Enhancing Whole-Brain Magnetic Field Homogeneity for 3D-Magnetic Resonance Spectroscopic Imaging with a Novel Unified Coil: A Preliminary Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dataset and Imaging Protocol
2.2. Coil Design for Whole-Brain Shimming
2.3. B0 Shim Field Analysis and 3D-MRSI Brain Coverage Analysis
2.4. Statistical Analysis
3. Results
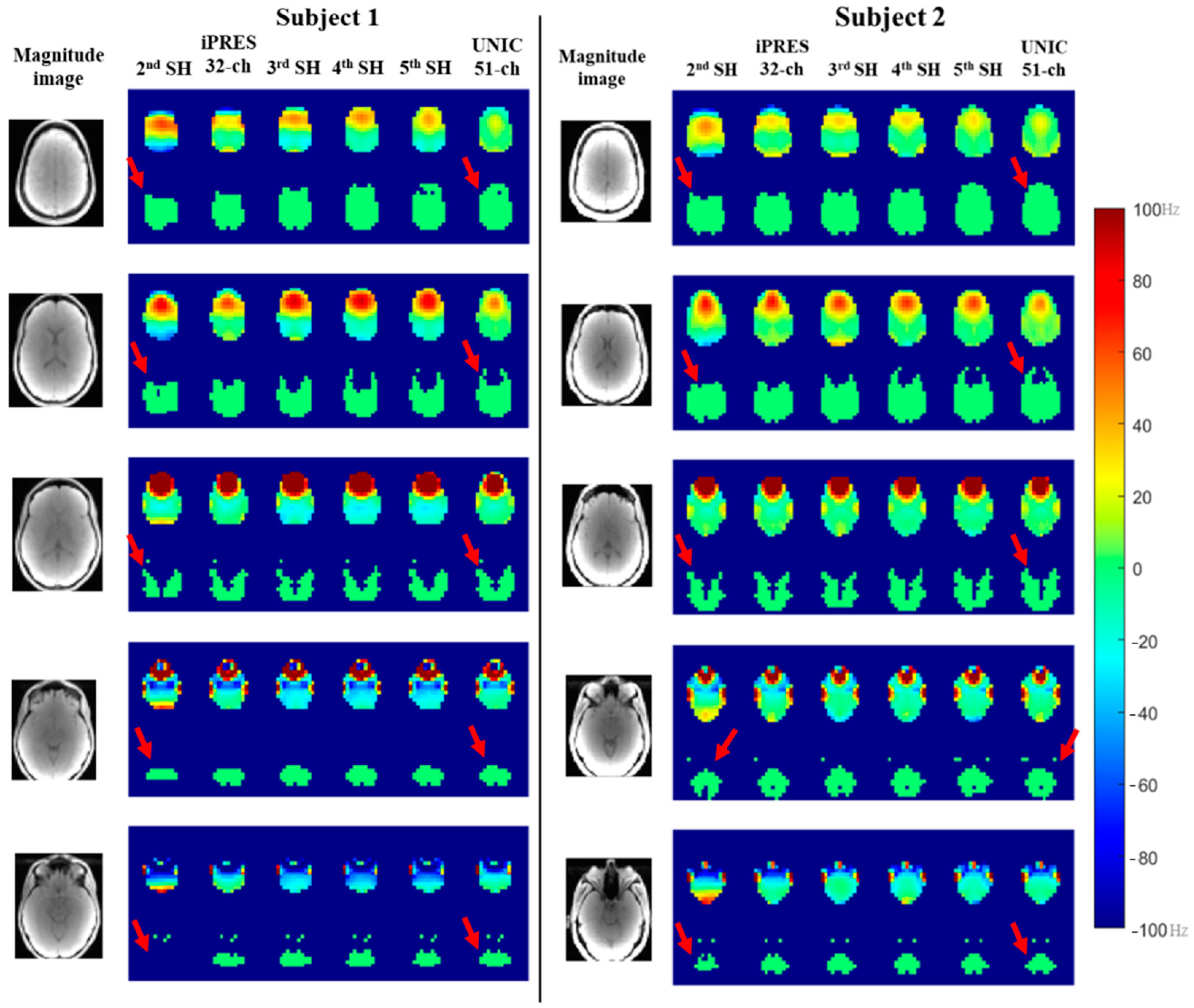
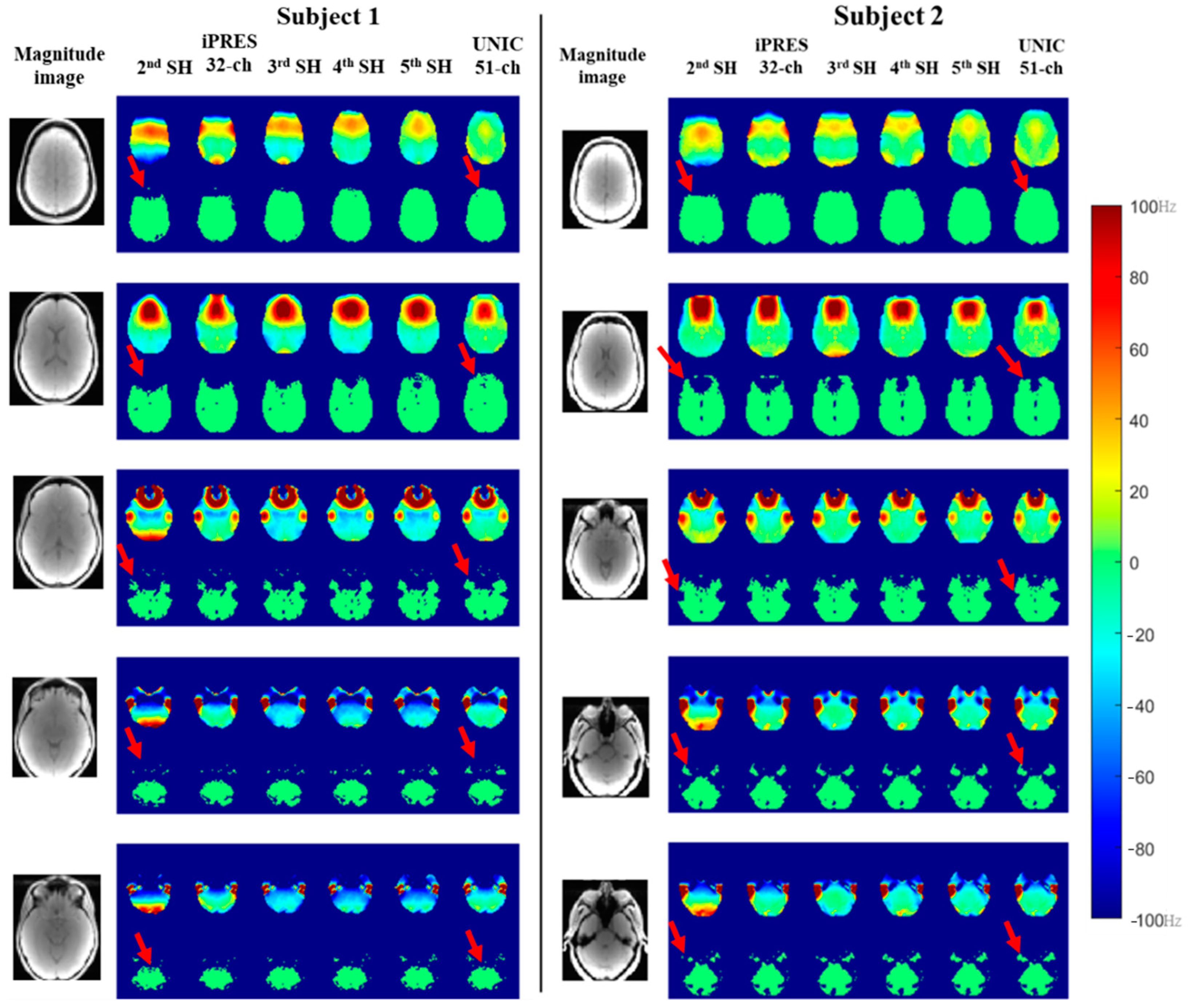
3.1. Whole-Brain B0 Shimming Performance of Spherical Harmonic (SH), iPRES, and UNIC Shim
3.2. Whole-Brain Coverage of 3D-MRSI with SH Shim, iPRES, and UNIC Shim
3.3. Region-Specific Brain Coverage of 3D-MRSI with SH Shim, iPRES, and UNIC Shim
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cordova, J.S.; Gurbani, S.S.; Olson, J.J.; Liang, Z.; Cooper, L.A.D.; Shu, H.-K.G.; Schreibmann, E.; Neill, S.G.; Hadjipanayis, C.G.; Holder, C.A.; et al. A Systematic Pipeline for the Objective Comparison of Whole-Brain Spectroscopic MRI with Histology in Biopsy Specimens from Grade 3 Glioma. Tomography 2016, 2, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Cordova, J.S.; Shu, H.-K.G.; Liang, Z.; Gurbani, S.S.; Cooper, L.A.D.; Holder, C.A.; Olson, J.J.; Kairdolf, B.; Schreibmann, E.; Neill, S.G.; et al. Whole-Brain Spectroscopic MRI Biomarkers Identify Infiltrating Margins in Glioblastoma Patients. Neuro-Oncology 2016, 18, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Posse, S.; Otazo, R.; Dager, S.R.; Alger, J. MR Spectroscopic Imaging: Principles and Recent Advances. J. Magn. Reson. Imaging 2013, 37, 1301–1325. [Google Scholar] [CrossRef] [PubMed]
- Bogner, W.; Gagoski, B.; Hess, A.T.; Bhat, H.; Tisdall, M.D.; van der Kouwe, A.J.W.; Strasser, B.; Marjańska, M.; Trattnig, S.; Grant, E.; et al. 3D GABA Imaging with Real-Time Motion Correction, Shim Update and Reacquisition of Adiabatic Spiral MRSI. NeuroImage 2014, 103, 290–302. [Google Scholar] [CrossRef]
