1. Introduction
Cancer is among the leading causes of death worldwide [
1,
2]. Radiotherapy is one of the traditional and promising areas for patients in the treatment of cancer, mainly due to its non-invasive nature and the low incidence of side effects, as well as its suitability for patients who are resistant to current drug therapies [
3,
4]. Immunotherapy plus radiotherapy is also developing rapidly [
5,
6]. However, exposure to ionizing radiation (IR) from radiotherapy is a major concern due to the resultant adverse effects on people’s tissues and functions induced by IR [
7,
8]. Damage induced by ionizing radiation can be caused in two ways. One is direct injury from high-energy radiation, which can cause damage to various biological macromolecules, such as DNA, proteins, and mitochondria. The other type is indirect damage resulting from water ionization, leading to a series of free radical chain reactions, producing various free radicals, such as hydrogen peroxide (H
2O
2), hydroxyl radicals (∙OH), and superoxide anions (O
2•) [
9]. Reactive oxygen species (ROS) can act on the mitochondrial membrane potential, cause changes in cellular physiology and biochemistry, and induce cell apoptosis. The complex reactions of biomolecules caused by these processes lead to widespread damage to normal tissues and functions.
Radiation therapy cures patients, reduces physical pain, and prolongs patient lifespans by applying high-energy radiation to tumors to shrink or remove them and/or to prevent cancer recurrence. However, this treatment method is not specific to cancer cells, which may lead to acute and chronic side effects in adjacent normal organs, including intestinal inflammation, bleeding or diarrhea, tissue fibrosis, secondary cancer, and infertility [
10].
Some endogenous antioxidant enzymes, such as superoxide dismutase, catalase and glutathione peroxidase, have the ability to scavenge ROS and play an important role in protecting mitochondrial function and repairing the DNA damage induced by reactive oxygen species [
10]. Recently, exploring radioprotective agents that can effectively protect against radiation damage has attracted widespread attention. So far, some radioactive protective agents in clinical use have been approved by the Food and Drug Administration (FDA), such as amifostine (WR2721, Cumberland Pharmaceuticals Inc., Nashville, TN, USA), Leukine
® (sargramostim, Sanofi-Aventis, Paris, France), Neulasta
® (pegfilgrastim, Amgen, Thousand Oaks, CA, USA), and Neupogen
® (filgratism, Amgen, Thousand Oaks, CA, USA); among these agents, amifostine is the most widely used [
11]. However, WR2721 has some serious side effects, including hypotension, vomiting, and nausea. In addition to sulfhydryl compounds, other compounds that protect against radiation, such as vitamins, amines, hormones, biological agents, and traditional Chinese medicine, are also the focus of research [
12,
13]. Researchers have recently investigated the efficacy of many natural or synthetic/semisynthetic chemicals to reduce the adverse effects of ionizing radiation, due to the inherent toxicity of some synthetic chemicals at the concentration required for protection against radiation. However, it is necessary to develop radioprotective agents with improved activity and less toxic side effects.
Nitronyl nitroxide radicals (NRs) are stable free radical compounds that have single-spin electrons. NRs are classified into two types: TEMPO (2,2,6,6-tetramethyl piperidine-
N-oxyl) and nitronyl nitroxyl radicals (NITs). In recent years, it has been proven that NRs are effective free radical scavengers and have a certain protective effect against radiation. NRs can react with ROS produced by catalytically ionizing radiation and prevent biomacromolecules and biofilms from being attacked by ROS to protect the body against oxidative damage [
14,
15].
NPs are widely used in the clinic for therapeutic purposes. Sarkar Siddique et al. reviewed the application of nanomaterials in biomedical imaging and cancer therapy [
16,
17]. Therapeutic NPs improve the accumulation and release of pharmacologically active agents at the pathological site, which increases therapeutic efficacy and reduces the incidence and intensity of side effects overall. Poly(lactic-co-glycolic-acid) (PLGA), a biodegradable nanodrug carrier with a high degree of biological safety, is formed by the copolymerization of two monomers, poly(lactic acid) and poly(glycolic acid), in different proportions. It has been recognized as a widely used nanodrug carrier and tissue engineering scaffolding material by the FDA and the European Medicines Agency.
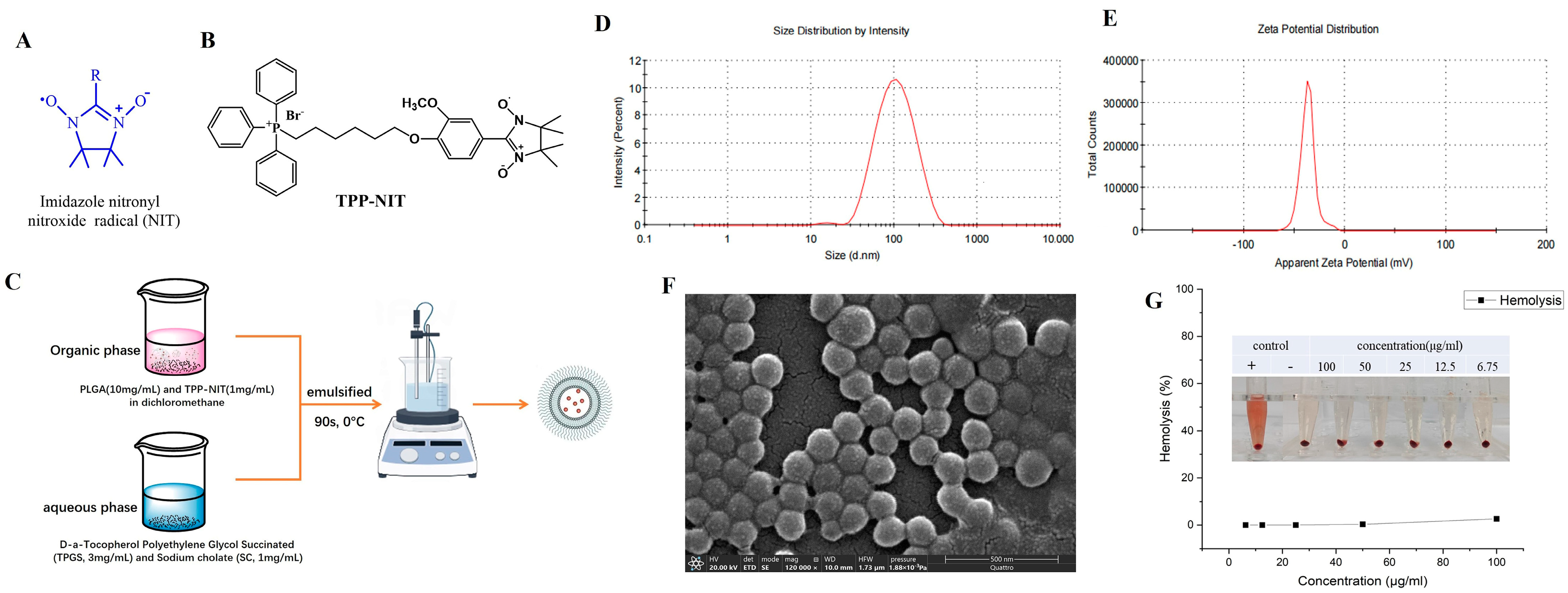
Therefore, with the aim of developing agents that protect against IR more effectively and with less toxicity, we synthesized a new NIT radical derivative with a triphenylphosphine ion (TPP-NIT;
Figure 1B). Furthermore, we used PLGA as a nanocarrier to encapsulate the mitochondrial-targeting NIT radical to form nanoparticles (NPs-TPP-NIT;
Figure 1C). Furthermore, we studied the suppressive effect of NPs-TPP-NIT on the oxidative stress damage in vitro and in vivo. The role of nitronyl nitroxide radical nanoparticles (NPs-TPP-NIT) in the protection of normal cells and healthy tissues from irradiation was studied [
18]. Therefore, in this study, we developed a protocol to apply NPs-TPP-NIT as radiotherapy in order to reduce the side effects of X-radiation on healthy tissues and organs.
