Adaptive Radiotherapy: Next-Generation Radiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Evolution of Radiotherapy and Why We Need ART
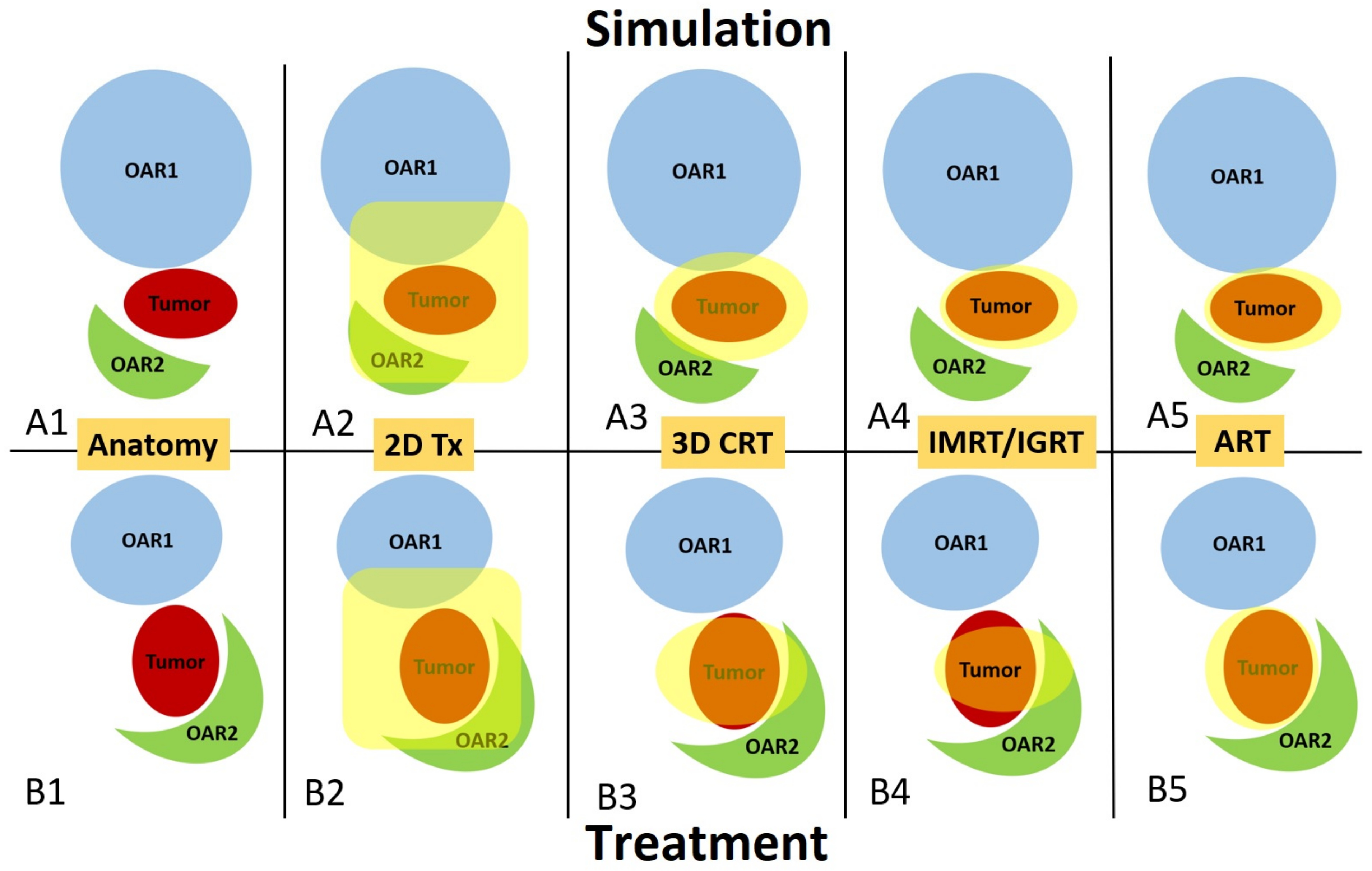
3. Frequency, General Workflow, and Offline vs. Online ART
3.1. Offline ART
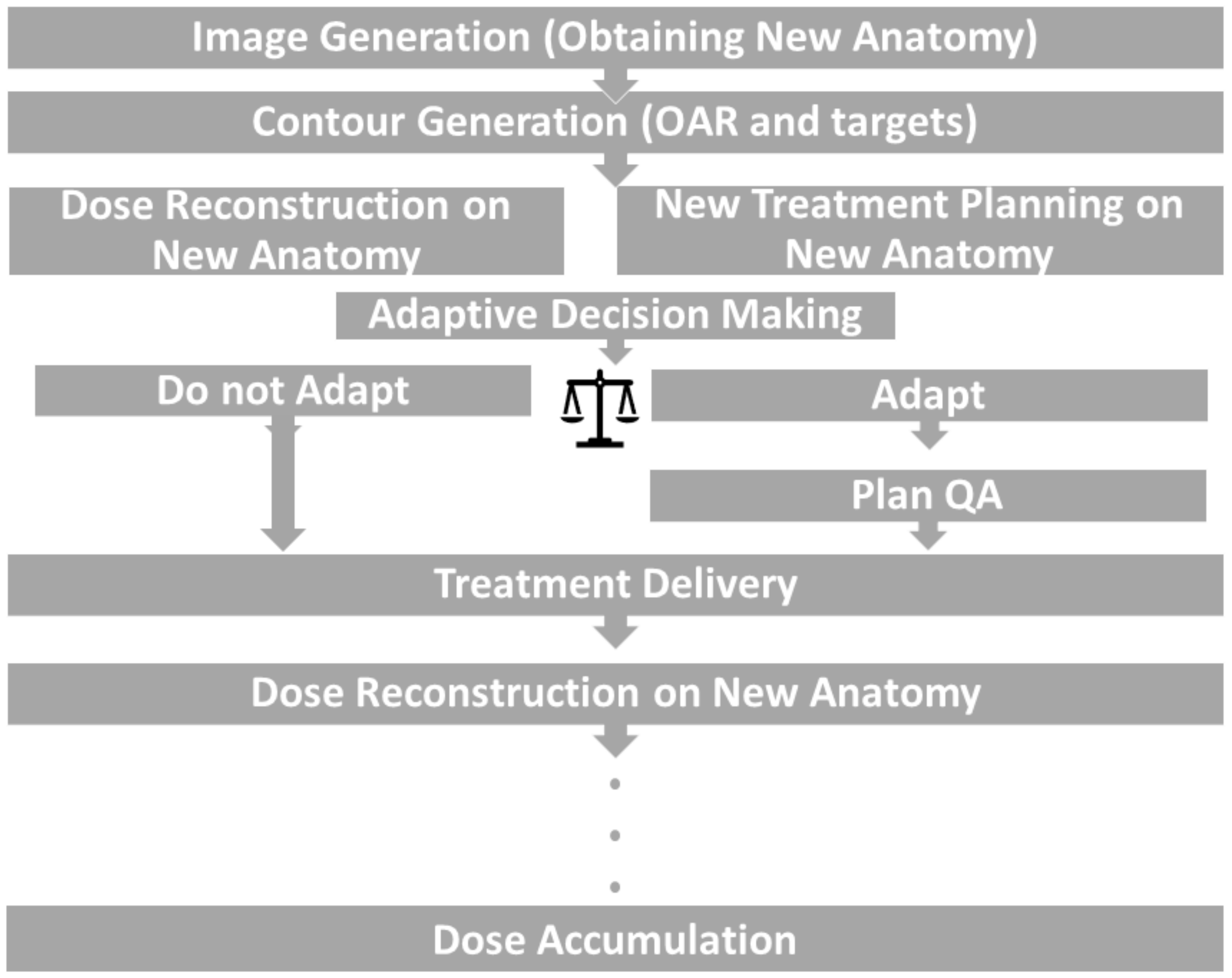
3.2. Online ART
3.3. Resource Considerations and Role of AI
4. Three Major Imaging Modalities for Online ART
4.1. MRI-Based Online ART
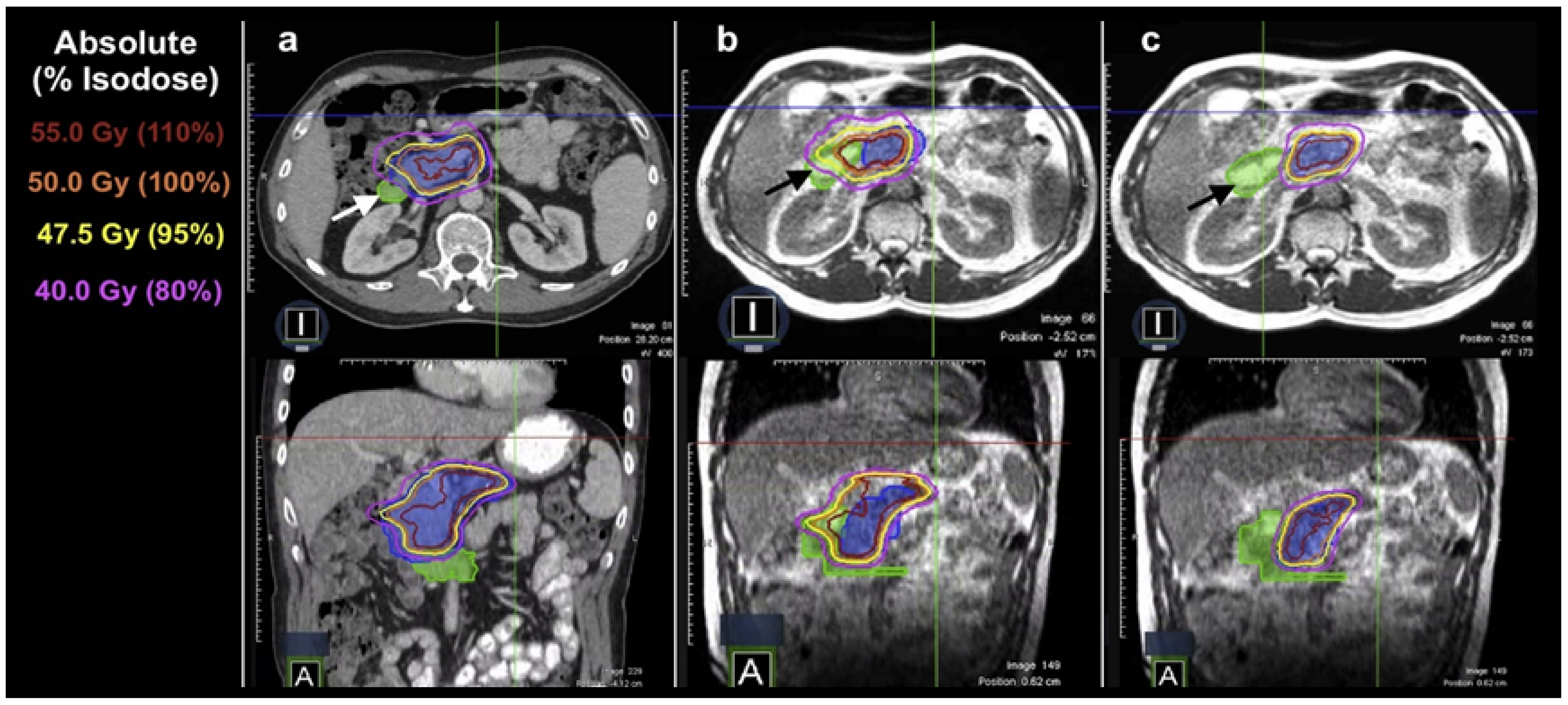
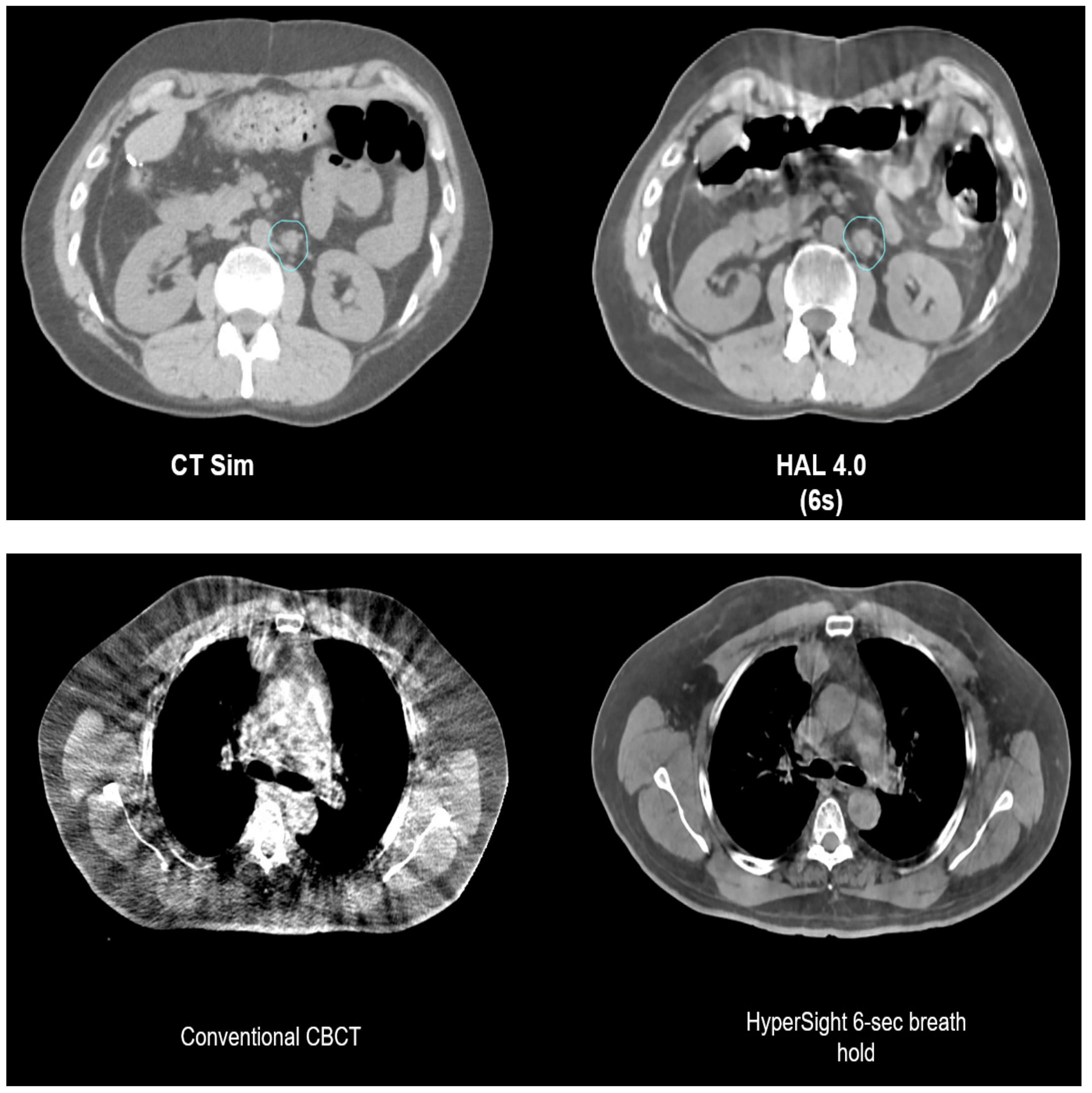
4.2. CBCT-Based Online ART
4.3. PET-Based ART
5. Clinical Results of ART
5.1. Cervical Cancer
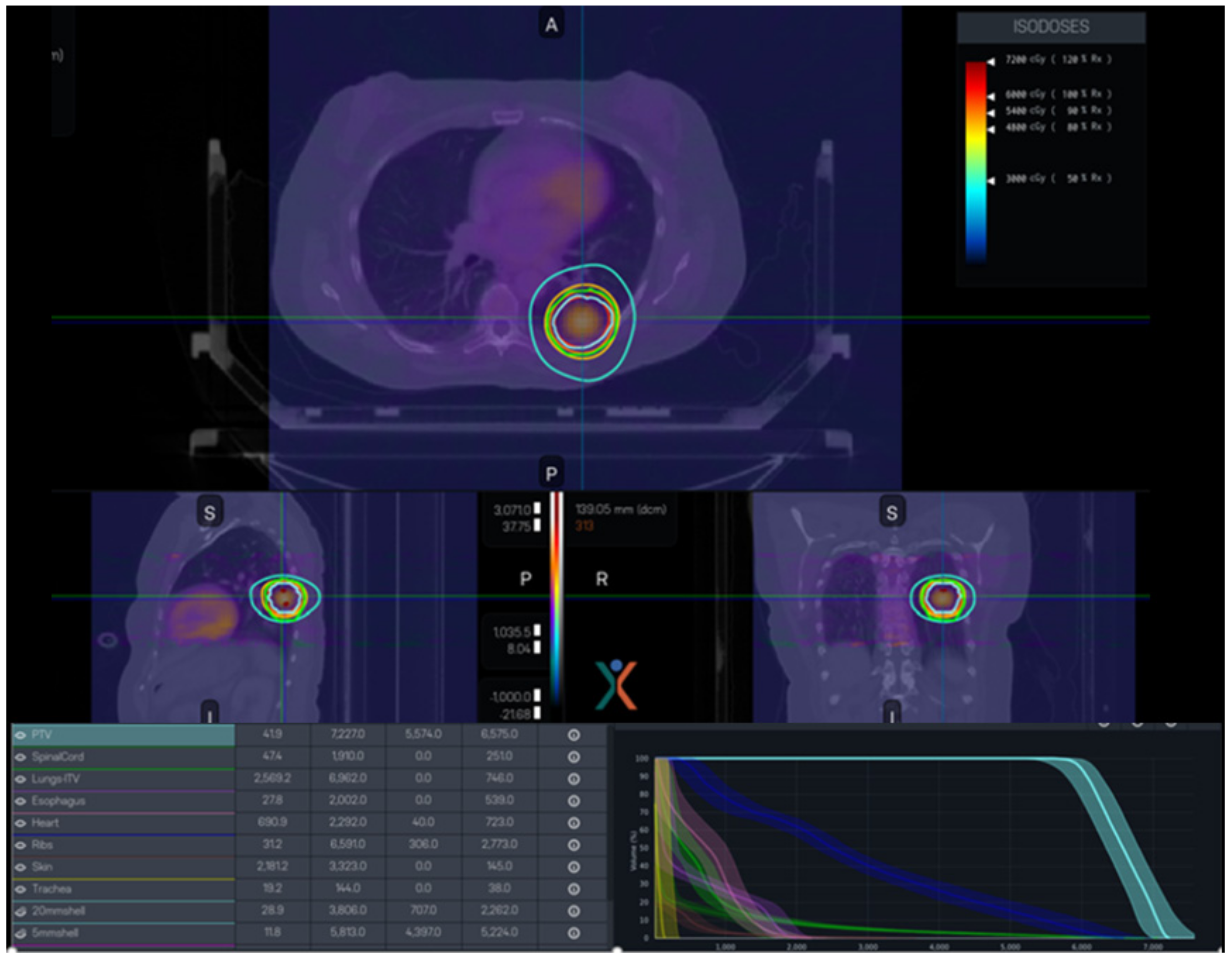
5.2. Lung Cancer
5.3. Prostate Cancer
5.4. Bladder Cancer
5.5. Pancreatic Cancer, Liver Cancer, and Abdominal Oligometastasis
5.6. Head and Neck Cancer
6. Challenges and Outlook
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| ART | Adaptive radiotherapy |
| ATP | Adapt to position |
| ATS | Adapt to shape |
| CBCT | Cone-beam computed tomography |
| CT | Computed tomography |
| CTV | Clinical target volume |
| DIR | Deformable image registration |
| DVH | Dose–volume histogram |
| EF5 | Pentafluorinated etanidazole |
| FAZA | Fluoroazomycin arabinoside |
| FMISO | Fluoromisonidazole |
| GI | Gastrointestinal |
| GU | Genitourinary |
| IGRT | Image-guided radiotherapy |
| IMRT | Intensity-modulated radiotherapy |
| IOE | Intelligent optimization engine |
| MRI | Magnetic resonance imaging |
| OAR | Organ at risk |
| PET | Positron emission tomography |
| PTV | Planning target volume |
| QA | Quality assurance |
| SBRT | Stereotactic body radiotherapy |
| SMART | MRI-guided stereotactic body radiation treatment |
| VMAT | Volumetric-modulated arc therapy |
References
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Duke, S.; Jena, R.; Williams, M.V.; Burnet, N.G. Advances in radiotherapy. BMJ 2012, 345, e7765. [Google Scholar] [CrossRef] [PubMed]
- Connell, P.P.; Hellman, S. Advances in Radiotherapy and Implications for the Next Century: A Historical Perspective. Cancer Res. 2009, 69, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Antolak, J.A.; Rosen, I.I.; Childress, C.H.; Zagars, G.K.; Pollack, A. Prostate target volume variations during a course of radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 661–672. [Google Scholar] [CrossRef] [PubMed]
- de Crevoisier, R.; Melancon, A.D.; Kuban, D.A.; Lee, A.K.; Cheung, R.M.; Tucker, S.L.; Kudchadker, R.J.; Newhauser, W.D.; Zhang, L.; Mohan, R.; et al. Changes in the Pelvic Anatomy After an IMRT Treatment Fraction of Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- McDermott, L.N.; Wendling, M.; Sonke, J.-J.; van Herk, M.; Mijnheer, B.J. Anatomy changes in radiotherapy detected using portal imaging. Radiother. Oncol. 2006, 79, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Noble, D.J.; Yeap, P.-L.; Seah SY, K.; Harrison, K.; Shelley LE, A.; Romanchikova, M.; Bates, A.M.; Zheng, Y.; Barnett, G.C.; Benson, R.J.; et al. Anatomical change during radiotherapy for head and neck cancer, and its effect on delivered dose to the spinal cord. Radiother. Oncol. 2019, 130, 32–38. [Google Scholar] [CrossRef]
