Exploratory Assessment of Galectin-1, -3, and -9 in Non-Small Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. Patient Information
2.3. Galectin Profiling
2.4. Data Analysis
3. Results
3.1. Patient Characteristics Correlations with Galectin-1, -3, and -9 Levels
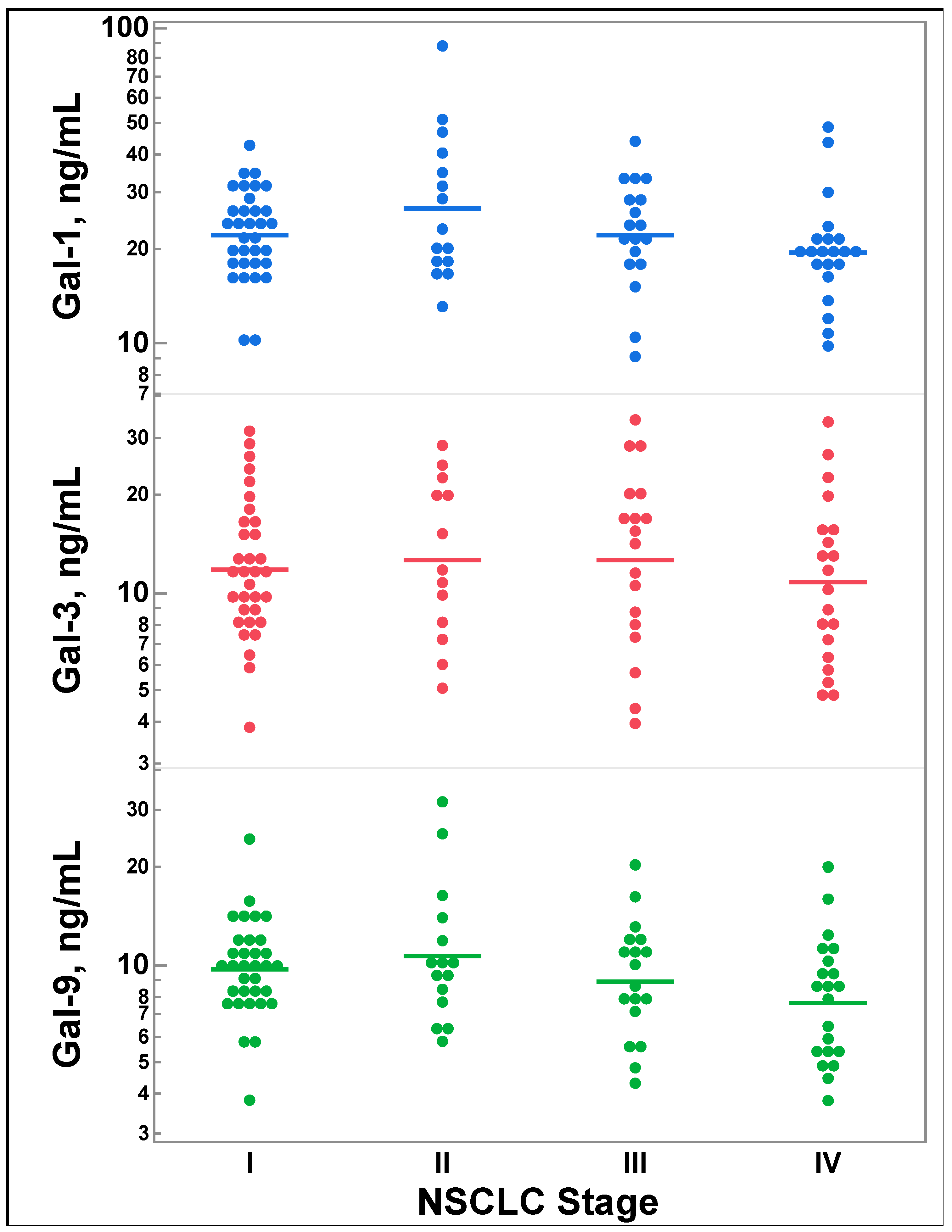
3.2. Galectin-1, -3, and -9 Levels Do Not Differ by Lung Cancer Stage
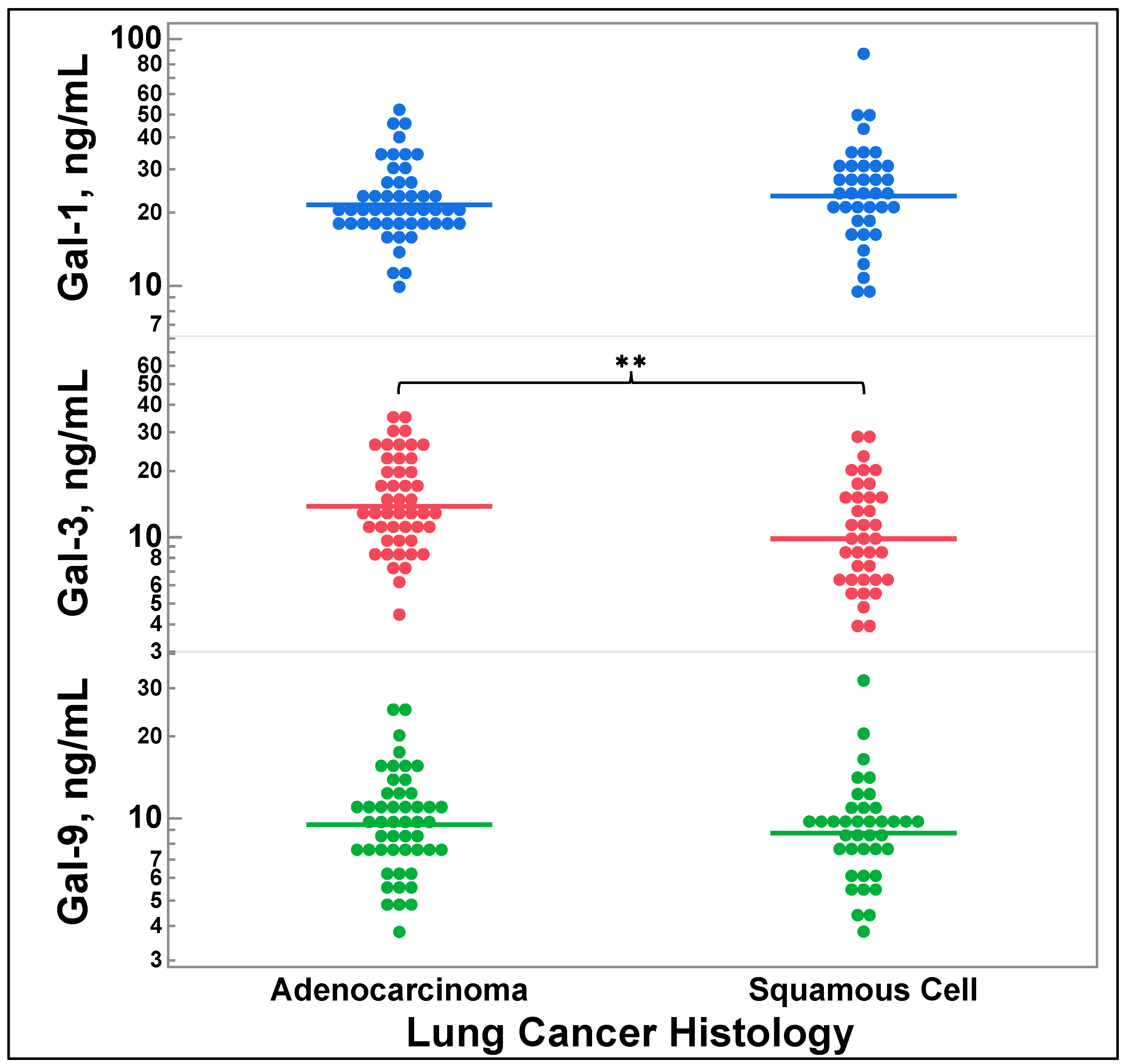
3.3. Galectin-3 Levels Vary by Lung Cancer Histology
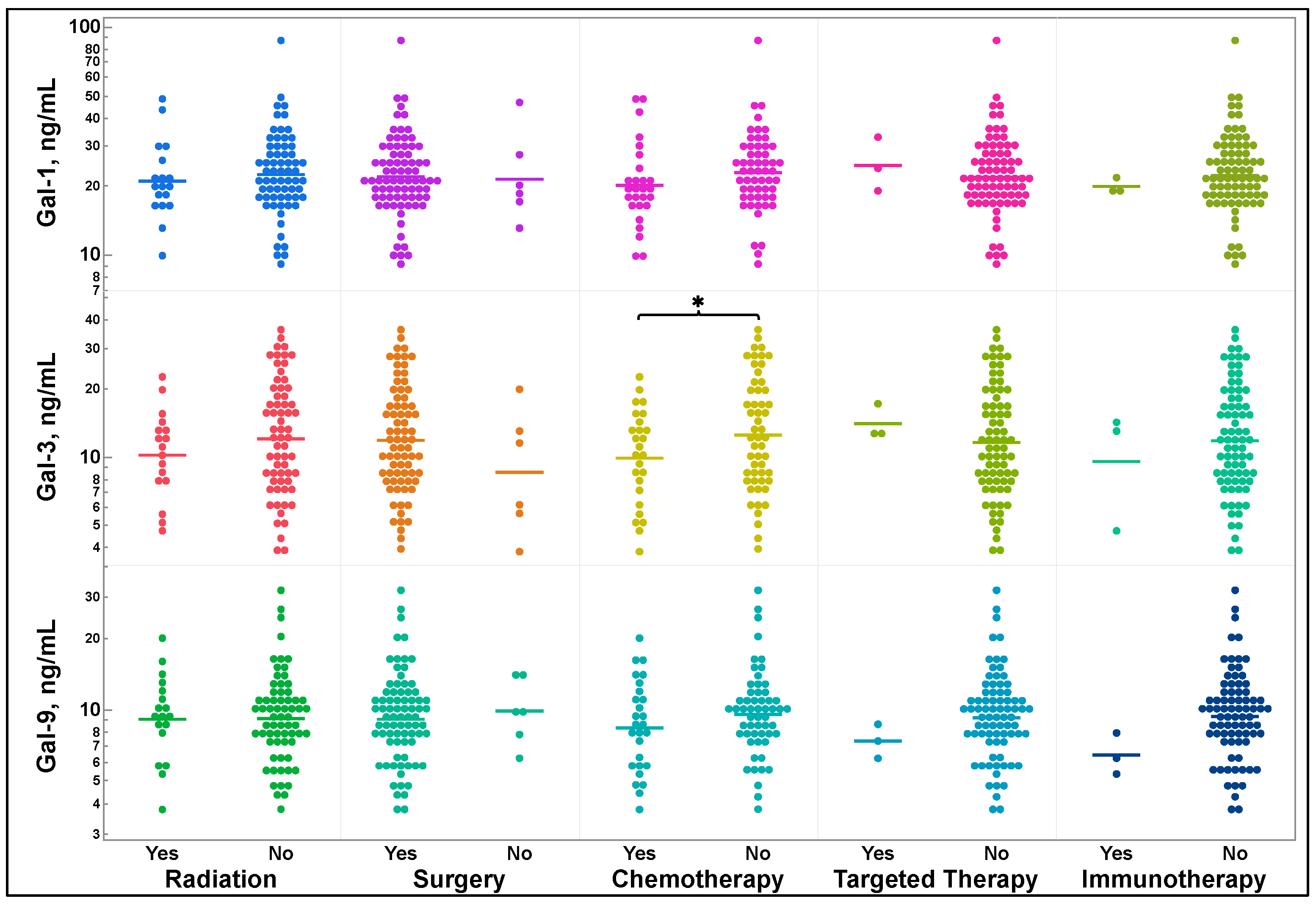
3.4. Galectin-1, -3, and -9 Levels and Previous Treatment Status
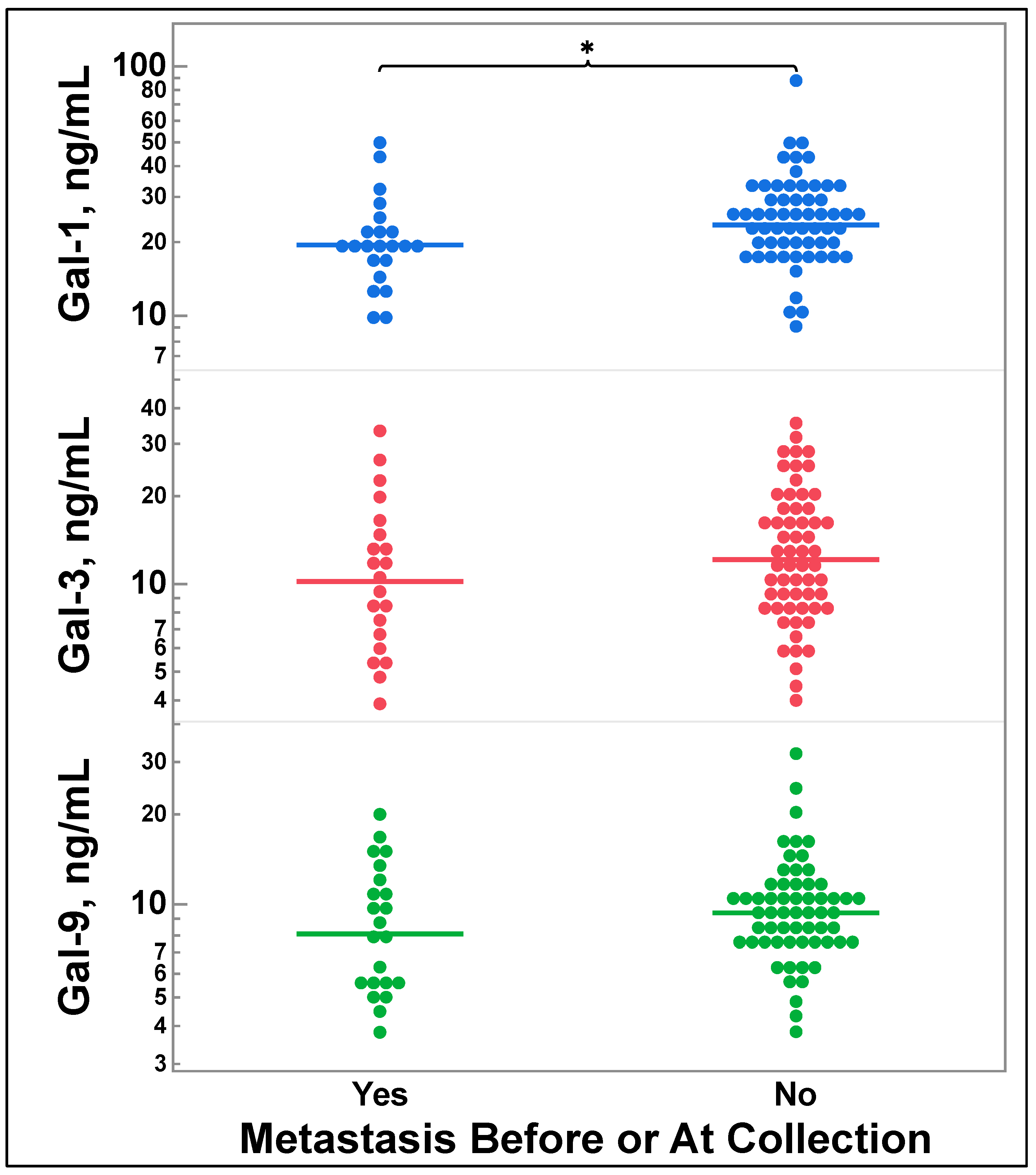
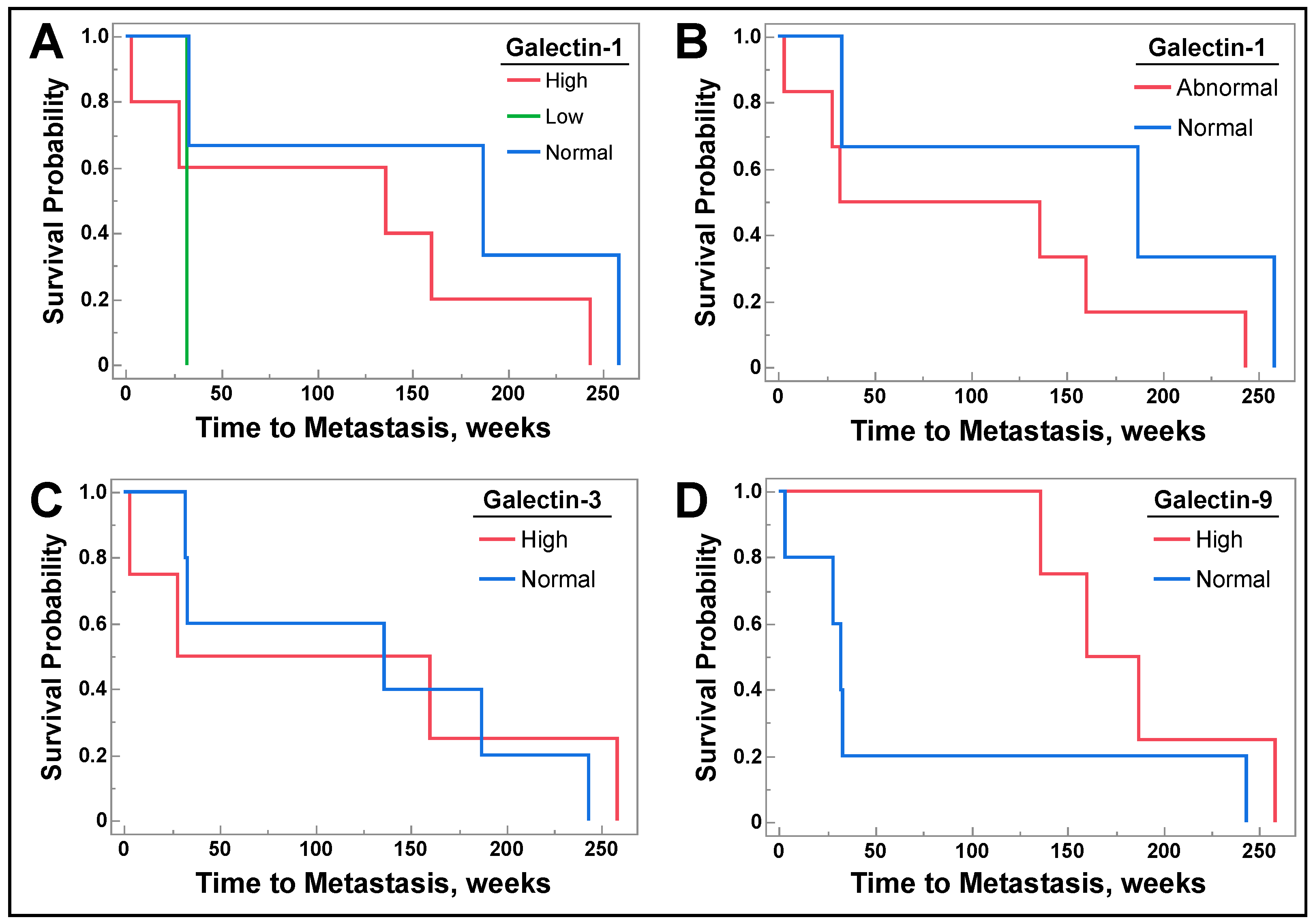
3.5. Galectin-1, -3, and -9 Levels Do Not Correlate with Future Metastasis
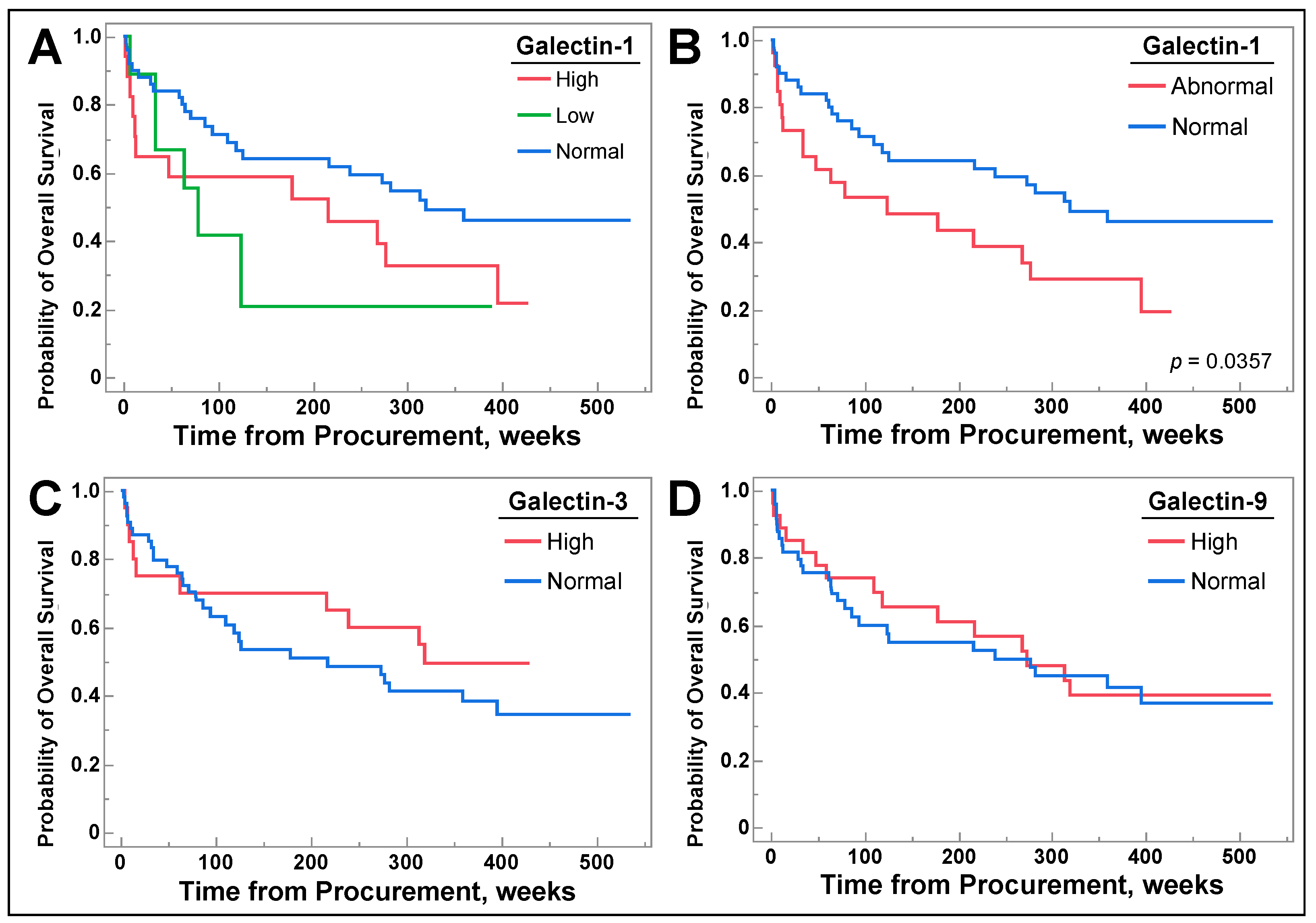
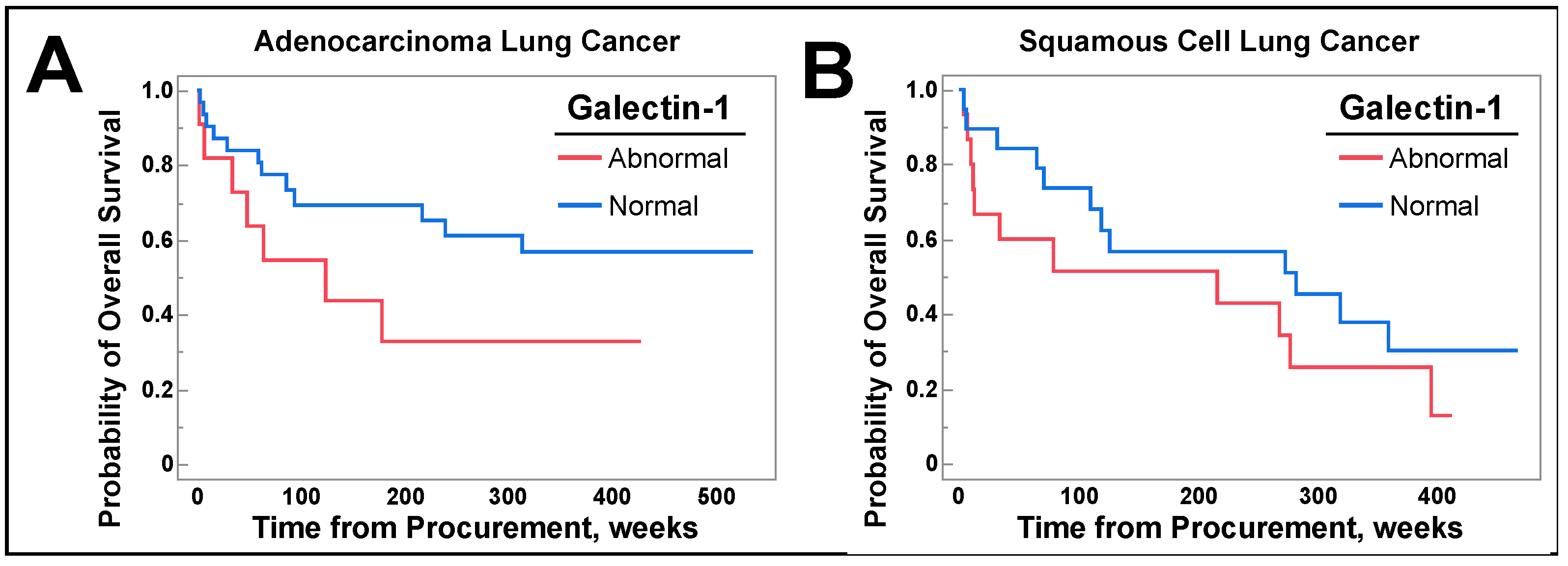
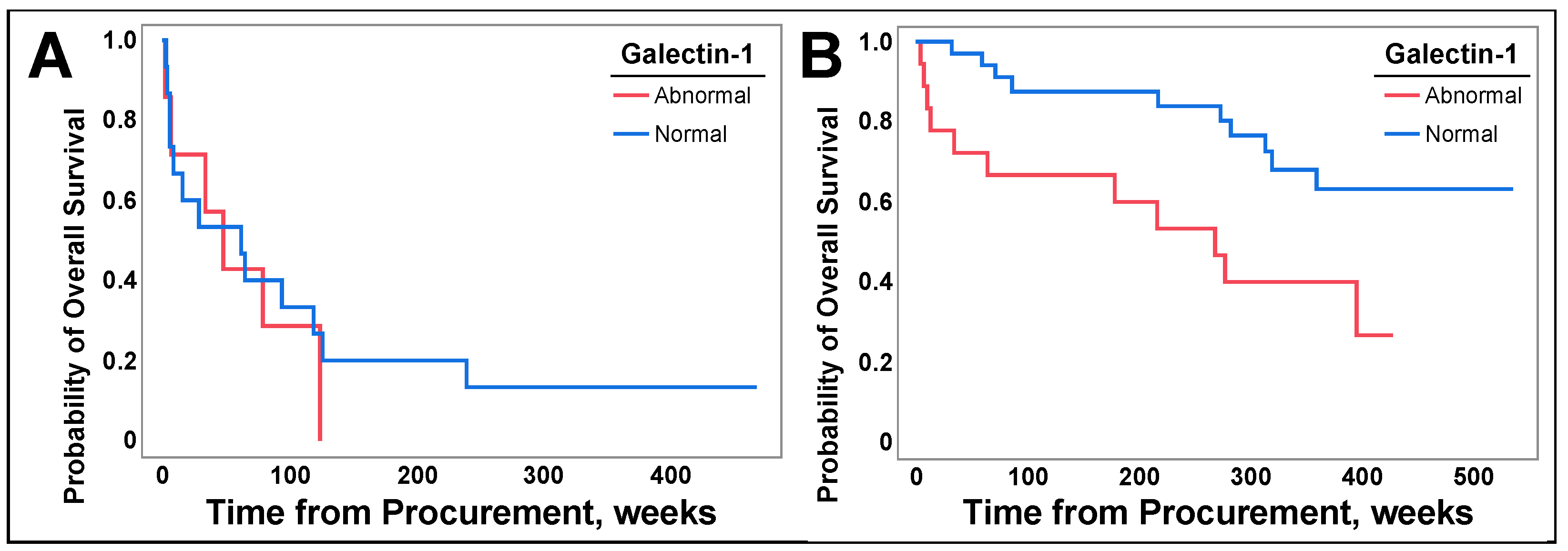
3.6. Galetin-1 May Be a Prognostic Biomarker for Select Populations of NSCLC
4. Discussion
4.1. Findings
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliver, A.L. Lung Cancer: Epidemiology and Screening. Surg. Clin. N. Am. 2022, 102, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J. The impact of lung cancer on patients and carers. Chron. Respir. Dis. 2012, 9, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, A.B.; Yabroff, K.R.; Shao, Y.; Feuer, E.J.; Brown, M.L. Projections of the cost of cancer care in the United States: 2010–2020. J. Natl. Cancer Inst. 2011, 103, 117–128. