Application of Nanoparticles for Magnetic Hyperthermia for Cancer Treatment—The Current State of Knowledge
Abstract
Simple Summary
Abstract
1. Introduction
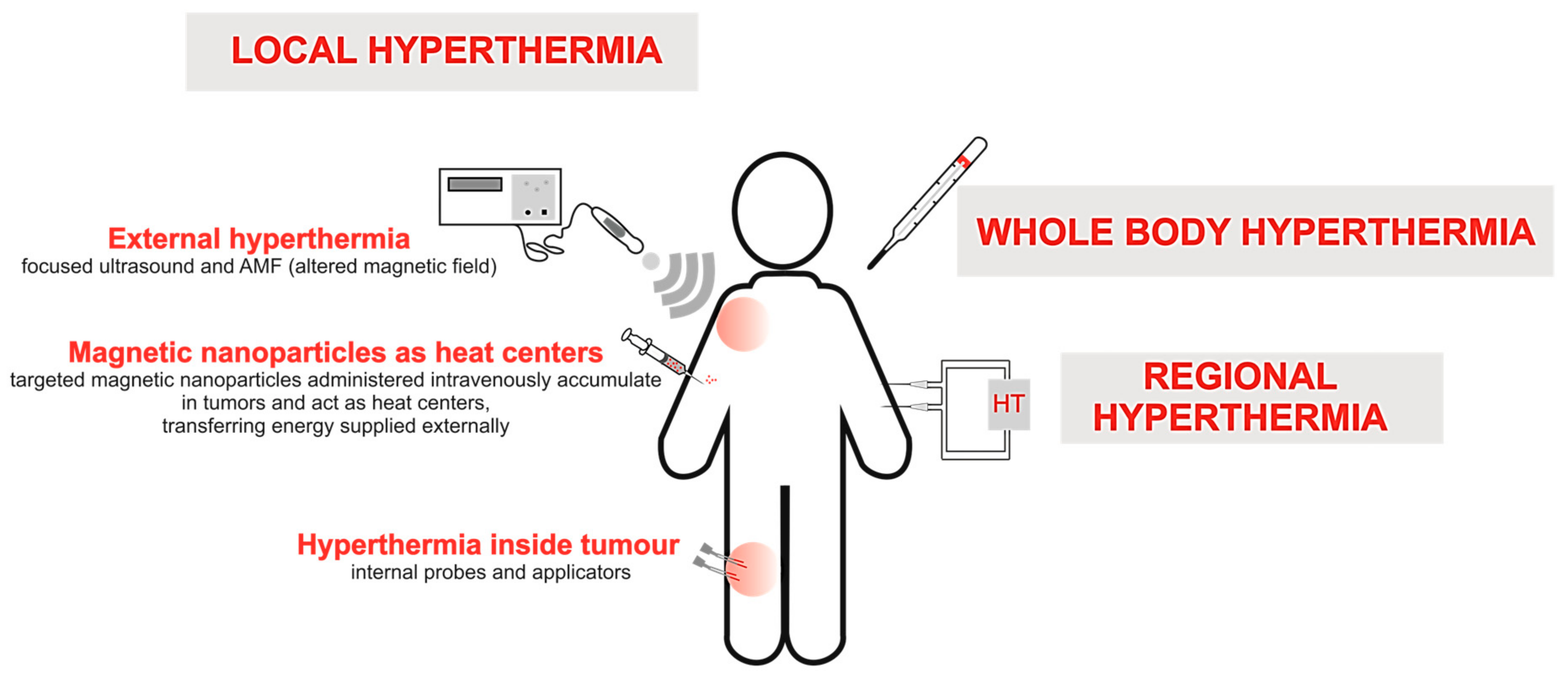
2. Types of Conventional HT Methods
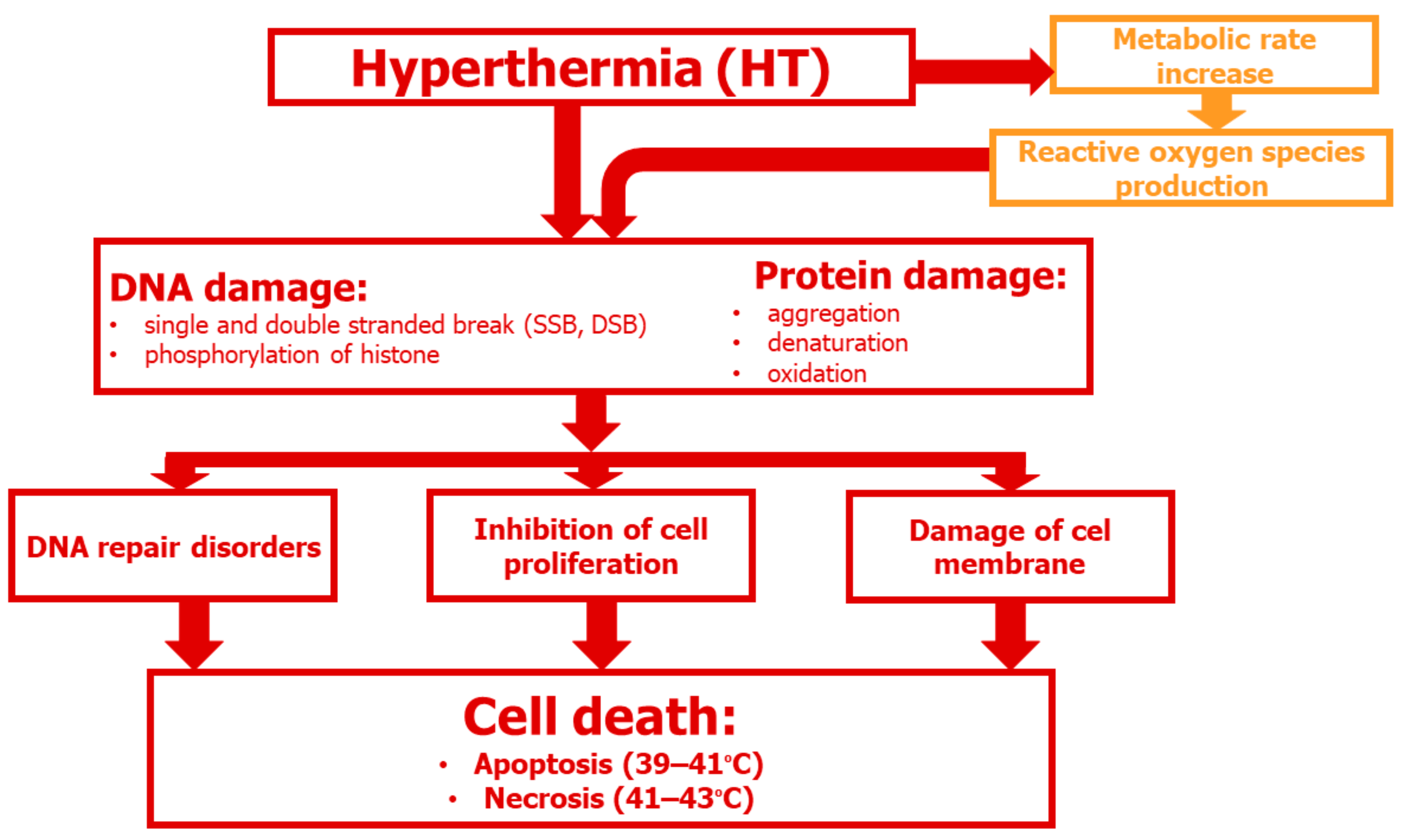
3. Cellular and Molecular Aspects of HT
3.1. DNA Damage
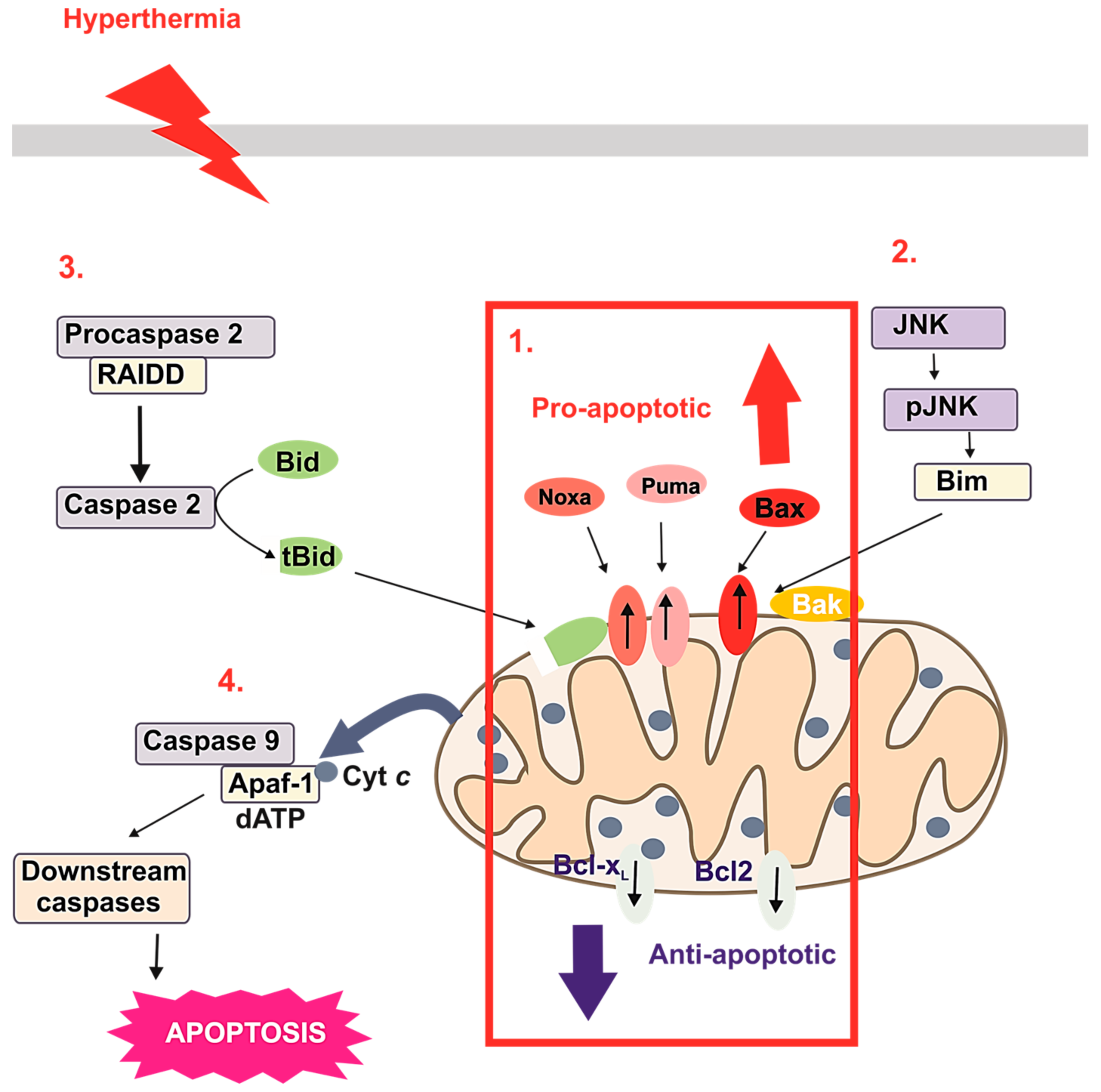
3.2. HT-Induced Apoptosis
4. Use of MNPs with Locally Induced HT
5. Bioconjugates of MNPs as Potential Passive or Targeted Delivery Systems
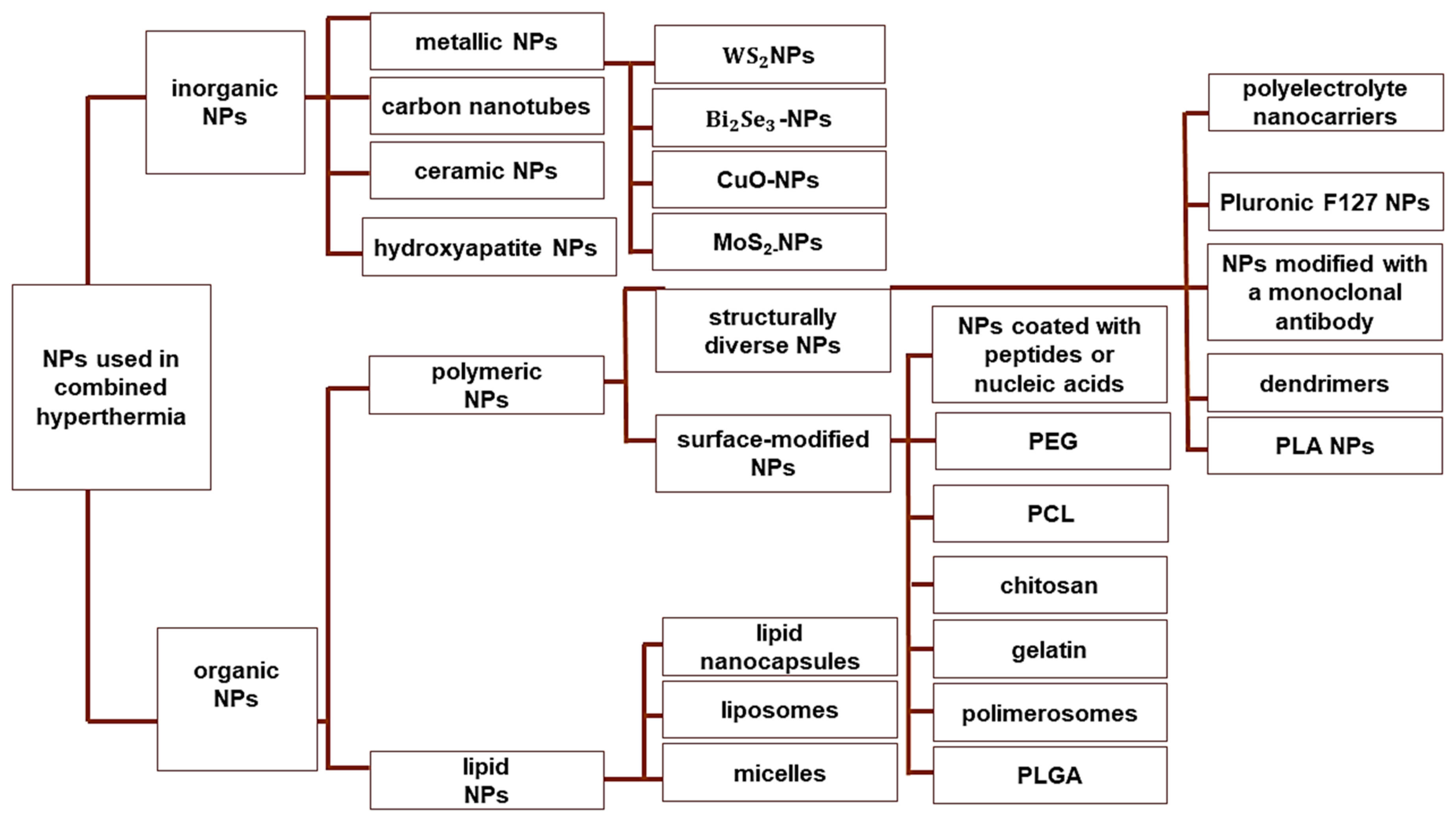
6. Combination Therapies Using NP-Based MHT
6.1. CT
6.2. RT
6.3. Gene Therapy
6.4. Photothermal Therapy
6.5. Immunotherapy
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- Kang, J.K.; Kim, J.C.; Shin, Y.; Han, S.M.; Won, W.R.; Her, J.; Park, J.Y.; Oh, K.T. Principles and applications of nanomaterial-based hyperthermia in cancer therapy. Arch. Pharm. Res. 2020, 43, 46–57. [Google Scholar] [CrossRef]
- Bucharskaya, A.B.; Khlebtsov, N.G.; Khlebtsov, B.N.; Maslyakova, G.N.; Navolokin, N.A.; Genin, V.D.; Tuchin, V.V. Photothermal and Photodynamic Therapy of tumors with plasmonic nanoparticles: Challenges and prospects. Materials 2022, 15, 1606. [Google Scholar] [CrossRef]
- Hader, M.; Frey, B.; Fietkau, R.; Hecht, M.; Gaipl, U.S. Immune biological rationales for the design of combined radio- and immunotherapies. Cancer Immunol. Immunother. 2020, 69, 293–306. [Google Scholar] [CrossRef]
- Farzin, L.; Saber, R.; Sadjadi, S.; Mohagheghpour, E.; Sheini, A. Nanomaterials-based hyperthermia: A literature review from concept to applications in chemistry and biomedicine. J. Therm. Biol. 2022, 104, 103201. [Google Scholar] [CrossRef]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; Ter Haar, G.; Wust, P.; Crezee, J. Heating technology for malignant tumors: A review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.A.; Kinsman, K.A.; Schmit, G.D.; Atwell, T.D.; Schmitz, J.J.; Welch, B.T.; Callstrom, M.R.; Geske, J.R.; Kurup, A.N. Thermal ablation of intrahepatic cholangiocarcinoma: Safety, efficacy, and factors affecting local tumor progression. Abdom. Radiol. 2018, 43, 3487–3492. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hou, Y. New types of magnetic nanoparticles for stimuli-responsive theranostic nanoplatforms. Adv. Sci. 2024, 11, e2305459. [Google Scholar] [CrossRef] [PubMed]
- Liebl, C.M.; Kutschan, S.; Dörfler, J.; Käsmann, L.; Hübner, J. Systematic review about complementary medical hyperthermia in oncology. Clin. Exp. Med. 2022, 22, 519–565. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.P.; Moreira, J.A.; Monteiro, F.J.; Laranjeira, M.S. Nanomaterials in cancer: Reviewing the combination of hyperthermia and triggered chemotherapy. J. Control Release 2022, 347, 89–103. [Google Scholar] [CrossRef]
- Toro-Córdova, A.; Llaguno-Munive, M.; Jurado, R.; Garcia-Lopez, P. The therapeutic potential of chemo/thermotherapy with magnetoliposomes for cancer treatment. Pharmaceutics 2022, 14, 2443. [Google Scholar] [CrossRef]
- Eltigani, F.; Ahmed, S.; Yahya, M.; Ahmed, M. Modeling of interstitial microwave hyperthermia for hepatic tumors using floating sleeve antenna. Phys. Eng. Sci. Med. 2022, 45, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Roti Roti, J.L. Cellular responses to hyperthermia (40–46 °C): Cell killing and molecular events. Int. J. Hyperth. 2008, 24, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Souiade, L.; Domingo-Diez, J.; Alcaide, C.; Gámez, B.; Gámez, L.; Ramos, M.; Serrano Olmedo, J.J. Improving the efficacy of magnetic nanoparticle-mediated hyperthermia using trap-ezoidal pulsed electromagnetic fields as an in vitro anticancer treatment in melanoma and glioblastoma multiforme cell lines. Int. J. Mol. Sci. 2023, 24, 15933. [Google Scholar] [CrossRef]
- Dewey, W.C.; Hopwood, L.E.; Sapareto, S.A.; Gerweck, L.E. Cellular responses to combinations of hyperthermia and radiation. Radiology 1977, 123, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Coss, R.A.; Dewey, W.C.; Bamburg, J.R. Effects of hyperthermia (41.5 degrees) on Chinese hamster ovary cells analyzed in motisis. Cancer Res. 1979, 39 Pt 1, 1911–1918. [Google Scholar]
- Lepock, J.R. How do cells respond to their thermal environment? Int. J. Hyperth. 2005, 21, 681–687. [Google Scholar] [CrossRef]
