growth of A2B5-Defined Glioma Progenitor Cells and Their Derived Gliomas in Vivo. Oncogene 2016, 35, 3817–3828. [Google Scholar] [CrossRef]
- Krenzlin, H.; Lorenz, V.; Alessandri, B. The Involvement of Thrombin in the Pathogenesis of Glioblastoma. J. Neurosci. Res. 2017, 95, 2080–2085. [Google Scholar] [CrossRef]
- Jeon, H.-M.; Kim, J.-Y.; Cho, H.J.; Lee, W.J.; Nguyen, D.; Kim, S.S.; Oh, Y.T.; Kim, H.-J.; Jung, C.-W.; Pinero, G.; et al. Tissue Factor Is a Critical Regulator of Radiation Therapy-Induced Glioblastoma Remodeling. Cancer Cell 2023, 41, 1480–1497.e9. [Google Scholar] [CrossRef]
- Mu, W.; Schabath, M.B.; Gillies, R.J. Images Are Data: Challenges and Opportunities in the Clinical Translation of Radiomics. Cancer Res. 2022, 82, 2066–2068. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Jang, J.; Kim, B.-S.; Ahn, K.-J. Pretreatment MR-Based Radiomics in Patients with Glioblastoma: A Systematic Review and Meta-Analysis of Prognostic Endpoints. Eur. J. Radiol. 2023, 168, 111130. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Han, K.; Ahn, S.S.; Bae, S.; Choi, Y.S.; Chang, J.H.; Kim, S.H.; Kang, S.-G.; Lee, S.-K. Prediction of IDH1-Mutation and 1p/19q-Codeletion Status Using Preoperative MR Imaging Phenotypes in Lower Grade Gliomas. AJNR Am. J. Neuroradiol. 2018, 39, 37–42. [Google Scholar] [CrossRef]
- Park, C.J.; Han, K.; Kim, H.; Ahn, S.S.; Choi, D.; Park, Y.W.; Chang, J.H.; Kim, S.H.; Cha, S.; Lee, S.-K. MRI Features May Predict Molecular Features of Glioblastoma in Isocitrate Dehydrogenase Wild-Type Lower-Grade Gliomas. AJNR Am. J. Neuroradiol. 2021, 42, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ammari, S.; Lawrance, L.; Quillent, A.; Assi, T.; Lassau, N.; Chouzenoux, E. Radiomics-Based Method for Predicting the Glioma Subtype as Defined by Tumor Grade, IDH Mutation, and 1p/19q Codeletion. Cancers 2022, 14, 1778. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Guo, F.; Xu, Z.; Li, C.; Wei, W.; Tian, P.; Liu, T.; Liu, L.; Chen, G.; Ye, J.; et al. Radiomics Signature: A Potential Biomarker for the Prediction of MGMT Promoter Methylation in Glioblastoma. J. Magn. Reson. Imaging 2018, 47, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Beig, N.; Patel, J.; Prasanna, P.; Hill, V.; Gupta, A.; Correa, R.; Bera, K.; Singh, S.; Partovi, S.; Varadan, V.; et al. Radiogenomic Analysis of Hypoxia Pathway Is Predictive of Overall Survival in Glioblastoma. Sci. Rep. 2018, 8, 7. [Google Scholar] [CrossRef]
- Fan, X.; Li, J.; Huang, B.; Lu, H.; Lu, C.; Pan, M.; Wang, X.; Zhang, H.; You, Y.; Wang, X.; et al. Noninvasive Radiomics Model Reveals Macrophage Infiltration in Glioma. Cancer Lett. 2023, 573, 216380. [Google Scholar] [CrossRef]
- Li, G.; Li, L.; Li, Y.; Qian, Z.; Wu, F.; He, Y.; Jiang, H.; Li, R.; Wang, D.; Zhai, Y.; et al. An MRI Radiomics Approach to Predict Survival and Tumour-Infiltrating Macrophages in Gliomas. Brain 2022, 145, 1151–1161. [Google Scholar] [CrossRef]
- Liu, D.; Chen, J.; Ge, H.; Yan, Z.; Luo, B.; Hu, X.; Yang, K.; Liu, Y.; Liu, H.; Zhang, W. Radiogenomics to Characterize the Immune-Related Prognostic Signature Associated with Biological Functions in Glioblastoma. Eur. Radiol. 2022, 33, 209–220. [Google Scholar] [CrossRef]