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.R.; Wagner, H. Small cell lung cancer. Chest 2003, 123, 259S–271S. [Google Scholar] [CrossRef] [PubMed]
- Ganti, A.K.; Klein, A.B.; Cotarla, I.; Seal, B.; Chou, E. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients with Non-Small Cell Lung Cancer in the US. JAMA Oncol. 2021, 7, 1824–1832. [Google Scholar] [CrossRef]
- Cummings, R.D.; Liu, F.T.; Rabinovich, G.A.; Stowell, S.A.; Vasta, G.R. Galectins. In Essentials of Glycobiology [Internet], 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022. [Google Scholar]
- Vasta, G.R. Galectins as pattern recognition receptors: Structure, function, and evolution. Adv. Exp. Med. Biol. 2012, 946, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Hogan, V.; Balan, V.; Raz, A. Chimeric galectin-3 and collagens: Biomarkers and potential therapeutic targets in fibroproliferative diseases. J. Biol. Chem. 2022, 298, 102622. [Google Scholar] [CrossRef]
- Ko, F.C.F.; Yan, S.; Lee, K.W.; Lam, S.K.; Ho, J.C.M. Chimera and Tandem-Repeat Type Galectins: The New Targets for Cancer Immunotherapy. Biomolecules 2023, 13, 902. [Google Scholar] [CrossRef]
- Troncoso, M.F.; Elola, M.T.; Croci, D.O.; Rabinovich, G.A. Integrating structure and function of ‘tandem-repeat’ galectins. Front. Biosci. (Schol. Ed.) 2012, 4, 864–887. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef]