2. Materials and Methods
2.1. Preparation and Characterization of NPs-TPP-NIT
2.1.1. Chemical Materials
The (4-hydroxy-3-methoxyphenyl)-4,4,5,5-tetramethyl-4,5-dihydro imidazole radical (TPP-NIT) was synthesized in our laboratory [
19]. Amifostine (WR2721) was purchased from MedChemExpress LLC. (Shanghai, China). We also used 6-bromohexyl (triphenyl) phosphanium (CAS No. 83152-22-1) Jskchem Ltd. (Shanghai, China). The other chemical reagents, including K
2CO
3, CH
2Cl
2, and KBr, were also purchased from Aladdin Ltd. (Shanghai, China). CH
2Cl
2 was distilled in the presence of CaH
2, and THF was distilled with sodium/benzophenone under nitrogen protection. All chemicals were of commercial analytical reagent grade.
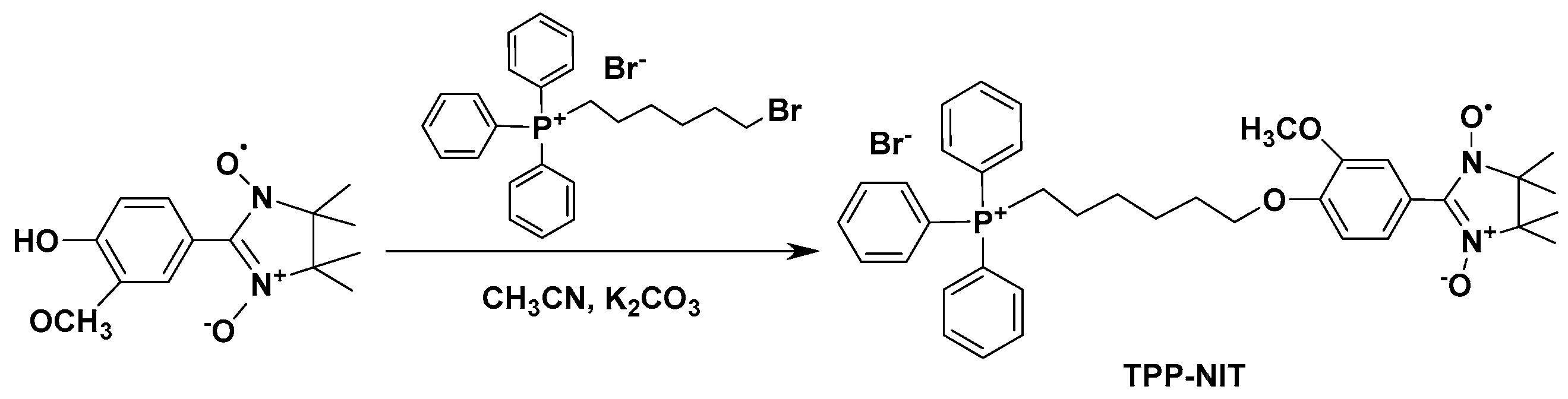
2.1.2. Synthesis of (4-(6-(Triphenylphosphonio)hexyl)oxy)phenyl)-3-methoxyphenyl)-4,4,5,5-tetramethyl-4,5-dihydro Imidazole Radical (TPP-NIT)
The synthetic route for the synthesis of the (4-(6-(triphenylphosphonio)hexyl)oxy)phenyl)-3-methoxyphenyl)-4,4,5,5-tetramethyl-4,5-dihydro imidazole radical (TPP-NIT) is shown in
Scheme 1 (the complete synthesis method is shown in
Supplementary Scheme S1). A total of 0.199 g (0.8 mmol) of the (4-hydroxy-3-methoxyphenyl)-4,4,5,5-tetramethyl-4,5-dihydro imidazole radical (see
Supplementary Figures S1 and S2), 0.28 g (1.0 mmol) of 6-bromohexyl(triphenyl) phosphanium, and 0.138 g K
2CO
3 (1.0 mmol) were all added to 30 mL of acetonitrile and stirred at 60 °C. The reaction process was monitored via thin-layer chromatography (TLC). After reacting for approximately 8 h, the mixture was infiltrated to remove the precipitate, and a crude blue liquid was obtained. Using column chromatography, the crude blue liquid was purified (dichloromethane:methanol = 12:1) to obtain a dark blue oil (TPP-NIT) with a yield of 72.5%. IR(KBr): ν 1357, 1265 (N-O), 1114 (C-O) cm
−1 (see
Supplementary Figure S3). HRMS (ESI):
m/
z [M-Br]
+ calcd for C
28H
28ClN
4O
2: 624.3111, found: 624.3101 (see
Supplementary Figure S4).
