Cancer Screening: Present Recommendations, the Development of Multi-Cancer Early Development Tests, and the Prospect of Universal Cancer Screening
Abstract
Simple Summary
Abstract
1. Introduction
2. Status Quo of Cancer Screening
2.1. Colorectal Cancer
2.2. Breast Cancer
2.3. Prostate Cancer
2.4. Lung Cancer
2.5. Cervical Cancer
2.6. Oral Cancer Screening
2.7. Screening for Other Cancers
3. Fantastic Biomarkers and Where to Find Them
3.1. Blood Tumor Markers
3.1.1. Circulating Tumor Cells
3.1.2. Plasma Cell-Free Nucleic Acids
3.1.3. Extracellular Vesicle-Based Tests for MCED
3.2. Non-Invasive Early Cancer Detection Using Indirect Media—Saliva, Tears, Urine, Breath
3.3. Tumor Exfoliation
| Trial ID | Trial Name | MCED Test | Sponsor | Status (as of February 2024) | Est. Completion | Enrolled | Publications |
|---|---|---|---|---|---|---|---|
| NCT02889978 | CCGA, GRAIL-001 | Galleri | GRAIL, LLC | Active, not recruiting | March 2024 | 15,254 | [135,155] |
| NCT03085888 | STRIVE, GRAIL-002 | Galleri | GRAIL, LLC | Active, not recruiting | May 2025 | 99,481 | |
| NCT03934866 | SUMMIT | Galleri (combined with LDCT) | University College London and Grail | Active, not recruiting | August 2030 | 13,035 | [156] |
| NCT04241796 | PATHFINDER, GRAIL-007 | Galleri | GRAIL, LLC | Completed | NA | 6662 | [157,158,159] |
| NCT05155605 | PATHFINDER2 | Galleri | GRAIL, LLC | Recruiting | January 2028 | 35,000 | |
| NCT05205967 | REFLECTION | Galleri | GRAIL, LLC | Recruiting | August 2026 | 17,000 | |
| NCT05611632,ISRCTN91431511 | NHS-Galleri, GRAIL-009 | Galleri | GRAIL, Bio UK Ltd. | Active, not recruiting | February 2026 | 140,000 | [160,161,162] |
| NCT05235009 | LEV87A | GAGome | Elypta | Recruiting | March 2025 | 9170 | [163] |
| NCT05295017 | LEV93A | GAGome | Elypta | Recruiting | March 2025 | 1256 | |
| NCT05780957 | Multi-Cancer Early Detection (MCED) of Firefighters | GAGome | Elypta | Recruiting | January 2031 | 2000 (estimated) | |
| NCT05227534 | PREVENT | OverC | Burning Rock Dx | Recruiting | January 2029 | 12,500 | - |
| NCT04972201 | PROMISE | OverC | Chinese Academy of Medical Sciences and Burning Rock Dx | Completed | NA | 2305 | [164] |
| NCT04822792 | PRESCIENT | OverC | Chinese Academy of Medical Sciences and Burning Rock Dx | Completed | NA | 11,879 | - |
| NCT04820868 | THUNDER | OverC | Shanghai Zhongshan Hospital and Burning Rock Dx | Completed | NA | 2508 | [165,166] |
| NCT04817306 | PREDICT | OverC | Shanghai Zhongshan Hospital and Burning Rock Dx | Completed | NA | 14,206 | - |
| NCT03517332 | - | DEEPGEN | Quantgene Inc. | Completed | NA | 675 | [167] |
| NCT03967652 | - | Na-nose | Anhui Medical University | - | NA | NA | [168,169] |
| NCT05366881 | CAMPERR | - | Adela, Inc. | Active, recruiting | December 2026 | 7000 | [170] |
| NCT05254834 | Vallania | - | Freenome Holdings Inc. | Active, recruiting | June 2025 | 5400 | - |
| NCT05227261 | K-DETEK | SPOT-MAS | Gene Solutions | Active, recruiting | - | 10,000 | [171,172] |
| NCT05159544 | FuSion | - | Singlera Genomics Inc. | - | - | 60,000 | [173] |
| NCT04405557 | PREDICT | - | Geneplus-Beijing Co. | Completed | NA | 757 | Article under review |
| NCT02662621 | EXODIAG | - | Centre Georges Francois Leclerc | Completed | NA | 80 | [174] |
| NCT04197414 | - | - | Yonsei University | Active, recruiting | December 2029 | 3000 | - |
| NCT03951428 | - | - | LifeStory Health Inc. | Completed | NA | 30 | - |
| NCT03869814 | - | - | Bluestar Genomics Inc. | Completed | NA | 660 | - |
| NCT02612350 | - | - | Pathway Genomics | Completed | NA | 1106 | - |
| NCT06011694 | The Jinling Cohort | MERCURY test | Nanjing Shihejiyin Technology, Inc. | Recruiting | May 2027 | 15,000 (estimated) | [175] |
| NCT05633342 | CADENCE | MiRXES MCST | MiRXES Pte Ltd. | Recruiting | May 2025 | 15,000 (estimated) | - |
| NCT04825834 | DELFI-L101 | DELFI | Delfi Diagnostics Inc. | Recruiting | June 2026 | 2660 (estimated) | [176] |
| NCT05306288 | DELFI-L201 (CASCADE-LUNG) | Delfi Diagnostics Inc. | Active, not recruiting | March 2025 | 15,000 (estimated) | - | |
| NCT05492617 | DETECT | Rigshospitalet, Denmark | Completed | NA | 3628 | [177] | |
| NCT05435066 | CORE-HH | Harbringer Hx | Harbringer Health | Recruiting | July 2025 | 10,000 (estimated) | [178] |
| NCT05117840 | SHIELD | LUNAR-2 | Guardant Health, Inc. | Recruiting | January 2026 | 9000 (estimated) | [179,180] |
| NCT04136002 | ECLIPSE | January 2024 | 40,000 | [181] |
| MCED Test | Sponsor | Publications | Sample | Method of Detection | Tumor Types |
|---|---|---|---|---|---|
| Galleri | GRAIL, LLC | [135,155] | plasma | Targeted methylation assay (cfDNA), combined with a machine learning classifier for detecting (54.9% sensitivity, 99.3% specificity) and discriminating TOO (93% accuracy) [134,135]. | <40 different tumor types: GI, hematological, head and neck, lung, ovary, etc. |
| GAGome | Elypta | [163] | plasma and urine | Free glycosaminoglycan profiles (GAGome) | 14 different cancers: gliomas, genito-urinary, etc. |
| OverC | Burning Rock Dx | [164] | plasma | Targeted methylation sequencing assay (ELSA-seq) combined with machine learning for cancer detection (80.6% sensitivity, 98.3% specificity) and TOO discrimination (81.0% accuracy) | Lung, colorectal, liver, ovarian, pancreatic, esophageal [182] |
| DEEPGEN | Quantgene Inc. | [167] | Plasma | NGS combined with machine learning for cancer detection (57% sensitivity, 95% specificity) | Lung, breast, colorectal, prostate, bladder, pancreatic, and liver |
| Na-nose | Anhui Medical University | [168,169] | Exhaled breath | Nanoparticle sensors for Volatile Organic Compounds found in exhaled breath | Head and neck, lung, breast, colorectal, etc. |
| - | Adela, Inc. | [170] | Plasma | cfMeDIP-seq [183] combined with machine learning | Kidney, pancreas, lung, gliomas, etc. |
| SPOT-MAS | Gene Solutions | [171,172] | Plasma | NGS sequencing of ctDNA to detect genome-wide hypomethylation, targeted hypermethylated regions, genome-wide copy number variation, length profile of ctDNA | Gastric, lung, breast, etc. |
| - | Singlera Genomics Inc. | [173] | Plasma | DNA methylation | lung, esophagus, stomach, liver, pancreatic and colorectal |
| - | Geneplus-Beijing Co. | Article under review | Plasma | cfDNA analysis | Hepatic, pancreatic, ovarian, breast, colorectal and gastric cancer. |
| - | Centre Georges Francois Leclerc | [174] | Blood and urine | Analysis of HSP70-exosomes | Breast, lung, ovarian |
| - | Yonsei University | - | Blood and urine | ctDNA analysis | Urological malignancies |
| - | LifeStory Health Inc. | - | Menstrual blood | Breast, endometrial, lung | |
| - | Bluestar Genomics Inc. | - | Plasma | cfDNA analysis | Pancreatic |
| - | Pathway Genomics | - | Plasma | cfDNA analysis | Pancancer |
| MERCURY test | Nanjing Shihejiyin Technology, Inc. | [175] | plasma | cfDNA analysis | Liver, lung, colorectal |
| MiRXES MCST | MiRXES Pte Ltd. | - | plasma | miRNA expression in tandem with DNA methylation | lung, breast, colorectal, liver, stomach, esophageal, ovarian, pancreatic, and prostate |
| DELFI | Delfi Diagnostics Inc. | [176] | plasma | cfDNA analysis | Lung, liver cancer |
| Harbringer Hx | Harbringer Health | [178] | plasma | cfDNA analysis | Variety of solid and hematological cancers |
| LUNAR-2 | Guardant Health, Inc. | [179,180] | plasma | ctDNA analysis | Colon, lung cancer |
4. Expanding the Horizons towards Universal Cancer Screening
4.1. Improved Imaging Techniques
4.2. Volatile Organic Compounds
4.3. Always on PATROL for Early Lung Cancer
5. Discussion on the Prospect of Universal Cancer Screening
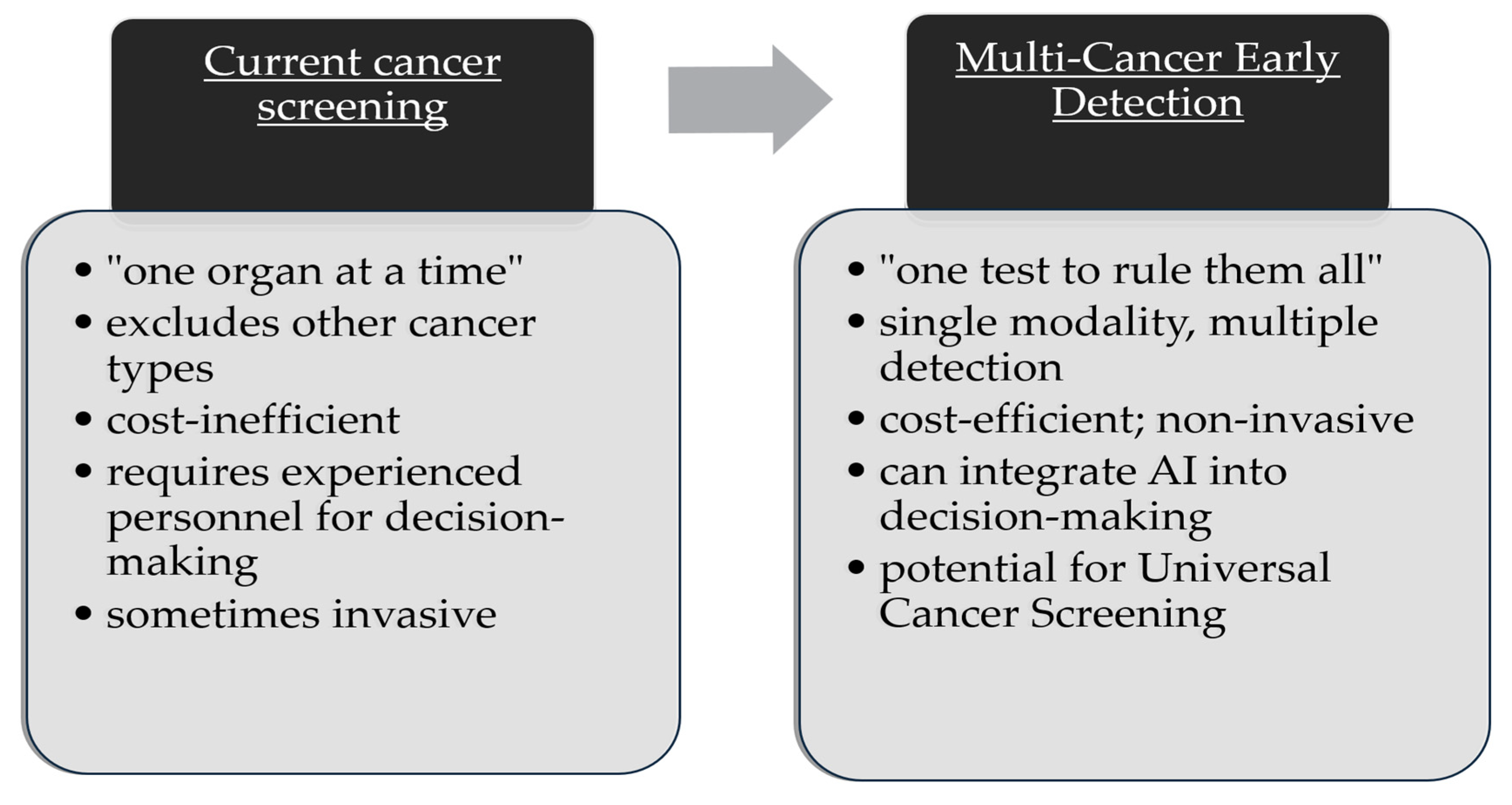
5.1. “One Test to Rule Them All” vs. “One Organ at a Time”
5.2. Aggregate Prevalence
5.3. Manpower Considerations
5.4. Cost-Effectiveness
5.5. Bioinformatics and Artificial Intelligence (AI)
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European Cancer Burden in 2020: Incidence and Mortality Estimates for 40 Countries and 25 Major Cancers. Eur J Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Basu, P.; Ponti, A.; Anttila, A.; Ronco, G.; Senore, C.; Vale, D.B.; Segnan, N.; Tomatis, M.; Soerjomataram, I.; Primic Žakelj, M.; et al. Status of Implementation and Organization of Cancer Screening in The European Union Member States—Summary Results from the Second European Screening Report. Int. J. Cancer 2018, 142, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.A.; Cronin, K.A.; Plevritis, S.K.; Fryback, D.G.; Clarke, L.; Zelen, M.; Mandelblatt, J.S.; Yakovlev, A.Y.; Habbema, J.D.F.; Feuer, E.J. Effect of Screening and Adjuvant Therapy on Mortality from Breast Cancer. N. Engl. J. Med. 2005, 353, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Di Luccio, E.; Morishita, M.; Hirotsu, T.C. Elegans as a Powerful Tool for Cancer Screening. Biomedicines 2022, 10, 2371. [Google Scholar] [CrossRef] [PubMed]