- Newlaczyl, A.U.; Yu, L.G. Galectin-3—A jack-of-all-trades in cancer. Cancer Lett. 2011, 313, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.R.; Shankar, J.; Dennis, J.W. The galectin lattice at a glance. J. Cell Sci. 2015, 128, 2213–2219. [Google Scholar] [CrossRef]
- Chang, W.A.; Tsai, M.J.; Kuo, P.L.; Hung, J.Y. Role of galectins in lung cancer. Oncol. Lett. 2017, 14, 5077–5084. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Wu, C.Y.; Hung, J.Y.; Lin, Y.S.; Huang, M.S.; Kuo, P.L. Galectin-1 promotes lung cancer tumor metastasis by potentiating integrin α6β4 and Notch1/Jagged2 signaling pathway. Carcinogenesis 2013, 34, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, D.; Wang, X.; Zhang, B.; Zhu, H.; Zhao, J. Galectin-1 is overexpressed in CD133+ human lung adenocarcinoma cells and promotes their growth and invasiveness. Oncotarget 2015, 6, 3111–3122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chung, L.Y.; Tang, S.J.; Wu, Y.C.; Sun, G.H.; Liu, H.Y.; Sun, K.H. Galectin-3 augments tumor initiating property and tumorigenicity of lung cancer through interaction with β-catenin. Oncotarget 2015, 6, 4936–4952. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jia, K.; Dziadziuszko, R.; Zhao, S.; Zhang, X.; Deng, J.; Wang, H.; Hirsch, F.R.; Zhou, C. Galectin-9 in non-small cell lung cancer. Lung Cancer 2019, 136, 80–85. [Google Scholar] [CrossRef]
