De-Escalation Surgery in cT3-4 Breast Cancer Patients after Neoadjuvant Therapy: Predictors of Breast Conservation and Comparison of Long-Term Oncological Outcomes with Mastectomy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Management
2.2. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Comparison of Characteristics between Surgical Groups (Breast-Conserving Surgery versus Mastectomy) and Predictive Factors of Breast Conservation
3.3. Comparison of Long-Term Oncological Outcomes between Surgical Groups (Breast-Conserving Surgery versus Mastectomy)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choudhary, P.; Gogia, A.; Deo, S.; Mathur, S.; Sharma, D. Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer: Clinicopathological Characteristics and Correlation with Pathological Complete Response. J. Clin. Oncol. 2020, 38, e12658. [Google Scholar] [CrossRef]
- Wang, M.; Hou, L.; Chen, M.; Zhou, Y.; Liang, Y.; Wang, S.; Jiang, J.; Zhang, Y. Neoadjuvant Chemotherapy Creates Surgery Opportunities for Inoperable Locally Advanced Breast Cancer. Sci. Rep. 2017, 7, 44673. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Ames, F.C.; Buzdar, A.U.; Kau, S.W.; McNeese, M.D.; Paulus, D.; Hug, V.; Holmes, F.A.; Romsdahl, M.M.; Fraschini, G.; et al. Management of Stage III Primary Breast Cancer with Primary Chemotherapy, Surgery, and Radiation Therapy. Cancer 1988, 62, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Blohmer, J.U.; Costa, S.D.; Denkert, C.; Eidtmann, H.; Eiermann, W.; Gerber, B.; Hanusch, C.; Hilfrich, J.; Huober, J.; et al. Response-Guided Neoadjuvant Chemotherapy for Breast Cancer. J. Clin. Oncol. 2013, 31, 3623–3630. [Google Scholar] [CrossRef] [PubMed]
- Gentile, D.; Sagona, A.; De Carlo, C.; Fernandes, B.; Barbieri, E.; Di Maria Grimaldi, S.; Jacobs, F.; Vatteroni, G.; Scardina, L.; Biondi, E.; et al. Pathologic Response and Residual Tumor Cellularity after Neo-Adjuvant Chemotherapy Predict Prognosis in Breast Cancer Patients. Breast 2023, 69, 323–329. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and Impact of Pathologic Complete Response on Prognosis after Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef]
- Yoshioka, T.; Hosoda, M.; Yamamoto, M.; Taguchi, K.; Hatanaka, K.C.; Takakuwa, E.; Hatanaka, Y.; Matsuno, Y.; Yamashita, H. Prognostic Significance of Pathologic Complete Response and Ki67 Expression after Neoadjuvant Chemotherapy in Breast Cancer. Breast Cancer 2015, 22, 185–191. [Google Scholar] [CrossRef]
- Broglio, K.R.; Quintana, M.; Foster, M.; Olinger, M.; McGlothlin, A.; Berry, S.M.; Boileau, J.F.; Brezden-Masley, C.; Chia, S.; Dent, S.; et al. Association of Pathologic Complete Response to Neoadjuvant Therapy in HER2-Positive Breast Cancer with Long-Term Outcomes Ameta-Analysis. JAMA Oncol. 2016, 2, 751–760. [Google Scholar] [CrossRef]