- Barsouk, A.; Saginala, K.; Aluru, J.S.; Rawla, P.; Barsouk, A. US Cancer Screening Recommendations: Developments and the Impact of COVID-19. Med. Sci. 2022, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Armaroli, P.; Villain, P.; Suonio, E.; Almonte, M.; Anttila, A.; Atkin, W.S.; Dean, P.B.; de Koning, H.J.; Dillner, L.; Herrero, R.; et al. European Code against Cancer, 4th Edition: Cancer Screening. Cancer Epidemiol. 2015, 39, S139–S152. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1901–1913. [CrossRef]
- Wadsworth, L.P.; Wessman, I.; Björnsson, A.S.; Jonsdottir, G.; Kristinsson, S.Y. The Half-Painted Picture: Reviewing the Mental Health Impacts of Cancer Screening. Medicine 2022, 101, e30479. [Google Scholar] [CrossRef]
- Wu, G.X.; Raz, D.J. Lung Cancer Screening. Cancer Treat. Res. 2016, 170, 1–23. [Google Scholar] [CrossRef]
- Independent UK Panel on Breast Cancer Screening. The Benefits and Harms of Breast Cancer Screening: An Independent Review. Lancet 2012, 380, 1778–1786. [Google Scholar] [CrossRef]
- Barry, M.J.; Simmons, L.H. Prevention of Prostate Cancer Morbidity and Mortality: Primary Prevention and Early Detection. Med. Clin. North. Am. 2017, 101, 787–806. [Google Scholar] [CrossRef] [PubMed]
- Geneve, N.; Kairys, D.; Bean, B.; Provost, T.; Mathew, R.; Taheri, N. Colorectal Cancer Screening. Prim. Care 2019, 46, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J. Carcinoembryonic Antigen as a Marker for Colorectal Cancer: Is It Clinically Useful? Clin. Chem. 2001, 47, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Ahlquist, D.A. Universal Cancer Screening: Revolutionary, Rational, and Realizable. NPJ Precis. Onc. 2018, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Brito-Rocha, T.; Constâncio, V.; Henrique, R.; Jerónimo, C. Shifting the Cancer Screening Paradigm: The Rising Potential of Blood-Based Multi-Cancer Early Detection Tests. Cells 2023, 12, 935. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, A.; Cohen, S.S.; Reichert, H.; Kansal, A.R.; Chung, K.C.; Ofman, J.J. Estimating the Population Health Impact of a Multi-Cancer Early Detection Genomic Blood Test to Complement Existing Screening in the US and UK. Br. J. Cancer 2021, 125, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Moleyar-Narayana, P.; Ranganathan, S. Cancer Screening. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Issa, I.A.; Noureddine, M. Colorectal Cancer Screening: An Updated Review of the Available Options. World J. Gastroenterol. 2017, 23, 5086–5096. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.H. Fecal Occult Blood Test Screening for Colorectal Cancer. Gastrointest. Endosc. Clin. N. Am. 2002, 12, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Meklin, J.; Syrjänen, K.; Eskelinen, M. Colorectal Cancer Screening with Traditional and New-Generation Fecal Immunochemical Tests: A Critical Review of Fecal Occult Blood Tests. Anticancer. Res. 2020, 40, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Nugent, Z.; Demers, A.A.; Kliewer, E.V.; Mahmud, S.M.; Bernstein, C.N. The Reduction in Colorectal Cancer Mortality after Colonoscopy Varies by Site of the Cancer. Gastroenterology 2010, 139, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.F.; Regula, J.; Kraszewska, E.; Polkowski, M.; Wojciechowska, U.; Didkowska, J.; Zwierko, M.; Rupinski, M.; Nowacki, M.P.; Butruk, E. Quality Indicators for Colonoscopy and the Risk of Interval Cancer. N. Engl. J. Med. 2010, 362, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Lakemeyer, L.; Sander, S.; Wittau, M.; Henne-Bruns, D.; Kornmann, M.; Lemke, J. Diagnostic and Prognostic Value of CEA and CA19-9 in Colorectal Cancer. Diseases 2021, 9, 21. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar] [CrossRef]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.-C.T.; et al. Colorectal Cancer Screening for Average-Risk Adults: 2018 Guideline Update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef]
- Ebell, M.H.; Thai, T.N.; Royalty, K.J. Cancer Screening Recommendations: An International Comparison of High Income Countries. Public Health Rev. 2018, 39, 7. [Google Scholar] [CrossRef]
- Schneider, R.; Syrogiannouli, L.; Bissig, S.; Scharf, T.; Bulliard, J.-L.; Ducros, C.; Del Giovane, C.; Tal, K.; Zwahlen, M.; Selby, K.; et al. Ten-Year Changes in Colorectal Cancer Screening in Switzerland: The Swiss Health Interview Survey 2007, 2012 and 2017. Prev. Med. Rep. 2022, 27, 101815. [Google Scholar] [CrossRef] [PubMed]
- Bretthauer, M.; Løberg, M.; Wieszczy, P.; Kalager, M.; Emilsson, L.; Garborg, K.; Rupinski, M.; Dekker, E.; Spaander, M.; Bugajski, M.; et al. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N. Engl. J. Med. 2022, 387, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, M. Interim Evaluation of the Colorectal Cancer Screening Programme in the Netherlands. Lancet Gastroenterol. Hepatol. 2022, 7, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, M.; Diedrich, L.; Krauth, C.; Robra, B.-P.; Stahmeyer, J.T.; Dreier, M. General Populations’ Preferences for Colorectal Cancer Screening: Rationale and Protocol for the Discrete Choice Experiment in the SIGMO Study. BMJ Open 2021, 11, e042399. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.; Saraste, D.; Törnberg, S.; Jonsson, H. Routine Fecal Occult Blood Screening and Colorectal Cancer Mortality in Sweden. JAMA Netw. Open 2024, 7, e240516. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, D.; Sheahan, K.; MacMathuna, P.; Stephens, R.B.; Fenlon, H.; Morrin, M.; Mooney, J.; Fahy, L.E.; Mooney, T.; Smith, A. A National Bowel Cancer Screening Programme Using FIT: Achievements and Challenges. Cancer Prev. Res. 2019, 12, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Gartlehner, G.; Schernhammer, E.; Lax, S.F.; Preusser, M.; Bachler, H.; Titzer, H.; Kletecka-Pulker, M.; Turnher, H.; Siebert, U. Screening for Colorectal Cancer. Wien. Klin. Wochenschr. 2023, 135, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Olesen, T.B.; Jensen, H.; Møller, H.; Jensen, J.W.; Andersen, B.; Rasmussen, M. Nationwide Participation in FIT-Based Colorectal Cancer Screening in Denmark during the COVID-19 Pandemic: An Observational Study. eLife 2023, 12, e81808. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Hoeck, S.; De Schutter, H.; Janssens, S.; Peeters, M.; Van Hal, G. The Impact of a Six-Year Existing Screening Programme Using the Faecal Immunochemical Test in Flanders (Belgium) on Colorectal Cancer Incidence, Mortality and Survival: A Population-Based Study. Int. J. Environ. Res. Public Health 2023, 20, 1654. [Google Scholar] [CrossRef]
- Canadian Task Force on Preventive Health Care. Recommendations on Screening for Colorectal Cancer in Primary Care. CMAJ 2016, 188, 340–348. [Google Scholar] [CrossRef]
- Clinical Practice Guidelines for the Prevention, Early Detection, and Management of Colorectal Cancer: Population Screening. Available online: https://app.magicapp.org/#/guideline/j1Q1Xj (accessed on 4 March 2024).
- Seitz, J.-F.; Lapalus, D.; Arlotto, S.; Gentile, S.; Ettori, F.; Rinaldi, Y.; Grandval, P.; Delasalle, P. Colorectal Cancer Screening by Fecal Immunochemical Test or Colonoscopy in France: How Many People Are Actually Covered? Focus on the Provence-Alpes-Côte d’Azur Region. Eur. J. Gastroenterol. Hepatol. 2022, 34, 405–410. [Google Scholar] [CrossRef]
- Saito, Y.; Oka, S.; Kawamura, T.; Shimoda, R.; Sekiguchi, M.; Tamai, N.; Hotta, K.; Matsuda, T.; Misawa, M.; Tanaka, S.; et al. Colonoscopy Screening and Surveillance Guidelines. Dig. Endosc. 2021, 33, 486–519. [Google Scholar] [CrossRef]
- Bowel Cancer-UK National Screening Committee (UK NSC)-GOV.UK. Available online: https://view-health-screening-recommendations.service.gov.uk/bowel-cancer/ (accessed on 4 March 2024).
- Clinical Practice Guidelines for Bowel Screening in New Zealand—Health New Zealand|Te Whatu Ora. Available online: https://www.tewhatuora.govt.nz/publications/clinical-practice-guidelines-for-bowel-screening-in-new-zealand/ (accessed on 4 March 2024).