- Yang, R.; Sun, L.; Li, C.-F.; Wang, Y.-H.; Yao, J.; Li, H.; Yan, M.; Chang, W.-C.; Hsu, J.-M.; Cha, J.-H.; et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 2021, 12, 832. [Google Scholar] [CrossRef] [PubMed]
- Carlini, M.J.; Roitman, P.; Nuñez, M.; Pallotta, M.G.; Boggio, G.; Smith, D.; Salatino, M.; Bal de Kier Joffé, E.D.; Rabinovich, G.A.; Puricelli, L.I. Clinical relevance of galectin-1 expression in non-small cell lung cancer patients. Lung Cancer 2014, 84, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.; Monaghan, H.; Salter, D.M.; Sethi, T. Galectin-3: Differential expression between small-cell and non-small-cell lung cancer. Histopathology 2004, 44, 339–344. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, Y.; Li, H.; Wang, R.; Qian, K. Expression and Clinical Significance of Galcetin-3 in the Serum of Non-small Cell Lung Cancer Patients. Zhongguo Fei Ai Za Zhi 2020, 23, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Igarashi, T.; Ohshio, Y.; Fujita, T.; Hanaoka, J. Predictive importance of galectin-3 for recurrence of non-small cell lung cancer. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 704–711. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, L.; Zhang, W.; Sun, C.; Wu, C.; He, Y.; Zhou, C. Galectin-9-based immune risk score model helps to predict relapse in stage I-III small cell lung cancer. J. Immunother. Cancer 2020, 8, e001391. [Google Scholar] [CrossRef]