- Tinterri, C.; Sagona, A.; Barbieri, E.; Di Maria Grimaldi, S.; Caraceni, G.; Ambrogi, G.; Jacobs, F.; Biondi, E.; Scardina, L.; Gentile, D. Sentinel Lymph Node Biopsy in Breast Cancer Patients Undergoing Neo-Adjuvant Chemotherapy: Clinical Experience with Node-Negative and Node-Positive Disease Prior to Systemic Therapy. Cancers 2023, 15, 1719. [Google Scholar] [CrossRef]
- Boughey, J.C.; Yu, H.; Dugan, C.L.; Piltin, M.A.; Postlewait, L.; Son, J.D.; Edmiston, K.K.; Godellas, C.V.; Lee, M.C.; Carr, M.J.; et al. Changes in Surgical Management of the Axilla Over 11 Years—Report on More Than 1500 Breast Cancer Patients Treated with Neoadjuvant Chemotherapy on the Prospective I-SPY2 Trial. Ann. Surg. Oncol. 2023, 30, 6401–6410. [Google Scholar] [CrossRef]
- Clough, K.B.; Acosta-Marín, V.; Nos, C.; Alran, S.; Rouanet, P.; Garbay, J.R.; Giard, S.; Verhaeghe, J.L.; Houvenaeghel, G.; Flipo, B.; et al. Rates of Neoadjuvant Chemotherapy and Oncoplastic Surgery for Breast Cancer Surgery: A French National Survey. Ann. Surg. Oncol. 2015, 22, 3504–3511. [Google Scholar] [CrossRef]
- Vugts, G.; Maaskant-Braat, A.J.G.; Nieuwenhuijzen, G.A.P.; Roumen, R.M.H.; Luiten, E.J.T.; Voogd, A.C. Patterns of Care in the Administration of Neo-Adjuvant Chemotherapy for Breast Cancer. A Population-Based Study. Breast J. 2016, 22, 316–321. [Google Scholar] [CrossRef]
- Golshan, M.; Cirrincione, C.T.; Sikov, W.M.; Berry, D.A.; Jasinski, S.; Weisberg, T.F.; Somlo, G.; Hudis, C.; Winer, E.; Ollila, D.W. Impact of Neoadjuvant Chemotherapy in Stage II-III Triple Negative Breast Cancer on Eligibility for Breast-Conserving Surgery and Breast Conservation Rates: Surgical Results from CALGB 40603 (Alliance). Ann. Surg. 2015, 262, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Gentile, D.; Bottini, A.; Sagona, A.; Gatzemeier, W.; Losurdo, A.; Fernandes, B.; Tinterri, C. Neo-Adjuvant Chemotherapy in Luminal, Node Positive Breast Cancer: Characteristics, Treatment and Oncological Outcomes: A Single Center’s Experience. Eur. J. Breast Health 2021, 17, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-Year Follow-up of a Randomized Study Comparing Breast-Conserving Surgery with Radical Mastectomy for Early Breast Cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef]
- Agarwal, S.; Pappas, L.; Neumayer, L.; Kokeny, K.; Agarwal, J. Effect of Breast Conservation Therapy vs Mastectomy on Disease-Specific Survival for Early-Stage Breast Cancer. JAMA Surg. 2014, 149, 267. [Google Scholar] [CrossRef]
- De la Cruz Ku, G.; Karamchandani, M.; Chambergo-Michilot, D.; Narvaez-Rojas, A.R.; Jonczyk, M.; Príncipe-Meneses, F.S.; Posawatz, D.; Nardello, S.; Chatterjee, A. Does Breast-Conserving Surgery with Radiotherapy Have a Better Survival than Mastectomy? A Meta-Analysis of More than 1,500,000 Patients. Ann. Surg. Oncol. 2022, 29, 6163–6188. [Google Scholar] [CrossRef]