- Mackey, M.A.; Ianzini, F. Enhancement of radiation-induced mitotic catastrophe by moderate hyperthermia. Int. J. Radiat. Biol. 2000, 76, 273–280. [Google Scholar]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Bettaieb, A.; Averill-Bates, D.A. Thermotolerance induced at a mild temperature of 40 degrees C protects cells against heat shock-induced apoptosis. J. Cell Physiol. 2005, 205, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Sonna, L.A.; Fujita, J.; Gaffin, S.L.; Lilly, C.M. Invited review: Effects of heat and cold stress on mammalian gene expression. J. Appl. Physiol. 2002, 92, 1725–1742. [Google Scholar] [CrossRef] [PubMed]
- Imashiro, C.; Takeshita, H.; Morikura, T.; Miyata, S.; Takemura, K.; Komotori, J. Development of accurate temperature regulation culture system with metallic culture vessel demonstrates different thermal cytotoxicity in cancer and normal cells. Sci. Rep. 2021, 11, 21466. [Google Scholar] [CrossRef] [PubMed]
- Kase, K.; Hahn, G.M. Differential heat response of normal and transformed human cells in tissue culture. Nature 1975, 255, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Tabuchi, Y.; Kondo, T. Hyperthermia: An effective strategy to induce apoptosis in cancer cells. Apoptosis 2015, 20, 1411–1419. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.; Wu, Q.; Hu, P.; Shi, J. Mild magnetic hyperthermia-activated innate immunity for liver cancer therapy. J. Am. Chem. Soc. 2021, 143, 8116–8128. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.W.; Sun, W.; Guo, Y.; Jia, H.R.; Yu, X.W.; Pan, G.Y.; Wu, F.G. Molecular targeting-mediated mild-temperature photothermal therapy with a smart albumin-based nanodrug. Small 2019, 15, e1900501. [Google Scholar] [CrossRef]
- Yagawa, Y.; Tanigawa, K.; Kobayashi, Y.; Yamamoto, M. Cancer immunity and therapy using hyperthermia with immunotherapy, radiotherapy, chemotherapy, and surgery. J. Cancer Metastasis Treat. 2017, 3, 218. [Google Scholar] [CrossRef]
- Frey, B.; Weiss, E.M.; Rubner, Y.; Wunderlich, R.; Ott, O.J.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Old and new facts about hyperthermia-induced modulations of the immune system. Int. J. Hyperth. 2012, 28, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, G.; Lorkowski, M.E.; Sims, H.M.; Loutrianakis, G.; Rahmy, A.; Cha, A.; Abenojar, E.; Wickramasinghe, S.; Moon, T.F.; Samia, A.C.S.; et al. Hyperthermia-mediated changes in the tumor immune microenvironment using iron oxide nanoparticles. Nanoscale Adv. 2021, 3, 5890–5899. [Google Scholar] [CrossRef] [PubMed]
- Mikucki, M.E.; Fisher, D.T.; Ku, A.W.; Appenheimer, M.M.; Muhitch, J.B.; Evans, S.S. Preconditioning thermal therapy: Flipping the switch on IL-6 for anti-tumour immunity. Int. J. Hyperth. 2013, 29, 464–473. [Google Scholar] [CrossRef]
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat shock proteins and cancer. Trends Pharmacol. Sci. 2017, 38, 226–256. [Google Scholar] [CrossRef]
- Watanabe, M.; Suzuki, K.; Kodama, S.; Sugahara, T. Normal human cells at confluence get heat resistance by efficient accumulation of hsp72 in nucleus. Carcinogenesis 1995, 16, 2373–2380. [Google Scholar] [CrossRef]
- Oei, A.L.; Vriend, L.E.M.; Krawczyk, P.M.; Horsman, M.R.; Franken, N.A.P.; Crezee, J. Targeting therapy-resistant cancer stem cells by hyperthermia. Int. J. Hyperth. 2017, 33, 419–427. [Google Scholar] [CrossRef]
- Hurwitz, M.; Stauffer, P. Hyperthermia, radiation and chemotherapy: The role of heat in multidisciplinary cancer care. Semin. Oncol. 2014, 41, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Bewicke-Copley, F.; Mulcahy, L.A.; Jacobs, L.A.; Samuel, P.; Akbar, N.; Pink, R.C.; Carter, D.R.F. Extracellular vesicles released following heat stress induce bystander effect in unstressed populations. J. Extracell. Vesicles 2017, 6, 1340746. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Scheper, W.; Kvistborg, P. Cancer neoantigens. Annu. Rev. Immunol. 2019, 37, 173–200. [Google Scholar] [CrossRef]
- Poe, B.S.; O’Neill, K.L. Inhibition of protein synthesis sensitizes thermotolerant cells to heat shock induced apoptosis. Apoptosis 1997, 2, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Averill-Bates, D.A. Thermotolerance induced at a fever temperature of 40 °C protects cells against hyperthermia-induced apoptosis mediated by death receptor signalling. Biochem. Cell Biol. 2008, 86, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Signore, M.; Ricci-Vitiani, L.; De Maria, R. Targeting apoptosis pathways in cancer stem cells. Cancer Lett. 2013, 332, 374–382. [Google Scholar] [CrossRef]