- Funkhouser, A.T.; Strigenz, A.M.; Blair, B.B.; Miller, A.P.; Shealy, J.C.; Ewing, J.A.; Martin, J.C.; Funk, C.R.; Edenfield, W.J.; Blenda, A.V. KIT Mutations Correlate with Higher Galectin Levels and Brain Metastasis in Breast and Non-Small Cell Lung Cancer. Cancers 2022, 14, 2781. [Google Scholar] [CrossRef]
- Blair, B.B.; Funkhouser, A.T.; Goodwin, J.L.; Strigenz, A.M.; Chaballout, B.H.; Martin, J.C.; Arthur, C.M.; Funk, C.R.; Edenfield, W.J.; Blenda, A.V. Increased Circulating Levels of Galectin Proteins in Patients with Breast, Colon, and Lung Cancer. Cancers 2021, 13, 4819. [Google Scholar] [CrossRef] [PubMed]
- Gluck, W.L.; Callahan, S.P.; Brevetta, R.A.; Stenbit, A.E.; Smith, W.M.; Martin, J.C.; Blenda, A.V.; Arce, S.; Edenfield, W.J. Efficacy of therapeutic plasma exchange in the treatment of penn class 3 and 4 cytokine release syndrome complicating COVID-19. Respir. Med. 2020, 175, 106188. [Google Scholar] [CrossRef] [PubMed]
- Shuford, S.; Lipinski, L.; Abad, A.; Smith, A.M.; Rayner, M.; O’Donnell, L.; Stuart, J.; Mechtler, L.L.; Fabiano, A.J.; Edenfield, J.; et al. Prospective prediction of clinical drug response in high-grade gliomas