- Beig, N.; Bera, K.; Tiwari, P. Introduction to Radiomics and Radiogenomics in Neuro-Oncology: Implications and Challenges. Neuro-Oncol. Adv. 2020, 2, iv3–iv14. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Manjila, S.; Sakla, N.; True, A.; Wardeh, A.H.; Beig, N.; Vaysberg, A.; Matthews, J.; Prasanna, P.; Spektor, V. Radiomics and Radiogenomics in Gliomas: A Contemporary Update. Br. J. Cancer 2021, 125, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Chirica, C.; Haba, D.; Cojocaru, E.; Mazga, A.I.; Eva, L.; Dobrovat, B.I.; Chirica, S.I.; Stirban, I.; Rotundu, A.; Leon, M.M. One Step Forward-The Current Role of Artificial Intelligence in Glioblastoma Imaging. Life 2023, 13, 1561. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive Genomic Characterization Defines Human Glioblastoma Genes and Core Pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Barthel, F.P.; Malta, T.M.; Sabedot, T.S.; Salama, S.R.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 2016, 164, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Gusev, Y.; Bhuvaneshwar, K.; Song, L.; Zenklusen, J.-C.; Fine, H.; Madhavan, S. The REMBRANDT Study, a Large Collection of Genomic Data from Brain Cancer Patients. Sci. Data 2018, 5, 180158. [Google Scholar] [CrossRef] [PubMed]
- Bakas, S.; Akbari, H.; Sotiras, A.; Bilello, M.; Rozycki, M.; Kirby, J.S.; Freymann, J.B.; Farahani, K.; Davatzikos, C. Advancing The Cancer Genome Atlas Glioma MRI Collections with Expert Segmentation Labels and Radiomic Features. Sci. Data 2017, 4, 170117. [Google Scholar] [CrossRef] [PubMed]
- Sayah, A.; Bencheqroun, C.; Bhuvaneshwar, K.; Belouali, A.; Bakas, S.; Sako, C.; Davatzikos, C.; Alaoui, A.; Madhavan, S.; Gusev, Y. Enhancing the REMBRANDT MRI Collection with Expert Segmentation Labels and Quantitative Radiomic Features. Sci. Data 2022, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.J.; Spiliopoulou, A.; Goldmann, K.; Pitzalis, C.; McKeigue, P.; Barnes, M.R. Nestedcv: An R Package for Fast Implementation of Nested Cross-Validation with Embedded Feature Selection Designed for Transcriptomics and High-Dimensional Data. Bioinforma. Adv. 2023, 3, vbad048. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Carter, S.L.; Eklund, A.C.; Kohane, I.S.; Harris, L.N.; Szallasi, Z. A Signature of Chromosomal Instability Inferred from Gene Expression Profiles Predicts Clinical Outcome in Multiple Human Cancers. Nat. Genet. 2006, 38, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.E.; Sathe, A.A.; Xing, C.; Mirchia, K.; Viapiano, M.S.; Snuderl, M.; Abdullah, K.G.; Hatanpaa, K.J.; Walker, J.M. Molecular Signatures of Chromosomal Instability Correlate with Copy Number Variation Patterns and Patient Outcome in IDH-Mutant and IDH-Wildtype Astrocytomas. J. Neuropathol. Exp. Neurol. 2021, 80, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Shahmoradgoli, M.; Martínez, E.; Vegesna, R.; Kim, H.; Torres-Garcia, W.; Treviño, V.; Shen, H.; Laird, P.W.; Levine, D.A. Inferring Tumour Purity and Stromal and Immune Cell Admixture from Expression Data. Nat. Commun. 2013, 4, 2612. [Google Scholar] [CrossRef]
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. In Cancer Systems Biology; Von Stechow, L., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1711, pp. 243–259. ISBN 978-1-4939-7492-4. [Google Scholar]