2.1.3. Preparation of Nanoparticles (NPs-TPP-NIT)
The preparation of nanoparticles is shown in
Figure 1C. TPP-NIT was encapsulated in PLGA using a modified emulsion solvent evaporation method. In brief, the organic phase was composed of PLGA (10 mg/mL; 50:50; CAS number: 34346-01-5, Sigma Aldrich, St Louis, MO, USA), TPP-NIT (1 mg/mL, synthesized by our laboratory, supporting information) and dichloromethane (DCM). The water phase included sodium cholate (SC; 1 mg/mL; CAS number: 361-09-1, TCI, Shanghai, China) and D-α-tocopherol polyethylene glycol succinate (TPGS; 3 mg/mL; CAS number: 9002-96-4, Sigma Aldrich, St Louis, MO, USA). The organic phase and aqueous phase of the above composition were mixed and emulsified in an ice bath for 90 s. Then, the mixture was stirred using a magnetic stirrer for 5 h to allow the solvent to continuously evaporate, thereby removing organic solvents and obtaining nanodispersions. Then, the nanodispersion was added to centrifugal filters with a molecular weight limit of 10 kD and collected via centrifugation. Finally, the nanosuspension was redispersed in double-distilled water (ddH
2O) to obtain the nanoparticles (NPs-TPP-NIT).
2.1.4. Characterization of NPs-TPP-NIT
Using a Nano ZSP Zetasizer (Malvern Instruments, Malvern, UK), dynamic light scattering (DLS) was carried out to measure the particle size, polydispersity index (PDI), and ζ electric potential at room temperature. The NP surface morphology was characterized using a scanning electron microscope (SEM—Quattro S; Thermo Fisher, Brno-Černovice, Czechia). The NP encapsulation efficiency (EE) was measured using a high-performance liquid chromatography system (Agilent Technologies, Inc., Santa Clara, CA, USA) with a reverse-phase C18 column (F90104; 4.6 × 250 mm; Capcell Pak MG, Beijing, China). The NP encapsulation efficiency (EE) and drug loading were measured using a high-performance liquid chromatography system (Agilent Technologies, Inc., Santa Clara, CA, USA) with a reverse-phase C18 column (F90104; 4.6 × 250 mm; Capcell Pak MG, Beijing, China) in triplicate. The hemolytic activity of NPs was measured on a spectrophotometer (WD-2102B; Beijing Liuyi Biotechnology, Beijing, China). To clarify the biological distribution of NPs, the nanoparticles labeled with phthalocyanine 5.5 (Cy5.5; HY-D0924; MedChem Express, Monmouth Junction, NJ, USA) were prepared using the method used for the PLGA-encapsulated TPP-NIT, except TPP-NIT was replaced with Cy5.5.
2.2. Cells
The human normal hepatocytes cell line (L-02), human liver cancer cells (HEPG2), human gastric epithelial cells (GES), human moderately well-differentiated gastric cancer cells (MKN28), and human poorly differentiated gastric cancer cells (MKN45) were provided by the Department of Chemistry, the Air Force Medical University (No. 169, Changle West Road, Xi’an, Shaanxi 710032, China). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) at 37 °C and a humidity of 5% CO2 supplemented with 10% fetal bovine serum, 50 μg/mL streptomycin, and 10,000 units/mL penicillin.