- Yan, D.; Vicini, F.; Wong, J.; Martinez, A. Adaptive radiation therapy. Phys. Med. Biol. 1997, 42, 123. [Google Scholar] [CrossRef]
- Dohopolski, M.; Choi, B.; Meng, B.; Visak, J.; Zhong, X.; Kim, J.S.; Inam, E.; Avkshtol, V.; Moon, D.H.; Sher, D.J.; et al. Dosimetric Impact of Simulated Daily Adaptive Radiotherapy with Significantly Reduced Setup Margins in the Definitive Treatment of Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, e590. [Google Scholar] [CrossRef]
- Guberina, M.; Santiago Garcia, A.; Khouya, A.; Pöttgen, C.; Holubyev, K.; Ringbaek, T.P.; Lachmuth, M.; Alberti, Y.; Hoffmann, C.; Hlouschek, J.; et al. Comparison of Online-Onboard Adaptive Intensity-Modulated Radiation Therapy or Volumetric-Modulated Arc Radiotherapy with Image-Guided Radiotherapy for Patients with Gynecologic Tumors in Dependence on Fractionation and the Planning Target Volume Margin. JAMA Netw. Open 2023, 6, e234066. [Google Scholar] [CrossRef]
- Winkel, D.; Bol, G.H.; Kroon, P.S.; van Asselen, B.; Hackett, S.S.; Werensteijn-Honingh, A.M.; Intven MP, W.; Eppinga WS, C.; Tijssen RH, N.; Kerkmeijer LG, W.; et al. Adaptive radiotherapy: The Elekta Unity MR-LINAC concept. Clin. Transl. Radiat. Oncol. 2019, 18, 54–59. [Google Scholar] [CrossRef]
- Green, O.L.; Henke, L.E.; Hugo, G.D. Practical Clinical Workflows for Online and Offline Adaptive Radiation Therapy. Semin. Radiat. Oncol. 2019, 29, 219–227. [Google Scholar] [CrossRef]
- Qin, A.; Sun, Y.; Liang, J.; Yan, D. Evaluation of Online/Offline Image Guidance/Adaptation Approaches for Prostate Cancer Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 1026–1033. [Google Scholar] [CrossRef]
- Weykamp, F.; Meixner, E.; Arians, N.; Hoegen-Saßmannshausen, P.; Kim, J.Y.; Tawk, B.; Knoll, M.; Huber, P.; König, L.; Sander, A.; et al. Daily AI-Based Treatment Adaptation under Weekly Offline MR Guidance in Chemoradiotherapy for Cervical Cancer 1: The AIM-C1 Trial. J. Clin. Med. 2024, 13, 957. [Google Scholar] [CrossRef]
- Vuong, W.; Gupta, S.; Weight, C.; Almassi, N.; Nikolaev, A.; Tendulkar, R.D.; Scott, J.G.; Chan, T.A.; Mian, O.Y. Trial in Progress: Adaptive RADiation Therapy with Concurrent Sacituzumab Govitecan (SG) for Bladder Preservation in Patients with MIBC (RAD-SG). Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, e447–e448. [Google Scholar] [CrossRef]
- Cardenas, C.E.; Yang, J.; Anderson, B.M.; Court, L.E.; Brock, K.B. Advances in Auto-Segmentation. Semin. Radiat. Oncol. 2019, 29, 185–197. [Google Scholar] [CrossRef]
- Fu, Y.; Lei, Y.; Wang, T.; Curran, W.J.; Liu, T.; Yang, X. Deep learning in medical image registration: A review. Phys. Med. Biol. 2020, 65, 20TR01. [Google Scholar] [CrossRef]
- Chen, L.; Liang, X.; Shen, C.; Jiang, S.; Wang, J. Synthetic CT generation from CBCT images via deep learning. Med. Phys. 2020, 47, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, X.; Hong, J.C.; Zheng, D. Artificial Intelligence in Radiotherapy Treatment Planning: Present and Future. Technol. Cancer Res. Treat. 2019, 18, 153303381987392. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.F.; Witztum, A.; Valdes, G. Integration of AI and Machine Learning in Radiotherapy QA. Front. Artif. Intell. 2020, 3, 577620. [Google Scholar] [CrossRef] [PubMed]