- Maudsley, A.A.; Andronesi, O.C.; Barker, P.B.; Bizzi, A.; Bogner, W.; Henning, A.; Nelson, S.J.; Posse, S.; Shungu, D.C.; Soher, B.J. Advanced Magnetic Resonance Spectroscopic Neuroimaging: Experts’ Consensus Recommendations. NMR Biomed. 2021, 34, e4309. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Lustig, M.; Chen, A.P.; Crane, J.; Kerr, A.; Kelley, D.A.C.; Hurd, R.; Kurhanewicz, J.; Nelson, S.J.; Pauly, J.M.; et al. Compressed Sensing for Resolution Enhancement of Hyperpolarized 13C Flyback 3D-MRSI. J. Magn. Reson. 2008, 192, 258–264. [Google Scholar] [CrossRef]
- Ma, J.; Wismans, C.; Cao, Z.; Klomp, D.W.J.; Wijnen, J.P.; Grissom, W.A. Tailored Spiral In-out Spectral-Spatial Water Suppression Pulses for Magnetic Resonance Spectroscopic Imaging. Magn. Reson. Med. 2018, 79, 31–40. [Google Scholar] [CrossRef]
- Weinberg, B.D.; Kuruva, M.; Shim, H.; Mullins, M.E. Clinical Applications of Magnetic Resonance Spectroscopy (MRS) in of Brain Tumors: From Diagnosis to Treatment. Radiol. Clin. N. Am. 2021, 59, 349–362. [Google Scholar] [CrossRef]
- Zhong, J.; Huang, V.; Gurbani, S.S.; Ramesh, K.; Scott Cordova, J.; Schreibmann, E.; Shu, H.-K.G.; Olson, J.; Han, H.; Giuffrida, A.; et al. 3D Whole-Brain Metabolite Imaging to Improve Characterization of Low-to-Intermediate Grade Gliomas. J. Neurooncol. 2021, 153, 303–311. [Google Scholar] [CrossRef]
- Horská, A.; Barker, P.B. Imaging of Brain Tumors: MR Spectroscopy and Metabolic Imaging. Neuroimaging Clin. N. Am. 2010, 20, 293–310. [Google Scholar] [CrossRef]
- Sabati, M.; Sheriff, S.; Gu, M.; Wei, J.; Zhu, H.; Barker, P.B.; Spielman, D.M.; Alger, J.R.; Maudsley, A.A. Multivendor Implementation and Comparison of Volumetric Whole-Brain Echo-Planar MR Spectroscopic Imaging. Magn. Reson. Med. 2015, 74, 1209–1220. [Google Scholar] [CrossRef]