2.3. Animals
BALB/c male mice (weight, 18–22 g; SPF grade) were provided by the Center for Experimental Animals at the Fourth Military Medical University. The mice were kept in a constant environment. The temperature was maintained at 23 ± 2 °C, the relative humidity was 60 ± 5%, and the light conditions consisted of a 12 h light/12 h dark cycle. The mice were allowed free access to tap water and pellet food. The number of animals used and the pain experienced by the animals was minimized. Animal experiments were approved by the Animal Care and Use Committee of the AFMU (Certificate No. KY20194116) following the Guidelines for the Care and Use of Laboratory Animals in China.
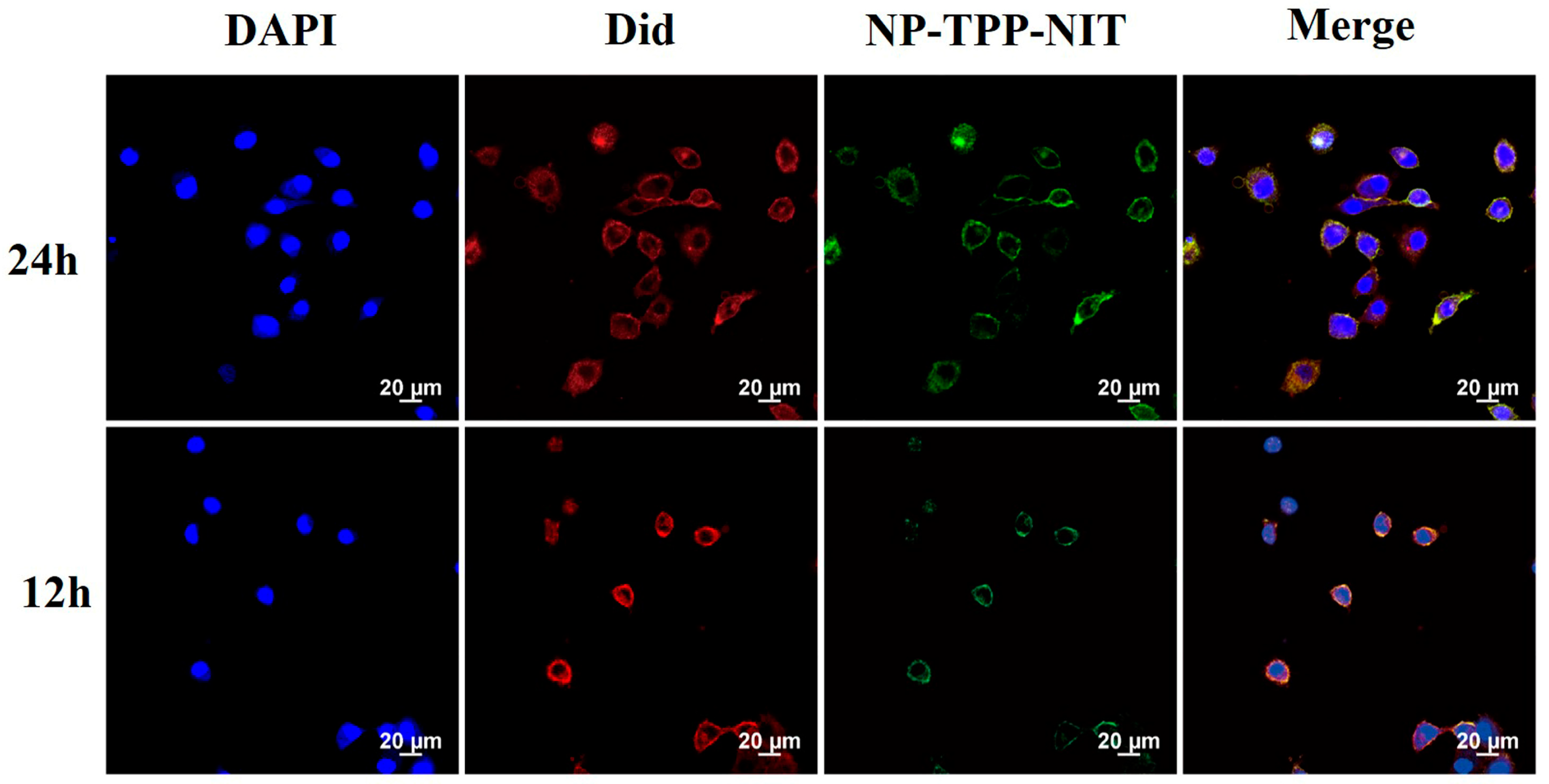
2.4. Cell Uptake and Distribution In Vivo of NPs-TPP-NIT
L-02 cells were inoculated into a 6 cm culture dish and cultured for 24 h. Then, 1 mg/mL Cy5.5 labeled nanoparticles were dissolved in 10 mL of 1640 complete culture medium (serum-free) and the L-02 cells were incubated with the above nanoparticle suspension for 12 and 24 h. The cells were digested with trypsin after incubation. Then, the cells were inoculated into a 20 mm confocal culture dish. After the cells adhered to the wall and grew for 12 h, they were washed with PBS 4 times, and the culture medium was removed. Afterwards, PBS was absorbed and 480 mL of DiD working solution (C1995s, Beyotime, Haimen, China) was added and incubated at 37 °C for 15 min. The working solution was removed and the cells were deeply washed 4 times with PBS. Then, cells were fixed using a 4% paraformaldehyde fixative for 15 min. After washing with PBS three times, 250 μL of DAPI was added and incubated for 15 min. The DAPI (DAPI; C10012, Beyotime, Haimen, China) was then removed and fixed with paraformaldehyde for 10 min for observation under an Olympus DP80 fluorescence microscope. Sample images were observed at excitation wavelengths of 644 nm and emission wavelengths of 655 nm.
The Cy5.5-labeled NPs (10 mg/kg−1 in 200 μL PBS) were administered to male BALB/c mice (body weight, 18–22 g; specific-pathogen-free (SPF) grade) intravenously (i.v.). Then, at 0, 1, 6, 12, 24, and 48 h, primary organs, including the spleens, livers, lungs, and kidneys, were harvested from the sacrificed mice. We used a Kodak in vivo imaging system to image Cy5.5 fluorescence in these organs (IVIS Spectrum; PerkinElmer Corp., Waltham, MA, USA).
2.5. Cytotoxicity Assay
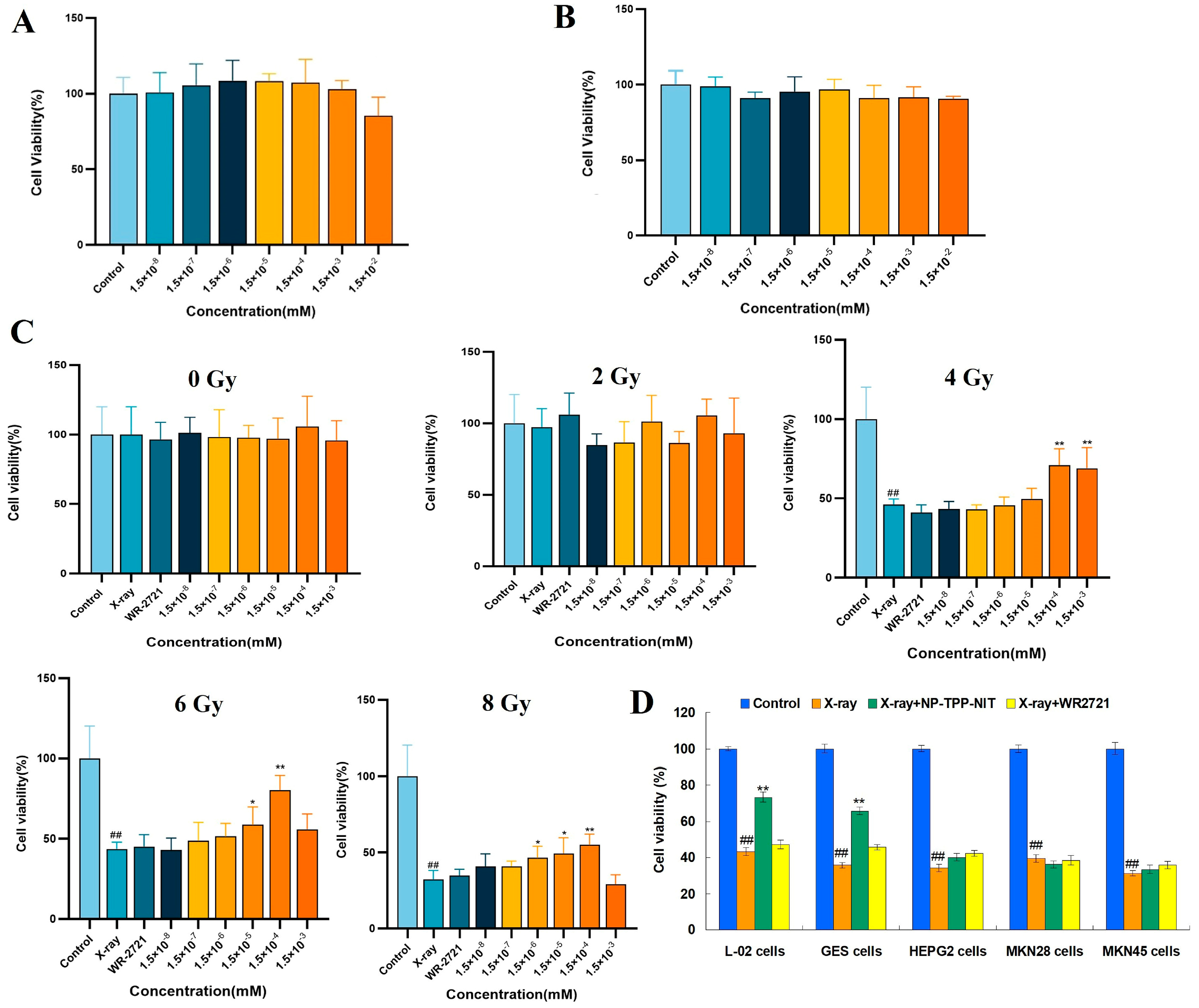