- Gwark, S.; Kim, H.J.; Kim, J.; Chung, I.Y.; Kim, H.J.; Ko, B.S.; Lee, J.W.; Son, B.H.; Ahn, S.H.; Lee, S.B. Survival After Breast-Conserving Surgery Compared with That After Mastectomy in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2023, 30, 2845–2853. [Google Scholar] [CrossRef]
- Simons, J.M.; Jacobs, J.G.; Roijers, J.P.; Beek, M.A.; Boonman-de Winter, L.J.M.; Rijken, A.M.; Gobardhan, P.D.; Wijsman, J.H.; Tetteroo, E.; Heijns, J.B.; et al. Disease-Free and Overall Survival after Neoadjuvant Chemotherapy in Breast Cancer: Breast-Conserving Surgery Compared to Mastectomy in a Large Single-Centre Cohort Study. Breast Cancer Res. Treat. 2021, 185, 441–451. [Google Scholar] [CrossRef]
- Agrawal, S.K.; Patel, D.; Shenoy, P.; Ahmed, R.; Arun, I.; Chatterjee, S. Oncologic Safety of Breast Conservation Following NACT in Women with Locally Advanced Breast Cancer. Ecancermedicalscience 2023, 17, 1554. [Google Scholar] [CrossRef]
- Pfob, A.; Dubsky, P. The Underused Potential of Breast Conserving Therapy after Neoadjuvant System Treatment—Causes and Solutions. Breast 2023, 67, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Matuschek, C.; Nestle-Kraemling, C.; Haussmann, J.; Bölke, E.; Wollandt, S.; Speer, V.; Djiepmo Njanang, F.J.; Tamaskovics, B.; Gerber, P.A.; Orth, K.; et al. Long-Term Cosmetic Outcome after Preoperative Radio-/Chemotherapy in Locally Advanced Breast Cancer Patients. Strahlenther. Onkol. 2019, 195, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Caudle, A.S.; Kuerer, H.M. Breast Conservation Therapy after Neoadjuvant Chemotherapy: Optimization of a Multimodality Approach. J. Surg. Oncol. 2014, 110, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Gusic, L.H.; Walsh, K.; Flippo-Morton, T.; Sarantou, T.; Boselli, D.; White, R.L. Rationale for Mastectomy after Neoadjuvant Chemotherapy. Am. Surg. 2018, 84, 126–132. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Spronk, P.E.R.; Volders, J.H.; van den Tol, P.; Smorenburg, C.H.; Vrancken Peeters, M.-J.T.F.D. Breast Conserving Therapy after Neoadjuvant Chemotherapy; Data from the Dutch Breast Cancer Audit. Eur. J. Surg. Oncol. 2019, 45, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Curigliano, G.; Burstein, H.J.; Wong, S.; Esposito, A.; Viale, G.; Giuliano, M.; Veronesi, U.; Santangelo, M.; Golshan, M. Breast Conservation Following Neoadjuvant Therapy for Breast Cancer in the Modern Era: Are We Losing the Opportunity? Eur. J. Surg. Oncol. 2016, 42, 1780–1786. [Google Scholar] [CrossRef]