- Desideri, I.; Loi, M.; Francolini, G.; Becherini, C.; Livi, L.; Bonomo, P. Application of Radiomics for the Prediction of Radiation-Induced Toxicity in the IMRT Era: Current State-of-the-Art. Front. Oncol. 2020, 10, 1708. [Google Scholar] [CrossRef]
- Isaksson, L.J.; Pepa, M.; Zaffaroni, M.; Marvaso, G.; Alterio, D.; Volpe, S.; Corrao, G.; Augugliaro, M.; Starzyńska, A.; Leonardi, M.C.; et al. Machine Learning-Based Models for Prediction of Toxicity Outcomes in Radiotherapy. Front. Oncol. 2020, 10, 790. [Google Scholar] [CrossRef]
- Henke, L.; Kashani, R.; Yang, D.; Zhao, T.; Green, O.; Olsen, L.; Rodriguez, V.; Wooten, H.O.; Li, H.H.; Hu, Y.; et al. Simulated Online Adaptive Magnetic Resonance–Guided Stereotactic Body Radiation Therapy for the Treatment of Oligometastatic Disease of the Abdomen and Central Thorax: Characterization of Potential Advantages. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 1078–1086. [Google Scholar] [CrossRef]
- Keall, P.J.; Glide-Hurst, C.K.; Cao, M.; Lee, P.; Murray, B.; Raaymakers, B.W.; Tree, A.; van der Heide, U.A. ICRU REPORT 97: MRI-Guided Radiation Therapy Using MRI-Linear Accelerators. J. ICRU 2022, 22, 1–100. [Google Scholar] [CrossRef]
- Bohoudi, O.; Bruynzeel, A.; Senan, S.; Cuijpers, J.; Slotman, B.; Lagerwaard, F.; Palacios, M. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother. Oncol. 2017, 125, 439–444. [Google Scholar] [CrossRef]
- Güngör, G.; Serbez, I.; Temur, B.; Gür, G.; Kayalılar, N.; Mustafayev, T.Z.; Korkmaz, L.; Aydın, G.; Yapıcı, B.; Atalar, B.; et al. Time Analysis of Online Adaptive Magnetic Resonance–Guided Radiation Therapy Workflow According to Anatomical Sites. Pract. Radiat. Oncol. 2021, 11, e11–e21. [Google Scholar] [CrossRef]
- Lim, S.B.; Godoy Scripes, P.; Napolitano, M.; Subashi, E.; Tyagi, N.; Cervino Arriba, L.; Lovelock, D.M. An investigation of using log-file analysis for automated patient-specific quality assurance in MRgRT. J. Appl. Clin. Med. Phys. 2021, 22, 183–188. [Google Scholar] [CrossRef]
- Kontaxis, C.; de Muinck Keizer, D.M.; Kerkmeijer, L.G.W.; Willigenburg, T.; den Hartogh, M.D.; van der Voort van Zyp, J.R.N.; Hes, J.; Raaymakers, B.W.; Lagendijk, J.J.; de Boer, H.C. Delivered dose quantification in prostate radiotherapy using online 3D cine imaging and treatment log files on a combined 1.5T magnetic resonance imaging and linear accelerator system. Phys. Imaging Radiat. Oncol. 2020, 15, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, D.; Lenkowicz, J.; Votta, C.; Boldrini, L.; Placidi, L.; Catucci, F.; Dinapoli, N.; Antonelli, M.V.; Romano, A.; De Luca, V.; et al. A deep learning approach to generate synthetic CT in low field MR-guided adaptive radiotherapy for abdominal and pelvic cases. Radiother. Oncol. 2020, 153, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.U.; Han, F.; Zhou, Z.; Cao, M.; Kaprealian, T.; Kamrava, M.; Wang, C.; Neylon, J.; Low, D.A.; Yang, Y.; et al. Distortion-free diffusion MRI using an MRI-guided Tri-Cobalt 60 radiotherapy system: Sequence verification and preliminary clinical experience. Med. Phys. 2017, 44, 5357–5366. [Google Scholar] [CrossRef] [PubMed]