The cell counting kit 8 (CCK-8) method was conducted to perform the cytotoxicity testing. L-02 cells were cultured in DMEM/F12 containing a 10% fetal bovine serum until the logarithmic growth phase was reached; then, they were digested by 0.25% trypsin. Cells were inoculated into a 96-well plate (1 × 104 cells/well). Then, the cells were cultured under suitable conditions for 24 h at 37 °C in a humidified atmosphere with 5% CO2. Amifostine (WR2721) or NPs-TPP-NIT were dissolved in 1640 complete culture medium at the final concentrations of 1.5 × 10−8, 1.5 × 10−7, 1.5 × 10−6, 1.5 × 10−5, 1.5 × 10−4, 1.5 × 10−3, and 1.5 × 10−2 mM. The cells were cultivated with these solutions at 100 μL per well in a 96-well plate for 24 h. Each concentration had 5 duplicate wells in one plate.
2.6. Protective Effects of NPs-TPP-NIT on L-02 Cells after X-ray Irradiation
The protective effect of NPs-TPP-NIT on radiation damage in L-02 cells was measured using the CCK-8 method. The L-02 single-cell suspension was diluted to a cell concentration of 5000 cells/mL using a complete culture medium containing 20% fetal bovine serum 1640; then, 100 μL of the suspension per well was added to the cells and the cells were cultured for 24 h in a humidified atmosphere with 5% CO2 at 37 °C. Afterward, the culture medium was removed and 100 μL of the NP-TPP-NIT solution at different concentrations (1.5 × 10−8, 1.5 × 10−7, 1.5 × 10−6, 1.5 × 10−5, 1.5 × 10−4, and 1.5 × 10−3 mM) was added, and the concentration of WR2721 was 1.5 × 10−3 mM. After incubation for 2 h, the cells were irradiated with 2 Gy, 4 Gy, 6 Gy, and 8 Gy X-rays, respectively. The dosage rate of the X-ray irradiation was approximately 1.414 Gy·min−1. The cells continued to be cultured for 72 h after irradiation. Then, the cells were incubated with 100 µL of 10% CCK-8 for 1 h. Using PBS as a control, the optical density (OD) at 570 nm was determined using a spectrophotometer. Five replicates were used for each concentration. In addition, the influence of NPs-TPP-NIT on the cell viability of GES, HEPG2, MKN28, and MKN45 cells after 6 Gy X-ray irradiation was estimated using the CCK-8 method. The pretreatment method of NPs-TPP-NIT (0.15 µM) and WR2721 (1.5 µM) on these cells was the same as the L-02 cell culture method mentioned above. After 2 h of treatment, the cells were exposed to a dose of 6 Gy X-ray radiation. Then, the cell viability was determined using the method mentioned. Five replicates were used for each concentration.
2.7. Cell Apoptosis
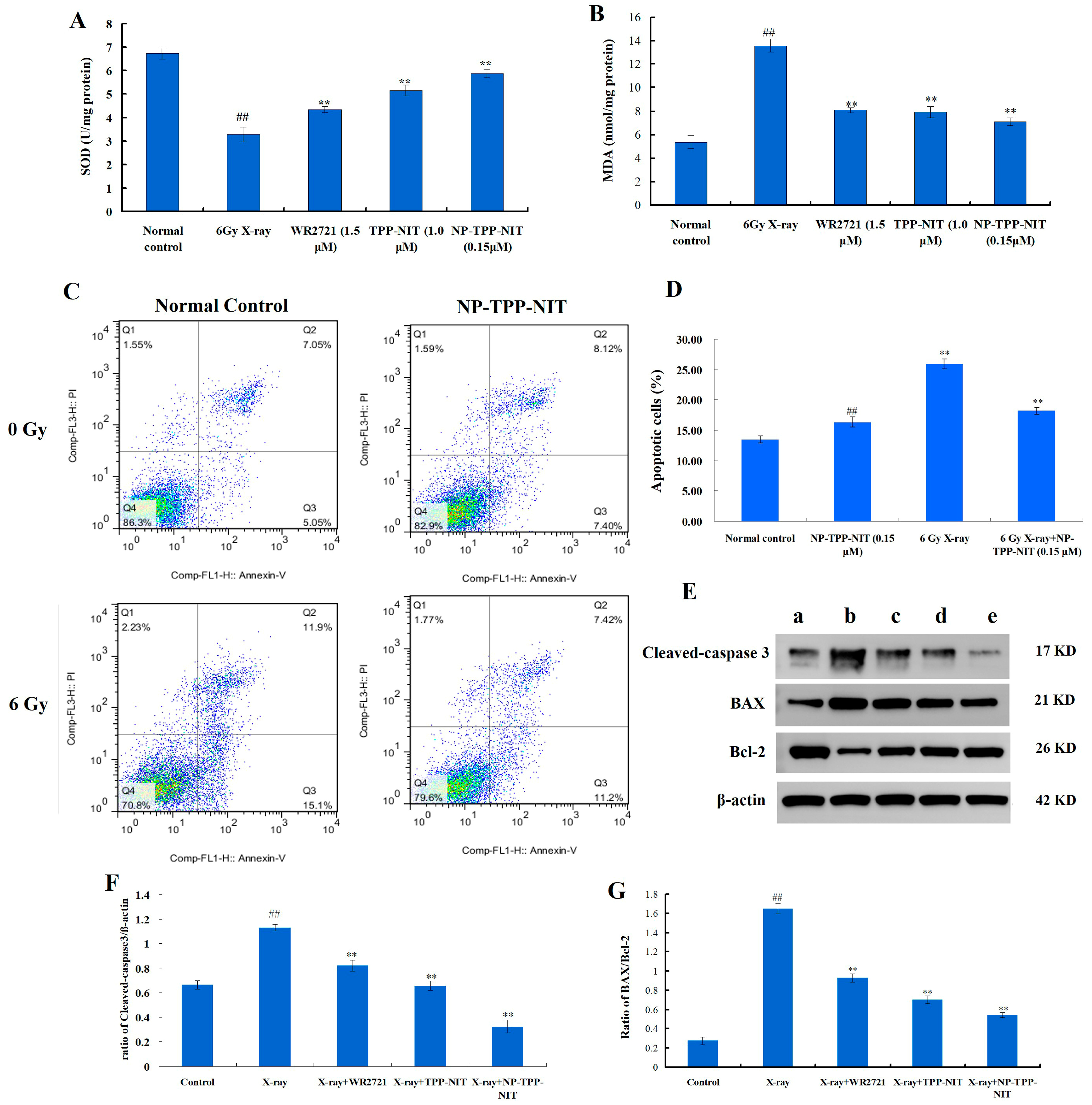