- Moriyama-Gonda, N.; Igawa, M.; Shiina, H.; Urakami, S.; Shigeno, K.; Terashima, M. Modulation of heat-induced cell death in PC-3 prostate cancer cells by the antioxidant inhibitor diethyldithiocarbamate. BJU Int. 2002, 90, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, T.; Bouchier-Hayes, L.; Chipuk, J.E.; Bonzon, C.; Sullivan, B.A.; Green, D.R.; Newmeyer, D.D. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell 2005, 17, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.G.; Piao, J.L.; Rehman, M.U.; Ogawa, R.; Li, P.; Zhao, Q.L.; Kondo, T.; Inadera, H. Molecular mechanisms of hyperthermia-induced apoptosis enhanced by withaferin A. Eur. J. Pharmacol. 2014, 723, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Narmani, A.; Salehi, M.; Bagheri, H.; Farhood, B.; Haghi-Aminjan, H.; Masoud, N. Synergic effects of nanoparticles-mediated hyperthermia in radiotherapy/chemotherapy of cancer. Life Sci. 2021, 269, 119020. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.P.; Gómez-Pastora, J.; et al. Magnetic nanoparticles: A review on synthesis, characterization, functionalization, and biomedical applications. Small 2024, 20, e2304848. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control Release 2016, 235, 205–221. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as drug delivery systems: A review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Rahban, D.; Doostan, M.; Salimi, A. Cancer therapy; prospects for application of nanoparticles for magnetic-based hyperthermia. Cancer Investig. 2020, 38, 507–521. [Google Scholar] [CrossRef]
- Lanier, O.L.; Korotych, O.I.; Monsalve, A.G.; Wable, D.; Savliwala, S.; Grooms, N.W.F.; Nacea Ch Tuitt, O.R.; Dobson, J. Evaluation of magnetic nanoparticles for magnetic fluid hyperthermia. Int. J. Hyperth. 2019, 36, 687–701. [Google Scholar] [CrossRef]
- Di Corato, R.; Espinosa, A.; Lartigue, L.; Tharaud, M.; Chat, S.; Pellegrino, T.; Ménager, C.; Gazeau, F.; Wilhelm, C. Magnetic hyperthermia efficiency in the cellular environment for different nanoparticle designs. Biomaterials 2014, 35, 6400–6411. [Google Scholar] [CrossRef]
- Usov, N.A.; Gubanova, E.M. Application of magnetosomes in magnetic hyperthermia. Nanomaterials 2020, 10, 1320. [Google Scholar] [CrossRef]
- Snigdhadev, C.; Gokul, N.; Gunaseelan, M.; Srestha, R.; Muruga, L.; Jayesh, G.; Priyankan, D.; Pallab, S.M.; Basudev, R. Facets of optically and magnetically induced heating in ferromagnetically doped-NaYF4 particle. J. Phys. Commun. 2022, 7, 065008. [Google Scholar]
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic iron oxide nanoparticles (SPION): From fundamentals to state of the art Innovative applications for cancer therapy. Pharmaceutics 2023, 15, 236. [Google Scholar] [CrossRef]
- Schemberg, J.; Abbassi, A.E.; Lindenbauer, A.; Chen, L.Y.; Grodrian, A.; Nakos, X.; Apte, G.; Khan, N.; Kraupner, A.; Nguyen, T.H.; et al. Synthesis of biocompatible superparamagnetic iron oxide nanoparticles (SPION) under different microfluidic regimes. ACS Appl. Mater. Interfaces 2022, 14, 48011–48028. [Google Scholar] [CrossRef]
- Carrera Espinoza, M.J.; Lin, K.S.; Weng, M.T.; Kunene, S.C.; Lin, Y.S.; Wu, C.M. Synthesis and characterization of supermagnetic nanocomposites coated with pluronic F127 as a contrast agent for biomedical applications. Pharmaceutics 2023, 15, 740. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Degl’Innocenti, A.; Belenli Gümüş, M.; Ciofani, G. Superparamagnetic iron oxide nanoparticles for magnetic hyperthermia: Recent advancements, molecular effects, and future directions in the omics era. Biomater. Sci. 2022, 10, 2103–2121. [Google Scholar] [CrossRef] [PubMed]
- Grauer, O.; Jaber, M.; Hess, K.; Weckesser, M.; Schwindt, W.; Maring, S.; Wölfer, J.; Stummer, W. Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients. J. Neurooncol. 2019, 141, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.R.; Lee, Y.H.; Bat-Ulzii, A.; Bhattacharya, M.; Chakraborty, C.; Lee, S.S. Recent advances of metal-based nanoparticles in nucleic acid delivery for therapeutic applications. J. Nanobiotechnol. 2022, 20, 501. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, M.; Martella, D.; Barrera, G.; Celegato, F.; Coïsson, M.; Ferrero, R.; Olivetti, E.S.; Troia, A.; Sözeri, H.; Parmeggiani, C.; et al. Improvement of hyperthermia properties of iron oxide nanoparticles by surface coating. ACS Omega 2023, 8, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hajebrahimi, S.; Tong, S.; Gao, X.; Cheng, H.; Zhang, Q.; Hinojosa, D.T.; Jiang, K.; Hong, L.; Huard, J.; et al. Force-Mediated Endocytosis of iron oxide nanoparticles for magnetic targeting of stem cells. ACS Appl. Mater. Interfaces, 2023; ahead of print. [Google Scholar] [CrossRef]
