Responses to Medical Treatment in 192 Patients with Pancreatic Neuroendocrine Neoplasms Referred to the Copenhagen Neuroendocrine Tumour Centre in 2000–2020
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Acquisition
2.3. Outcome
2.4. Statistics
3. Results
3.1. Baseline Characteristics
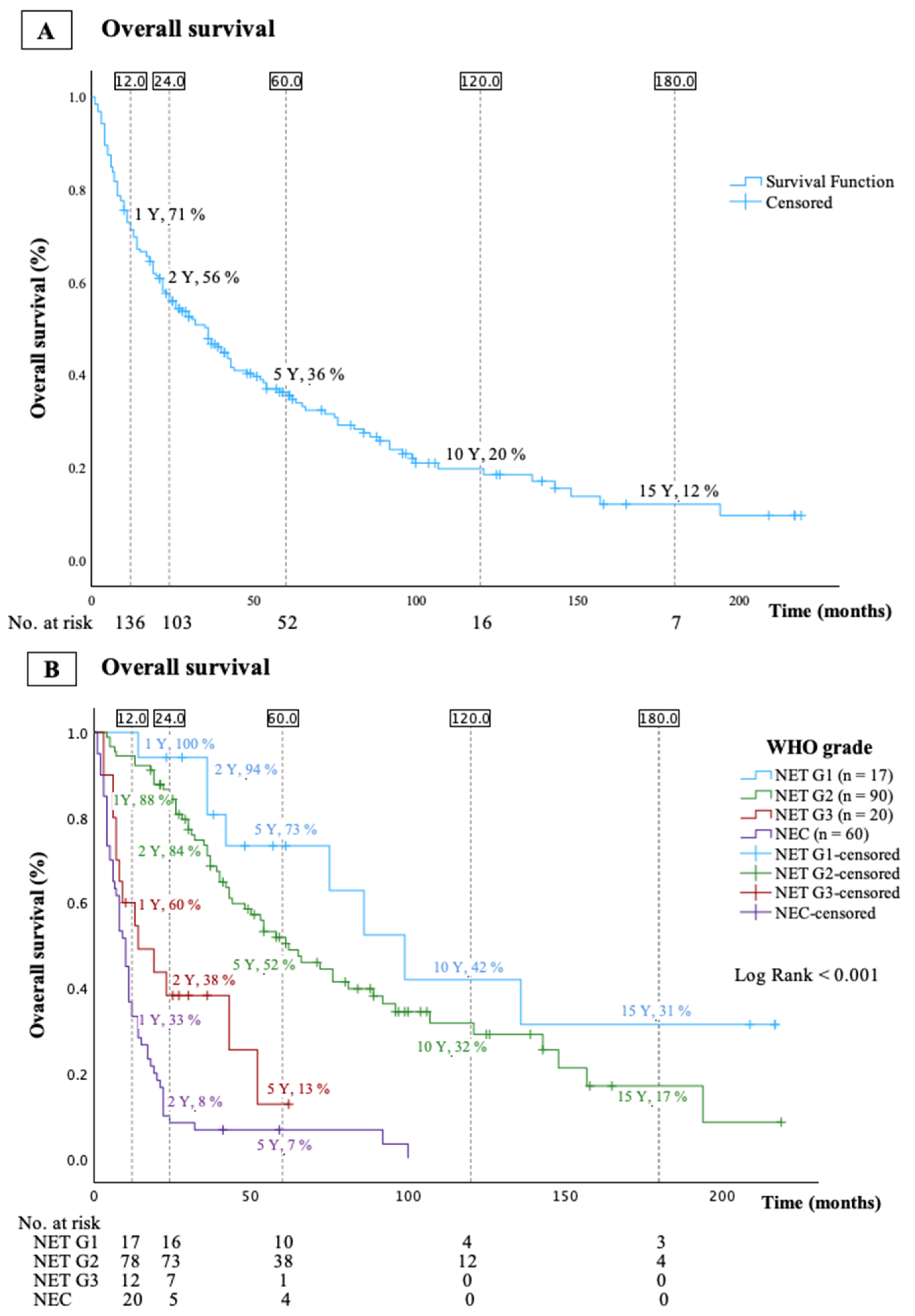
3.2. Overall Survival (OS)
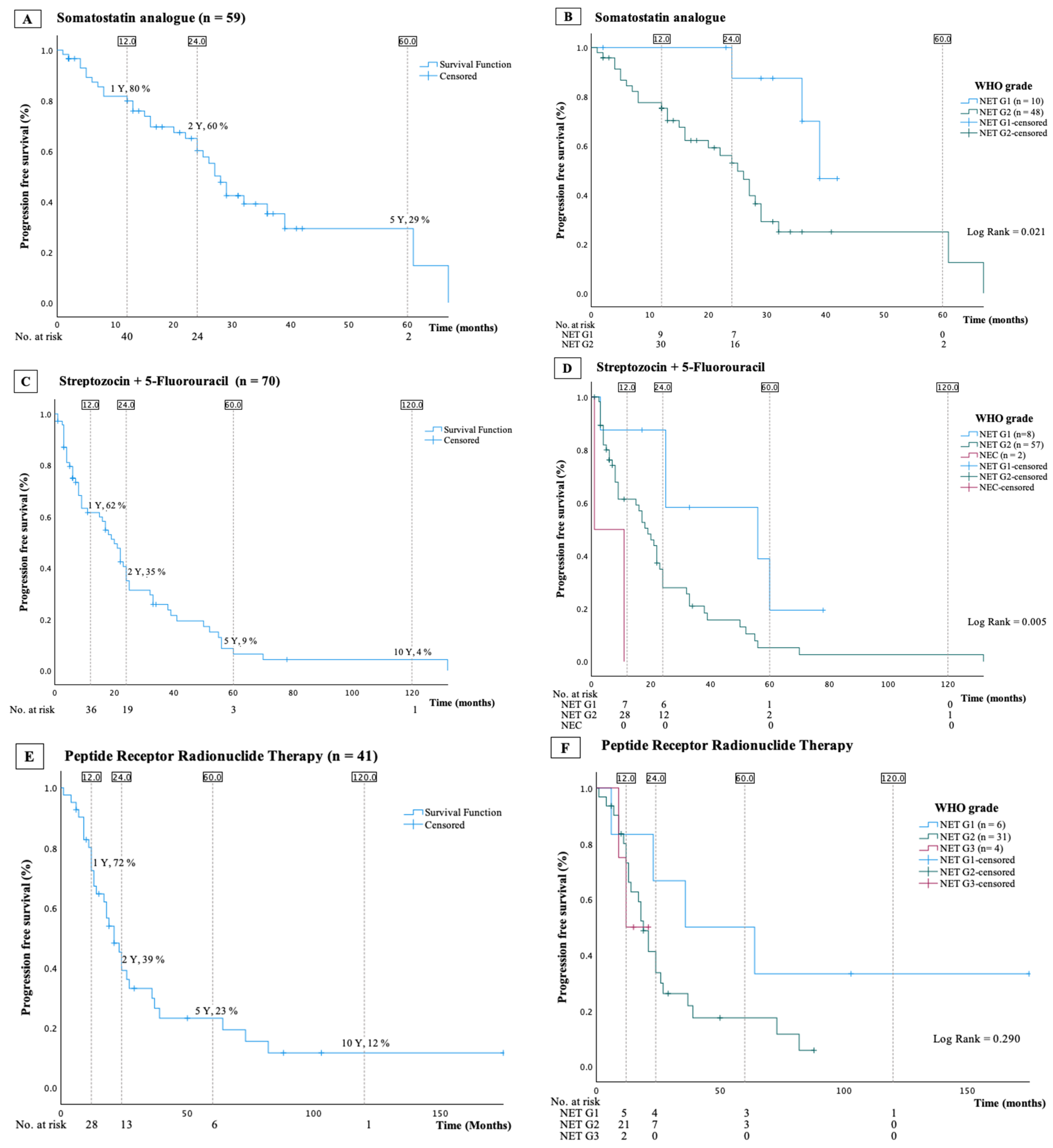
3.3. Somatostatin Analogues
3.4. Streptozocin and 5-Fluorouracil
3.5. Peptide Receptor Radionuclide Therapy
3.6. Everolimus
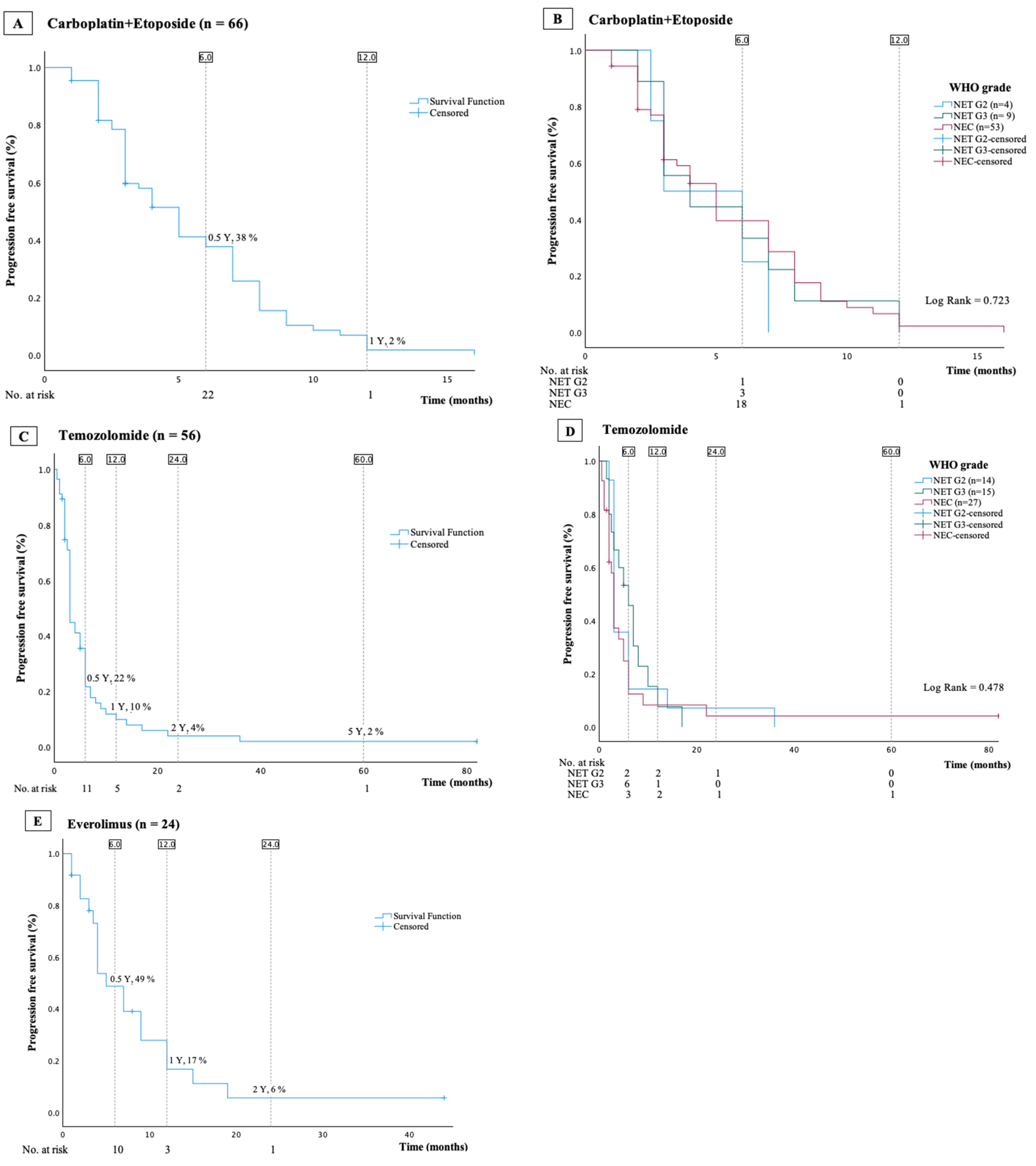
3.7. Temozolomide
3.8. Other Treatments
3.9. Carboplatin and Etoposide
3.10. Post Hoc Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Hofland, J.; Falconi, M.; Christ, E.; Castaño, J.P.; Faggiano, A.; Lamarca, A.; Perren, A.; Petrucci, S.; Prasad, V.; Ruszniewski, P.; et al. European Neuroendocrine Tumor Society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes. J. Neuroendocrinol. 2023, 35, e13318. [Google Scholar] [CrossRef]
- Mohindroo, C.; McAllister, F.; De Jesus-Acosta, A. Genetics of Pancreatic Neuroendocrine Tumors. Hematol. Oncol. Clin. N. Am. 2022, 36, 1033–1051. [Google Scholar] [CrossRef]
- Ito, T.; Igarashi, H.; Jensen, R.T. Pancreatic neuroendocrine tumors: Clinical features, diagnosis and medical treatment: Advances. Best. Pract. Res. Clin. Gastroenterol. 2012, 26, 737–753. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; Castaño, J.P.; Denecke, T.; Grande, E.; Kjaer, A.; Koumarianou, A.; de Mestier, L.; Partelli, S.; Perren, A.; Stättner, S.; et al. European Neuroendocrine Tumour Society (ENETS) 2023 guidance paper for nonfunctioning pancreatic neuroendocrine tumours. J. Neuroendocrinol. 2023, 35, e13343. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O’Dorisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.A.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 863–881. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Hope, T.A.; Pavel, M.; Bergsland, E.K. Neuroendocrine Tumors and Peptide Receptor Radionuclide Therapy: When Is the Right Time? J. Clin. Oncol. 2022, 40, 2818–2829. [Google Scholar] [CrossRef]
