Splenectomy as Part of Maximal-Effort Cytoreductive Surgery in Advanced Epithelial Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Definitions
Evaluated Outcomes
2.3. Statistical Analysis
3. Results
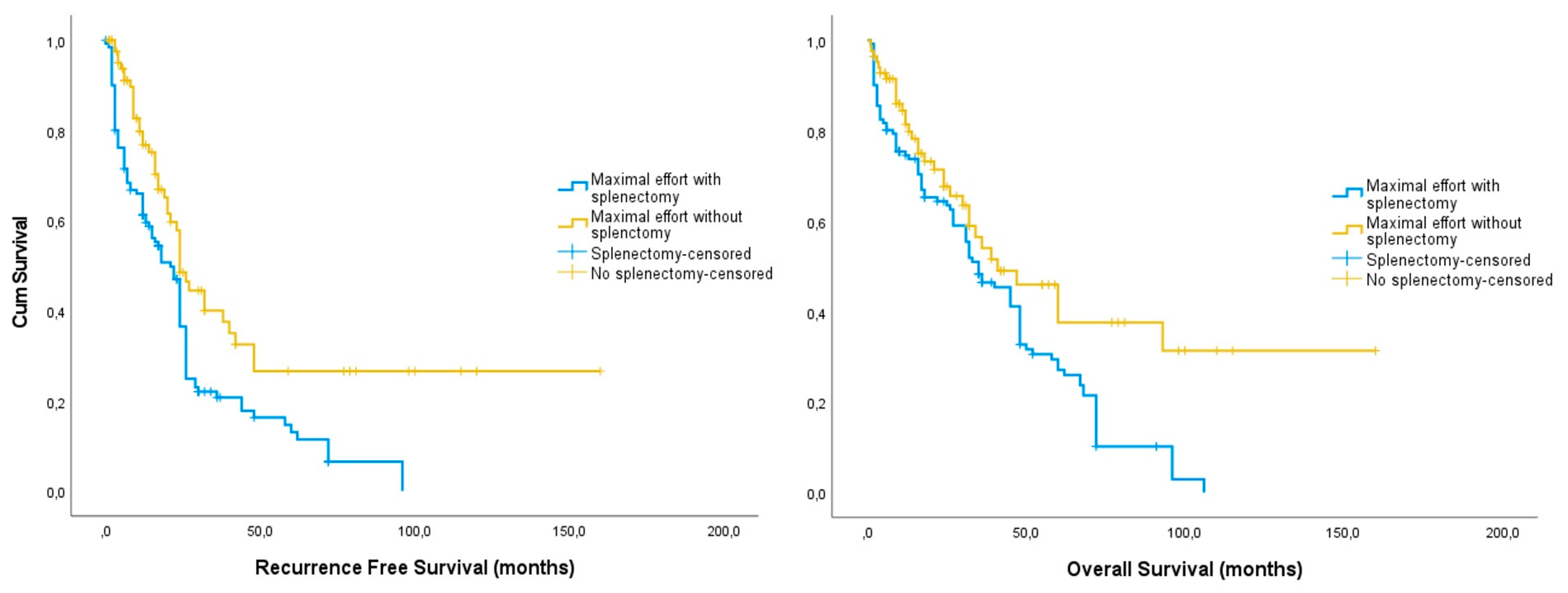
3.1. Whole Cohort
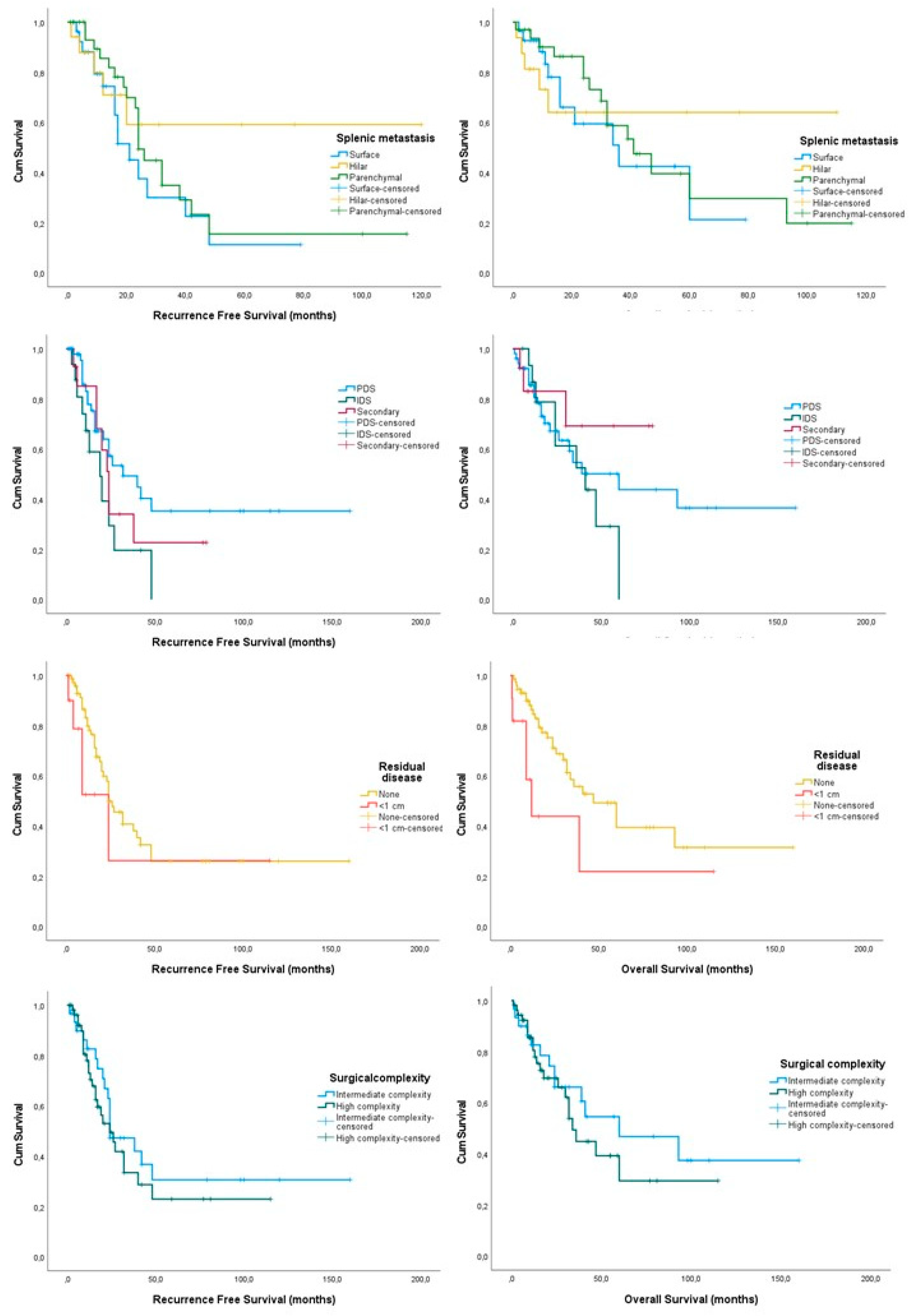
3.2. Splenectomy Cases Only
4. Discussion
4.1. Study Findings
4.2. Comparison with Existing Knowledge
4.3. Strengths and Limitations
4.4. Implications and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Glob. Cancer Stat. 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- Gaona-Luviano, P.; Medina-Gaona, L.A.; Magaña-Pérez, K. Epidemiology of ovarian cancer. Chin. Clin. Oncol. 2020, 9, 47. [Google Scholar] [CrossRef]
- Dilley, J.; Burnell, M.; Gentry-Maharaj, A.; Ryan, A.; Neophytou, C.; Apostolidou, S.; Karpinskyj, C.; Kalsi, J.; Mould, T.; Woolas, R.; et al. Ovarian cancer symptoms, routes to diagnosis and survival—Population cohort study in the ‘no screen’ arm of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Gynecol. Oncol. 2020, 158, 316–322. [Google Scholar] [CrossRef]
- Matz, M.; Coleman, M.P.; Sant, M.; Chirlaque, M.D.; Visser, O.; Gore, M.; Allemani, C. The histology of ovarian cancer: Worldwide distribution and implications for international survival comparisons (CONCORD-2). Gynecol. Oncol. 2017, 144, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Sessa, C.; Bois, A.D.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Greer, B.E.; Swensen, R.E.; Gray, H.J. Surgery for ovarian cancer: Rationale and guidelines. J. Natl. Compr. Cancer Netw. 2004, 2, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Ferrandina, G.; Vizzielli, G.; Fanfani, F.; Gallotta, V.; Chiantera, V.; Costantini, B.; Margariti, P.A.; Alletti, S.G.; Cosentino, F.; et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur. J. Cancer 2016, 59, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Coens, C.; Nankivell, M.; Kristensen, G.B.; Parmar, M.K.B.; Ehlen, T.; Jayson, G.C.; Johnson, N.; Swart, A.M.; Verheijen, R.; et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: Pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018, 19, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.; Nicolais, O.; Shahin, M. Surgery in Advanced Ovary Cancer: Primary versus Interval Cytoreduction. Diagnostics 2022, 12, 988. [Google Scholar] [CrossRef]
- Al Rawahi, T.; Lopes, A.D.; Bristow, R.E.; Bryant, A.; Elattar, A.; Chattopadhyay, S.; Galaal, K. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst. Rev. 2013, 2013, Cd008765. [Google Scholar] [CrossRef] [PubMed]