In 6-well plates, L-02 cells at a concentration of 5 × 103 cells per well were allowed to attach for 24 h. Before being exposed to 6 Gy X-ray irradiation (1.414 Gy·min−1), the cells were treated with a 0.15 µM NP-TPP-NIT solution for 1 h. After 0.5 h of X-ray irradiation, the cells were washed with PBS three times and then incubated in DMEM culture for 24 h. Then, the apoptosis rate of L-02 cells was determined by a FACS flow cytometer, using the annexin V-fluorescein isothiocyanate (annexin V-FITC) apoptosis detection assay kit (Beyotime Biotech Inc., Shanghai, China).
2.8. Western Blot Analysis
We used the BCA assay kit to determine the protein concentrations and separated proteins of total extracts on 10% SDS gels (W003-1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Separated proteins were electrophoretically transferred onto polyvinylidene difluoride membrane (PVDF) (WGPVDF45, Servicebio, Wuhan, China) and blocked with 5% non-fat milk in phosphate-buffered saline (PBS)-Tween for 2 h. The primary antibodies were polyclonal rabbit anti-Bcl-2 (1:1000, GB124830, Servicebio, China), anti-BAX (1:1000, GB12690, Servicebio, China), anti-cleaved-caspase 3 (1:1000, GB11767C, Servicebio, China) and β-actin (1:2000, GB11001, Servicebio, China). They were incubated with the membranes at 4 °C overnight. The membranes were incubated with a secondary antibody conjugated to horseradish peroxidase (Beyotime, Shanghai, China) after being washed with TBST three times for 10 min each time. Bands were visualized using an ECL system (G2014-50ML, Servicebio, China). The ChemiDoc-It2 610 imaging system (UVP, LLC, Upland, CA, USA) was used to conduct the visualization, and Image-Pro Plus 6.0 (Media Cybernetics, Inc., Bethesda, MD, USA) was used for quantification. All experiments were independently replicated three times.
2.9. In Vivo Toxicity Assay of NPs-TPP-NIT
The BALB/c male mice were assigned to 4 groups (6 mice in each group): (i) control group, (ii) 7-day NP-TPP-NIT, (iii) 14-day control, and (iv) 14-day NP-TPP-NIT. The mice in groups (ii) and (iv) were treated with NPs-TPP-NIT (5 mg/kg) through intraperitoneal injection once a day, for either 7 days or 14 days consecutively. After 7 or 14 days of administration, blood samples from each group of mice were collected for blood biochemical examination, and major organs including the heart, liver, spleen, lungs, and kidneys were collected for histopathological examination.
(1) Hematology and blood biochemistry examinations. Approximately 100 µL of blood was collected through the eye socket and was maintained at 4 °C for 4 h. The blood sample was centrifuged at 1500 rpm for 10 min and tested for alanine aminotransferase (ALT), aspartate aminotransferase, total protein (TP), blood urea nitrogen (BUN), and albumin (ALB).
(2) Histopathology examinations. The main organs of the mice were fixed with formaldehyde (4%) and processed into paraffin blocks. After staining with hematoxylin and eosin (H&E), the slides were imaged using a fluorescence inverted microscope.