- Bucchi, L.; Mancini, S.; Baldacchini, F.; Ravaioli, A.; Giuliani, O.; Vattiato, R.; Zamagni, F.; Giorgi Rossi, P.; Campari, C.; Canuti, D.; et al. How a Faecal Immunochemical Test Screening Programme Changes Annual Colorectal Cancer Incidence Rates: An Italian Intention-to-Screen Study. Br. J. Cancer 2022, 127, 541–548. [Google Scholar] [CrossRef]
- Nouni-García, R.; Lara-López, Á.; Carratalá-Munuera, C.; Gil-Guillén, V.F.; López-Pineda, A.; Orozco-Beltrán, D.; Quesada, J.A. Factors Associated with Colorectal Cancer Screening in Spain: Results of the 2017 National Health Survey. Int. J. Environ. Res. Public Health 2022, 19, 5460. [Google Scholar] [CrossRef]
- Budh, D.P.; Sapra, A. Breast Cancer Screening. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Autier, P.; Boniol, M. Mammography Screening: A Major Issue in Medicine. Eur. J. Cancer 2018, 90, 34–62. [Google Scholar] [CrossRef]
- Choi, J.W.; Moon, B.-I.; Lee, J.W.; Kim, H.J.; Jin, Y.; Kim, H.-J. Use of CA15-3 for Screening Breast Cancer: An Antibody-Lectin Sandwich Assay for Detecting Glycosylation of CA15-3 in Sera. Oncol. Rep. 2018, 40, 145–154. [Google Scholar] [CrossRef]
- Duffy, M.J.; Evoy, D.; McDermott, E.W. CA 15-3: Uses and Limitation as a Biomarker for Breast Cancer. Clin. Chim. Acta 2010, 411, 1869–1874. [Google Scholar] [CrossRef]
- Davisson, L. USPSTF Breast Cancer Screening Guidelines. W. V. Med. J. 2016, 112, 29–31. [Google Scholar]
- Oeffinger, K.C.; Fontham, E.T.H.; Etzioni, R.; Herzig, A.; Michaelson, J.S.; Shih, Y.-C.T.; Walter, L.C.; Church, T.R.; Flowers, C.R.; LaMonte, S.J.; et al. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update from the American Cancer Society. JAMA 2015, 314, 1599–1614. [Google Scholar] [CrossRef]
- Draft Recommendation: Breast Cancer: Screening|United States Preventive Services Taskforce. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/draft-recommendation/breast-cancer-screening-adults#bcei-recommendation-title-area (accessed on 4 March 2024).
- Saslow, D.; Boetes, C.; Burke, W.; Harms, S.; Leach, M.O.; Lehman, C.D.; Morris, E.; Pisano, E.; Schnall, M.; Sener, S.; et al. American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. CA Cancer J. Clin. 2007, 57, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Risk Assessment and Screening in Average-Risk Women. Available online: https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2017/07/breast-cancer-risk-assessment-and-screening-in-average-risk-women (accessed on 4 March 2024).
- Monticciolo, D.L.; Malak, S.F.; Friedewald, S.M.; Eby, P.R.; Newell, M.S.; Moy, L.; Destounis, S.; Leung, J.W.T.; Hendrick, R.E.; Smetherman, D. Breast Cancer Screening Recommendations Inclusive of All Women at Average Risk: Update from the ACR and Society of Breast Imaging. J. Am. Coll. Radiol. 2021, 18, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Monticciolo, D.L.; Newell, M.S.; Moy, L.; Lee, C.S.; Destounis, S.V. Breast Cancer Screening for Women at Higher-Than-Average Risk: Updated Recommendations from the ACR. J. Am. Coll. Radiol. 2023, 20, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Vounatsou, P.; Thürlimann, B.; Probst-Hensch, N.; Rothermundt, C.; Ess, S. Impact of Mammography Screening Programmes on Breast Cancer Mortality in Switzerland, a Country with Different Regional Screening Policies. BMJ Open 2018, 8, e017806. [Google Scholar] [CrossRef] [PubMed]
- Bringedal, B.; Fretheim, A.; Nilsen, S.; Isaksson Rø, K. Do You Recommend Cancer Screening to Your Patients? A Cross-Sectional Study of Norwegian Doctors. BMJ Open 2019, 9, e029739. [Google Scholar] [CrossRef] [PubMed]
- Kregting, L.M.; Olthof, E.M.G.; Breekveldt, E.C.H.; Aitken, C.A.; Heijnsdijk, E.A.M.; Toes-Zoutendijk, E.; De Koning, H.J.; Van Ravesteyn, N.T. Concurrent Participation in Breast, Cervical, and Colorectal Cancer Screening in the Netherlands. Eur. J. Cancer 2022, 175, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Heinig, M.; Schäfer, W.; Langner, I.; Zeeb, H.; Haug, U. German Mammography Screening Program: Adherence, Characteristics of (Non-)Participants and Utilization of Non-Screening Mammography—A Longitudinal Analysis. BMC Public Health 2023, 23, 1678. [Google Scholar] [CrossRef] [PubMed]
- Lagerlund, M.; Åkesson, A.; Zackrisson, S. Population-Based Mammography Screening Attendance in Sweden 2017–2018: A Cross-Sectional Register Study to Assess the Impact of Sociodemographic Factors. Breast 2021, 59, 16–26. [Google Scholar] [CrossRef]
- O’Sullivan, K.; O’Donovan, A. Factors Associated with Breast Cancer Mammography Screening and Breast Self-Examination in Irish Women: Results from the Irish Longitudinal Study on Ageing (TILDA). Acta Oncol. 2022, 61, 1301–1308. [Google Scholar] [CrossRef]
- Schiller-Fruehwirth, I.; Jahn, B.; Einzinger, P.; Zauner, G.; Urach, C.; Siebert, U. The Long-Term Effectiveness and Cost Effectiveness of Organized versus Opportunistic Screening for Breast Cancer in Austria. Value Health 2017, 20, 1048–1057. [Google Scholar] [CrossRef]
- Lynge, E.; Bak, M.; von Euler-Chelpin, M.; Kroman, N.; Lernevall, A.; Mogensen, N.B.; Schwartz, W.; Wronecki, A.J.; Vejborg, I. Outcome of Breast Cancer Screening in Denmark. BMC Cancer 2017, 17, 897. [Google Scholar] [CrossRef]
- De Troeyer, K.; Silversmit, G.; Rosskamp, M.; Truyen, I.; Van Herck, K.; Goossens, M.M.; Martens, P.; Kellen, E.; Hendrickx, E.; Rummens, E.; et al. The Effect of the Flemish Breast Cancer Screening Program on Breast Cancer-Specific Mortality: A Case-Referent Study. Cancer Epidemiol. 2023, 82, 102320. [Google Scholar] [CrossRef]
- Klarenbach, S.; Sims-Jones, N.; Lewin, G.; Singh, H.; Thériault, G.; Tonelli, M.; Doull, M.; Courage, S.; Garcia, A.J.; Thombs, B.D. Recommendations on Screening for Breast Cancer in Women Aged 40–74 Years Who Are Not at Increased Risk for Breast Cancer. CMAJ 2018, 190, E1441–E1451. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. BreastScreen Australia Monitoring Report. 2022. Available online: https://www.breastcheck.ie/_fileupload/documents/BC-PR-PM-12-Rev0-BreastCheck-Programme-Report_2018_and_2019.pdf (accessed on 4 March 2024).
- Nickson, C.; Velentzis, L.; Brennan, P.; Mann, G.B.; Houssami, N. Improving Breast Cancer Screening in Australia: A Public Health Perspective. Public Health Res. Pract. 2019, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Seradour, B. The French breast cancer screening program in 2022: Ensuring progress. Bull Cancer 2022, 109, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, T.; Izumori, A.; Moon, W.K. Overcoming the Limitations of Screening Mammography in Japan and Korea: A Paradigm Shift to Personalized Breast Cancer Screening Based on Ultrasonography. Ultrasonography 2023, 42, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Njor, S.H.; Heinävaara, S.; Stefansdóttir, H.; Nygård, M.; Guðmundsdóttir, E.M.; Bhargava, S.; Leivonen, A.; Campbell, S.; Søborg, B.; Hofvind, S.; et al. Differences in Mammography Screening Attendance among Non-Western Immigrants in Denmark, Finland, Iceland and Norway. Prev. Med. Rep. 2023, 36, 102516. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Phillips, S.; Seedat, F.; Kijauskaite, G.; Marshall, J.; Halligan, S.; Hyde, C.; Given-Wilson, R.; Wilkinson, L.; Denniston, A.K.; Glocker, B.; et al. UK National Screening Committee’s Approach to Reviewing Evidence on Artificial Intelligence in Breast Cancer Screening. Lancet Digit. Health 2022, 4, e558–e565. [Google Scholar] [CrossRef]
- Taylor, R.; Gregory, M.; Sexton, K.; Wharton, J.; Sharma, N.; Amoyal, G.; Morrell, S. Breast Cancer Mortality and Screening Mammography in New Zealand: Incidence-Based and Aggregate Analyses. J. Med. Screen 2019, 26, 35–43. [Google Scholar] [CrossRef]
- Pelullo, C.P.; Cantore, F.; Lisciotto, A.; Di Giuseppe, G.; Pavia, M. Organized Breast and Cervical Cancer Screening: Attendance and Determinants in Southern Italy. Cancers 2021, 13, 1578. [Google Scholar] [CrossRef]
- Portero de la Cruz, S.; Béjar, L.M.; Cebrino, J. Temporal Evolution and Associated Factors of Adherence to Mammography Screening among Women in Spain: Results from Two National Health Surveys (2017–2020). Healthcare 2023, 11, 2934. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Djulbegovic, M.; Jung, J.H.; Hwang, E.C.; Zhou, Q.; Cleves, A.; Agoritsas, T.; Dahm, P. Prostate Cancer Screening with Prostate-Specific Antigen (PSA) Test: A Systematic Review and Meta-Analysis. BMJ 2018, 362, k3519. [Google Scholar] [CrossRef] [PubMed]
- Balk, S.P.; Ko, Y.-J.; Bubley, G.J. Biology of Prostate-Specific Antigen. JCO 2003, 21, 383–391. [Google Scholar] [CrossRef]
- David, M.K.; Leslie, S.W. Prostate Specific Antigen. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- London, J.W.; Fazio-Eynullayeva, E.; Palchuk, M.B.; Sankey, P.; McNair, C. Effects of the COVID-19 Pandemic on Cancer-Related Patient Encounters. JCO Clin. Cancer Inform. 2020, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Raman, J.D. Impact of the Evolving United States Preventative Services Task Force Policy Statements on Incidence and Distribution of Prostate Cancer over 15 Years in a Statewide Cancer Registry. Prostate Int. 2021, 9, 12–17. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research Team; Church, T.R.; Black, W.C.; Aberle, D.R.; Berg, C.D.; Clingan, K.L.; Duan, F.; Fagerstrom, R.M.; Gareen, I.F.; Gierada, D.S.; et al. Results of Initial Low-Dose Computed Tomographic Screening for Lung Cancer. N. Engl. J. Med. 2013, 368, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Pastorino, U.; Sverzellati, N. Lung Cancer Screening with Low-Dose CT in Europe: Strength and Weakness of Diverse Independent Screening Trials. Clin. Radiol. 2017, 72, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Amicizia, D.; Piazza, M.F.; Marchini, F.; Astengo, M.; Grammatico, F.; Battaglini, A.; Schenone, I.; Sticchi, C.; Lavieri, R.; Di Silverio, B.; et al. Systematic Review of Lung Cancer Screening: Advancements and Strategies for Implementation. Healthcare 2023, 11, 2085. [Google Scholar] [CrossRef]
- Bartlett, E.C.; Silva, M.; Callister, M.E.; Devaraj, A. False-Negative Results in Lung Cancer Screening-Evidence and Controversies. J. Thorac. Oncol. 2021, 16, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Colson, Y.L.; Shepard, J.-A.O.; Lennes, I.T. New USPSTF Guidelines for Lung Cancer Screening: Better but Not Enough. JAMA Surg. 2021, 156, 513–514. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.D.; Oeffinger, K.C.; Shih, T.Y.-C.; Walter, L.C.; Church, T.R.; Fontham, E.T.H.; Elkin, E.B.; Etzioni, R.D.; Guerra, C.E.; Perkins, R.B.; et al. Screening for Lung Cancer: 2023 Guideline Update from the American Cancer Society. CA Cancer J. Clin. 2024, 74, 50–81. [Google Scholar] [CrossRef]
- Jansen, E.E.L.; Zielonke, N.; Gini, A.; Anttila, A.; Segnan, N.; Vokó, Z.; Ivanuš, U.; McKee, M.; de Koning, H.J.; de Kok, I.M.C.M.; et al. Effect of Organised Cervical Cancer Screening on Cervical Cancer Mortality in Europe: A Systematic Review. Eur. J. Cancer 2020, 127, 207–223. [Google Scholar] [CrossRef]
- Melnikow, J.; Henderson, J.T.; Burda, B.U.; Senger, C.A.; Durbin, S.; Soulsby, M.A. Screening for Cervical Cancer with High-Risk Human Papillomavirus Testing: A Systematic Evidence Review for the U.S. Preventive Services Task Force; U.S. In Preventive Services Task Force Evidence Syntheses, Formerly Systematic Evidence Reviews; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2018. [Google Scholar]
- Fontham, E.T.H.; Wolf, A.M.D.; Church, T.R.; Etzioni, R.; Flowers, C.R.; Herzig, A.; Guerra, C.E.; Oeffinger, K.C.; Shih, Y.-C.T.; Walter, L.C.; et al. Cervical Cancer Screening for Individuals at Average Risk: 2020 Guideline Update from the American Cancer Society. CA Cancer J. Clin. 2020, 70, 321–346. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; et al. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Updated Cervical Cancer Screening Guidelines. Available online: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/04/updated-cervical-cancer-screening-guidelines (accessed on 5 March 2024).