- Møller, S.; Langer, S.W.; Slott, C.; Krogh, J.; Hansen, C.P.; Kjaer, A.; Holmager, P.; Klose, M.; Garbyal, R.S.; Knigge, U.; et al. Recurrence-Free Survival and Disease-Specific Survival in Patients with Pancreatic Neuroendocrine Neoplasms: A Single-Center Retrospective Study of 413 Patients. Cancers 2023, 16, 100. [Google Scholar] [CrossRef]
- Bodei, L.; Mueller-Brand, J.; Baum, R.P.; Pavel, M.E.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisio, T.M.; Howe, J.R.; Cremonesi, M.; Kwekkeboom, D.J.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef]
- Groth, S.; Aasted, M. 51Cr-EDTA clearance determined by one plasma sample. Clin. Physiol. 1981, 1, 417–425. [Google Scholar] [CrossRef]
- Michael, M.; Garcia-Carbonero, R.; Weber, M.M.; Lombard-Bohas, C.; Toumpanakis, C.; Hicks, R.J. The Antiproliferative Role of Lanreotide in Controlling Growth of Neuroendocrine Tumors: A Systematic Review. Oncologist 2017, 22, 272–285. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Phan, A.T.; Ćwikła, J.B.; Sedláčková, E.; Thanh, X.T.; Wolin, E.M.; Ruszniewski, P. Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: Final results of the CLARINET open-label extension study. Endocrine 2021, 71, 502–513. [Google Scholar] [CrossRef]
- Merola, E.; Alonso Gordoa, T.; Zhang, P.; Al-Toubah, T.; Pellè, E.; Kolasińska-Ćwikła, A.; Zandee, W.; Laskaratos, F.; de Mestier, L.; Lamarca, A.; et al. Somatostatin Analogs for Pancreatic Neuroendocrine Tumors: Any Benefit When Ki-67 Is ≥10%? Oncologist 2021, 26, 294–301. [Google Scholar] [CrossRef]
- Capdevila, J.; Ducreux, M.; García Carbonero, R.; Grande, E.; Halfdanarson, T.; Pavel, M.; Tafuto, S.; Welin, S.; Valentí, V.; Salazar, R. Streptozotocin, 1982-2022: Forty Years from the FDA’s Approval to Treat Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2022, 112, 1155–1167. [Google Scholar] [CrossRef]