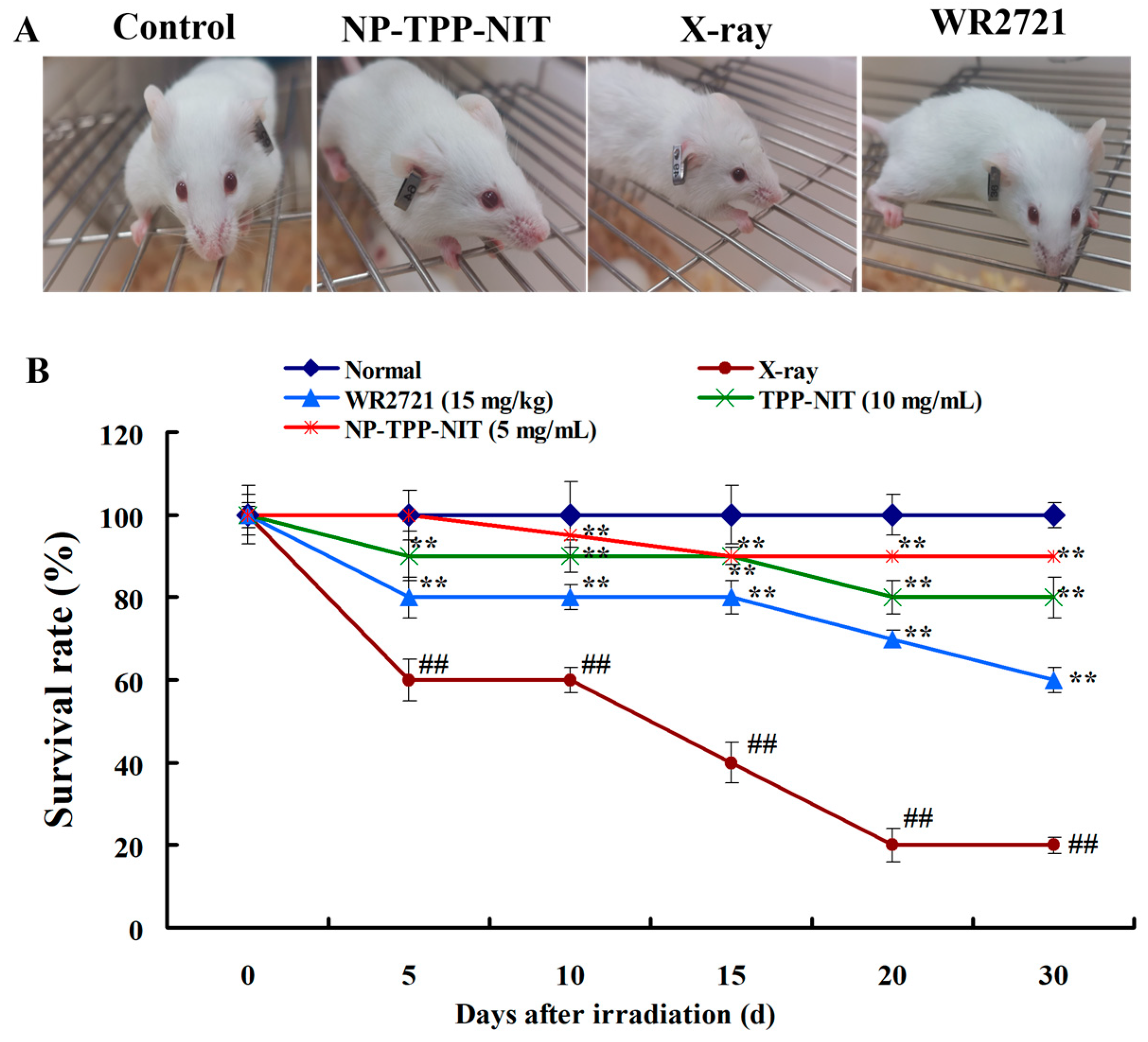
2.10. Survival Assays In Vivo
Male mice were randomly divided into a normal group, an IR exposure group, and an NP-TPP-NIT (5 mg/kg) pre-treatment group (n = 10). A total of 30 min prior to irradiation exposure, the drug solution was administered to mice via intravenous injection. The mice in the normal group and the IR group were given an equal amount of distilled water. Thirty minutes after administration, all mice except for those in the normal group received a single dose of 6-Gy X-ray total body irradiation at the Radiation Center of AFMU, with an irradiation duration of 8 min and a dose rate of 1.414 Gy·min−1. The overall health status, including fur condition, behavior, food intake, and bowel movements, was measured. The survival rates of mice in different groups were observed over 30 days. In addition, the protective effect of NPs-TPP-NIT on the main organs of mice irradiated with 6 Gy X-ray was also observed on the 7th day. The organs of the 6 Gy X-ray group and the 6 Gy X-ray + NP-TPP-NIT group were fixed with 10% (v/v) buffered formalin for 24 h, dehydrated in ethanol, and embedded in paraffin. Next, the organs were sliced into 5 µm thick sections, stained with hematoxylin and eosin (H&E), and observed under an Olympus BX41 microscope (Olympus, Tokyo, Japan). The hematological examination experiment was conducted as follows: on the 7th and 14th days after irradiation, approximately 100 µL of the blood from the eyes of mice from different groups (n = 6) was collected into anticoagulant tubes (potassium EDTA) for hematological examinations to estimate the effect of NPs-TPP-NIT on the level of WBCs, RBCs and PLTs of irradiated mice.
2.11. Statistical Analysis
The significant differences between the groups were determined by analyzing data using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). The mean standard deviation was used to represent the experimental results. In addition, one-way analysis of variance was also tested, as well as the Tukey multiple comparison test using the least significant difference (LSD) test. In all cases, p < 0.05 indicates a significant difference.
4. Discussion
Radiotherapy is one of the traditional and promising treatments for patients with cancer, but it may also cause severe side effects [
3,
4]. Exposure to IR is known to result in organ injury. Radiotherapy using ionizing radiation is becoming increasingly widespread, and the associated side effects experienced by organisms have also become a problem worth studying. The development of radioprotective agents has been a focal point for a long time. Direct or indirect irradiation (through the radiolysis of water-producing reactive oxygen species, ROS) interacts with macromolecules (such as DNA, lipids, and proteins) causing oxidative damage to tissues, and finally, organ dysfunction, through a series of biochemical and physical disturbances [
20]. This disturbance is observed in terms of the increased production of ROS leading to lipid peroxidation, the oxidation of DNA and proteins, as well as the activation of pro-inflammatory factors that subsequently lead to the occurrence of diseases [
21,
22]. Thus, the search for radioprotectors to mitigate radiation damage is necessary and will be of extensive use in radiotherapy [
23].