- Tirri, B.F.; Petignat, P.; Jacot-Guillarmod, M.; Mueller, M.D.; Fehr, M.; Kind, A.B. Recommandations pour la prévention du cancer du col de l’utérus. Avis, D’expert 2018, 50, 1–5. [Google Scholar]
- Bjørge, T.; Engesæter, B.; Skare, G.B.; Tropé, A. CervicalScreen Norway—A Screening Programme in Transition. Nor. J. Epidemiol. 2022, 30, 55–60. [Google Scholar] [CrossRef]
- Großmann, L.M.; Napierala, H.; Herrmann, W.J. Differences in Breast and Cervical Cancer Screening between West and East Germany: A Secondary Analysis of a German Nationwide Health Survey. BMC Public Health 2023, 23, 1931. [Google Scholar] [CrossRef] [PubMed]
- Neis, F.; Holleczek, B.; Henes, M.; Juhasz-Böss, I.; Wallwiener, D.; Neis, K.J. Proposal for a Descriptive and Differentiated Presentation of the Longitudinal Impact of the New Organized Cancer Screening Guideline and HPV Vaccination in Germany. Arch. Gynecol. Obstet. 2022, 307, 1125–1136. [Google Scholar] [CrossRef]
- Wang, W.; Arcà, E.; Sinha, A.; Hartl, K.; Houwing, N.; Kothari, S. Cervical Cancer Screening Guidelines and Screening Practices in 11 Countries: A Systematic Literature Review. Prev. Med. Rep. 2022, 28, 101813. [Google Scholar] [CrossRef]
- Cervical Cancer Screening|Irish Cancer Society. Available online: https://www.cancer.ie/cancer-information-and-support/cancer-types/cervical-cancer/cervical-cancer-screening (accessed on 5 March 2024).
- Brzoska, P.; Wahidie, D.; Yilmaz-Aslan, Y. An Intersectional Perspective on the Utilization of Cervical Cancer Screening among Migrants. A Cross-Sectional Analysis of Survey Data from Austria. Cancers 2021, 13, 6082. [Google Scholar] [CrossRef]
- Bonde, J.; Schroll, J.B.; Kj, B. Differentieret implementering af HPV-baseret screening i dansk livmoderhalskræftscreening. Ugeskr. Læger 2022, 184, V04210327. [Google Scholar]
- Olesen, T.B.; Jensen, H.; Møller, H.; Jensen, J.W.; Waldstrøm, M.; Andersen, B. Participation in the Nationwide Cervical Cancer Screening Programme in Denmark during the COVID-19 Pandemic: An Observational Study. eLife 2023, 12, e81522. [Google Scholar] [CrossRef]
- Dombrowski, C.; Bourgain, C.; Ma, Y.; Meiwald, A.; Pinsent, A.; Weynand, B.; Turner, K.M.; Huntington, S.; Adams, E.J.; Bogers, J.; et al. An Economic Evaluation of Two Cervical Screening Algorithms in Belgium: HR-HPV Primary Compared to HR-HPV and Liquid-Based Cytology (LBC) Co-Testing. Eur. J. Cancer Prev. 2023, 10, 1097. [Google Scholar] [CrossRef]
- Canadian Task Force on Preventive Health Care Recommendations on Screening for Cervical Cancer. CMAJ 2013, 185, 35–45. [CrossRef]
- Cervical Cancer Screening in Australia and New Zealand. Available online: https://ranzcog.edu.au/wp-content/uploads/2022/05/Cervical-cancer-screening-in-Australia-and-New-Zealand-C-Gyn-19-Amended-July-2020.pdf (accessed on 5 March 2024).
- Hamers, F.F.; Poullié, A.-I.; Arbyn, M. Updated Evidence-Based Recommendations for Cervical Cancer Screening in France. Eur. J. Cancer Prev. 2022, 31, 279–286. [Google Scholar] [CrossRef]
- Palmer, M.R.; Saito, E.; Katanoda, K.; Sakamoto, H.; Hocking, J.S.; Brotherton, J.M.L.; Ong, J.J. The Impact of Alternate HPV Vaccination and Cervical Screening Strategies in Japan: A Cost-Effectiveness Analysis. Lancet Reg. Health—West. Pac. 2024, 44, 101018. [Google Scholar] [CrossRef]
- Cervical Cancer—UK National Screening Committee (UK NSC)—GOV.UK. Available online: https://view-health-screening-recommendations.service.gov.uk/cervical-cancer/ (accessed on 5 March 2024).
- Finland: Human Papillomavirus and Related Cancers, Fact Sheet 2023. Fact Sheet. 2023. Available online: https://hpvcentre.net/statistics/reports/FIN_FS.pdf (accessed on 5 March 2024).
- Portero de la Cruz, S.; Cebrino, J. Trends and Determinants in Uptake of Cervical Cancer Screening in Spain: An Analysis of National Surveys from 2017 and 2020. Cancers 2022, 14, 2481. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Warnakulasuriya, S. Screening for Oral Cancer: Future Prospects, Research and Policy Development for Asia. Oral Oncol. 2020, 105, 104632. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Ramadas, K.; Thara, S.; Muwonge, R.; Thomas, G.; Anju, G.; Mathew, B. Long Term Effect of Visual Screening on Oral Cancer Incidence and Mortality in a Randomized Trial in Kerala, India. Oral Oncol. 2013, 49, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.F.A.; Oliveira, M.C.M.; Leite, A.C.; Bruzinga, F.F.B.; Mendes, P.A.; Grossmann, S.d.M.C.; de Araújo, V.E.; Souto, G.R. Assessment of Screening Programs as a Strategy for Early Detection of Oral Cancer: A Systematic Review. Oral Oncol. 2022, 130, 105936. [Google Scholar] [CrossRef]
- Liberto, J.M.; Chen, S.-Y.; Shih, I.-M.; Wang, T.-H.; Wang, T.-L.; Pisanic, T.R. Current and Emerging Methods for Ovarian Cancer Screening and Diagnostics: A Comprehensive Review. Cancers 2022, 14, 2885. [Google Scholar] [CrossRef] [PubMed]
- Waleleng, B.J.; Adiwinata, R.; Wenas, N.T.; Haroen, H.; Rotty, L.; Gosal, F.; Rotty, L.; Winarta, J.; Waleleng, A.; Simadibrata, M. Screening of Pancreatic Cancer: Target Population, Optimal Timing and How? Ann. Med. Surg. 2022, 84, 104814. [Google Scholar] [CrossRef] [PubMed]
- Rovito, M.J.; Manjelievskaia, J.; Leone, J.E.; Lutz, M.J.; Nangia, A. From ‘D’ to ‘I’: A Critique of the Current United States Preventive Services Task Force Recommendation for Testicular Cancer Screening. Prev. Med. Rep. 2016, 3, 361–366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- D’Souza-Schorey, C.; Clancy, J.W. Tumor-Derived Microvesicles: Shedding Light on Novel Microenvironment Modulators and Prospective Cancer Biomarkers. Genes. Dev. 2012, 26, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.L.; Martin, S.S.; Alpaugh, R.K.; Charpentier, M.; Tsai, S.; Bergan, R.C.; Ogden, I.M.; Catalona, W.; Chumsri, S.; Tang, C.-M.; et al. Circulating Giant Macrophages as a Potential Biomarker of Solid Tumors. Proc. Natl. Acad. Sci. USA 2014, 111, 3514–3519. [Google Scholar] [CrossRef]
- Macdonald, I.K.; Parsy-Kowalska, C.B.; Chapman, C.J. Autoantibodies: Opportunities for Early Cancer Detection. Trends Cancer 2017, 3, 198–213. [Google Scholar] [CrossRef]
- Vasseur, A.; Kiavue, N.; Bidard, F.; Pierga, J.; Cabel, L. Clinical Utility of Circulating Tumor Cells: An Update. Mol. Oncol. 2021, 15, 1647–1666. [Google Scholar] [CrossRef]
- Flores, B.C.T.; Correia, M.P.; Rodríguez, J.G.; Henrique, R.; Jerónimo, C. Bridging the Gaps between Circulating Tumor Cells and DNA Methylation in Prostate Cancer. Cancers 2021, 13, 4209. [Google Scholar] [CrossRef] [PubMed]
- Crook, T.; Leonard, R.; Mokbel, K.; Thompson, A.; Michell, M.; Page, R.; Vaid, A.; Mehrotra, R.; Ranade, A.; Limaye, S.; et al. Accurate Screening for Early-Stage Breast Cancer by Detection and Profiling of Circulating Tumor Cells. Cancers 2022, 14, 3341. [Google Scholar] [CrossRef]
- Ilie, M.; Hofman, V.; Long-Mira, E.; Selva, E.; Vignaud, J.-M.; Padovani, B.; Mouroux, J.; Marquette, C.-H.; Hofman, P. “Sentinel” Circulating Tumor Cells Allow Early Diagnosis of Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease. PLoS ONE 2014, 9, e111597. [Google Scholar] [CrossRef]
- Huang, C.; Ding, S.; Huang, C.; Pan, F.; Liu, X.; Zhang, H.; Zhou, J.; Liang, X.; Wang, X.; Song, P. Distribution and Clinical Analysis of EpCAM+/Vimentin+ Circulating Tumor Cells in High-Risk Population and Cancer Patients. Front. Oncol. 2021, 11, 642971. [Google Scholar] [CrossRef]
- Hillig, T.; Horn, P.; Nygaard, A.-B.; Haugaard, A.S.; Nejlund, S.; Brandslund, I.; Sölétormos, G. In Vitro Detection of Circulating Tumor Cells Compared by the CytoTrack and CellSearch Methods. Tumour Biol. 2015, 36, 4597–4601. [Google Scholar] [CrossRef]
- Akolkar, D.; Patil, D.; Crook, T.; Limaye, S.; Page, R.; Datta, V.; Patil, R.; Sims, C.; Ranade, A.; Fulmali, P.; et al. Circulating Ensembles of Tumor-Associated Cells: A Redoubtable New Systemic Hallmark of Cancer. Int. J. Cancer 2020, 146, 3485–3494. [Google Scholar] [CrossRef] [PubMed]
- Gaya, A.; Crook, T.; Plowman, N.; Ranade, A.; Limaye, S.; Bhatt, A.; Page, R.; Patil, R.; Fulmali, P.; Datta, V.; et al. Evaluation of Circulating Tumor Cell Clusters for Pan-cancer Noninvasive Diagnostic Triaging. Cancer Cytopathol. 2021, 129, 226–238. [Google Scholar] [CrossRef]
- Inaba, S.; Shimozono, N.; Yabuki, H.; Enomoto, M.; Morishita, M.; Hirotsu, T.; di Luccio, E. Accuracy Evaluation of the C. Elegans Cancer Test (N-NOSE) Using a New Combined Method. Cancer Treat. Res. Commun. 2021, 27, 100370. [Google Scholar] [CrossRef] [PubMed]
- Markou, A.; Tzanikou, E.; Lianidou, E. The Potential of Liquid Biopsy in the Management of Cancer Patients. Semin. Cancer Biol. 2022, 84, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Poulet, G.; Massias, J.; Taly, V. Liquid Biopsy: General Concepts. Acta Cytol. 2019, 63, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid Biopsy Enters the Clinic—Implementation Issues and Future Challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef]
- Cohen, S.; Mehrabi, S.; Yao, X.; Millingen, S.; Aikhionbare, F.O. Reactive Oxygen Species and Serous Epithelial Ovarian Adenocarcinoma. Cancer Res. J. 2016, 4, 106–114. [Google Scholar] [CrossRef]