using an ex vivo 3D cell culture assay. Neuro-Oncol. Adv. 2021, 3, vdab065. [Google Scholar] [CrossRef]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.M. Lung cancer health disparities. Carcinogenesis 2018, 39, 741–751. [Google Scholar] [PubMed]
- Arora, P.; Agarwal, Z.; Venkatraman, A.; Callas, P.; Kissela, B.M.; Jenny, N.S.; Judd, S.E.; Zakai, N.A.; Cushman, M. Galectin-3 and risk of ischaemic stroke: Reasons for Geographic and Racial Differences in Stroke cohort. Eur. J. Neurol. 2017, 24, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Pokhare, S.; Sharma, U.C.; Attwood, K.; Mansoor, S. Clinical Significance of Galectin-3 Expression in Squamous Cell Carcinoma of Lung. J. Cancer Sci. Clin. Ther. 2022, 6, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Lababede, O.; Meziane, M.A. The Eighth Edition of TNM Staging of Lung Cancer: Reference Chart and Diagrams. Oncologist 2018, 23, 844–848. [Google Scholar] [CrossRef] [PubMed]
- GR-MD-02 Plus Pembrolizumab in Melanoma, Non-small Cell Lung Cancer, and Squamous Cell Head and Neck Cancer Patients. ClinicalTrials.gov Identifier: NCT02575404. Updated 5 June 2023. GR-MD-02 Plus Pembrolizumab in Melanoma, Non-small Cell Lung Cancer, and Squamous Cell Head and Neck Cancer Patients—Full Text View. Available online: https://clinicaltrials.gov/study/NCT02575404 (accessed on 22 October 2023).