- Van der Hage, J.A.; Van de Velde, C.J.H.; Julien, J.P.; Tubiana-Hulin, M.; Vandervelden, C.; Duchateau, L. Preoperative Chemotherapy in Primary Operable Breast Cancer: Results from the European Organization for Research and Treatment of Cancer Trial 10902. J. Clin. Oncol. 2001, 19, 4224–4237. [Google Scholar] [CrossRef] [PubMed]
- Caudle, A.S.; Gonzalez-Angulo, A.M.; Hunt, K.K.; Pusztai, L.; Kuerer, H.M.; Mittendorf, E.A.; Hortobagyi, G.N.; Meric-Bernstam, F. Impact of Progression During Neoadjuvant Chemotherapy on Surgical Management of Breast Cancer. Ann. Surg. Oncol. 2011, 18, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Gonzalez-Angulo, A.; Sneige, N.; Kau, S.-W.; Broglio, K.; Theriault, R.L.; Valero, V.; Buzdar, A.U.; Kuerer, H.; Buccholz, T.A.; et al. Invasive Lobular Carcinoma Classic Type: Response to Primary Chemotherapy and Survival Outcomes. J. Clin. Oncol. 2005, 23, 41–48. [Google Scholar] [CrossRef]
- Zarba Meli, E.; De Santis, A.; Cortese, G.; Manna, E.; Mastropietro, T.; La Pinta, M.; Loreti, A.; Arelli, F.; Scavina, P.; Minelli, M.; et al. Nipple-Sparing Mastectomy After Neoadjuvant Chemotherapy: Definitive Results with a Long-Term Follow-Up Evaluation. Ann. Surg. Oncol. 2023, 30, 2163–2172. [Google Scholar] [CrossRef]
- Li, X.; Yan, C.; Xiao, J.; Xu, X.; Li, Y.; Wen, X.; Wei, H. Factors Associated with Surgical Modality Following Neoadjuvant Chemotherapy in Patients with Breast Cancer. Clin. Breast Cancer 2021, 21, e611–e617. [Google Scholar] [CrossRef]
- Criscitiello, C.; Golshan, M.; Barry, W.T.; Viale, G.; Wong, S.; Santangelo, M.; Curigliano, G. Impact of Neoadjuvant Chemotherapy and Pathological Complete Response on Eligibility for Breast-Conserving Surgery in Patients with Early Breast Cancer: A Meta-Analysis. Eur. J. Cancer 2018, 97, 1–6. [Google Scholar] [CrossRef]
- Asselain, B.; Barlow, W.; Bartlett, J.; Bergh, J.; Bergsten-Nordström, E.; Bliss, J.; Boccardo, F.; Boddington, C.; Bogaerts, J.; Bonadonna, G.; et al. Long-Term Outcomes for Neoadjuvant versus Adjuvant Chemotherapy in Early Breast Cancer: Meta-Analysis of Individual Patient Data from Ten Randomised Trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef]
- Mauriac, L.; MacGrogan, G.; Avril, A.; Durand, M.; Floquet, A.; Debled, M.; Dilhuydy, J.M.; Bonichon, F. Neoadjuvant Chemotherapy for Operable Breast Carcinoma Larger than 3 Cm: A Unicentre Randomized Trial with a 124-Month Median Follow-up. Institut Bergonié Bordeaux Groupe Sein (IBBGS). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1999, 10, 47–52. [Google Scholar] [CrossRef]
- Scholl, S.M.; Asselain, B.; Palangie, T.; Dorval, T.; Jouve, M.; Garcia Giralt, E.; Vilcoq, J.; Durand, J.C.; Pouillart, P. Neoadjuvant Chemotherapy in Operable Breast Cancer. Eur. J. Cancer 1991, 27, 1668–1671. [Google Scholar] [CrossRef]