- Pons-Belda, O.D.; Fernandez-Uriarte, A.; Diamandis, E.P. Multi Cancer Early Detection by Using Circulating Tumor DNA-The Galleri Test. Reply to Klein et al. The Promise of Multicancer Early Detection. Comment on “Pons-Belda et al. Can Circulating Tumor DNA Support a Successful Screening Test for Early Cancer Detection? The Grail Paradigm. Diagnostics 2021, 11, 2171”. Diagnostics 2022, 12, 1244. [Google Scholar] [CrossRef]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.; Seiden, M.V.; Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Smith, D.; Richards, D.; et al. Sensitive and Specific Multi-Cancer Detection and Localization Using Methylation Signatures in Cell-Free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef]
- Klein, E.A.; Richards, D.; Cohn, A.; Tummala, M.; Lapham, R.; Cosgrove, D.; Chung, G.; Clement, J.; Gao, J.; Hunkapiller, N.; et al. Clinical Validation of a Targeted Methylation-Based Multi-Cancer Early Detection Test Using an Independent Validation Set. Ann. Oncol. 2021, 32, 1167–1177. [Google Scholar] [CrossRef]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 2020, 182, 1044–1061.e18. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular Vesicles as Biomarkers and Therapeutic Targets for Cancer. Am. J. Physiol.-Cell Physiol. 2020, 318, C29–C39. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Hurley, J.; Roberts, D.; Chakrabortty, S.K.; Enderle, D.; Noerholm, M.; Breakefield, X.O.; Skog, J.K. Exosome-Based Liquid Biopsies in Cancer: Opportunities and Challenges. Ann. Oncol. 2021, 32, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Goldvaser, H.; Gutkin, A.; Beery, E.; Edel, Y.; Nordenberg, J.; Wolach, O.; Rabizadeh, E.; Uziel, O.; Lahav, M. Characterisation of Blood-Derived Exosomal hTERT mRNA Secretion in Cancer Patients: A Potential Pan-Cancer Marker. Br. J. Cancer 2017, 117, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Hinestrosa, J.P.; Kurzrock, R.; Lewis, J.M.; Schork, N.J.; Schroeder, G.; Kamat, A.M.; Lowy, A.M.; Eskander, R.N.; Perrera, O.; Searson, D.; et al. Early-Stage Multi-Cancer Detection Using an Extracellular Vesicle Protein-Based Blood Test. Commun. Med. 2022, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, J.; Zhang, H.; Zhu, Y.; Liu, W.; Zhang, K.; Zhang, Z. Localized Fluorescent Imaging of Multiple Proteins on Individual Extracellular Vesicles Using Rolling Circle Amplification for Cancer Diagnosis. J. Extracell. Vesicles 2020, 10, 12025. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, J.; Tian, F.; Cai, L.; Zhang, W.; Feng, Q.; Chang, J.; Wan, F.; Yang, Y.; Dai, B.; et al. Low-Cost Thermophoretic Profiling of Extracellular-Vesicle Surface Proteins for the Early Detection and Classification of Cancers. Nat. Biomed. Eng. 2019, 3, 183–193. [Google Scholar] [CrossRef]
- Yasui, T.; Yanagida, T.; Ito, S.; Konakade, Y.; Takeshita, D.; Naganawa, T.; Nagashima, K.; Shimada, T.; Kaji, N.; Nakamura, Y.; et al. Unveiling Massive Numbers of Cancer-Related Urinary-microRNA Candidates via Nanowires. Sci. Adv. 2017, 3, e1701133. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.E.; Martín Carreras-Presas, C.; Kaczor, T.; Tu, M.; Wei, F.; Garcia-Godoy, F.; Wong, D.T.W. Emerging Technologies for Salivaomics in Cancer Detection. J. Cell Mol. Med. 2017, 21, 640–647. [Google Scholar] [CrossRef]
- Daily, A.; Ravishankar, P.; Harms, S.; Klimberg, V.S. Using Tears as a Non-Invasive Source for Early Detection of Breast Cancer. PLoS ONE 2022, 17, e0267676. [Google Scholar] [CrossRef] [PubMed]
- Krilaviciute, A.; Heiss, J.A.; Leja, M.; Kupcinskas, J.; Haick, H.; Brenner, H. Detection of Cancer through Exhaled Breath: A Systematic Review. Oncotarget 2015, 6, 38643–38657. [Google Scholar] [CrossRef] [PubMed]
- Lidgard, G.P.; Domanico, M.J.; Bruinsma, J.J.; Light, J.; Gagrat, Z.D.; Oldham–Haltom, R.L.; Fourrier, K.D.; Allawi, H.; Yab, T.C.; Taylor, W.R.; et al. Clinical Performance of an Automated Stool DNA Assay for Detection of Colorectal Neoplasia. Clin. Gastroenterol. Hepatol. 2013, 11, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Redwood, D.G.; Asay, E.D.; Blake, I.D.; Sacco, P.E.; Christensen, C.M.; Sacco, F.D.; Tiesinga, J.J.; Devens, M.E.; Alberts, S.R.; Mahoney, D.W.; et al. Stool DNA Testing for Screening Detection of Colorectal Neoplasia in Alaska Native People. Mayo Clin. Proc. 2016, 91, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, T.F.; Ransohoff, D.F.; Itzkowitz, S.H.; Levin, T.R.; Lavin, P.; Lidgard, G.P.; Ahlquist, D.A.; Berger, B.M. Multitarget Stool DNA Testing for Colorectal-Cancer Screening. N. Engl. J. Med. 2014, 370, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Barnell, E.K.; Wurtzler, E.M.; La Rocca, J.; Fitzgerald, T.; Petrone, J.; Hao, Y.; Kang, Y.; Holmes, F.L.; Lieberman, D.A. Multitarget Stool RNA Test for Colorectal Cancer Screening. JAMA 2023, 330, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Currais, P.; Rosa, I.; Claro, I. Colorectal Cancer Carcinogenesis: From Bench to Bedside. World J. Gastrointest. Oncol. 2022, 14, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Bakkum-Gamez, J.N.; Wentzensen, N.; Maurer, M.J.; Hawthorne, K.M.; Voss, J.S.; Kroneman, T.N.; Famuyide, A.O.; Clayton, A.C.; Halling, K.C.; Kerr, S.E.; et al. Detection of Endometrial Cancer via Molecular Analysis of DNA Collected with Vaginal Tampons. Gynecol. Oncol. 2015, 137, 14–22. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Douville, C.; Cohen, J.D.; Yen, T.-T.; Kinde, I.; Sundfelt, K.; Kjær, S.K.; Hruban, R.H.; Shih, I.-M.; et al. Evaluation of Liquid from the Papanicolaou Test and Other Liquid Biopsies for the Detection of Endometrial and Ovarian Cancers. Sci. Transl. Med. 2018, 10, eaap8793. [Google Scholar] [CrossRef]
- Bryce, A.H.; Thiel, D.D.; Seiden, M.V.; Richards, D.; Luan, Y.; Coignet, M.; Zhang, Q.; Zhang, N.; Hubbell, E.; Kurtzman, K.N.; et al. Performance of a Cell-Free DNA-Based Multi-Cancer Detection Test in Individuals Presenting with Symptoms Suspicious for Cancers. JCO Precis. Oncol. 2023, 7, e2200679. [Google Scholar] [CrossRef]
- Bhamani, A.; Horst, C.; Bojang, F.; Quaife, S.L.; Dickson, J.L.; Tisi, S.; Hall, H.; Verghese, P.; Creamer, A.; Prendecki, R.; et al. The SUMMIT Study: Utilising a Written ‘Next Steps’ Information Booklet to Prepare Participants for Potential Lung Cancer Screening Results and Follow-Up. Lung Cancer 2023, 176, 75–81. [Google Scholar] [CrossRef]
- Nadauld, L.D.; McDonnell, C.H.; Beer, T.M.; Liu, M.C.; Klein, E.A.; Hudnut, A.; Whittington, R.A.; Taylor, B.; Oxnard, G.R.; Lipson, J.; et al. The PATHFINDER Study: Assessment of the Implementation of an Investigational Multi-Cancer Early Detection Test into Clinical Practice. Cancers 2021, 13, 3501. [Google Scholar] [CrossRef]
- Schrag, D.; Beer, T.M.; McDonnell, C.H.; Nadauld, L.; Dilaveri, C.A.; Reid, R.; Marinac, C.R.; Chung, K.C.; Lopatin, M.; Fung, E.T.; et al. Blood-Based Tests for Multicancer Early Detection (PATHFINDER): A Prospective Cohort Study. Lancet 2023, 402, 1251–1260. [Google Scholar] [CrossRef]
- Westgate, C.; Salles, G.; Shih, S.; Lopatin, M.; Kurtzman, K.N.; Kappes, K.; Venn, O.; Parekh, S.; Venstrom, J.M.; Younes, A.; et al. Multi-Cancer Early Detection Test Is Sensitive and Accurate in Detecting Shared DNA Methylation Signal in a Variety of Lymphoid and Plasma Cell Neoplasms. Blood 2023, 142, 3646. [Google Scholar] [CrossRef]
- Neal, R.D.; Johnson, P.; Clarke, C.A.; Hamilton, S.A.; Zhang, N.; Kumar, H.; Swanton, C.; Sasieni, P. Cell-Free DNA–Based Multi-Cancer Early Detection Test in an Asymptomatic Screening Population (NHS-Galleri): Design of a Pragmatic, Prospective Randomised Controlled Trial. Cancers 2022, 14, 4818. [Google Scholar] [CrossRef]
- Sasieni, P.; Smittenaar, R.; Hubbell, E.; Broggio, J.; Neal, R.D.; Swanton, C. Modelled Mortality Benefits of Multi-Cancer Early Detection Screening in England. Br. J. Cancer 2023, 129, 72–80. [Google Scholar] [CrossRef]
- Brentnall, A.R.; Mathews, C.; Beare, S.; Ching, J.; Sleeth, M.; Sasieni, P. Dynamic Data-Enabled Stratified Sampling for Trial Invitations with Application in NHS-Galleri. Clin. Trials 2023, 20, 425–433. [Google Scholar] [CrossRef]
- Bratulic, S.; Limeta, A.; Dabestani, S.; Birgisson, H.; Enblad, G.; Stålberg, K.; Hesselager, G.; Häggman, M.; Höglund, M.; Simonson, O.E.; et al. Noninvasive Detection of Any-Stage Cancer Using Free Glycosaminoglycans. Proc. Natl. Acad. Sci. USA 2022, 119, e2115328119. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wang, C.; Yang, X.; Fang, S.; Zhang, Y.; Wang, G.; Liu, F.; Wen, X.; Zhao, J.; Zhou, G.; et al. 909P A Multi-Cancer Early Detection Model Based on Liquid Biopsy of Multi-Omics Biomarkers: A Proof of Concept Study (PROMISE Study). Ann. Oncol. 2022, 33, S963–S964. [Google Scholar] [CrossRef]
- Gao, Q.; Lin, Y.P.; Li, B.S.; Wang, G.Q.; Dong, L.Q.; Shen, B.Y.; Lou, W.H.; Wu, W.C.; Ge, D.; Zhu, Q.L.; et al. Unintrusive Multi-Cancer Detection by Circulating Cell-Free DNA Methylation Sequencing (THUNDER): Development and Independent Validation Studies. Ann. Oncol. 2023, 34, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, B.; Cai, S.; Xu, J.; Wang, C.; Fang, S.; Qiu, F.; Su, J.; Xu, F.; Wen, X.; et al. LBA3 Early Detection and Localization of Multiple Cancers Using a Blood-Based Methylation Assay (ELSA-Seq). Ann. Oncol. 2020, 31, S1358. [Google Scholar] [CrossRef]
- Ris, F.; Hellan, M.; Douissard, J.; Nieva, J.J.; Triponez, F.; Woo, Y.; Geller, D.; Buchs, N.C.; Buehler, L.; Moenig, S.; et al. Blood-Based Multi-Cancer Detection Using a Novel Variant Calling Assay (DEEPGENTM): Early Clinical Results. Cancers 2021, 13, 4104. [Google Scholar] [CrossRef]
- Hakim, M.; Billan, S.; Tisch, U.; Peng, G.; Dvrokind, I.; Marom, O.; Abdah-Bortnyak, R.; Kuten, A.; Haick, H. Diagnosis of Head-and-Neck Cancer from Exhaled Breath. Br. J. Cancer 2011, 104, 1649–1655. [Google Scholar] [CrossRef]