To date, the FDA has approved some radioprotective drugs for clinical use, such as amifostine (WR2721), filgrastim, pegfilgrastim, and sargramostim [
24]. WR2721 is an organic thiophosphate that can be metabolized to generate compounds containing sulfhydryl groups. The thiol metabolites of WR2721 can clear ROS, which allows WR2721 to selectively protect normal tissues from acute and late radiation damage, but it does not have a protective effect on tumor tissues [
25]. However, the side effects of WR2721 seriously limit its clinical use [
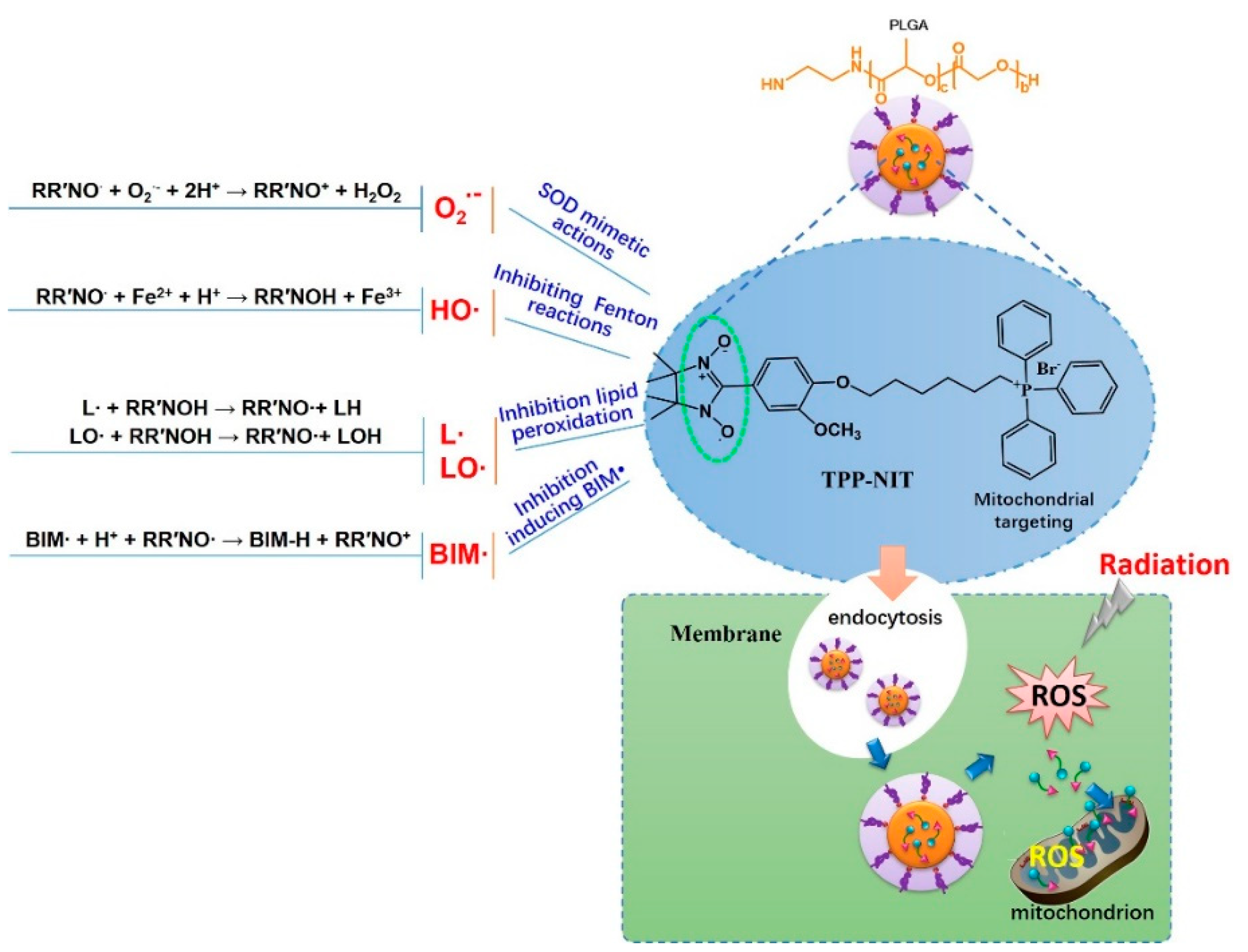
26]. Imidazole nitronyl nitroxide radicals (NITs,
Figure 1A) are new and efficient scavengers that have been developed in recent years. They have been shown to reduce oxidative damage in some experimental models. This is due to their imidazole ring nitrogen atom and the oxygen atom bonded to it, which can receive or contribute one to two electrons, causing them to transition between three different chemical structures: nitrogen oxide radicals, reduced hydroxylamines, and oxidized ammonium oxide cations (
Figure 8). NIT has a similar free-radical scavenging effect to endogenous SOD, and it is not consumed during the decomposition of O
2ˉ
· into O
2 and H
2O
2. It is also known as a catalytic free-radical scavenger [
27]. NITs can react with the membrane lipid free radicals L∙ and LO∙ to protect the cell membrane, mitochondrial membrane, and other biomembranes [
28]. NIT can also prevent Fenton reactions via accepting electrons from reducing metal complexes, thereby inhibiting the generation of ·OH radicals.
The nano-delivery systems developed in the past decade have obvious advantages, such as significantly improving the stability of drugs in the body, prolonging the cycle and metabolism time of drugs, increasing the sustained action time of drugs against damage, and reducing the dosage of drugs used. For example, Nguyen et al. prepared a stable nanomelanin and found that X-rays only caused mild splenic fibrosis in 40% of nanomelanin-protected mice, whereas severe fibrosis was observed in 100% of mice treated with X-rays alone [
29]. The nanomelanin also partly rescued the volume and weight of mouse spleens from irradiation by promoting the transcription levels of splenic interleukin 2 (IL-2) and tumor necrosis factor alpha (TNF-α). In this study, we successfully loaded a TPP-NIT onto PLGA and obtained nanoscale particleswith the size range of 80–200 nm (
Figure 1D). This increased the effective distribution in the absorption of radiation, and provided excellent antioxidant activity for nitronyl nitroxide radicals to scavenge ROS generated during and after X-ray irradiation. These characteristics of NPs-TPP-NIT may have contributed to the partial protection of mouse spleens from X-ray irradiation attack.
Although our research demonstrated that NPs-TPP-NIT have a protective effect in reducing IR-induced damage, there are still some limitations. For example, the relationships between the protective effect of NPs-TPP-NIT and the IR-induced redox imbalance and the oxidative damage and apoptosis signaling pathways remain to be explored. Furthermore, more in vivo tumor models need to verify the ability of NPs-TPP-NIT to confer protection against radiation in addition to the safety of radiotherapy treatment.