- Archambault, Y.; Boylan, C.; Bullock, D.; Morgas, T.; Peltola, J.; Ruokokoski, E.; Genghi, A.; Haas, B.; Suhonen, P.; Thompson, S. Making on-line adaptive radiotherapy possible using artificial intelligence and machine learning for efficient daily re-planning. Med. Phys. Intl. J. 2020, 8, 77–86. [Google Scholar]
- Liu, H.; Schaal, D.; Curry, H.; Clark, R.; Magliari, A.; Kupelian, P.; Khuntia, D.; Beriwal, S. Review of cone beam computed tomography based online adaptive radiotherapy: Current trend and future direction. Radiat. Oncol. 2023, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, C.J.; Bird, D.; Al-Qaisieh, B.; Speight, R. Assessment of CBCT–based synthetic CT generation accuracy for adaptive radiotherapy planning. J. Appl. Clin. Med. Phys. 2022, 23, S342–S343. [Google Scholar] [CrossRef] [PubMed]
- Schiff, J.P.; Price, A.T.; Stowe, H.B.; Laugeman, E.; Chin, R.-I.; Hatscher, C.; Pryser, E.; Cai, B.; Hugo, G.D.; Kim, H.; et al. Simulated computed tomography-guided stereotactic adaptive radiotherapy (CT-STAR) for the treatment of locally advanced pancreatic cancer. Radiother. Oncol. 2022, 175, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.; Archibald-Heeren, B.; Hu, Y.; Teh, A.; Beserminji, R.; Cai, E.; Liu, G.; Yates, A.; Rijken, J.; Collett, N.; et al. Varian ethos online adaptive radiotherapy for prostate cancer: Early results of contouring accuracy, treatment plan quality, and treatment time. J. Appl. Clin. Med. Phys. 2022, 23, e13479. [Google Scholar] [CrossRef]
- Moazzezi, M.; Rose, B.; Kisling, K.; Moore, K.L.; Ray, X. Prospects for daily online adaptive radiotherapy via ethos for prostate cancer patients without nodal involvement using unedited CBCT auto-segmentation. J. Appl. Clin. Med. Phys. 2021, 22, 82–93. [Google Scholar] [CrossRef]
- Branco, D.; Mayadev, J.; Moore, K.; Ray, X. Dosimetric and feasibility evaluation of a CBCT-based daily adaptive radiotherapy protocol for locally advanced cervical cancer. J. Appl. Clin. Med. Phys. 2023, 24, e13783. [Google Scholar] [CrossRef]
- Håkansson, K.; Giannoulis, E.; Lindegaard, A.; Friborg, J.; Vogelius, I. CBCT-based online adaptive radiotherapy for head and neck cancer—Dosimetric evaluation of first clinical experience. Acta Oncol. 2023, 62, 1369–1374. [Google Scholar] [CrossRef]
- Jadon, R.; Pembroke, C.; Hanna, C.; Palaniappan, N.; Evans, M.; Cleves, A.E.; Staffurth, J. A Systematic Review of Organ Motion and Image-guided Strategies in External Beam Radiotherapy for Cervical Cancer. Clin. Oncol. 2014, 26, 185–196. [Google Scholar] [CrossRef]
- van de Bunt, L.; Jürgenliemk-Schulz, I.M.; de Kort, G.A.P.; Roesink, J.M.; Tersteeg, R.J.H.A.; van der Heide, U.A. Motion and deformation of the target volumes during IMRT for cervical cancer: What margins do we need? Radiother. Oncol. 2008, 88, 233–240. [Google Scholar] [CrossRef]
- Yen, A.; Choi, B.; Inam, E.; Yeh, A.; Lin, M.; Park, C.; Hrycushko, B.; Nwachukwu, C.; Albuquerque, K. Spare the Bowel, Don’t Spoil the Target: Optimal Margin Assessment for Online Cone Beam Adaptive Radiation Therapy (OnC-ART) of the Cervix. Pract. Radiat. Oncol. 2023, 13, e176–e183. [Google Scholar] [CrossRef]