- Peng, G.; Hakim, M.; Broza, Y.Y.; Billan, S.; Abdah-Bortnyak, R.; Kuten, A.; Tisch, U.; Haick, H. Detection of Lung, Breast, Colorectal, and Prostate Cancers from Exhaled Breath Using a Single Array of Nanosensors. Br. J. Cancer 2010, 103, 542–551. [Google Scholar] [CrossRef]
- Park, B.; Shen, S.Y.; Min, J.; Fleshner, N.; Knox, J.; May, T.; Ailles, L.; Newton, Y.; Zhang, J.; Singhania, R.; et al. Abstract 1030: Development of a Genome-Wide Methylome Enrichment Platform for Multi-Cancer Early Detection (MCED). Cancer Res. 2023, 83, 1030. [Google Scholar] [CrossRef]
- Nguyen, H.T.-H.; Tran, L.S. Clinical Validation of a ctDNA-Based Assay for Multi-Cancer Detection: An Interim Report from a Vietnamese Longitudinal Prospective Cohort Study of 2795 Participants. JCO Glob. Oncol. 2023, 9, 135. [Google Scholar] [CrossRef]
- Tran, L.S.; Nguyen, L.H.D.; Nguyen, T.H.H. LBA2 Analytical and Clinical Validation of a ctDNA-Based Assay for Multi-Cancer Detection. Ann. Oncol. 2023, 34, S1467. [Google Scholar] [CrossRef]
- Suo, C.; Zhao, R.; Jiang, Y.; Zhang, Y.; He, Q.; Su, Z.; Liu, R.; Jin, L.; Chen, X. Abstract 4194: The FuSion Project of Pan-Cancer Early Screening in Chinese– An Integrative Study by Fudan University and Singlera. Cancer Res. 2023, 83, 4194. [Google Scholar] [CrossRef]
- Chanteloup, G.; Cordonnier, M.; Isambert, N.; Bertaut, A.; Marcion, G.; Garrido, C.; Gobbo, J. Membrane-Bound Exosomal HSP70 as a Biomarker for Detection and Monitoring of Malignant Solid Tumours: A Pilot Study. Pilot. Feasibility Stud. 2020, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Wang, Z.; Ma, X.; Guo, W.; Zhang, X.; Tang, W.; Chen, X.; Wang, X.; Chen, Y.; Mo, S.; et al. Letter to the Editor: An Ultra-Sensitive Assay Using Cell-Free DNA Fragmentomics for Multi-Cancer Early Detection. Mol. Cancer 2022, 21, 129. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J.; Wong, K.-K.; Tsay, J.-C.J.; Pass, H.I.; Vachani, A.; Ryan, A.; Carey, J.; Jakubowski, D.; Wu, T.; Zong, Y.; et al. Abstract 5766: Prospective Evaluation of Cell-Free DNA Fragmentomes for Lung Cancer Detection. Cancer Res. 2023, 83, 5766. [Google Scholar] [CrossRef]
- Pommergaard, H.-C. Early Detection of de Novo Cancer in Liver Transplant Recipients—A ScandiaTransplant Collaboration. Available online: https://clinicaltrials.gov/study/NCT05492617 (accessed on 3 March 2024).
- Harbinger Health Cancer ORigin Epigenetics-Harbinger Health—Collection of Blood and Tissue Samples From Cancer and Non-Cancer Subjects for Validation of a Novel Blood-Based Multi-Cancer Screening Test. Available online: https://clinicaltrials.gov/study/NCT05435066 (accessed on 3 March 2024).
- Nguyen, H.; Raymond, V.M.; Vento-Gaudens, E.; Marino, E.; Lang, K.; Eagle, C. Screening for High Frequency Malignant Disease (SHIELD). JCO 2022, 40, TPS1602. [Google Scholar] [CrossRef]
- Raymond, V.; Nguyen, H.; Cotton, L.; Vento-Gaudens, E.; Eagle, C. PP01.20 Trial in Progress: Screening for High Frequency Malignant Disease (SHIELD). J. Thorac. Oncol. 2023, 18, e19. [Google Scholar] [CrossRef]
- Raymond, V.M.; Higashi, L.; Marino, E.; Lang, K. Evaluation of the ctDNA LUNAR-2 Test in an Average Patient Screening Episode (ECLIPSE). JCO 2021, 39, TPS142. [Google Scholar] [CrossRef]
- Hackshaw, A.; Clarke, C.A.; Hartman, A.-R. New Genomic Technologies for Multi-Cancer Early Detection: Rethinking the Scope of Cancer Screening. Cancer Cell 2022, 40, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.Y.; Burgener, J.M.; Bratman, S.V.; De Carvalho, D.D. Preparation of cfMeDIP-Seq Libraries for Methylome Profiling of Plasma Cell-Free DNA. Nat. Protoc. 2019, 14, 2749–2780. [Google Scholar] [CrossRef] [PubMed]
- Twombly, R. Full-Body CT Screening: Preventing or Producing Cancer? JNCI J. Natl. Cancer Inst. 2004, 96, 1650–1651. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Welch, H.G.; Skinner, J.S.; Schroeck, F.R.; Zhou, W.; Black, W.C. Regional Variation of Computed Tomographic Imaging in the United States and the Risk of Nephrectomy. JAMA Intern. Med. 2018, 178, 221–227. [Google Scholar] [CrossRef]
- Tipirneni, K.E.; Rosenthal, E.L.; Moore, L.S.; Haskins, A.D.; Udayakumar, N.; Jani, A.H.; Carroll, W.R.; Morlandt, A.B.; Bogyo, M.; Rao, J.; et al. Fluorescence Imaging for Cancer Screening and Surveillance. Mol. Imaging Biol. 2017, 19, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.Y.; Bishop, M.; Zheng, B.; Ferguson, R.M.; Khandhar, A.P.; Kemp, S.J.; Krishnan, K.M.; Goodwill, P.W.; Conolly, S.M. Magnetic Particle Imaging: A Novel in Vivo Imaging Platform for Cancer Detection. Nano Lett. 2017, 17, 1648–1654. [Google Scholar] [CrossRef]
- Cheng, Z.; Yan, X.; Sun, X.; Shen, B.; Gambhir, S.S. Tumor Molecular Imaging with Nanoparticles. Engineering 2016, 2, 132–140. [Google Scholar] [CrossRef]
- Shumilov, E.; Flach, J.; Pabst, T.; Fiedler, M.; Angelillo-Scherrer, A.; Trümper, L.; Joncourt, R.; Kohlmann, A.; Bacher, U. Genetic Alterations Crossing the Borders of Distinct Hematopoetic Lineages and Solid Tumors: Diagnostic Challenges in the Era of High-Throughput Sequencing in Hemato-Oncology. Crit. Rev. Oncol. Hematol. 2018, 126, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Tivey, A.; Church, M.; Rothwell, D.; Dive, C.; Cook, N. Circulating Tumour DNA—Looking beyond the Blood. Nat. Rev. Clin. Oncol. 2022, 19, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Boshier, P.; Myridakis, A.; Belluomo, I.; Hanna, G.B. Urinary Volatile Organic Compound Analysis for the Diagnosis of Cancer: A Systematic Literature Review and Quality Assessment. Metabolites 2020, 11, 17. [Google Scholar] [CrossRef]
- Saalberg, Y.; Wolff, M. VOC Breath Biomarkers in Lung Cancer. Clin. Chim. Acta 2016, 459, 5–9. [Google Scholar] [CrossRef]
- Feil, C.; Staib, F.; Berger, M.R.; Stein, T.; Schmidtmann, I.; Forster, A.; Schimanski, C.C. Sniffer Dogs Can Identify Lung Cancer Patients from Breath and Urine Samples. BMC Cancer 2021, 21, 917. [Google Scholar] [CrossRef]
- Mallafré-Muro, C.; Llambrich, M.; Cumeras, R.; Pardo, A.; Brezmes, J.; Marco, S.; Gumà, J. Comprehensive Volatilome and Metabolome Signatures of Colorectal Cancer in Urine: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 2534. [Google Scholar] [CrossRef]
- Lima, A.R.; Pinto, J.; Carvalho-Maia, C.; Jerónimo, C.; Henrique, R.; Bastos, M.d.L.; Carvalho, M.; Guedes de Pinho, P. A Panel of Urinary Volatile Biomarkers for Differential Diagnosis of Prostate Cancer from Other Urological Cancers. Cancers 2020, 12, 2017. [Google Scholar] [CrossRef]
- Monteiro, M.; Moreira, N.; Pinto, J.; Pires-Luís, A.S.; Henrique, R.; Jerónimo, C.; Bastos, M. de L.; Gil, A.M.; Carvalho, M.; Guedes de Pinho, P. GC-MS Metabolomics-based Approach for the Identification of a Potential VOC-biomarker Panel in the Urine of Renal Cell Carcinoma Patients. J. Cell Mol. Med. 2017, 21, 2092–2105. [Google Scholar] [CrossRef]
- Giró Benet, J.; Seo, M.; Khine, M.; Gumà Padró, J.; Pardo Martnez, A.; Kurdahi, F. Breast Cancer Detection by Analyzing the Volatile Organic Compound (VOC) Signature in Human Urine. Sci. Rep. 2022, 12, 14873. [Google Scholar] [CrossRef]
- Iliff, A.J.; Xu, X.Z.S.C. Elegans: A Sensible Model for Sensory Biology. J. Neurogenet. 2020, 34, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Girard, L.R.; Fiedler, T.J.; Harris, T.W.; Carvalho, F.; Antoshechkin, I.; Han, M.; Sternberg, P.W.; Stein, L.D.; Chalfie, M. WormBook: The Online Review of Caenorhabditis Elegans Biology. Nucleic Acids Res. 2007, 35, D472–D475. [Google Scholar] [CrossRef]
- Bargmann, C.I. Comparative Chemosensation from Receptors to Ecology. Nature 2006, 444, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hattori, Y.; Saito, T.; Harada, Y. Pond Assay for the Sensory Systems of Caenorhabditis Elegans: A Novel Anesthesia-Free Method Enabling Detection of Responses to Extremely Low Chemical Concentrations. Biology 2022, 11, 335. [Google Scholar] [CrossRef]
- Hirotsu, T.; Sonoda, H.; Uozumi, T.; Shinden, Y.; Mimori, K.; Maehara, Y.; Ueda, N.; Hamakawa, M. A Highly Accurate Inclusive Cancer Screening Test Using Caenorhabditis Elegans Scent Detection. PLoS ONE 2015, 10, e0118699. [Google Scholar] [CrossRef]
- Bargmann, C.I.; Hartwieg, E.; Horvitz, H.R. Odorant-Selective Genes and Neurons Mediate Olfaction in C. Elegans. Cell 1993, 74, 515–527. [Google Scholar] [CrossRef]
- Zhong, Q.; Tan, E.K.W.; Martin-Alonso, C.; Parisi, T.; Hao, L.; Kirkpatrick, J.D.; Fadel, T.; Fleming, H.E.; Jacks, T.; Bhatia, S.N. Inhalable Point-of-Care Urinary Diagnostic Platform. Sci. Adv. 2024, 10, eadj9591. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, E.; Clarke, C.A.; Aravanis, A.M.; Berg, C.D. Modeled Reductions in Late-Stage Cancer with a Multi-Cancer Early Detection Test. Cancer Epidemiol. Biomark. Prev. 2021, 30, 460–468. [Google Scholar] [CrossRef]
- Brill, J.V. Screening for Cancer: The Economic, Medical, and Psychosocial Issues. Am. J. Manag. Care 2020, 26 (Suppl. S14), S300–S306. [Google Scholar]
- Tafazzoli, A.; Ramsey, S.D.; Shaul, A.; Chavan, A.; Ye, W.; Kansal, A.R.; Ofman, J.; Fendrick, A.M. The Potential Value-Based Price of a Multi-Cancer Early Detection Genomic Blood Test to Complement Current Single Cancer Screening in the USA. Pharm. Econ. 2022, 40, 1107–1117. [Google Scholar] [CrossRef]