- Lyons, Y.A.; Reyes, H.E.; McDonald, M.E.; Newtson, A.; Devor, E.; Bender, D.P.; Goodheart, M.J.; Bosquet, J.G. Interval debulking surgery is not worth the wait: A National Cancer Database study comparing primary cytoreductive surgery versus neoadjuvant chemotherapy. Gynecol. Oncol. 2019, 154, e5–e6. [Google Scholar] [CrossRef]
- Haidopoulos, D.; Pergialiotis, V.; Zachariou, E.; Sapantzoglou, I.; Thomakos, N.; Stamatakis, E.; Alexakis, N. Maximal Effort Cytoreduction in Epithelial Ovarian Cancer: Perioperative Complications and Survival Outcomes from a Retrospective Cohort. J. Clin. Med. 2023, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.; Singh, K.; Pounds, R.; Sundar, S.; Kehoe, S.; Nevin, J.; Elattar, A.; Balega, J. Predictive value of the age-adjusted Charlston co-morbidity index on peri-operative complications, adjuvant chemotherapy usage and survival in patients undergoing debulking surgery after neo-adjuvant chemotherapy for advanced epithelial ovarian cancer. J. Obstet. Gynaecol. 2017, 37, 1070–1075. [Google Scholar] [CrossRef]
- El Hajj, H.; Ferraioli, D.; Meus, P.; Beurrier, F.; Tredan, O.; Ray-Coquard, I.; Chopin, N. Splenectomy in epithelial ovarian cancer surgery. Int. J. Gynecol. Cancer 2023, 33, 944–950. [Google Scholar] [CrossRef]
- Sun, H.; Bi, X.; Cao, D.; Yang, J.; Wu, M.; Pan, L.; Huang, H.; Chen, G.; Shen, K. Splenectomy during cytoreductive surgery in epithelial ovarian cancer. Cancer Manag. Res. 2018, 10, 3473–3482. [Google Scholar] [CrossRef]
- Said, S.A.; van der Aa, M.A.; Veldmate, G.; de Hullu, J.A.; van Altena, A.M. Oncologic outcomes after splenectomy during initial cytoreductive surgery in advanced epithelial ovarian cancer: A nationwide population-based cohort study. Acta Obstet. Gynecol. Scand. 2022, 101, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Edgren, G.; Almqvist, R.; Hartman, M.; Utter, G.H. Splenectomy and the Risk of Sepsis: A Population-Based Cohort Study. Ann. Surg. 2014, 260, 1081–1087. [Google Scholar] [CrossRef]
- Camejo, L.; Nandeesha, N.; Phan, K.; Chharath, K.; Tran, T.; Ciesla, D.; Velanovich, V. Infectious outcomes after splenectomy for trauma, splenectomy for disease and splenectomy with distal pancreatectomy. Langenbecks Arch. Surg. 2022, 407, 1685–1691. [Google Scholar] [CrossRef]