- Yock, A.D.; Ahmed, M.; Ayala-Peacock, D.; Chakravarthy, A.B.; Price, M. Initial analysis of the dosimetric benefit and clinical resource cost of CBCT-based online adaptive radiotherapy for patients with cancers of the cervix or rectum. J. Appl. Clin. Med. Phys. 2021, 22, 210–221. [Google Scholar] [CrossRef]
- Shelley, C.E.; Bolt, M.A.; Hollingdale, R.; Chadwick, S.J.; Barnard, A.P.; Rashid, M.; Reinlo, S.C.; Fazel, N.; Thorpe, C.R.; Stewart, A.J.; et al. Implementing cone-beam computed tomography-guided online adaptive radiotherapy in cervical cancer. Clin. Transl. Radiat. Oncol. 2023, 40, 100596. [Google Scholar] [CrossRef] [PubMed]
- Dial, C.; Weiss, E.; Siebers, J.V.; Hugo, G.D. Benefits of adaptive radiation therapy in lung cancer as a function of replanning frequency. Med. Phys. 2016, 43, 1787–1794. [Google Scholar] [CrossRef]
- Mao, W.; Riess, J.; Kim, J.; Vance, S.; Chetty, I.J.; Movsas, B.; Kretzler, A. Evaluation of Auto-Contouring and Dose Distributions for Online Adaptive Radiation Therapy of Patients with Locally Advanced Lung Cancers. Pract. Radiat. Oncol. 2022, 12, e329–e338. [Google Scholar] [CrossRef]
- Nenoff, L.; Matter, M.; Amaya, E.J.; Josipovic, M.; Knopf, A.C.; Lomax, A.J.; Persson, G.F.; Ribeiro, C.O.; Visser, S.; Walser, M.; et al. Dosimetric influence of deformable image registration uncertainties on propagated structures for online daily adaptive proton therapy of lung cancer patients. Radiother. Oncol. 2021, 159, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, T.; Haasbeek, C.J.A.; Spoelstra, F.O.B.; Palacios, M.A.; Admiraal, M.A.; Bruynzeel, A.M.E.; Slotman, B.J.; Lagerwaard, F.J.; Senan, S. Clinical Outcomes of Stereotactic MR-Guided Adaptive Radiation Therapy for High-Risk Lung Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Henke, L.E.; Olsen, J.R.; Contreras, J.A.; Curcuru, A.; DeWees, T.A.; Green, O.L.; Michalski, J.; Mutic, S.; Roach, M.C.; Bradley, J.D.; et al. Stereotactic MR-Guided Online Adaptive Radiation Therapy (SMART) for Ultracentral Thorax Malignancies: Results of a Phase 1 Trial. Adv. Radiat. Oncol. 2019, 4, 201–209. [Google Scholar] [CrossRef]
- Tetar, S.U.; Bruynzeel, A.M.E.; Lagerwaard, F.J.; Slotman, B.J.; Bohoudi, O.; Palacios, M.A. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Phys. Imaging Radiat. Oncol. 2019, 9, 69–76. [Google Scholar] [CrossRef]
- Leeman, J.E.; Cagney, D.N.; Mak, R.H.; Huynh, M.A.; Tanguturi, S.K.; Singer, L.; Catalano, P.; Martin, N.E.; D’Amico, A.V.; Mouw, K.W.; et al. Magnetic Resonance–Guided Prostate Stereotactic Body Radiation Therapy with Daily Online Plan Adaptation: Results of a Prospective Phase 1 Trial and Supplemental Cohort. Adv. Radiat. Oncol. 2022, 7, 100934. [Google Scholar] [CrossRef]
- Bruynzeel, A.M.E.; Tetar, S.U.; Oei, S.S.; Senan, S.; Haasbeek, C.J.A.; Spoelstra, F.O.B.; Piet, A.H.; Meijnen, P.; van der Jagt, M.A.B.; Fraikin, T.; et al. A Prospective Single-Arm Phase 2 Study of Stereotactic Magnetic Resonance Guided Adaptive Radiation Therapy for Prostate Cancer: Early Toxicity Results. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1086–1094. [Google Scholar] [CrossRef]