- Tomczak, K.; Czerwińska, P.; Wiznerowicz, M. The Cancer Genome Atlas (TCGA): An Immeasurable Source of Knowledge. Contemp. Oncol. 2015, 19, A68–A77. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.; Craft, B.; Swatloski, T.; Ellrott, K.; Cline, M.; Diekhans, M.; Ma, S.; Wilks, C.; Stuart, J.; Haussler, D.; et al. The UCSC Cancer Genomics Browser: Update 2013. Nucleic Acids Res. 2013, 41, D949–D954. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer Analysis Project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- GTEx Consortium Human Genomics. The Genotype-Tissue Expression (GTEx) Pilot Analysis: Multitissue Gene Regulation in Humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Troup, D.B.; Wilhite, S.E.; Ledoux, P.; Rudnev, D.; Evangelista, C.; Kim, I.F.; Soboleva, A.; Tomashevsky, M.; Edgar, R. NCBI GEO: Mining Tens of Millions of Expression Profiles—Database and Tools Update. Nucleic Acids Res. 2007, 35, D760–D765. [Google Scholar] [CrossRef] [PubMed]
- Manoochehri, M.; Wu, Y.; Giese, N.A.; Strobel, O.; Kutschmann, S.; Haller, F.; Hoheisel, J.D.; Moskalev, E.A.; Hackert, T.; Bauer, A.S. SST Gene Hypermethylation Acts as a Pan-Cancer Marker for Pancreatic Ductal Adenocarcinoma and Multiple Other Tumors: Toward Its Use for Blood-Based Diagnosis. Mol. Oncol. 2020, 14, 1252–1267. [Google Scholar] [CrossRef]
- Chen, W.; Li, G.; Peng, J.; Dai, W.; Su, Q.; He, Y. Transcriptomic Analysis Reveals That Heat Shock Protein 90α Is a Potential Diagnostic and Prognostic Biomarker for Cancer. Eur. J. Cancer Prev. 2020, 29, 357–364. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Pan, X.; Li, M.; Yang, S.; Li, S.C. DNA Methylation Markers for Pan-Cancer Prediction by Deep Learning. Genes 2019, 10, 778. [Google Scholar] [CrossRef]
| Cancer | Screening Procedure | Screening Interval | % Eligible Adults | Adherence | Sensitivity | Specificity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| US | UK | US | UK | US | UK | US | UK | US | UK | US | UK | |
| Lung | Low-dose computed tomography | No Recommendation | Annually | NA | 7% | NA | 14.4% | NA | 84.9% | NA | 87.2% | NA |
| Colorectal | Cologuard | FIT | Every 3 years | Every 2 years | 93% | 68% | 67.7% | 57.7% | 92% | 79% | 87% | 94% |
| Breast | Bilateral mammography | Annually | Every 3 years | 90% of women | 77% of women | 77.6% | 70.5% | 87% | 87% | 89% | 97.2% | |
| Cervical | Cytological examination and hrHPV testing | Every 5 years | Every 5 years | 63% of women | 60% of women | 95% | 76.2% | 95% | 95% | 85% | 85% | |
| Country | Organization | Type | Year | Test | Eligible Population | Relevant Literature |
|---|---|---|---|---|---|---|
| United States | USPSTF | National Guideline Committee | 2021 | Shared decision-making between clinician and patient for one of the following strategies:
| All adults aged 45–75 years; selectively screen adults aged 76–85 years | [27] |
| ACS | Cancer Society | 2018 |
| All adults aged 45–75; selectively screen adults aged 76–85 years | [28] | |
| Luxembourg | Ministry of Health | National Guideline Committee | 2016 | FIT every 2 years. Colonoscopy if positive test. | All adults between 55–74 years old invited to participate | NA |
| Switzerland | League Against Cancer | Cancer Society | 2013 | Colonoscopy every 10 years—g-FOBT every two years. | All adults aged 50–69 | [30] |
| Norway | Cancer Registry of Norway | Cancer Society | 2022 | FIT every 2 years. Colonoscopy if positive test. | All adults aged 55–74 | [31] |
| Netherlands | NIPHE | National Guideline Committee | 2019 | FIT every 2 years. | All adults aged 55–75 | [32] |
| Germany | Federal Joint Committee | National Guideline Committee | 2019 | Women, 50–54: annual FIT. Women >55: FIT every 2 years or colonoscopy twice at an interval of 10 years. | All adults from 55 years onwards | [33] |
| Men, 50–54: annual FIT or colonoscopy twice at an interval of 10 years. Men, >55: FIT once every 2 years or colonoscopy twice at intervals of 10 years. | ||||||
| Sweden | National Board of Health and Welfare | National Guideline Committee | FIT once every 2 years. Colonoscopy if positive test. | All adults aged 60–74 | [34] | |
| Ireland | National Screening Service | National Guideline Committee | 2013 | Bowel Screen Programme: FIT once every 2 years. Colonoscopy if positive test. | All adults aged 59–69 | [35] |
| Austria | Austrian Cancer Care | Cancer Society | 2023 | Colonoscopy every 10 years, or FIT every 2 years. | All adults 45–75 years | [36] |
| Denmark | National Board of Health | National Guideline Committee | 2014 | FIT once every 2 years. Colonoscopy if positive test. | All adults aged 50–74 | [37] |
| Belgium | Foundation Against Cancer | Cancer Society | 2013 | FIT once every 2 years. Colonoscopy if positive test. | All adults aged 50–74 | [38] |
| Canada | CTFPHC | National Guideline Committee | 2016 | 50–59: g-FOBT/FIT every 2 years or flexible sigmoidoscopy every 10 years (weak recommendation). 60–74 years: g-FOBT/FIT every 2 years or flexible sigmoidoscopy every 10 years (strong recommendation). | All adults aged 50–74 years | [39] |
| Australia | Australian Government Department of Health | National Guideline Committee | 2023 | FIT once every 2 years. Colonoscopy if positive test. | All adults aged 50–74 years | [40] |
| France | National Cancer Institute | National Guideline Committee | 2015 | FIT once every 2 years. Colonoscopy if positive test. | All adults aged 50–74 years | [41] |
| Japan | National Cancer Center | National Guideline Committee | 2016 | g-FOBT once per year. | All adults aged 40 and over | [42] |
| Iceland | Icelandic Cancer Society | Cancer Society | 2015 | FIT once every 2 years. Colonoscopy if positive test. | All adults aged 60–69 | NA |
| UK | UK National Screening Committee | National Guideline Committee | 2020 | FIT once every 2 years. Colonoscopy if positive test. | All adults aged 50–74 | [43] |
| Finland | Cancer Society of Finland | Cancer Society | 2021 | FIT once every 2 years. Colonoscopy if positive test. | Adults aged 56–74 years old | NA |
| New Zealand | Ministry of Health | Cancer Society | 2023 | Time to Screen program offering FIT once every 2 years. Colonoscopy if the test is positive | Adults aged 60–74 | [44] |
| Italy | National Screening Observatory | National Guideline Committee | 2015 | FIT once every 2 years. Colonoscopy if the test is positive. | Adults aged 50–69 years | [45] |
| Spain | Cancer Strategy of National Health System | National Guideline Committee | 2009 | FIT once every 2 years. | Adults aged 50–69 | [46] |
| Country | Organization | Type | Year | Primary Group | Further Discussion | Relevant Literature |
|---|---|---|---|---|---|---|
| United States | USPSTF | National Guideline Committee | 2023 * | Women aged 40–75: biennial screening mammography | Insufficient evidence for mammography screening in women >75 | [53] |
| Insufficient evidence for breast US, or MRI in women identified with dense breasts on an otherwise negative screening mammogram | ||||||
| ACS | Cancer Society | 2015, 2007 | Women with average risk of breast cancer: ages 40–45—option to start yearly mammography ages 45–54—yearly mammography; ages ≥55—biennial/annual screening; continue mammography until life expectancy ≥10 years | Annual breast MRI screening in higher-risk patients: BRCA mutation, a first-degree relative of BRCA carrier (untested), the lifetime risk of ≥20–25% (as defined by BRCAPRO/other models), radiation to the chest between 10–30 years, Li–Fraumeni/Cowden/Bannayan–Riley–-Ruvalcaba syndrome and first-degree relatives | [52,54] | |
| ACOG | Specialty Society | 2017, reaffirmed 2021 | Offer mammography starting at 40 years. Initiate at ages 40–49 years after counseling. Recommend by no later than age 50 if the patient has not already been initiated. Continue until age 75. Beyond 75, consider life expectancy. | Annual or biennial screening. Clinical breast examination may be offered for women aged 25–39 years and annually for women 40 years and older. | [55] | |
| ACR | Specialty Society | 2023, 2021 | Risk assessment by age 25. Annual mammography starting at the age of 40. Screening should continue past age 74 unless severe comorbidities limit life expectancy. | Women with genetically based increased risk, or with a calculated lifetime risk of ≥20% are recommended to undergo breast MRI surveillance starting at ages 25–30 and annual mammography starting between ages 25–40, depending on the risk. | [56,57] | |
| Luxembourg | Ministry of Health | National Guideline Committee | NA | Programme Mammographie targets women aged 50–70, inviting them for mammography every 23 months. | ||
| Switzerland | Swiss Cancer League | Cancer Society | 2016 | Mammography once every 2 years for women aged 50–70 (74 in some cantons) | [58] | |
| Norway | Cancer Registry of Norway | Cancer Society | 2010 | Mammography once every 2 years for women 50–69 years of age | [59] | |
| Netherlands | NIPHE | National Guideline Committee | 2017 | Mammography once every 2 years for women 50–74 years of age | [60] | |
| Germany | Federal Joint Committee | National Guideline Committee | 2015 | Mammography once every 2 years for women 50–69 years of age | [61] | |
| Sweden | National Board of Health and Welfare | National Guideline Committee | 2013 | Mammography once every 2 years for women 40–74 years of age | [62] | |
| Ireland | National Screening Service | National Guideline Committee | 2015 | BreastCheck program offers mammography once every 2 years for all women aged 50–69 years. | [63] | |
| Austria | Austrian Cancer Aid Society | Cancer Society | 2014 | Mammography once every 2 years for women aged 45–69 years | [64] | |
| Denmark | National Board of Health | National Guideline Committee | 2014 | Mammography once every 2 years for women aged 50–69 years | [65] | |