- Zappi, A.; Persano, I.; Galvani, L.; Parlagreco, E.; Andrini, E.; Campana, D.; Brizzi, M.P.; Lamberti, G.; La Salvia, A. Chemotherapy in Well Differentiated Neuroendocrine Tumors (NET) G1, G2, and G3: A Narrative Review. J. Clin. Med. 2023, 12, 717. [Google Scholar] [CrossRef]
- Tilsed, C.M.; Fisher, S.A.; Nowak, A.K.; Lake, R.A.; Lesterhuis, W.J. Cancer chemotherapy: Insights into cellular and tumor microenvironmental mechanisms of action. Front. Oncol. 2022, 12, 960317. [Google Scholar] [CrossRef]
- Childs, A.; Kirkwood, A.; Edeline, J.; Luong, T.V.; Watkins, J.; Lamarca, A.; Alrifai, D.; Nsiah-Sarbeng, P.; Gillmore, R.; Mayer, A.; et al. Ki-67 index and response to chemotherapy in patients with neuroendocrine tumours. Endocr. Relat. Cancer 2016, 23, 563–570. [Google Scholar] [CrossRef]
- Dilz, L.M.; Denecke, T.; Steffen, I.G.; Prasad, V.; von Weikersthal, L.F.; Pape, U.F.; Wiedenmann, B.; Pavel, M. Streptozocin/5-fluorouracil chemotherapy is associated with durable response in patients with advanced pancreatic neuroendocrine tumours. Eur. J. Cancer 2015, 51, 1253–1262. [Google Scholar] [CrossRef]
- Clewemar Antonodimitrakis, P.; Sundin, A.; Wassberg, C.; Granberg, D.; Skogseid, B.; Eriksson, B. Streptozocin and 5-Fluorouracil for the Treatment of Pancreatic Neuroendocrine Tumors: Efficacy, Prognostic Factors and Toxicity. Neuroendocrinology 2016, 103, 345–353. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef]
- Faivre, S.; Niccoli, P.; Castellano, D.; Valle, J.W.; Hammel, P.; Raoul, J.L.; Vinik, A.; Van Cutsem, E.; Bang, Y.J.; Lee, S.H.; et al. Sunitinib in pancreatic neuroendocrine tumors: Updated progression-free survival and final overall survival from a phase III randomized study. Ann. Oncol. 2017, 28, 339–343. [Google Scholar] [CrossRef]
- Starr, J.S.; Sonbol, M.B.; Hobday, T.J.; Sharma, A.; Kendi, A.T.; Halfdanarson, T.R. Peptide Receptor Radionuclide Therapy for the Treatment of Pancreatic Neuroendocrine Tumors: Recent Insights. Onco Targets Ther. 2020, 13, 3545–3555. [Google Scholar] [CrossRef] [PubMed]