- Pos, F.J.; Koedooder, K.; Hulshof, M.C.C.M.; van Tienhoven, G.; González González, D. Influence of bladder and rectal volume on spatial variability of a bladder tumor during radical radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Meijer, G.J.; Rasch, C.; Remeijer, P.; Lebesque, J. Three-dimensional analysis of delineation errors, setup errors, and organ motion during radiotherapy of bladder cancer. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.D.; Leech, M.M. A review of plan library approaches in adaptive radiotherapy of bladder cancer. Acta Oncol. 2018, 57, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Kuyumcian, A.; Pham, D.; Thomas, J.M.; Law, A.; Willis, D.; Kron, T.; Foroudi, F. Adaptive radiotherapy for muscle-invasive bladder cancer: Optimisation of plan sizes. J. Med. Imaging Radiat. Oncol. 2012, 56, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Lalondrelle, S.; Huddart, R.; Warren-Oseni, K.; Hansen, V.N.; McNair, H.; Thomas, K.; Dearnaley, D.; Horwich, A.; Khoo, V. Adaptive-Predictive Organ Localization Using Cone-Beam Computed Tomography for Improved Accuracy in External Beam Radiotherapy for Bladder Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 705–712. [Google Scholar] [CrossRef]
- Foroudi, F.; Wong, J.; Kron, T.; Rolfo, A.; Haworth, A.; Roxby, P.; Thomas, J.; Herschtal, A.; Pham, D.; Williams, S.; et al. Online Adaptive Radiotherapy for Muscle-Invasive Bladder Cancer: Results of a Pilot Study. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 765–771. [Google Scholar] [CrossRef]
- Tuomikoski, L.; Collan, J.; Keyriläinen, J.; Visapää, H.; Saarilahti, K.; Tenhunen, M. Adaptive radiotherapy in muscle invasive urinary bladder cancer—An effective method to reduce the irradiated bowel volume. Radiother. Oncol. 2011, 99, 61–66. [Google Scholar] [CrossRef]
- Grønborg, C.; Vestergaard, A.; Høyer, M.; Söhn, M.; Pedersen, E.M.; Petersen, J.B.; Agerbæk, M.; Muren, L.P. Intra-fractional bladder motion and margins in adaptive radiotherapy for urinary bladder cancer. Acta Oncol. 2015, 54, 1461–1466. [Google Scholar] [CrossRef]
- Azzarouali, S.; Goudschaal, K.; Visser, J.; Hulshof, M.; Admiraal, M.; van Wieringen, N.; Nieuwenhuijzen, J.; Wiersma, J.; Daniëls, L.; Boer, D.D.; et al. Online adaptive radiotherapy for bladder cancer using a simultaneous integrated boost and fiducial markers. Radiat. Oncol. 2023, 18, 165. [Google Scholar] [CrossRef]
- de Leon, J.; Crawford, D.; Moutrie, Z.; Alvares, S.; Hogan, L.; Pagulayan, C.; Jelen, U.; Loo, C.; Aylward, J.D.; Condon, K.; et al. Early experience with MR-guided adaptive radiotherapy using a 1.5 T MR-Linac: First 6 months of operation using adapt to shape workflow. J. Med. Imaging Radiat. Oncol. 2022, 66, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Pöttgen, C.; Hoffmann, C.; Gauler, T.; Guberina, M.; Guberina, N.; Ringbaek, T.; Garcia, A.S.; Krafft, U.; Hadaschik, B.; Khouya, A.; et al. Fractionation versus Adaptation for Compensation of Target Volume Changes during Online Adaptive Radiotherapy for Bladder Cancer: Answers from a Prospective Registry. Cancers 2023, 15, 4933. [Google Scholar] [CrossRef]