- Dias, A.M.M.; Courteau, A.; Bellaye, P.S.; Kohli, E.; Oudot, A.; Doulain, P.E.; Petitot, C.; Walker, P.M.; Decréau, R.; Collin, B. Superparamagnetic iron oxide nanoparticles for immunotherapy of cancers through macrophages and magnetic hyperthermia. Pharmaceutics 2022, 14, 2388. [Google Scholar] [CrossRef]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In Vitro methods for assessing nanoparticle toxicity. Methods Mol. Biol. 2019, 1894, 1–29. [Google Scholar] [PubMed]
- Vroman, L. Effect of absorbed proteins on the wettability of hydrophilic and hydrophobic solids. Nature 1962, 196, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef]
- Akkewar, A.; Mahajan, N.; Kharwade, R.; Gangane, P. Liposomes in the targeted gene therapy of cancer: A critical review. Curr. Drug Deliv. 2023, 20, 350–370. [Google Scholar] [CrossRef] [PubMed]
- Fulton, M.D.; Najahi-Missaoui, W. Liposomes in cancer therapy: How did we start and where are we now. Int. J. Mol. Sci. 2023, 24, 6615. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.S.; Pershina, L.V.; Nikolaev, K.G.; Skorb, E.V. Recent progress of layer-by-layer assembly, free-standing film and hydrogel based on polyelectrolytes. Macromol. Biosci. 2021, 21, e2100117. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Colombo, M.; Prosperi, D. Recent advances in magnetic fluid hyperthermia for cancer therapy. Colloids Surf. B Biointerfaces 2019, 174, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, V.; Carvalho, F.; Espiña, B. Magnetic Hyperthermia for cancer treatment: Main parameters affecting the outcome of in vitro and in vivo studies. Molecules 2020, 25, 2874. [Google Scholar] [CrossRef]
- Sohail, A.; Ahmad, Z.; Bég, O.A.; Arshad, S.; Sherin, L. A review on hyperthermia via nanoparticle-mediated therapy. Bull. Cancer 2017, 104, 452–461. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Spirou, S.V.; Costa Lima, S.A.; Bouziotis, P.; Vranješ-Djurić, S.; Efthimiadou, E.; Laurenzana, A.; Barbosa, A.I.; Garcia-Alonso, I.; Jones, C.; Jankovic, D.; et al. Recommendations for in vitro and in vivo testing of magnetic nanoparticle hyperthermia combined with radiation therapy. Nanomaterials 2018, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic iron oxide nanoparticles-current and prospective medical applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Takada, K.; Kashiwagi, S.; Asano, Y.; Goto, W.; Kouhashi, R.; Yabumoto, A.; Morisaki, T.; Shibutani, M.; Takashima, T.; Fujita, H. Prediction of lymph node metastasis by tumor-infiltrating lymphocytes in T1 breast cancer. BMC Cancer 2020, 20, 598. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control Release 2016, 244 Pt A, 108–121. [Google Scholar] [CrossRef]
- Ahmed, A.; Sarwar, S.; Hu, Y.; Munir, M.U.; Nisar, M.F.; Ikram, F.; Asif, A.; Rahman, S.U.; Chaudhry, A.A.; Rehman, I.U. Surface-modified polymeric nanoparticles for drug delivery to cancer cells. Expert Opin. Drug Deliv. 2021, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.; Wang, J. Role of ligand distribution in the cytoskeleton-associated endocytosis of ellipsoidal nanoparticles. Membranes 2021, 11, 993. [Google Scholar] [CrossRef] [PubMed]
- Ndong, C.; Tate, J.A.; Kett, W.C.; Batra, J.; Demidenko, E.; Lewis, L.D.; Hoopes, P.J.; Gerngross, T.U.; Griswold, K.E. Affiliations expand. Tumor cell targeting by iron oxide nanoparticles is dominated by different factors in vitro versus in vivo. PLoS ONE 2015, 10, e0115636. [Google Scholar] [CrossRef]
- Abdelkhaliq, A.; van der Zande, M.; Punt, A.; Helsdingen, R.; Boeren, S.; Vervoort, J.J.M.; Rietjens, I.M.C.M.; Bouwmeester, H. Impact of nanoparticle surface functionalization on the protein corona and cellular adhesion, uptake and transport. J. Nanobiotechnol. 2018, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, A.B.; Khot, V.M.; Pawar, S.H. Magnetic hyperthermia with magnetic nanoparticles: A status review. Curr. Top. Med. Chem. 2014, 14, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Manigandan, A.; Handi, V.; Sundaramoorthy, N.S.; Dhandapani, R.; Radhakrishnan, J.; Sethuraman, S.; Subramanian, A. Responsive nanomicellar theranostic cages for metastatic breast cancer. Bioconjug. Chem. 2018, 29, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, R.; Yuan, C.; An, Y.; Tang, Q.; Chen, D. Preparation of folic acid-targeted temperature-sensitive magnetoliposomes and their antitumor effects in vitro and in vivo. Target Oncol. 2018, 13, 481–494. [Google Scholar] [CrossRef]
- Khan, M.M.; Madni, A.; Filipczak, N.; Pan, J.; Rehman, M.; Rai, N.; Attia, S.A.; Torchilin, V.P. Folate targeted lipid chitosan hybrid nanoparticles for enhanced anti-tumor efficacy. Nanomedicine 2020, 28, 102228. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liang, N.; Yang, H.; Liu, H.; Li, S. Temozolomide encapsulated and folic acid decorated chitosan nanoparticles for lung tumor targeting: Improving therapeutic efficacy both in vitro and in vivo. Oncotarget 2017, 8, 111318–111332. [Google Scholar] [CrossRef] [PubMed]