- Fancellu, A.; Houssami, N.; Sanna, V.; Porcu, A.; Ninniri, C.; Marinovich, M.L. Outcomes after Breast-Conserving Surgery or Mastectomy in Patients with Triple-Negative Breast Cancer: Meta-Analysis. Br. J. Surg. 2021, 108, 760–768. [Google Scholar] [CrossRef]
- Werutsky, G.; Untch, M.; Hanusch, C.; Fasching, P.A.; Blohmer, J.-U.; Seiler, S.; Denkert, C.; Tesch, H.; Jackisch, C.; Gerber, B.; et al. Locoregional Recurrence Risk after Neoadjuvant Chemotherapy: A Pooled Analysis of Nine Prospective Neoadjuvant Breast Cancer Trials. Eur. J. Cancer 2020, 130, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, R.A.; Chau, H.; Woriax, H.; Piltin, M.; Ahrendt, G.; Tchou, J.; Yu, H.; Ding, Q.; Dugan, C.L.; Sheade, J.; et al. Breast Conservation Surgery and Mastectomy Have Similar Locoregional Recurrence After Neoadjuvant Chemotherapy. Ann. Surg. 2023, 278, 320–327. [Google Scholar] [CrossRef]
- Arlow, R.L.; Paddock, L.E.; Niu, X.; Kirstein, L.; Haffty, B.G.; Goyal, S.; Kearney, T.; Toppmeyer, D.; Stroup, A.M.; Khan, A.J. Breast-Conservation Therapy After Neoadjuvant Chemotherapy Does Not Compromise 10-Year Breast Cancer–Specific Mortality. Am. J. Clin. Oncol. 2018, 41, 1246–1251. [Google Scholar] [CrossRef]
- Levy, A.; Borget, I.; Bahri, M.; Arnedos, M.; Rivin, E.; Vielh, P.; Balleyguier, C.; Rimareix, F.; Bourgier, C. Loco-Regional Control After Neo-Adjuvant Chemotherapy and Conservative Treatment for Locally Advanced Breast Cancer Patients. Breast J. 2014, 20, 381–387. [Google Scholar] [CrossRef]
- Cho, J.H.; Park, J.M.; Park, H.S.; Park, S.; Kim, S.I.; Park, B. Oncologic Safety of Breast-conserving Surgery Compared to Mastectomy in Patients Receiving Neoadjuvant Chemotherapy for Locally Advanced Breast Cancer. J. Surg. Oncol. 2013, 108, 531–536. [Google Scholar] [CrossRef]
- Carrara, G.F.A.; Scapulatempo-Neto, C.; Abrahão-Machado, L.F.; Brentani, M.M.; Nunes, J.S.; Folgueira, M.A.A.K.; da Costa Vieira, R.A. Breast-Conserving Surgery in Locally Advanced Breast Cancer Submitted to Neoadjuvant Chemotherapy. Safety and Effectiveness Based on Ipsilateral Breast Tumor Recurrence and Long-Term Follow-Up. Clinics 2017, 72, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-C.; Han, W.; Moon, H.-G.; Im, S.-A.; Moon, W.K.; Park, I.-A.; Park, S.J.; Noh, D.-Y. Breast-Conserving Surgery After Tumor Downstaging by Neoadjuvant Chemotherapy Is Oncologically Safe for Stage III Breast Cancer Patients. Ann. Surg. Oncol. 2013, 20, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Masood, M.; Shahid, A.; Mirza, Z.R.; Cheema, F.E.; Fatima, I. Sonographically Guided Metalic Clip Placement for Tumour Localization in Early Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. J. Pak. Med. Assoc. 2019, 69, 1501–1504. [Google Scholar]