- Aletti, G.D.; Dowdy, S.C.; Gostout, B.S.; Jones, M.B.; Stanhope, R.C.; Wilson, T.O.; Podratz, K.C.; Cliby, W.A. Quality improvement in the surgical approach to advanced ovarian cancer: The Mayo Clinic experience. J. Am. Coll. Surg. 2009, 208, 614–620. [Google Scholar] [CrossRef]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 61–85. [Google Scholar] [CrossRef]
- Chang, S.J.; Son, J.H.; Shim, J.H.; Kim, J.; Kim, S.; Kong, T.W.; Paek, J.; Ryu, H.S. Prognostic analysis of splenic parenchymal metastasis in advanced ovarian cancer: Does parenchymal metastasis matter? Gynecol. Oncol. 2020, 159, 91–92. [Google Scholar] [CrossRef]
- Ayhan, A.; Kucukyildiz, I.; Akilli, H.; Gunakan, E.; Serbetcioglu, G.C.; Haberal, N. What is the Effect of Splenic Involvement Sites in Advanced Ovarian, Tubal and Peritoneal Epithelial Cancer on Overall Survival? Arch. Gynecol. Obstet. 2021. under review. [Google Scholar] [CrossRef]
- Aguilar-Nascimento, J.E.; Zampieri-Filho, J.P.; Bordin, J.O. Implications of perioperative allogeneic red blood cell transfusion on the immune-inflammatory response. Hematol. Transfus. Cell Ther. 2021, 43, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Fragkou, P.C.; Torrance, H.D.; Pearse, R.M.; Ackland, G.L.; Prowle, J.R.; Owen, H.C.; Hinds, C.J.; O’Dwyer, M.J. Perioperative blood transfusion is associated with a gene transcription profile characteristic of immunosuppression: A prospective cohort study. Crit. Care 2014, 18, 541. [Google Scholar] [CrossRef]
- Prescott, L.S.; Vergote, I.; Sun, C.C.; Bodurka, D.C.; Coleman, R.L. Transfusion use and effect on progression-free, overall survival, and quality of life in upfront treatment of advanced epithelial ovarian cancer: Evaluation of the European Organization for Research and Treatment EORTC-55971 Cohort. Int. J. Gynecol. Cancer 2023, 33, 1–9. [Google Scholar] [CrossRef]
- Sehouli, J.; Könsgen, D.; Mustea, A.; Oskay-Ozcelik, G.; Katsares, I.; Weidemann, H.; Lichtenegger, W. “IMO”—Intraoperative mapping of ovarian cancer. Zentralblatt Gynakol. 2003, 125, 129–135. [Google Scholar]
- Zapardiel, I.; Peiretti, M.; Zanagnolo, V.; Biffi, R.; Bocciolone, L.; Landoni, F.; Aletti, G.; Colombo, N.; Maggioni, A. Splenectomy as part of primary cytoreductive surgery for advanced ovarian cancer: A retrospective cohort study. Int. J. Gynecol. Cancer 2012, 22, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Bacalbasa, N.; Balescu, I.; Dima, S.; Brasoveanu, V.; Popescu, I. Splenectomy as Part of Cytoreductive Surgery in Recurrent Epithelial Ovarian Cancer. Anticancer. Res. 2015, 35, 5097–5101. [Google Scholar]