- Baudin, E.; Walter, T.; Docao, C.; Haissaguerre, M.; Hadoux, J.; Taieb, D.; Ansquer, C.; Dierickx, L.; De Mestier, L.; Deshayes, E.; et al. First multicentric randomized phase II trial investigating the antitumor efficacy of peptide receptor radionuclide therapy with 177Lutetium–Octreotate (OCLU) in unresectable progressive neuroendocrine pancreatic tumor: Results of the OCLURANDOM trial, on behalf of the ENDOCAN RENATEN network and GTE. Ann. d’Endocrinol. 2022, 83, 289–290. [Google Scholar]
- Clement, D.; Navalkissoor, S.; Srirajaskanthan, R.; Courbon, F.; Dierickx, L.; Eccles, A.; Lewington, V.; Mitjavila, M.; Percovich, J.C.; Lequoy, B.; et al. Efficacy and safety of (177)Lu-DOTATATE in patients with advanced pancreatic neuroendocrine tumours: Data from the NETTER-R international, retrospective study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3529–3537. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Kong, G.; Grozinsky-Glasberg, S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr. Relat. Cancer 2020, 27, R67–R77. [Google Scholar] [CrossRef] [PubMed]
- Noronha, I.L.; Santa-Catharina, G.P.; Andrade, L.; Coelho, V.A.; Jacob-Filho, W.; Elias, R.M. Glomerular filtration in the aging population. Front. Med. 2022, 9, 769329. [Google Scholar] [CrossRef] [PubMed]
- Valkema, R.; Pauwels, S.A.; Kvols, L.K.; Kwekkeboom, D.J.; Jamar, F.; de Jong, M.; Barone, R.; Walrand, S.; Kooij, P.P.; Bakker, W.H.; et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0),Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J. Nucl. Med. 2005, 46 (Suppl. 1), 83s–91s. [Google Scholar] [PubMed]
- Elvebakken, H.; Perren, A.; Scoazec, J.Y.; Tang, L.H.; Federspiel, B.; Klimstra, D.S.; Vestermark, L.W.; Ali, A.S.; Zlobec, I.; Myklebust, T.; et al. A Consensus-Developed Morphological Re-Evaluation of 196 High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms and Its Clinical Correlations. Neuroendocrinology 2021, 111, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.L.; Graham, N.T.; Catalano, P.J.; Nimeiri, H.S.