- Youn, I.; Choi, S.H.; Kook, S.H.; Choi, Y.J.; Park, C.H.; Park, Y.L.; Kim, D.H. Ultrasonography-Guided Surgical Clip Placement for Tumor Localization in Patients Undergoing Neoadjuvant Chemotherapy for Breast Cancer. J. Breast Cancer 2015, 18, 44. [Google Scholar] [CrossRef]
- Minella, C.; Villasco, A.; D’Alonzo, M.; Cellini, L.; Accomasso, F.; Actis, S.; Biglia, N. Surgery after Neoadjuvant Chemotherapy: A Clip-Based Technique to Improve Surgical Outcomes, a Single-Center Experience. Cancers 2022, 14, 2229. [Google Scholar] [CrossRef]

| Characteristics | Number (%)/Median (Range) |
|---|---|
| Patients | |
| Age (years) | 50 (20–76) |
| Post-menopausal | 63 (55.3%) |
| Pre-operative staging | |
| Mammography | 80 (70.2%) |
| Breast and axillary US | 114 (100%) |
| MRI | 40 (35.1%) |
| PET | 57 (50.0%) |
| Size pre-NAT (mm) | 51 (11–115) |
| Single nodule | 89 (78.1%) |
| Stage pre-NAT | |
| cT3 | 73 (64.0%) |
| cT4 | 41 (36.0%) |
| cN0 | 30 (26.3%) |
| cN+ | 84 (73.7%) |
| cM1 | 19 (16.7%) |
| NAT with anthracycline only | 21 (13.9%) |
| NAT with anthracycline and taxanes | 87 (86.1%) |
| Trastuzumab | 45 (39.5%) |
| Tumor | |
| Subtype | |
| Luminal-like | 45 (39.5%) |
| HER2-positive | 49 (43.0%) |
| Triple-negative | 20 (17.5%) |
| Histotype | |
| Ductal | 105 (92.1%) |
| Lobular | 6 (5.3%) |
| Mucinous | 3 (2.6%) |
| Vascular invasion | 41 (36.0%) |
| pCR | 23 (20.2%) |
| Size post-NAT (mm) | 13.5 (0–110) |
| Stage post-NAT | |
| ypT0 | 24 (21.1%) |
| ypTis | 7 (6.1%) |
| ypTmi | 4 (3.5%) |
| ypT1a | 8 (7.0%) |
| ypT1b | 4 (3.5%) |
| ypT1c | 25 (21.9%) |
| ypT2 | 20 (17.5%) |
| ypT3 | 11 (9.7%) |
| ypT4 | 11 (9.7%) |
| ypN0 | 61 (53.5%) |
| ypNmi | 2 (1.8%) |
| ypN1 | 17 (14.9%) |
| ypN2 | 17 (14.9%) |
| ypN3 | 17 (14.9%) |
| Surgical treatment | |
| BCS | 37 (32.5%) |
| Mastectomy | 77 (67.5%) |
| SLNB not followed by ALND | 37 (32.5%) |
| SLNB followed by ALND | 10 (8.8%) |
| ALND | 67 (58.7%) |
| Post-operative treatment | |
| Taxanes | 9 (7.9%) |
| Capecitabine | 4 (3.5%) |
| Radiotherapy | 95 (83.3%) |
| Endocrine | 72 (63.2%) |
| T-DM1 | 33 (29.0%) |
| Characteristics | BCS (No. 37) Tot. (%)/Mean (SD) | Mastectomy (No. 77) Tot. (%)/Mean (SD) | Univariate Analysis p-Value | Multivariate Analysis p-Value OR (95% CI) |
|---|---|---|---|---|
| Demographic | ||||
| Age (years) | 51.1 (11.9) | 52.4 (11.2) | 0.570 | - |
| Post-menopausal | 20 (54.1) | 43 (55.8) | 0.857 | - |
| Pre-operative staging | ||||
| Mammography | 26 (70.3) | 54 (70.1) | 0.988 | - |
| MRI | 15 (40.5) | 25 (32.5) | 0.398 | - |
| PET | 14 (37.8) | 43 (55.8) | 0.072 | - |
| Size pre-NAT (mm) | 54.6 (13.6) | 57.7 (25.0) | 0.001 a | 0.508 0.442 (51.390–60.870) |
| Single nodule | 24 (64.9) | 65 (84.4) | 0.059 | - |
| Stage pre-NAT | ||||
| cT3 | 29 (78.4) | 44 (57.1) | 0.027 a | 0.138 2.249 (3.187–3.393) |