- Merzel, R.L.; Frey, C.; Chen, J.; Garn, R.; van Dongen, M.; Dougherty, C.A.; Kandaluru, A.K.; Low, P.S.; Marsh, E.N.G.; Banaszak Holl, M.M. Conjugation dependent interaction of folic acid with folate binding protein. Bioconjug. Chem. 2017, 28, 2350–2360. [Google Scholar] [CrossRef]
- Narmani, A.; Rezvani, M.; Farhood, B.; Darkhor, P.; Mohammadnejad, J.; Amini, B.; Refahi, S.; Abdi Goushbolagh, N. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev. Res. 2019, 80, 404–424. [Google Scholar] [CrossRef]
- Bonvin, D.; Bastiaansen, J.A.M.; Stuber, M.; Hofmann, H.; Mionić Ebersold, M. Folic acid on iron oxide nanoparticles: Platform with high potential for simultaneous targeting, MRI detection and hyperthermia treatment of lymph node metastases of prostate cancer. Dalton Trans. 2017, 46, 12692–12704. [Google Scholar] [CrossRef]
- Liu, F.; Le, W.; Mei, T.; Wang, T.; Chen, L.; Lei, Y.; Cui, S.; Chen, B.; Cui, Z.; Shao, C. In Vitro and in vivo targeting imaging of pancreatic cancer using a Fe3O4@SiO2 nanoprobe modified with anti-mesothelin antibody. Int. J. Nanomed. 2016, 11, 2195–2207. [Google Scholar]
- Kasprzak, A.; Grudzinski, I.P.; Bamburowicz-Klimkowska, M.; Parzonko, A.; Gawlak, M.; Poplawska, M. New insight into the synthesis and Biological activity of the polymeric materials consisting of folic acid and β-Cyclodextrin. Macromol. Biosci. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Hamzehalipour Almaki, J.; Nasiri, R.; Idris, A.; Nasiri, M.; Abdul Majid, F.A.; Losic, D. Trastuzumab-decorated nanoparticles for in vitro and in vivo tumor-targeting hyperthermia of HER2+ breast cancer. J. Mater. Chem. B 2017, 5, 7369–7383. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Jeong, H.; Noh, S.H.; Lee, J.H.; Cheon, J. Magnetically triggered dual functional nanoparticles for resistance-free apoptotic hyperthermia. Angew. Chem. Int. Ed. Engl. 2013, 52, 13047–13051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, Q.; Du, M.; Vermorken, A.; Cui, Y.; Zhang, L.; Guo, L.; Ma, L.; Chen, M. Cetuximab and doxorubicin loaded dextran-coated Fe3O4 magnetic nanoparticles as novel targeted nanocarriers for non-small cell lung cancer. J. Magn. Magn. Mater. 2019, 481, 122–128. [Google Scholar] [CrossRef]
- Sadhukha, T.; Wiedmann, T.S.; Panyam, J. Inhalable magnetic nanoparticles for targeted hyperthermia in lung cancer therapy. Biomaterials 2013, 34, 5163–5171. [Google Scholar] [CrossRef]
- Irvine, D.J.; Dane, E.L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 2020, 20, 321–334. [Google Scholar] [CrossRef]
- Clerc, P.; Jeanjean, P.; Hallali, N.; Gougeon, M.; Pipy, B.; Carrey, J.; Fourmy, D.; Gigoux, V. Targeted magnetic intra-lysosomal hyperthermia produces lysosomal reactive oxygen species and causes Caspase-1 dependent cell death. J. Control Release 2018, 270, 120–134. [Google Scholar] [CrossRef]
- Domenech, M.; Marrero-Berrios, I.; Torres-Lugo, M.; Rinaldi, C. Lysosomal membrane permeabilization by targeted magnetic nanoparticles in alternating magnetic fields. ACS Nano 2013, 7, 5091–5101. [Google Scholar] [CrossRef]
- Shah, B.P.; Pasquale, N.; De, G.; Tan, T.; Ma, J.; Lee, K.B. Core-shell nanoparticle-based peptide therapeutics and combined hyperthermia for enhanced cancer cell apoptosis. ACS Nano 2014, 8, 9379–9387. [Google Scholar] [CrossRef]
- Phung, D.C.; Nguyen, H.T.; Phuong Tran, T.T.; Jin, S.G.; Yong, C.S.; Truong, D.H.; Tran, T.H.; Kim, J.O. Combined hyperthermia and chemotherapy as a synergistic anticancer treatment. J. Pharm. Investig. 2019, 49, 519–526. [Google Scholar] [CrossRef]
- Cazares-Cortes, E.; Cabana, S.; Boitard, C.; Nehlig, E.; Griffete, N.; Fresnais, J.; Wilhelm, C.; Abou-Hassan, A.; Ménager, C. Recent insights in magnetic hyperthermia: From the “hot-spot” effect for local delivery to combined magneto-photo-thermia using magneto-plasmonic hybrids. Adv. Drug Deliv. Rev. 2019, 138, 233–246. [Google Scholar] [CrossRef]
- Mertz, D.; Sandre, O.; Bégin-Colin, S. Drug releasing nanoplatforms activated by alternating magnetic fields. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1617–1641. [Google Scholar] [CrossRef]
- Wigner, P.; Zielinski, K.; Labieniec-Watala, M.; Marczak, A.; Szwed, M. Doxorubicin-transferrin conjugate alters mitochondrial homeostasis and energy metabolism in human breast cancer cells. Sci. Rep. 2021, 11, 4544. [Google Scholar] [CrossRef]