- Vuong, L.; Kouverianou, E.; Rooney, C.M.; McHugh, B.J.; Howie, S.E.M.; Gregory, C.D.; Forbes, S.J.; Henderson, N.C.; Zetterberg, F.R.; Nilsson, U.J.; et al. An Orally Active Galectin-3 Antagonist Inhibits Lung Adenocarcinoma Growth and Augments Response to PD-L1 Blockade. Cancer Res. 2019, 79, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US food and drug administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung Cancer. New Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Rodrigues-Pereira, J.; Kim, J.H.; Magallanes, M.; Lee, D.H.; Wang, J.; Ganju, V.; Martínez-Barrera, L.; Barraclough, H.; van Kooten, M.; Orlando, M. A randomized phase 3 trial comparing pemetrexed/carboplatin and docetaxel/carboplatin as first-line treatment for advanced, nonsquamous non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, M.; Vansteenkiste, J.; Fischer, J.R.; Eberhardt, W.E.; Zabeck, H.; Kollmeier, J.; Serke, M.; Frickhofen, N.; Reck, M.; Engel-Riedel, W.; et al. Three-Year Follow-Up of a Randomized Phase II Trial on Refinement of Early-Stage NSCLC Adjuvant Chemotherapy with Cisplatin and Pemetrexed versus Cisplatin and Vinorelbine (the TREAT Study). J. Thorac. Oncol. 2016, 11, 85–93. [Google Scholar] [CrossRef]
- Fossella, F.; Pereira, J.R.; von Pawel, J.; Pluzanska, A.; Gorbounova, V.; Kaukel, E.; Mattson, K.V.; Ramlau, R.; Szczesna, A.; Fidias, P.; et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: The TAX 326 study group. J. Clin. Oncol. 2003, 21, 3016–3024. [Google Scholar] [CrossRef] [PubMed]
- Pérol, M.; Chouaid, C.; Pérol, D.; Barlési, F.; Gervais, R.; Westeel, V.; Crequit, J.; Léna, H.; Vergnenègre, A.; Zalcman, G.; et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J. Clin. Oncol. 2012, 30, 3516–3524. [Google Scholar] [CrossRef]
- Funkhouser, A.; Shuster, H.; Martin, J.C.; Edenfield, W.J.; Blenda, A.V. Pattern Analysis of Serum Galectins-1, -3, and -9 in Breast Cancer. Cancers 2023, 15, 3809. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gao, D.; Xue, J.; Zuo, Z. Galectin-3 and Myeloperoxidase May Monitor Cancer-Therapy-Related Cardiotoxicity? A Systematic Review and Meta-Analysis. Biomolecules 2022, 12, 1788. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.; Bratman, S.V.; Shultz, D.B.; von Eyben, R.; Chan, C.; Wang, Z.; Say, C.; Gupta, A.; Loo, B.W., Jr.; Giaccia, A.J.; et al. Galectin-1 mediates radiation-related lymphopenia and attenuates NSCLC radiation response. Clin. Cancer Res. 2014, 20, 5558–5569. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chuang, I.-C.; Su, Y.-L.; Luo, H.-L.; Chang, Y.-C.; Chen, J.-Y.; Hsiao, C.-C.; Huang, E.-Y. Prognostic Significance of Galectin-1 but Not Galectin-3 in Patients with Lung Adenocarcinoma After Radiation Therapy. Front. Oncol. 2022, 12, 834749. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.C.; Rosen, J.E.; Wang, Z.; Arnold, B.N.; Thomas, D.C.; Herbst, R.S.; Kim, A.W. Association of Delayed Adjuvant Chemotherapy with Survival After Lung Cancer Surgery. JAMA Oncol. 2017, 3, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, A.; Moore, J.; Suleman, A.; Faiz, S.; Farooq, O.; Arshad, A.; Tehseen, M.; Zafar, A.; Ali, S.H.; Din, N.U.; et al. Elevated Soluble Galectin-3 as a Marker of Chemotherapy Efficacy in Breast Cancer Patients: A Prospective Study. Int. J. Breast Cancer. 2020, 2020, 4824813. [Google Scholar] [CrossRef]
| Characteristics | Galectin-1: Mean (SD), n | Galectin-3: Mean (SD), n | Galectin-9: Mean (SD), n |
|---|---|---|---|
| Age | n = 87 | n = 84 | n = 87 |
| 3.088 (0.3980), n = 87 | 2.463 (0.5432), n = 84 | 2.204 (0.4171), n = 87 | |
| p-value | 0.0850 | 0.3580 | 0.3972 |
| Sex | n = 86 | n = 83 | n = 86 |
| Female | 3.149 (0.0665), n = 36 | 2.595 (0.0939), n = 33 | 2.321 (0.0682), n = 36 |
| Male | 3.043 (0.0564), n = 50 | 2.377 (0.0763), n = 50 | 2.117 (0.0579), n = 50 |
| p-value | 0.1140 | 0.0377 | 0.0124 |