| cT4 | 8 (21.6) | 33 (42.9) | - | - |
| cN+ | 29 (78.4) | 55 (71.4) | 0.430 | - |
| cM1 | 4 (10.8) | 15 (19.5) | 0.245 | - |
| Subtype | ||||
| Luminal-like | 12 (32.4) | 33 (42.9) | 0.441 | - |
| HER2-positive | 19 (51.4) | 30 (39.0) | - | - |
| Triple-negative | 6 (16.2) | 14 (18.1) | - | - |
| Histotype | ||||
| Ductal | 35 (94.6) | 70 (90.9) | 0.698 | - |
| Lobular | 1 (2.7) | 5 (6.5) | - | - |
| Mucinous | 1 (2.7) | 2 (2.6) | - | - |
| Histopathological | ||||
| Vascular invasion | 8 (21.6) | 33 (42.9) | 0.027 a | 0.004 a 8.723 (0.232–0.440) |
| Size post-NAT (mm) | 13.0 (17.2) | 29.4 (38.9) | 0.004 a | 0.011 a 6.811 (13.984–29.449) |
| ypT0 | 15 (40.5) | 9 (11.7) | <0.001 a | <0.001 a 15.470 (0.189–0.367) |
| Ki67 (%) | 17.1 (17.2) | 21.5 (23.3) | 0.301 | - |
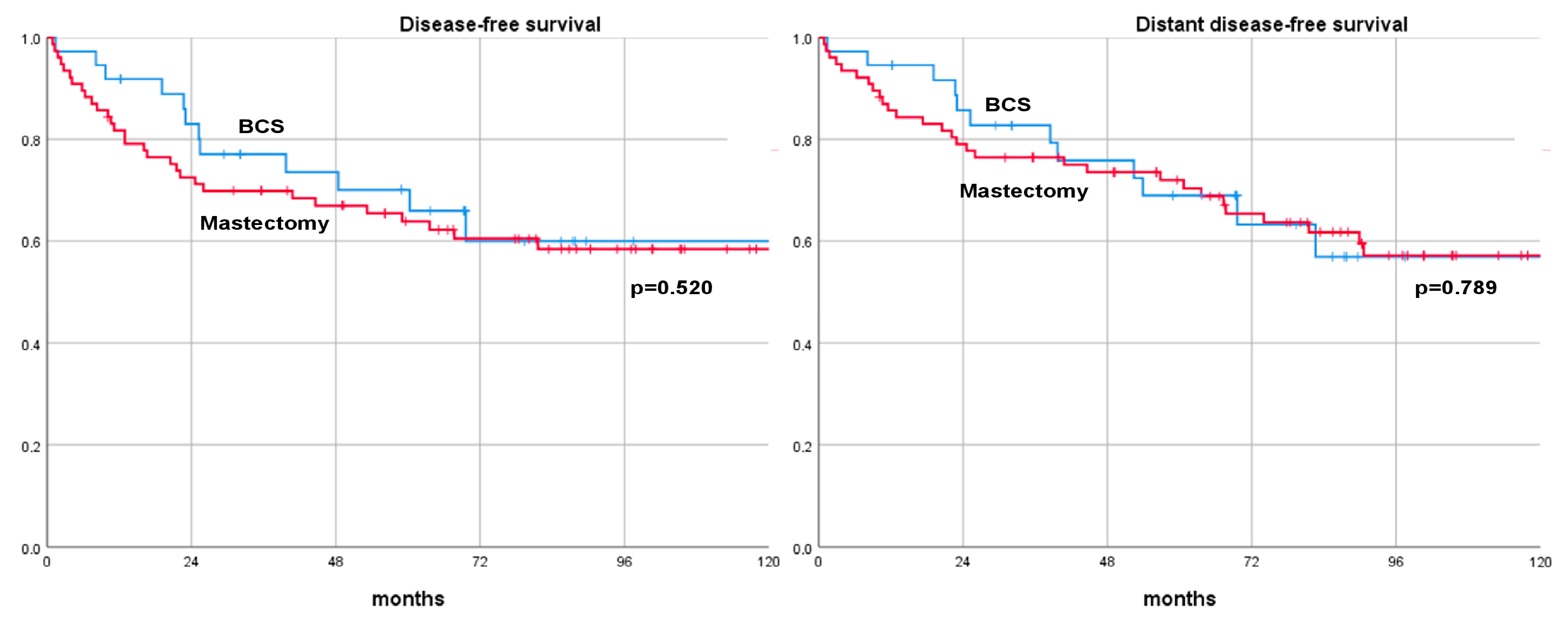
| Outcomes | BCS | Mastectomy | Log-Rank Test |
|---|---|---|---|
| DFS rate | 0.520 | ||
| 3-year | 77.1% | 69.9% | |
| 5-year | 65.9% | 63.9% | |
| 10-year | 59.9% | 58.4% | |
| DDFS rate | 0.789 | ||
| 3-year | 82.8% | 76.4% | |
| 5-year | 69.0% | 70.4% | |
| 10-year | 60.9% | 59.5% | |
| OS rate | 0.216 | ||
| 3-year | 93.4% | 83.1% | |
| 5-year | 85.9% | 74.7% | |
| 10-year | 61.4% | 65.6% | |
| BCSS rate | 0.559 | ||
| 3-year | 93.4% | 86.9% | |
| 5-year | 85.9% | 81.0% | |
| 10-year | 61.4% | 73.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinterri, C.; Barbieri, E.; Sagona, A.; Bottini, A.; Canavese, G.; Gentile, D. De-Escalation Surgery in cT3-4 Breast Cancer Patients after Neoadjuvant Therapy: Predictors of Breast Conservation and Comparison of Long-Term Oncological Outcomes with Mastectomy. Cancers 2024, 16, 1169. https://doi.org/10.3390/cancers16061169
Tinterri C, Barbieri E, Sagona A, Bottini A, Canavese G, Gentile D. De-Escalation Surgery in cT3-4 Breast Cancer Patients after Neoadjuvant Therapy: Predictors of Breast Conservation and Comparison of Long-Term Oncological Outcomes with Mastectomy. Cancers. 2024; 16(6):1169. https://doi.org/10.3390/cancers16061169
Chicago/Turabian StyleTinterri, Corrado, Erika Barbieri, Andrea Sagona, Alberto Bottini, Giuseppe Canavese, and Damiano Gentile. 2024. "De-Escalation Surgery in cT3-4 Breast Cancer Patients after Neoadjuvant Therapy: Predictors of Breast Conservation and Comparison of Long-Term Oncological Outcomes with Mastectomy" Cancers 16, no. 6: 1169. https://doi.org/10.3390/cancers16061169
APA StyleTinterri, C., Barbieri, E., Sagona, A., Bottini, A., Canavese, G., & Gentile, D. (2024). De-Escalation Surgery in cT3-4 Breast Cancer Patients after Neoadjuvant Therapy: Predictors of Breast Conservation and Comparison of Long-Term Oncological Outcomes with Mastectomy. Cancers, 16(6), 1169. https://doi.org/10.3390/cancers16061169