- Bavli, Y.; Winkler, I.; Chen, B.M.; Roffler, S.; Cohen, R.; Szebeni, J.; Barenholz, Y. Doxebo (doxorubicin-free Doxil-like liposomes) is safe to use as a pre-treatment to prevent infusion reactions to PEGylated nanodrugs. J. Control Release 2019, 306, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Mai, B.T.; Balakrishnan, P.B.; Barthel, M.J.; Piccardi, F.; Niculaes, D.; Marinaro, F.; Fernandes, S.; Curcio, A.; Kakwere, H.; Autret, G.; et al. Thermoresponsive iron oxide nanocubes for an effective clinical translation of magnetic hyperthermia and heat-mediated chemotherapy. ACS Appl. Mater. Interfaces 2019, 11, 5727–5739. [Google Scholar] [CrossRef]
- Thirunavukkarasu, G.K.; Cherukula, K.; Lee, H.; Jeong, Y.Y.; Park, I.K.; Lee, J.Y. Magnetic field-inducible drug-eluting nanoparticles for image-guided thermo-chemotherapy. Biomaterials 2018, 180, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Cazares-Cortes, E.; Espinosa, A.; Guigner, J.M.; Michel, A.; Griffete, N.; Wilhelm, C.; Ménager, C. Doxorubicin intracellular remote release from biocompatible oligo(ethylene glycol) methyl ether methacrylate-based magnetic nanogels triggered by magnetic hyperthermia. ACS Appl. Mater. Interfaces 2017, 9, 25775–25788. [Google Scholar] [CrossRef]
- Sharifi, M.; Hasan, A.; Nanakali, N.M.Q.; Salihi, A.; Qadir, F.A.; Muhammad, H.A.; Shekha, M.S.; Aziz, F.M.; Amen, K.M.; Najafi, F.; et al. Combined chemo-magnetic field-photothermal breast cancer therapy based on porous magnetite nanospheres. Sci. Rep. 2020, 10, 5925. [Google Scholar] [CrossRef] [PubMed]
- Chaurawal, N.; Misra, C.; Raza, K. Lipid-based nanocarriers loaded with taxanes for the management of breast cancer: Promises and challenges. Curr. Drug Targets 2022, 23, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Han, J.; Jin, Z.; Kim, C.S.; Park, S.; Kim, K.P.; Park, J.O.; Choi, E. Dual tumor-targeted multifunctional magnetic hyaluronic acid micelles for enhanced MR imaging and combined photothermal-chemotherapy. Colloids Surf. B Biointerfaces 2018, 164, 424–435. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Guo, X.; Guo, X.; Zhang, Z. Multi-functional magnetic nanoparticles as an effective drug carrier for the controlled anti-tumor treatment. J. Biomater. Appl. 2018, 32, 967–976. [Google Scholar] [CrossRef]
- Kumar, A.; Harsha, C.; Parama, D.; Girisa, S.; Daimary, U.D.; Mao, X.; Kunnumakkara, A.B. Current clinical developments in curcumin-based therapeutics for cancer and chronic diseases. Phytother. Res. 2021, 35, 6768–6801. [Google Scholar] [CrossRef] [PubMed]
- Senturk, F.; Cakmak, S.; Kocum, I.C.; Gumusderelioglu, M.; Ozturk, G.G. GRGDS-conjugated and curcumin-loaded magnetic polymeric nanoparticles for the hyperthermia treatment of glioblastoma cells. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126648. [Google Scholar] [CrossRef]
- Sudame, A.; Kandasamy, G.; Singh, D.; Tomy, C.V.; Maity, D. Symbiotic thermo-chemotherapy for enhanced HepG2 cancer treatment via magneto-drugs encapsulated polymeric nanocarriers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125355. [Google Scholar] [CrossRef]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bäuerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539–544. [Google Scholar] [CrossRef]
- Shestovskaya, M.V.; Luss, A.L.; Bezborodova, O.A.; Makarov, V.V.; Keskinov, A.A. Iron oxide nanoparticles in cancer treatment: Cell responses and the potency to improve radiosensitivity. Pharmaceutics 2023, 15, 2406. [Google Scholar] [CrossRef]
- Rezaie, P.; Khoei, S.; Khoee, S.; Shirvalilou, S.; Mahdavi, S.R. Evaluation of combined effect of hyperthermia and ionizing radiation on cytotoxic damages induced by IUdR-loaded PCL-PEG-coated magnetic nanoparticles in spheroid culture of U87MG glioblastoma cell line. Int. J. Radiat. Biol. 2018, 94, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Attaluri, A.; Kandala, S.K.; Wabler, M.; Zhou, H.; Cornejo, C.; Armour, M.; Hedayati, M.; Zhang, Y.; DeWeese, T.L.; Herman, C.; et al. Magnetic nanoparticle hyperthermia enhances radiation therapy: A study in mouse models of human prostate cancer. Int. J. Hyperth. 2015, 31, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.S.; Tsai, H.Y.; Drake, P.; Wang, F.N.; Chiang, C.S. Gadolinium-doped iron oxide nanoparticles induced magnetic field hyperthermia combined with radiotherapy increases tumour response by vascular disruption and improved oxygenation. Int. J. Hyperth. 2017, 33, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Denkbas, E.B.; Çelik, E.; Erdal, E.; Kavaz Da Akbal, Ö.; Kara, G.; Bayram, C. Magnetically based nanocarriers in drug delivery. Nanobiomater. Drug Deliv. 2016, 9, 285–331. [Google Scholar]