- Rush, S.K.; Lees, B.F.; Huang, D.S.; Peterson, M.F.; Al-Niaimi, A. Splenectomy at the time of primary or interval cytoreductive surgery for epithelial ovarian carcinoma: A review of outcomes. Gynecol. Oncol. 2022, 167, 283–288. [Google Scholar] [CrossRef]
- Sonoda, K.; Izumi, K.; Matsui, Y.; Inomata, M.; Shiraishi, N.; Kitano, S. Decreased growth rate of lung metastatic lesions after splenectomy in mice. Eur. Surg. Res. 2006, 38, 469–475. [Google Scholar] [CrossRef]
- Sato, N.; Michaelides, M.C.; Wallack, M.K. Effect of splenectomy on the growth of murine colon tumors. J. Surg. Oncol. 1983, 22, 73–76. [Google Scholar] [CrossRef]
- Prehn, R.T. The paradoxical effects of splenectomy on tumor growth. Theor. Biol. Med. Model. 2006, 3, 23. [Google Scholar] [CrossRef]
- Ben-Hur, H.; Kossoy, G.; Lifschitz, O.; Zusman, I. Splenectomy, chemically-induced mammary tumors and parathymic lymph nodes in rats: Experimental and morphological studies. In Vivo 2002, 16, 275–280. [Google Scholar] [PubMed]
- Higashijima, J.; Shimada, M.; Chikakiyo, M.; Miyatani, T.; Yoshikawa, K.; Nishioka, M.; Iwata, T.; Kurita, N. Effect of splenectomy on antitumor immune system in mice. Anticancer. Res. 2009, 29, 385–393. [Google Scholar]
- Weil, B.R.; Madenci, A.L.; Liu, Q.; Howell, R.M.; Gibson, T.M.; Yasui, Y.; Neglia, J.P.; Leisenring, W.M.; Smith, S.A.; Tonorezos, E.S.; et al. Late Infection-Related Mortality in Asplenic Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2018, 36, 1571–1578. [Google Scholar] [CrossRef]
- Kristinsson, S.Y.; Gridley, G.; Hoover, R.N.; Check, D.; Landgren, O. Long-term risks after splenectomy among 8,149 cancer-free American veterans: A cohort study with up to 27 years follow-up. Haematologica 2014, 99, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Goto, T. Links between Inflammation and Postoperative Cancer Recurrence. J. Clin. Med. 2021, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Nespoli, A.; Gianotti, L.; Bovo, G.; Brivio, F.; Nespoli, L.; Totis, M. Impact of postoperative infections on survival in colon cancer patients. Surg. Infect. 2006, 7 (Suppl. 2), s-41–s-43. [Google Scholar] [CrossRef]
- Mokart, D.; Giaoui, E.; Barbier, L.; Lambert, J.; Sannini, A.; Chow-Chine, L.; Brun, J.P.; Faucher, M.; Guiramand, J.; Ewald, J.; et al. Postoperative sepsis in cancer patients undergoing major elective digestive surgery is associated with increased long-term mortality. J. Crit. Care 2016, 31, 48–53. [Google Scholar] [CrossRef]