- Juchem, C.; de Graaf, R.A. B0 Magnetic Field Homogeneity and Shimming for In Vivo MR Spectroscopy. Anal. Biochem. 2017, 529, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Fleysher, R.; Fleysher, L.; Liu, S.; Gonen, O. On the Voxel Size and Magnetic Field Strength Dependence of Spectral Resolution in Magnetic Resonance Spectroscopy. Magn. Reson. Imaging 2009, 27, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Laprie, A.; Noel, G.; Chaltiel, L.; Truc, G.; Sunyach, M.-P.; Charissoux, M.; Magne, N.; Auberdiac, P.; Biau, J.; Ken, S.; et al. Randomized Phase III Trial of Metabolic Imaging-Guided Dose Escalation of Radio-Chemotherapy in Patients with Newly Diagnosed Glioblastoma (SPECTRO GLIO Trial). Neuro-Oncology 2023, 26, noad119. [Google Scholar] [CrossRef]
- Shu, H.-K.G.; Shim, H. SPECTRO GLIO Trial Aftermath: Where Do We Go from Here? Neuro-Oncology 2023, 26, noad166. [Google Scholar] [CrossRef] [PubMed]
- Mellon, E. Whole Brain Spectroscopic MRI for RT Dose Escalation in Glioblastoma. In Proceedings of the ASTRO Annual Meeting, Chicago, IL, USA, 24–27 October 2021. [Google Scholar]
- Pan, J.W.; Lo, K.-M.; Hetherington, H.P. Role of Very High Order and Degree B0 Shimming for Spectroscopic Imaging of the Human Brain at 7 Tesla. Magn. Reson. Med. 2012, 68, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Boer, V.O.; Andersen, M.; Lind, A.; Lee, N.G.; Marsman, A.; Petersen, E.T. MR Spectroscopy Using Static Higher Order Shimming with Dynamic Linear Terms (HOS-DLT) for Improved Water Suppression, Interleaved MRS-fMRI, and Navigator-based Motion Correction at 7T. Magn. Reson. Med. 2020, 84, 1101–1112. [Google Scholar] [CrossRef]
- Stockmann, J.P.; Wald, L.L. In Vivo B0 Field Shimming Methods for MRI at 7T. NeuroImage 2018, 168, 71–87. [Google Scholar] [CrossRef]