- Moros, M.; Idiago-López, J.; Asín, L.; Moreno-Antolín, E.; Beola, L.; Grazú, V.; Fratila, R.M.; Gutiérrez, L.; de la Fuente, J.M. Triggering antitumoural drug release and gene expression by magnetic hyperthermia. Adv. Drug Deliv. Rev. 2019, 138, 326–343. [Google Scholar] [CrossRef]
- Majidi, S.; Sehrig, F.Z.; Samiei, M.; Milani, M.; Abbasi, E.; Dadashzadeh, K.; Akbarzadeh, A. Magnetic nanoparticles: Applications in gene delivery and gene therapy. J. Mater. Chem. B 2021, 9, 4267–4286. [Google Scholar] [CrossRef] [PubMed]
- Huth, S.; Lausier, J.; Gersting, S.W.; Carsten, R.; Plank Ch Welsch, U.; Rosenecker, J. Insights into the mechanism of magnetofection using PEI-based magnetofectins for gene transfer. J. Gene Med. 2004, 6, 923–936. [Google Scholar] [CrossRef]
- Rarokar, N.; Yadav, S.; Saoji, S.; Bramhe, P.; Agade, R.; Gurav, S.; Khedekar, P.; Subramaniyan, V.; Wong, L.S.; Kumarasamy, V. Magnetic nanosystem a tool for targeted delivery and diagnostic application: Current challenges and recent advancement. Int. J. Pharm. X 2024, 7, 100231. [Google Scholar] [CrossRef]
- Saini, J.; Sharma, P.K. Clinical, Prognostic and therapeutic significance of heat shock proteins in cancer. Curr. Drug Targets 2018, 19, 1478–1490. [Google Scholar] [CrossRef]
- Tang, Q.S.; Zhang, D.S.; Cong, X.M.; Wan, M.L.; Jin, L.Q. Using thermal energy produced by irradiation of Mn-Zn ferrite magnetic nanoparticles (MZF-NPs) for heat-inducible gene expression. Biomaterials 2008, 29, 2673–2679. [Google Scholar] [CrossRef]
- Marinozzi, M.R.; Pandolfi, L.; Malatesta, M.; Colombo, M.; Collico, V.; Lievens, P.M.; Tambalo, S.; Lasconi, C.; Vurro, F.; Boschi, F.; et al. Innovative approach to safely induce controlled lipolysis by superparamagnetic iron oxide nanoparticles-mediated hyperthermic treatment. Int. J. Biochem. Cell Biol. 2017, 93, 62–73. [Google Scholar] [CrossRef]
- Yin, P.T.; Shah, S.; Pasquale, N.J.; Garbuzenko, O.B.; Minko, T.; Lee, K.B. Stem cell-based gene therapy activated using magnetic hyperthermia to enhance the treatment of cancer. Biomaterials 2016, 81, 46–57. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Kim, Y.M.; Song, S.C. Injectable and quadruple-functional hydrogel as an alternative to intravenous delivery for enhanced tumor targeting. ACS Appl. Mater. Interfaces 2019, 11, 34634–34644. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, Y.; Li, Y.; Xie, X.; Wei, X.; Yang, G.; Zhang, H.; Li, N.; Li, T.; Qin, X.; et al. Aptamer-dendrimer functionalized magnetic nano-octahedrons: Theranostic drug/gene delivery platform for near-infrared/magnetic resonance imaging-guided magnetochemotherapy. ACS Nano 2021, 15, 16683–16696. [Google Scholar] [CrossRef] [PubMed]
- Sadhasivam, J.; Sugumaran, A. Magnetic nanocarriers: Emerging tool for the effective targeted treatment of lung cancer. J. Drug Deliv. Sci. Technol. 2020, 55, 101493. [Google Scholar] [CrossRef]
- Wen, C.; Cheng, R.; Gong, T.; Huang, Y.; Li, D.; Zhao, X.; Yu, B.; Su, D.; Song, Z.; Liang, W. β-Cyclodextrin-cholic acid-hyaluronic acid polymer coated Fe3O4-graphene oxide nanohybrids as local chemo-photothermal synergistic agents for enhanced liver tumor therapy. Colloids Surf. B Biointerfaces 2021, 199, 111510. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of iron oxide nanoparticles in cancer therapy: Amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Shang, W.; Sun, X.; Zhao, L.; Wang, J.; Xiong, Z.; Yuan, J.; Zhang, R.; Huang, Q.; Wang, K.; et al. “All-in-One” nanoparticles for trimodality imaging-guided intracellular photo-magnetic hyperthermia therapy under intravenous administration. Adv. Funct. Mater. 2018, 28, 1705710. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Yang, C.; Gong, S.; Jiang, H.; Sun, M.; Qian, C. A pH-sensitive coordination polymer network-based nanoplatform for magnetic resonance imaging-guided cancer chemo-photothermal synergistic therapy. Nanomedicine 2020, 23, 102071. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Liu, X.-L.; Ma, H.; Li, G.; Li, Y.; Gao, F.; Peng, M.; Fan, H.M.; Liang, X.J. Fe3O4–Pd Janus nanoparticles with amplified dual-mode hyperthermia and enhanced ROS generation for breast cancer treatment. Nanoscale Horiz. 2019, 4, 1450–1459. [Google Scholar] [CrossRef]
- Li, Z.; Deng, J.; Sun, J.; Ma, Y. Hyperthermia targeting the tumor microenvironment facilitates immune checkpoint inhibitors. Front. Immunol. 2020, 11, 595207. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, W.; Zhang, J.; Liu, T.; Xing, J.; Zhang, H.; Tang, D. Nanomaterial-based drug delivery systems: A new weapon for cancer immunotherapy. Int. J. Nanomed. 2022, 17, 4677–4696. [Google Scholar] [CrossRef]