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Martin, B.A.; Yao, J.C.; Kulke, M.H.; et al. Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients With Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211). J. Clin. Oncol. 2023, 41, 1359–1369. [Google Scholar] [CrossRef]
- Chan, D.L.; Bergsland, E.K.; Chan, J.A.; Gadgil, R.; Halfdanarson, T.R.; Hornbacker, K.; Kelly, V.; Kunz, P.L.; McGarrah, P.W.; Raj, N.P.; et al. Temozolomide in Grade 3 Gastroenteropancreatic Neuroendocrine Neoplasms: A Multicenter Retrospective Review. Oncologist 2021, 26, 950–955. [Google Scholar] [CrossRef] [PubMed]


| Valid Cases | Medical Treatment | ||
|---|---|---|---|
| Mean age (Y ± SD) | 192 | 64 ± 13 | |
| Gender | Male | 192 | 108 (56%) |
| Year of diagnosis | 192 | ||
| 2000–2009 | 46 (24%) | ||
| 2010–2020 | 146 (76%) | ||
| Incidentaloma | 185 | 59 (32%) | |
| Functional tumor | 192 | ||
| Insulinoma | 7 (4%) | ||
| Gastrinoma | 5 (3%) | ||
| Other | 6 (3%) | ||
| Total functional tumors | 18 (9%) | ||
| Ki-67 index, median | 187 | 16 (8–50) | |
| CgA, pmol/L, median | 173 | 322 (98–1020) | |
| Stage | 192 | ||
| Local | 26 (14%) | ||
| Regional | 22 (11%) | ||
| Metastatic | 144 (75%) | ||
| WHO Grade | 187 | ||
| NET G1 | 17 (9%) | ||
| NET G2 | 90 (48%) | ||
| NET G3 | 20 (11%) | ||
| NEC | 60 (32%) | ||
| Location in pancreas | 159 | ||
| Caput | 87 (56%) | ||
| Cauda/corpus | 72 (45%) | ||
| Surgery | Primary surgery | 192 | 45 (23%) |
| Palliative surgery | 192 | 7 (4%) |
| SSA Progression-Free Survival | |||
|---|---|---|---|
| Univariable Analysis | |||
| Variables | HR | 95% CI | p-value |
| Age | 1.0 | 1.0–1.0 | 0.979 |
| Sex (ref. female) | 1.1 | 0.5–2.2 | 0.877 |
| WHO Grade (ref. NET G1) | 0.101 | ||
| NET G2 | 3.7 | 1.1–12.5 | 0.032 |
| Log2(Ki-67) | 1.6 | 1.1–2.3 | 0.025 |
| Stage (ref. localized) | 0.135 | ||
| Regional | 0.3 | 0.0–2.8 | 0.313 |
| Disseminated | 1.8 | 0.7–4.4 | 0.198 |
| Size primary tumor | 1.2 | 1.0–1.3 | 0.010 |