- Chang, P.; Nassirpour, S.; Henning, A. Modeling Real Shim Fields for Very High Degree (and Order) B0 Shimming of the Human Brain at 9.4 T. Magn. Reson. Med. 2018, 79, 529–540. [Google Scholar] [CrossRef]
- Hetherington, H.P.; Chu, W.-J.; Gonen, O.; Pan, J.W. Robust Fully Automated Shimming of the Human Brain for High-Field 1H Spectroscopic Imaging. Magn. Reson. Med. 2006, 56, 26–33. [Google Scholar] [CrossRef]
- Juchem, C.; Nixon, T.W.; McIntyre, S.; Boer, V.O.; Rothman, D.L.; de Graaf, R.A. Dynamic Multi-Coil Shimming of the Human Brain at 7T. J. Magn. Reson. 2011, 212, 280–288. [Google Scholar] [CrossRef]
- Juchem, C.; Umesh Rudrapatna, S.; Nixon, T.W.; de Graaf, R.A. Dynamic Multi-Coil Technique (DYNAMITE) Shimming for Echo-Planar Imaging of the Human Brain at 7 Tesla. NeuroImage 2015, 105, 462–472. [Google Scholar] [CrossRef]
- Han, H.; Song, A.W.; Truong, T.-K. Integrated Parallel Reception, Excitation, and Shimming (iPRES). Magn. Reson. Med. 2013, 70, 241–247. [Google Scholar] [CrossRef]
- Stockmann, J.P.; Witzel, T.; Keil, B.; Polimeni, J.R.; Mareyam, A.; LaPierre, C.; Setsompop, K.; Wald, L.L. A 32-Channel Combined RF and B0 Shim Array for 3T Brain Imaging. Magn. Reson. Med. 2016, 75, 441–451. [Google Scholar] [CrossRef]
- Truong, T.-K.; Darnell, D.; Song, A.W. Integrated RF/Shim Coil Array for Parallel Reception and Localized B0 Shimming in the Human Brain. NeuroImage 2014, 103, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, D.A.; Setsompop, K. Ultra-Fast MRI of the Human Brain with Simultaneous Multi-Slice Imaging. J. Magn. Reson. 2013, 229, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lustig, M.; Grissom, W.A. Root-Flipped Multiband Refocusing Pulses. Magn. Reson. Med. 2016, 75, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; Serry, F.; Hu, P.; Fan, Z.; Shim, H.; Christodoulou, A.; Wang, N.; Kwan, A.; Xie, Y.; Huang, Y.; et al. Ultra-Homogeneous B0 Field for High-Field Body Magnetic Resonance Imaging with Unified Shim-RF Coil. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Han, H. Multi-Coil B0 Shimming in the Body. In Proceedings of the 28th Annual Meeting of the ISMRM 2020, Virtual, 8–14 August 2020. E0912. [Google Scholar]