- Hunt, A.; Hansen, V.N.; Oelfke, U.; Nill, S.; Hafeez, S. Adaptive radiotherapy enabled by MRI guidance. Clin. Oncol. 2018, 30, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Placidi, L.; Romano, A.; Chiloiro, G.; Cusumano, D.; Boldrini, L.; Cellini, F.; Mattiucci, G.C.; Valentini, V. On-line adaptive MR guided radiotherapy for locally advanced pancreatic cancer: Clinical and dosimetric considerations. Tech. Innov. Patient Support Radiat. Oncol. 2020, 15, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.M.; Luterstein, E.; Chu, F.; Cao, M.; Lamb, J.; Agazaryan, N.; Low, D.; Raldow, A.; Steinberg, M.L.; Lee, P. Clinical outcomes of stereotactic magnetic resonance image-guided adaptive radiotherapy for primary and metastatic tumors in the abdomen and pelvis. Cancer Med. 2021, 10, 5897–5906. [Google Scholar] [CrossRef] [PubMed]
- Witt, J.S.; Rosenberg, S.A.; Bassetti, M.F. MRI-guided adaptive radiotherapy for liver tumours: Visualising the future. Lancet Oncol. 2020, 21, e74–e82. [Google Scholar] [CrossRef] [PubMed]
- Bulens, P.; Thomas, M.; Deroose, C.; Haustermans, K. PET imaging in adaptive radiotherapy of gastrointestinal tumors. Q. J. Nucl. Med. Mol. Imaging 2018, 62, 385–403. [Google Scholar] [CrossRef]
- Ogawa, A.; Nakamura, M.; Iramina, H.; Yoshimura, M.; Mizowaki, T. Potential utility of cone-beam CT-guided adaptive radiotherapy under end-exhalation breath-hold conditions for pancreatic cancer. J. Appl. Clin. Med. Phys. 2023, 24, e13827. [Google Scholar] [CrossRef]
- Castadot, P.; Lee, J.A.; Geets, X.; Grégoire, V. Adaptive radiotherapy of head and neck cancer. In Seminars in Radiation Oncology; WB Saunders: Philadelphia, PA, USA, 2010; Volume 20, pp. 84–93. [Google Scholar]
- Byrne, M.; Teh, A.Y.M.; Archibald-Heeren, B.; Hu, Y.; Rijken, J.; Luo, S.; Aland, T.; Greer, P. Intrafraction Motion and Margin Assessment for Ethos Online Adaptive Radiotherapy Treatments of the Prostate and Seminal Vesicles. Adv. Radiat. Oncol. 2023, 9, 101405. [Google Scholar] [CrossRef]
- Shepherd, M.; Graham, S.; Ward, A.; Zwart, L.; Cai, B.; Shelley, C.; Booth, J. Pathway for radiation therapists online advanced adapter training and credentialing. Tech. Innov. Patient Support Radiat. Oncol. 2021, 20, 54–60. [Google Scholar] [CrossRef]
- Taghizadeh-Hesary, F. “Reinforcement” by Tumor Microenvironment: The Seventh “R” of Radiobiology. Int. J. Radiat. Oncol. Biol. Phys. 2023. [Google Scholar] [CrossRef] [PubMed]
- Dolezel, M.; Slavik, M.; Blazek, T.; Kazda, T.; Koranda, P.; Veverkova, L.; Burkon, P.; Cvek, J. FMISO-Based Adaptive Radiotherapy in Head and Neck Cancer. J. Pers. Med. 2022, 12, 1245. [Google Scholar] [CrossRef] [PubMed]

| Offline ART | Online ART | |
|---|---|---|
| Frequency | Offline ART involves evaluation/adjustments to the treatment plan in between treatment sessions, with the patient off the table. Plan adjustments are based on anatomy imaged at a certain timepoint and applied for later sessions. It is often applied in lower frequency such as mid-treatment, biweekly, or weekly. | Online ART involves evaluations/adjustments based on the session anatomy, while the patient stays on the treatment table, and is applied for the treatment of the same session. It is currently more often applied in each treatment session. |