| Line of treatment (ref. 1. line) | 3.4 | 1.7–7.0 | <0.001 |
| Multivariable analysis | |||
| (incl. Ki-67 index) | |||
| Variables | HR | 95% CI | p-value |
| Line of treatment (ref. 1. line) | 4.1 | 1.9–9.1 | <0.001 |
| Multivariable analysis | |||
| (incl.WHO Grade) | |||
| Variables | HR | 95% CI | p-value |
| Line of treatment (ref. 1. line) | 3.1 | 1.4–6.9 | 0.006 |
| STZ/5FU Progression-Free Survival | |||
|---|---|---|---|
| Univariable Analysis | |||
| Variables | HR | 95% CI | p-value |
| Age | 1.0 | 1.0–1.0 | 0.568 |
| Sex (ref. female) | 1.0 | 0.6–1.7 | 0.874 |
| WHO Grade (ref. NET G1) | 0.030 | ||
| NET G2 | 2.8 | 1.1–7.2 | 0.031 |
| NEC | 11.1 | 2.1–60.9 | 0.005 |
| Log2(Ki67) | 1.3 | 1.1–1.6 | 0.008 |
| Stage (ref. localized) | 0.057 | ||
| Regional | 3.9 | 1.3–12.1 | 0.018 |
| Disseminated | 1.7 | 0.8–3.8 | 0.208 |
| Primary operation | 0.6 | 0.2–1.3 | 0.174 |
| Multivariable analysis | |||
| (incl. Ki-67 index) | |||
| Variables | HR | 95% CI | p-value |
| Log2(Ki67) | 1.3 | 1.1–1.6 | 0.008 |
| Multivariable analysis | |||
| (incl.WHO Grade) | |||
| Variables | HR | 95% CI | p-value |
| WHO Grade (ref. NET G1) | 0.030 | ||
| NET G2 | 2.8 | 1.1–7.2 | 0.031 |
| NEC | 11.1 | 2.0–60.9 | 0.005 |
| PRRT Progression-Free Survival | |||
|---|---|---|---|
| Univariable Analysis | |||
| Variables | HR | 95% CI | p-value |
| Age | 1.0 | 1.0–1.0 | 0.975 |
| Sex (ref. female) | 1.2 | 0.6–2.4 | 0.655 |
| WHO Grade (ref. NET G1) | 0.316 | ||
| NET G2 | 2.3 | 0.8–6.6 | 0.135 |
| NET G3 | 2.6 | 0.4–14.9 | 0.297 |
| Log2(CgA) | 1.2 | 1.0–1.4 | 0.055 |
| Log2(Ki-67) | 1.2 | 0.9–1.6 | 0.147 |
| Stage (ref. localized) | 0.250 | ||
| Regional | 1.4 | 0.3–6.2 | 0.672 |
| Disseminated | 2.3 | 0.8–6.8 | 0.123 |
| Multivariable analysis | |||
| (incl. Ki-67 index) | |||
| Variables | HR | 95% CI | p-value |
| Log2(CgA) | 1.2 | 1.0–1.4 | 0.011 |
| Log2(Ki-67) | 1.4 | 1.0–1.8 | 0.052 |
| Multivariable analysis | |||
| (incl.WHO Grade) | |||
| Variables | HR | 95% CI | p-value |
| Log2(CgA) | 1.2 | 1.0–1.4 | 0.055 |