- Yang, H.; Stager, J.; Azab, L.; Liu, W.; Lu, M.; Huang, Y.; Han, H. Whole Heart High-Order B0 Shimming at 3T Using a UNIfied Coil (UNIC) for RF Receive and Shimming. In Proceedings of the 28th Annual Meeting of the ISMRM 2020, Virtual, 8–14 August 2020; p. 2183. [Google Scholar]
- Wang, N.; Serry, F.; Ocasio, M.; Xie, Y.; Huang, Y.; Li, X.; Han, P.; Cao, T.; Ma, S.; Han, F.; et al. Integrated high-order B0 shimming for multiparametric quantitative liver imagingat 3T using a UNIfied Coil (UNIC). In Proceedings of the 31st Annual Meeting of the ISMRM-ESMRM 2022, London, UK, 7–12 May 2022; p. 0028. [Google Scholar]
- Serry, F.M.; Chen, J.; Christodoulou, A.G.; Huang, Y.; Han, F.; Bae, W.; Chung, C.; Handlin, R.; Stager, J.; Dausch, M.; et al. Improving MRI Near Metal with Local B0 Shimming using a Unified Shim-RF Coil (UNIC): First Case Study, Hip Prosthesis in Phantom. In Proceedings of the 28th Annual Meeting of the ISMRM 2020, Virtual, 8–14 August 2020; p. 3110. [Google Scholar]
- Stockmann, J.P.; Guerin, B.; Wald, L.L. Improving the Efficiency of Integrated RF-Shim Arrays Using Hybrid Coil Designs and Channel Placement and Compression via a Genetic Algorithm. In Proceedings of the 24th Annual Meeting of the ISMRM 2016, Singapore, 7–13 May 2016; p. 1153. [Google Scholar]
- Van Essen, D.C.; Ugurbil, K.; Auerbach, E.; Barch, D.; Behrens, T.E.J.; Bucholz, R.; Chang, A.; Chen, L.; Corbetta, M.; Curtiss, S.W.; et al. The Human Connectome Project: A Data Acquisition Perspective. NeuroImage 2012, 62, 2222–2231. [Google Scholar] [CrossRef]
- Smith, S.M. Fast Robust Automated Brain Extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef]