| Race | n = 87 | n = 84 | n = 87 |
| Black, African American | 3.014 (0.1016), n = 15 | 2.491 (0.1470), n = 14 | 2.075 (0.1068), n = 15 |
| Multicultural Cohort | 2.730 (0.2279), n = 3 | 2.355 (0.3172), n = 3 | 2.139 (0.2410), n = 3 |
| White | 3.120 (0.0474), n = 69 | 2.451 (0.0569), n = 67 | 2.234 (0.0498), n = 69 |
| p-value | 0.1846 | 0.9261 | 0.3949 |
| Ethnicity | (n = 87) | (n = 84) | (n = 87) |
| Non-Spanish, Non-Hispanic | 3.102 (0.0423), n = 85 | 2.461 (0.0603), n = 82 | 2.212 (0.0451)), n = 85 |
| Spanish, Hispanic | 2.516 (0.2760), n = 2 | 2.523 (0.3863), n = 2 | 1.833 (0.2938), n = 2 |
| p-value | 0.0195 | 0.5628 | 0.1029 |
| Smoking Status | (n = 87) | (n = 84) | (n = 87) |
| Current Smoker | 2.998 (0.0765), n = 27 | 2.442 (0.1049), n = 27 | 2.094 (0.0785), n = 27 |
| Never Smoker | 3.199 (0.1988), n = 4 | 2.835 (0.3147), n = 3 | 2.603 (0.2039), n = 4 |
| Previous Smoker | 3.124 (0.0531), n = 56 | 2.452 (0.0742), n = 54 | 2.228 (0.0545), n = 56 |
| p-value | 0.3481 | 0.4684 | 0.0553 |
| BMI | (n = 81) | (n = 78) | (n = 81) |
| Underweight | 2.919 (0.2394), n = 3 | 2.221 (0.3260), n = 3 | 2.003 (0.2473), n = 3 |
| Healthy | 3.090 (0.0784), n = 28 | 2.451 (0.1087), n = 27 | 2.125 (0.0810), n = 28 |
| Overweight | 3.065 (0.0701), n = 35 | 2.435 (0.0983), n = 33 | 2.213 (0.0724, n = 35 |
| Obese | 3.201 (0.1071), n = 15 | 2.502 (0.1458), n = 15 | 2.327 (0.1106), n = 15 |
| p-value | 0.6376 | 0.8879 | 0.4216 |
| Radiation | Surgery | Chemotherapy | Targeted Therapy | Immunotherapy | |
|---|---|---|---|---|---|
| Galectin-1 Mean (SD) | n = 84 | n = 84 | n = 83 | n = 81 | n = 82 |
| No Treatment | 3.107 (0.0450), n = 66 | 3.060 (0.1633), n = 6 | 3.127 (0.0532), n = 56 | 3.078 (0.0443), n = 78 | 3.096 (0.0448), n = 79 |
| Treatment | 3.041 (0.0957), n = 18 | 3.086 (0.0453), n = 78 | 2.998 (0.0765), n = 27 | 3.200 (0.2258), n = 3 | 2.988 (0.2301), n = 3 |
| T-test p value | 0.2703 | 0.5590 | 0.0852 | 0.7010 | 0.3223 |
| Galectin-3 Mean (SD) | n = 81 | n = 81 | n = 80 | n = 78 | n = 79 |
| No Treatment | 2.485 (0.0680), n = 64 | 2.146 (0.2210), n = 6 | 2.523 (0.0735), n = 54 | 2.447 (0.0634), n = 75 | 2.465 (0.0626), n = 76 |
| Treatment | 2.318 (0.1320), n = 17 | 2.470 (0.0625), n = 75 | 2.290 (0.1060), n = 26 | 2.637 (0.3168), n = 3 | 2.256 (0.3151), n = 3 |
| T-test p value | 0.1324 | 0.9186 | 0.0375 | 0.721 | 0.2579 |
| Galectin-9 Mean (SD) | n = 84 | n = 84 | n = 83 | n = 81 | n = 82 |
| No Treatment | 2.208 (0.0521), n = 66 | 2.282 (0.1729), n = 6 | 2.249 (0.0563), n = 56 | 2.217 (0.0471), n = 78 | 2.229 (0.0466), n = 79 |
| Treatment | 2.204 (0.0997), n = 18 | 2.204 (0.0480), n = 78 | 2.118 (0.0810), n = 27 | 1.991 (0.2401), n = 3 | 1.856 (0.2393), n = 3 |
| T-test p value | 0.4867 | 0.3322 | 0.0940 | 0.1797 | 0.0649 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuster, H.; Funkhouser, A.; Allen, L.; Heo, M.; Martin, J.C.; Edenfield, W.J.; Blenda, A.V. Exploratory Assessment of Galectin-1, -3, and -9 in Non-Small Cell Lung Cancer. Cancers 2024, 16, 1165. https://doi.org/10.3390/cancers16061165
Shuster H, Funkhouser A, Allen L, Heo M, Martin JC, Edenfield WJ, Blenda AV. Exploratory Assessment of Galectin-1, -3, and -9 in Non-Small Cell Lung Cancer. Cancers. 2024; 16(6):1165. https://doi.org/10.3390/cancers16061165
Chicago/Turabian StyleShuster, Hayden, Avery Funkhouser, Lorie Allen, Moonseong Heo, Julie C. Martin, W. Jeffery Edenfield, and Anna V. Blenda. 2024. "Exploratory Assessment of Galectin-1, -3, and -9 in Non-Small Cell Lung Cancer" Cancers 16, no. 6: 1165. https://doi.org/10.3390/cancers16061165
APA StyleShuster, H., Funkhouser, A., Allen, L., Heo, M., Martin, J. C., Edenfield, W. J., & Blenda, A. V. (2024). Exploratory Assessment of Galectin-1, -3, and -9 in Non-Small Cell Lung Cancer. Cancers, 16(6), 1165. https://doi.org/10.3390/cancers16061165