- Yuan, A.; Hsiao, Y.J.; Chen, H.Y.; Chen, H.W.; Ho, C.C.; Chen, Y.Y.; Liu, Y.C.; Hong, T.H.; Yu, S.L.; Chen, J.J.W.; et al. Opposite effects of M1 and M2 macrophage subtypes on lung cancer progression. Sci. Rep. 2015, 5, 14273. [Google Scholar] [CrossRef]
- Mills, C.D. Anatomy of a discovery: M1 and M2 macrophages. Front. Immunol. 2015, 6, 212. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, Z.; Duan, Y.; Fu, Z.; Bin, L. Reprogramming tumor microenvironment with photothermal therapy. Bioconjug. Chem. 2020, 31, 1268–1278. [Google Scholar] [CrossRef]
- Pondman, K.; Le Gac, S.; Kishore, U. Nanoparticle-induced immune response: Health risk versus treatment opportunity? Immunobiology 2023, 228, 152317. [Google Scholar] [CrossRef] [PubMed]
- Banda, N.K.; Mehta, G.; Chao, Y.; Wang, G.; Inturi, S.; Fossati-Jimack, L.; Botto, M.; Wu, L.P.; Moghimi, S.M.; Simberg, D. Mechanisms of complement activation by dextran-coated superparamagnetic iron oxide (SPIO) nanoworms in mouse versus human serum. Part Fibre Toxicol. 2014, 11, 64. [Google Scholar] [CrossRef]
- Park, E.J.; Oh, S.Y.; Lee, S.J.; Lee, K.; Kim, Y.; Lee, B.S.; Kim, J.S. Chronic pulmonary accumulation of iron oxide nanoparticles induced Th1-type immune response stimulating the function of antigen-presenting cells. Environ. Res. 2015, 143 Pt A, 138–147. [Google Scholar] [CrossRef]
- Escamilla-Rivera, V.; Uribe-Ramírez, M.; González-Pozos, S.; Lozano, O.; Lucas, S.; De Vizcaya-Ruiz, A. Protein corona acts as a protective shield against Fe3O4-PEG inflammation and ROS-induced toxicity in human macrophages. Toxicol. Lett. 2016, 240, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Mulens-Arias, V.; Rojas, J.M.; Pérez-Yagüe, S.; Morales, M.P.; Barber, D.F. Polyethylenimine-coated SPIONs trigger macrophage activation through TLR-4 signaling and ROS production and modulate podosome dynamics. Biomaterials 2015, 52, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.; Kaesler, S.; Posch, C.; Multhoff, G.; Biedermann, T. Magnetic nanoparticles in theranostics of malignant melanoma. EJNMMI Res. 2021, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.E.A.; Vernice, N.A.; Wagner, R.J.; Fiering, S.N.; Petryk, J.D.; Lowry, G.J.; Tau, S.S.; Yin, J.; Houde, G.R.; Chaudhry, A.S.; et al. Immunogenetic effects of low dose (CEM43 30) magnetic nanoparticle hyperthermia and radiation in melanoma cells. Int. J. Hyperth. 2019, 36 (Suppl. S1), 37–46. [Google Scholar] [CrossRef] [PubMed]
- Moy, A.J.; Tunnell, J.W. Combinatorial immunotherapy and nanoparticle mediated hyperthermia. Adv. Drug Deliv. Rev. 2017, 114, 175–183. [Google Scholar] [CrossRef]
- Stephen, Z.R.; Zhang, M. Recent progress in the synergistic combination of nanoparticle-mediated hyperthermia and immunotherapy for treatment of cancer. Adv. Healthc. Mater. 2021, 10, e2001415. [Google Scholar] [CrossRef]
- Hoopes, P.J.; Wagner, R.J.; Duval, K.; Kang, K.; Gladstone, D.J.; Moodie, K.L.; Crary-Burney, M.; Ariaspulido, H.; Veliz, F.A.; Steinmetz, N.F.; et al. Treatment of canine oral melanoma with nanotechnology-based immunotherapy and radiation. Mol. Pharm. 2018, 15, 3717–3722. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ran, Y.; Wang, Z.; Cheng, J.; Cao, Y.; Yang, C.; Liu, F.; Ran, H. Magnetic-responsive and targeted cancer nanotheranostics by PA/MR bimodal imaging-guided photothermally triggered immunotherapy. Biomaterials 2019, 219, 119370. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006, 5, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, G.; Wang, G.; Wu, L.; Monteiro-Riviere, N.A.; Li, Y. The synergistic strategies for the immuno-oncotherapy with photothermal nanoagents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1717. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwed, M.; Marczak, A. Application of Nanoparticles for Magnetic Hyperthermia for Cancer Treatment—The Current State of Knowledge. Cancers 2024, 16, 1156. https://doi.org/10.3390/cancers16061156
Szwed M, Marczak A. Application of Nanoparticles for Magnetic Hyperthermia for Cancer Treatment—The Current State of Knowledge. Cancers. 2024; 16(6):1156. https://doi.org/10.3390/cancers16061156
Chicago/Turabian StyleSzwed, Marzena, and Agnieszka Marczak. 2024. "Application of Nanoparticles for Magnetic Hyperthermia for Cancer Treatment—The Current State of Knowledge" Cancers 16, no. 6: 1156. https://doi.org/10.3390/cancers16061156
APA StyleSzwed, M., & Marczak, A. (2024). Application of Nanoparticles for Magnetic Hyperthermia for Cancer Treatment—The Current State of Knowledge. Cancers, 16(6), 1156. https://doi.org/10.3390/cancers16061156