- Tkáč, I.; Andersen, P.; Adriany, G.; Merkle, H.; Uǧurbil, K.; Gruetter, R. In Vivo 1H NMR Spectroscopy of the Human Brain at 7 T. Magn. Reson. Med. 2001, 46, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An Automated Labeling System for Subdividing the Human Cerebral Cortex on MRI Scans into Gyral Based Regions of Interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Hsu, J.-J.; Glover, G.H. Mitigation of Susceptibility-Induced Signal Loss in Neuroimaging Using Localized Shim Coils. Magn. Reson. Med. 2005, 53, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Stockmann, J.P.; Arango, N.; Witzel, T.; Scheffler, K.; Wald, L.L.; Lin, F.-H. An Orthogonal Shim Coil for 3T Brain Imaging. Magn. Reson. Med. 2020, 83, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Darnell, D.; Ma, Y.; Wang, H.; Robb, F.; Song, A.W.; Truong, T.-K. Adaptive Integrated Parallel Reception, Excitation, and Shimming (iPRES-A) with Microelectromechanical Systems Switches. Magn. Reson. Med. 2018, 80, 371–379. [Google Scholar] [CrossRef]
- Treadway, M.T.; Waskom, M.L.; Dillon, D.G.; Holmes, A.J.; Park, M.T.M.; Chakravarty, M.M.; Dutra, S.J.; Polli, F.E.; Iosifescu, D.V.; Fava, M.; et al. Illness Progression, Recent Stress, and Morphometry of Hippocampal Subfields and Medial Prefrontal Cortex in Major Depression. Biol. Psychiatry 2015, 77, 285–294. [Google Scholar] [CrossRef]
- Treadway, M.; Buckholtz, J.; Zald, D. Perceived Stress Predicts Altered Reward and Loss Feedback Processing in Medial Prefrontal Cortex. Front. Hum. Neurosci. 2013, 7, 180. [Google Scholar] [CrossRef]
- Pinho Meneses, B.; Stockmann, J.P.; Arango, N.; Gapais, P.-F.; Giacomini, E.; Mauconduit, F.; Gras, V.; Boulant, N.; Vignaud, A.; Luong, M.; et al. Shim Coils Tailored for Correcting B0 Inhomogeneity in the Human Brain (SCOTCH): Design Methodology and 48-Channel Prototype Assessment in 7-Tesla MRI. NeuroImage 2022, 261, 119498. [Google Scholar] [CrossRef]