| (A) Temozolomide progression-free survival | |||
| Univariable analysis | |||
| Variables | HR | 95% CI | p-value |
| Age | 1.0 | 1.0–1.0 | 0.543 |
| Sex (ref. female) | 1.2 | 0.7–2.2 | 0.467 |
| (B) Carboplatin + etoposide progression-free survival | |||
| Univariable analysis | |||
| Variables | HR | 95% CI | p-value |
| Age | 1.0 | 1.0–1.0 | 0.740 |
| Sex (ref. female) | 1.1 | 0.7–1.2 | 0.620 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petersen, S.S.; Møller, S.; Slott, C.; Krogh, J.; Hansen, C.P.; Kjaer, A.; Holmager, P.; Oturai, P.; Garbyal, R.S.; Langer, S.W.; et al. Responses to Medical Treatment in 192 Patients with Pancreatic Neuroendocrine Neoplasms Referred to the Copenhagen Neuroendocrine Tumour Centre in 2000–2020. Cancers 2024, 16, 1190. https://doi.org/10.3390/cancers16061190
Petersen SS, Møller S, Slott C, Krogh J, Hansen CP, Kjaer A, Holmager P, Oturai P, Garbyal RS, Langer SW, et al. Responses to Medical Treatment in 192 Patients with Pancreatic Neuroendocrine Neoplasms Referred to the Copenhagen Neuroendocrine Tumour Centre in 2000–2020. Cancers. 2024; 16(6):1190. https://doi.org/10.3390/cancers16061190
Chicago/Turabian StylePetersen, Sofie Skovlund, Stine Møller, Cecilie Slott, Jesper Krogh, Carsten Palnæs Hansen, Andreas Kjaer, Pernille Holmager, Peter Oturai, Rajendra Singh Garbyal, Seppo W. Langer, and et al. 2024. "Responses to Medical Treatment in 192 Patients with Pancreatic Neuroendocrine Neoplasms Referred to the Copenhagen Neuroendocrine Tumour Centre in 2000–2020" Cancers 16, no. 6: 1190. https://doi.org/10.3390/cancers16061190
APA StylePetersen, S. S., Møller, S., Slott, C., Krogh, J., Hansen, C. P., Kjaer, A., Holmager, P., Oturai, P., Garbyal, R. S., Langer, S. W., Knigge, U., & Andreassen, M. (2024). Responses to Medical Treatment in 192 Patients with Pancreatic Neuroendocrine Neoplasms Referred to the Copenhagen Neuroendocrine Tumour Centre in 2000–2020. Cancers, 16(6), 1190. https://doi.org/10.3390/cancers16061190