- Newman, A.M.; Steen, C.B.; Liu, C.L.; Gentles, A.J.; Chaudhuri, A.A.; Scherer, F.; Khodadoust, M.S.; Esfahani, M.S.; Luca, B.A.; Steiner, D.; et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotech. 2019, 37, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zou, C.; Guan, G.; Guo, Q.; Yan, Z.; Liu, T.; Shen, S.; Xu, X.; Chen, C.; Lin, Z.; et al. Development and Validation of an Interferon Signature Predicting Prognosis and Treatment Response for Glioblastoma. OncoImmunology 2019, 8, e1621677. [Google Scholar] [CrossRef]
- White, K.; Connor, K.; Meylan, M.; Bougoüin, A.; Salvucci, M.; Bielle, F.; O’Farrell, A.C.; Sweeney, K.; Weng, L.; Bergers, G.; et al. Identification, Validation and Biological Characterisation of Novel Glioblastoma Tumour Microenvironment Subtypes: Implications for Precision Immunotherapy. Ann. Oncol. 2023, 34, 300–314. [Google Scholar] [CrossRef]
- Gershon, R.; Polevikov, A.; Karepov, Y.; Shenkar, A.; Ben-Horin, I.; Alter Regev, T.; Dror-Levinsky, M.; Lipczyc, K.; Gasri-Plotnitsky, L.; Diamant, G.; et al. Frequencies of 4 Tumor-Infiltrating Lymphocytes Potently Predict Survival in Glioblastoma, an Immune Desert. Neuro-Oncology 2023, 26, 473–487. [Google Scholar] [CrossRef]

| Clinical Characteristics | TCGA (n = 136) | REMBRANDT (n = 39) |
|---|---|---|
| Age (years): mean, range | 48.8 (20–79) | 48 (18–87) |
| Sex (male, female, NA) | 69 (51%), 67 (49%), 0 (0%) | 21 (54%), 14 (36%), 4 (10%) |
| Histology: | ||
| Astrocytoma | 79 (58%) | 27 (69%) |
| Oligodendroglioma | 28 (21%) | 5 (13%) |
| Glioblastoma | 29 (21%) | 7 (18%) |
| Grade: II, III, IV | 48 (35%), 58 (43%), 29 (21%) | 20 (51%), 12 (31%), 7 (18%) |
| IDH1 status: | ||
| Mutated, WT, NA | 75 (55%), 51 (38%), 10 (7%) | 0, 0, 39 (100%) |
| Parameters | TCGA | REMBRANDT |
|---|---|---|
| AUC | 0.87 [0.81–0.94] | 0.78 [0.56–1.00] |
| Sensitivity | 0.89 [0.71–0.98] | 0.86 [0.42–1.00] |
| Specificity | 0.76 [0.67–0.84] | 0.72 [0.53–0.86] |
| Positive predictive value | 0.48 [0.34–0.63] | 0.40 [0.16–0.68] |
| Negative predictive value | 0.97 [0.90–0.99] | 0.96 [0.79–1.00] |
| Accuracy | 0.79 [0.71–0.85] | 0.74 [0.58–0.87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saidak, Z.; Laville, A.; Soudet, S.; Sevestre, M.-A.; Constans, J.-M.; Galmiche, A. An MRI Radiomics Approach to Predict the Hypercoagulable Status of Gliomas. Cancers 2024, 16, 1289. https://doi.org/10.3390/cancers16071289
Saidak Z, Laville A, Soudet S, Sevestre M-A, Constans J-M, Galmiche A. An MRI Radiomics Approach to Predict the Hypercoagulable Status of Gliomas. Cancers. 2024; 16(7):1289. https://doi.org/10.3390/cancers16071289
Chicago/Turabian StyleSaidak, Zuzana, Adrien Laville, Simon Soudet, Marie-Antoinette Sevestre, Jean-Marc Constans, and Antoine Galmiche. 2024. "An MRI Radiomics Approach to Predict the Hypercoagulable Status of Gliomas" Cancers 16, no. 7: 1289. https://doi.org/10.3390/cancers16071289
APA StyleSaidak, Z., Laville, A., Soudet, S., Sevestre, M.-A., Constans, J.-M., & Galmiche, A. (2024). An MRI Radiomics Approach to Predict the Hypercoagulable Status of Gliomas. Cancers, 16(7), 1289. https://doi.org/10.3390/cancers16071289