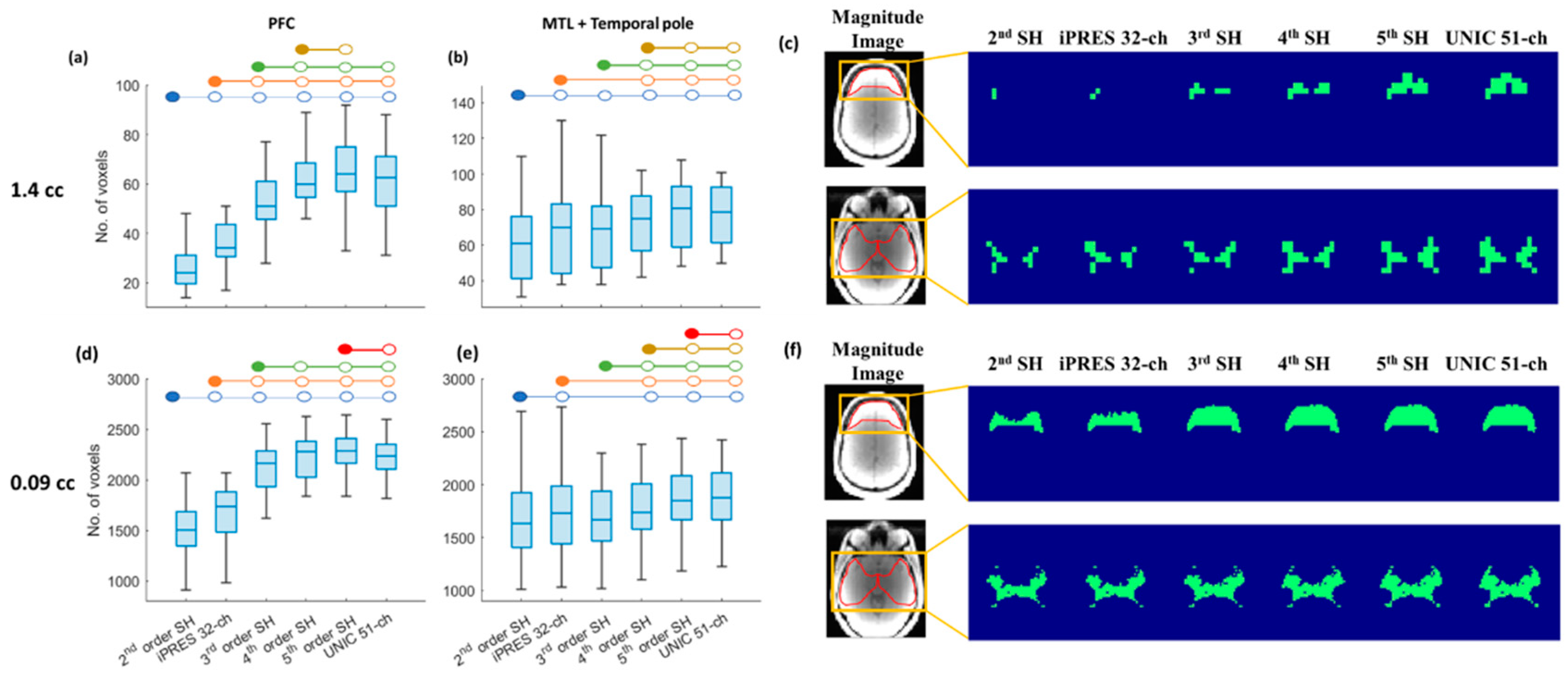
| B0 Field Shimming Methods | 1.44 cc ƚ | 0.09 cc ƚ |
|---|---|---|
| 2nd-order SH | 45 ± 7 | 74 ± 4 |
| iPRES 32-ch | 54 ± 6 | 77 ± 3 |
| 3rd-order SH | 56 ± 6 | 79 ± 3 |
| 4th-order SH | 60 ± 6 | 80 ± 3 |
| 5th-order SH | 62 ± 6 | 81 ± 3 |
| UNIC 51-ch | 61 ± 6 | 81 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malagi, A.V.; Li, X.; Zhang, N.; Liu, Y.; Huang, Y.; Serry, F.M.; Long, Z.; Yang, C.-C.; Shan, Y.; Cai, Y.; et al. Enhancing Whole-Brain Magnetic Field Homogeneity for 3D-Magnetic Resonance Spectroscopic Imaging with a Novel Unified Coil: A Preliminary Study. Cancers 2024, 16, 1233. https://doi.org/10.3390/cancers16061233
Malagi AV, Li X, Zhang N, Liu Y, Huang Y, Serry FM, Long Z, Yang C-C, Shan Y, Cai Y, et al. Enhancing Whole-Brain Magnetic Field Homogeneity for 3D-Magnetic Resonance Spectroscopic Imaging with a Novel Unified Coil: A Preliminary Study. Cancers. 2024; 16(6):1233. https://doi.org/10.3390/cancers16061233
Chicago/Turabian StyleMalagi, Archana Vadiraj, Xinqi Li, Na Zhang, Yucen Liu, Yuheng Huang, Fardad Michael Serry, Ziyang Long, Chia-Chi Yang, Yujie Shan, Yubin Cai, and et al. 2024. "Enhancing Whole-Brain Magnetic Field Homogeneity for 3D-Magnetic Resonance Spectroscopic Imaging with a Novel Unified Coil: A Preliminary Study" Cancers 16, no. 6: 1233. https://doi.org/10.3390/cancers16061233
APA StyleMalagi, A. V., Li, X., Zhang, N., Liu, Y., Huang, Y., Serry, F. M., Long, Z., Yang, C.-C., Shan, Y., Cai, Y., Zepeda, J., Binesh, N., Li, D., Yang, H.-J., & Han, H. (2024). Enhancing Whole-Brain Magnetic Field Homogeneity for 3D-Magnetic Resonance Spectroscopic Imaging with a Novel Unified Coil: A Preliminary Study. Cancers, 16(6), 1233. https://doi.org/10.3390/cancers16061233