| Belgium | Foundation Against Cancer | Cancer Society | 2017 | Mammography once every 2 years for women aged 50–69 years | [66] | |
| Canada | CTFPHC | National Guideline Committee | 2011 | Mammography once every 2–3 years for participants aged 50–74 | For women aged 40–49 years, recommendation against mammography (shared decision-making with healthcare provider) | [67] |
| Australia | Australian Government Department of Health | National Guideline Committee | 2015 | BreastScreen program offers mammography every 2 years for women aged 50–74 | Program available for women from age 40, with mammography every 2 years | [68,69] |
| France | National Cancer Institute | National Guideline Committee | 2015, 2016 | Mammography once every 2 years for participants aged 50–74. Screening also includes manual breast exam | Yearly MRI, mammography, and US for patients from the age of 30 onwards in high-risk cases (genetic factor, family history, personal history of radiation treatments) | [70] |
| Japan | National Cancer Center | National Guideline Committee | 2021 | Mammography once every two years to women aged 40–69 ± manual breast examination | [71] | |
| Iceland | Icelandic Cancer Society | Cancer Society | NA | Mammography once every 2 years for women aged 40–69 years. Invitation for women aged 70–74 to have mammography every three years | [72] | |
| UK | UK National Screening Committee | National Guideline Committee | 2012 | Mammography once every 3 years form women aged 50–70 years | [73] | |
| Finland | Cancer Society of Finland | Cancer Society | 2010 | Mammography once every 2 years for women aged 50–69 years | [72] | |
| New Zealand | Ministry of Health | Cancer Society | 2014 | BreastScreen Aotearoa program offers mammography every two years for women aged 45–69 | [74] | |
| Italy | National Screening Observatory | National Guideline Committee | 2015 | Mammography once every 2 years for women aged 50–69 years (45–69 in Campania region) | [75] | |
| Spain | Cancer Strategy of National Health System | National Guideline Committee | 2009 | Mammography once every 2 years for women aged 50–69 years | [76] |
| Country | Organization | Type | Year | Screening Method | Eligible Population | Relevant Literature |
|---|---|---|---|---|---|---|
| United States | USPSTF | National Guideline Committee | 2018 | Women aged 21–29: cytology alone every 3 years Women aged 30–65 years: cytology alone every 3 years, with HR-HPV testing alone every 5 years, or co-testing every 5 years | Women between years 21–65 | [91] |
| ACS | Cancer Society | 2020 | Screen with HR-HPV testing every 5 years If not available, co-testing every 5 years, or cytology every 3 years | Women between years 25–65 | [90] | |
| ACOG | Specialty Society | 2021 | Endorses USPSTF recommendations | Women between years 21–65 | [92] | |
| Luxembourg | Ministry of Health | National Guideline Committee | NA | NA | NA | NA |
| Switzerland | League Against Cancer | Cancer Society | 2018 | Women aged 21–29: cytological screening Women aged 30–70 years: cytological/HR-HPV screening every 3 years | Women between years 21–70 | [93] |
| Norway | Cancer Registry of Norway | Cancer Society | 2022 | Cytology screening from the age of 25, HR-HPV testing from the age of 34 | Women aged 25–69 years | [94] |
| Netherlands | NIPHE | National Guideline Committee | 2021 | HR-HPV screening every five years between 30–60 years | Women aged 30–60 | [60] |
| Germany | Federal Joint Committee | National Guideline Committee | 2020 | Women aged 21–35: annual genital examination, medical history, cytology Women aged >35 are offered HR-HPV and cytology co-testing | Women aged 20 | [95,96] |
| Sweden | National Board of Health and Welfare | National Guideline Committee | 2015 | Women aged 23–29: Cytology-based screening once every three years Women aged 30–49: HR-HPV testing every three years with a cytology co-test at age 41 Women aged 50–64: HR-HPV test every seven years | Women aged 23–64 | [97] |
| Ireland | National Screening Service | National Guideline Committee | 2020 | CervicalCheck program—women aged 25–29 years: cervical screening every 3 years Women aged 30–65 years: cervical screening every 5 years | Women between 25–65 years of age | [98] |
| Austria | Austrian Cancer Care | Cancer Society | 2014 | Cytology every 3 years | Women between 19–69 years of age | [99] |
| Denmark | National Board of Health | National Guideline Committee | 2022 | Women aged 23–49 years: co-testing every 3 years Women aged 50–64 years: co-testing every 5 years | Women between 23–64 years of age | [100,101] |
| Belgium | Foundation Against Cancer | Cancer Society | 2022 | Cytology tests every 3 years Women aged 30–64 years—HR-HPV test every five years | Women aged between 25–64 years | [102] |
| Canada | CTFPHC | National Guideline Committee | 2013 | Cytology every 3 years | Women aged between 25–69 years | [103] |
| Australia | Australian Government Department of Health | National Guideline Committee | 2022 | HR-HPV testing every 5 years with cytology for positive tests | Women aged 25–74 years of age | [104] |
| France | National Cancer Institute | National Guideline Committee | 2022 | Women 25–30 years: cytology testing every 3 years Women 30–65 years HR-HPV testing every 5 years | Women aged 25–65 years | [105] |
| Japan | National Cancer Center | National Guideline Committee | 2020 | Women aged 20–69 years: cytology testing once every 2 years | Women aged 20–69 | [106] |
| Iceland | Icelandic Cancer Society | Cancer Society | 2020 | Women aged 23–29 years: HR-HPV every three years Women aged 30–59 years: HR-HPV every five years Women aged 60–64 years: HR-HPV test—if a test is negative, women are discharged from screening | Women aged 23–64 | NA |
| UK | UK National Screening Committee | National Guideline Committee | 2019 | HR-HPV test once every 3 years | Women aged 25–64 | [107] |
| Finland | Cancer Society of Finland | Cancer Society | 2017 | Cytology once every five years (can be substituted with HR-HPV) | Women aged 25–65 | [108] |
| New Zealand | Ministry of Health | Cancer Society | 2023 | HR-HPV once every five years (once every 3 years if immunodeficient) screening extended to women aged 70–74 years if unscreened or under-screened | Women aged 25–69 | [104] |
| Italy | National Screening Observatory | National Guideline Committee | Women aged 25–30 years: Cytology test once every three years Women aged 30–65 years: HPV-based screening every five years | Women aged 25–65 years | [97] | |
| Spain | Cancer Strategy of National Health System | National Guideline Committee | 2019 | Women aged 25–34 years: cytology co-testing once every 3 years Women aged 35–65 years: HR-HPV testing every five years | Women aged 25–65 years | [109] |
| Current Screening Only | Incremental MCED Test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Analysis | Total Positives | True Positive Cancers (Diagnostic Yield per 1000) | True Positive/False Positive Ration | Cancer Detection Rate | Diagnostic cost per Confirmed Cancer Diagnosis | Total Positives | Diagnostic Yield per 1000 | True Positive/False Positive Ration | Cancer Detection Rate | Diagnostic Cost per Confirmed Cancer Diagnosis |
| 100% MCED uptake | 481,876 | 24,888 (3.43) | 1:18 | 12% | 10,452 | 244,153 | 92,817 (4.26) | 1:1.6 | 43% | 2175 |
| 20% decrease cancer incidence in screening population | 476,899 | 19,910 (2.74) | 1:23 | 9% | 12,921 | 246,220 | 94,884 (4.35) | 1:1.6 | 44% | 2134 |
| Difference | −4977 | −4978 | - | - | 2469 | 2067 | 2067 | - | - | −41 |
| Prostate cancer added | 635,797 | 27,754 (2.61%) | 1:22 | 13% | 12,917 | 243,586 | 92,250 (4.23%) | 1:1.6 | 43% | 2187 |
| Difference | 153,921 | 2866 | −2465 | −567 | −567 | - | - | 12 | ||
| 50% MCED uptake | - | - | - | - | - | 122,076 | 46,409 (4.23) | 1:1.6 | 22% | 2175 |
| Difference | 0 | 0 | - | - | 0 | −122,077 | −46,408 | - | - | 0 |
| 25% MCED uptake | - | - | - | - | - | 61,038 | 23,204 (4.23) | 1:1.6 | 11% | 2175 |
| Difference | 0 | 0 | - | - | 0 | −183,115 | −69,613 | - | - | 0 |
| 30% decline in standard-of-care screening, only receiving MCED test | - | - | - | - | - | 76,346 | 30,945 (4.72) | 1:1.5 | 14% | 1989 |
| Difference | - | - | - | - | - | −167,807 | −61,872 | - | - | −186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeș, L.N.; Păun, M.-A.; Anghel, R.M.; Trifănescu, O.G. Cancer Screening: Present Recommendations, the Development of Multi-Cancer Early Development Tests, and the Prospect of Universal Cancer Screening. Cancers 2024, 16, 1191. https://doi.org/10.3390/cancers16061191
Galeș LN, Păun M-A, Anghel RM, Trifănescu OG. Cancer Screening: Present Recommendations, the Development of Multi-Cancer Early Development Tests, and the Prospect of Universal Cancer Screening. Cancers. 2024; 16(6):1191. https://doi.org/10.3390/cancers16061191
Chicago/Turabian StyleGaleș, Laurenția Nicoleta, Mihai-Andrei Păun, Rodica Maricela Anghel, and Oana Gabriela Trifănescu. 2024. "Cancer Screening: Present Recommendations, the Development of Multi-Cancer Early Development Tests, and the Prospect of Universal Cancer Screening" Cancers 16, no. 6: 1191. https://doi.org/10.3390/cancers16061191
APA StyleGaleș, L. N., Păun, M.-A., Anghel, R. M., & Trifănescu, O. G. (2024). Cancer Screening: Present Recommendations, the Development of Multi-Cancer Early Development Tests, and the Prospect of Universal Cancer Screening. Cancers, 16(6), 1191. https://doi.org/10.3390/cancers16061191