| Complexity | When performed less frequently, it is generally less resource-intensive compared to online ART. At the same time, it could still be staff-time-demanding if offline ART has a less streamlined or automated workflow than available in online ART. | Online ART can be more complex and resource-intensive compared to offline ART because it requires specialized equipment and software and may be carried out more frequently. |
| Treatment planning | Offline ART is not conducted on patient images obtained in the session the adaptive plan is intended to be applied. Instead, planning is conducted offline on previously obtained images to apply in future sessions. | It allows for a highly individualized and precise treatment plan for each session, taking into account the new anatomy in each treatment session. The adaptive plan is made based on the session image and applied to the same session. |
| Clinical Applications | It is suitable for patients with tumors, OARs, and body habitus that are less likely to experience rapid anatomical changes and when the tumor is relatively distant from critical structures. It is commonly employed in situations such as head and neck cancers. Patient setup changes could also trigger the need for offline adaptation. | Used for cases where anatomical changes are expected on a daily basis. It is commonly employed in situations such as abdominal and pelvic malignancies. Based on the optimal trade-off between clinical benefits and required resources, the online ART platform may also be used for various disease sites to apply daily, weekly, or on-demand plan adaptation. |
| MRI | CBCT | PET | |
|---|---|---|---|
| Current systems | Elekta Unity 1.5 T MRI with a 7MV FFF LINAC ViewRay MRIdian (legacy system) 6MV FFF 0.35 T MRI | Varian Ethos 6MV FFF | RefleXion X1 6MV FFF |
| ART workflow | Unity: Adapt to position (ATP) and adapt to shape (ATS). MRIdian: Choice between scheduled vs. adaptive plans. | Choice between scheduled vs. adaptive plans. | Offline ART feasible; online ART under development. |
| Strengths |
|
|
|
| Limitations |
|
|
|
| Key clinical sites |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dona Lemus, O.M.; Cao, M.; Cai, B.; Cummings, M.; Zheng, D. Adaptive Radiotherapy: Next-Generation Radiotherapy. Cancers 2024, 16, 1206. https://doi.org/10.3390/cancers16061206
Dona Lemus OM, Cao M, Cai B, Cummings M, Zheng D. Adaptive Radiotherapy: Next-Generation Radiotherapy. Cancers. 2024; 16(6):1206. https://doi.org/10.3390/cancers16061206
Chicago/Turabian StyleDona Lemus, Olga Maria, Minsong Cao, Bin Cai, Michael Cummings, and Dandan Zheng. 2024. "Adaptive Radiotherapy: Next-Generation Radiotherapy" Cancers 16, no. 6: 1206. https://doi.org/10.3390/cancers16061206
APA StyleDona Lemus, O. M., Cao, M., Cai, B., Cummings, M., & Zheng, D. (2024). Adaptive Radiotherapy: Next-Generation Radiotherapy. Cancers, 16(6), 1206. https://doi.org/10.3390/cancers16061206