- Nasioudis, D.; Mastroyannis, S.A.; Ko, E.M.; Haggerty, A.F.; Cory, L.; Giuntoli, R.L., 2nd; Kim, S.H.; Morgan, M.A.; Latif, N.A. Delay in adjuvant chemotherapy administration for patients with FIGO stage I epithelial ovarian carcinoma is associated with worse survival; an analysis of the National Cancer Database. Gynecol. Oncol. 2022, 166, 263–268. [Google Scholar] [CrossRef]
- Elhadi, M.; Khaled, A.; Msherghi, A. Infectious diseases as a cause of death among cancer patients: A trend analysis and population-based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results database. Infect. Agents Cancer 2021, 16, 72. [Google Scholar] [CrossRef]
- Bacalbasa, N.; Balescu, I.; Dima, S.; Brasoveanu, V.; Popescu, I. Hematogenous Splenic Metastases as an Independent Negative Prognosis Factor at the Moment of Primary Cytoreduction in Advanced Stage Epithelial Ovarian Cancer—A Single Center Experience. Anticancer. Res. 2015, 35, 5649–5654. [Google Scholar]
- Durmuş, Y.; Bostancı, E.İ.; Çöteli, A.S.D.; Kayıkçıoğlu, F.; Boran, N. Metastasis patterns of the spleen and association with survival outcomes in advanced ovarian–tubal–peritoneal epithelial cancer. Arch. Gynecol. Obstet. 2019, 300, 1367–1375. [Google Scholar] [CrossRef]
- Spencer, N.J.; Spencer, J.A.; Perren, T.J.; Lane, G. CT appearances and prognostic significance of splenic metastasis in ovarian cancer. Clin. Radiol. 1998, 53, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, C.; Jones, B.P.; Savvatis, K.; Campbell, J.; Kyrgiou, M.; Farthing, A.; Brett, S.; Roux, R.; Hall, M.; Rustin, G.; et al. Maximal effort cytoreductive surgery for disseminated ovarian cancer in a UK setting: Challenges and possibilities. Arch. Gynecol. Obstet. 2016, 294, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am. J. Epidemiol. 2010, 172, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
| Comparison of Baseline Characteristics and Postoperative Outcome | |||
|---|---|---|---|
| Baseline Characteristics | |||
| Variable | Splenectomy | Control | p-Value |
| Age | 61 (32–78) | 67 (22–81) | 0.041 |
| BMI | 30 (18–34) | 32 (24–34) | 0.238 |
| ECOG status ECOG 0 ECOG 1 ECOG 2 | 7/91 12/91 72/91 | 4/132 17/132 111/132 | 0.281 |
| Stage IIIc IV | 41/74 33/74 | 62/104 42/104 | 0.575 |
| Histology Serous Other | 72/91 18/91 | 108/132 24/132 | 0.734 |
| Operation timing PDS IDS Secondary | 60/91 14/91 17/91 | 82/132 20/132 28/132 | 0.819 |
| Residual disease None <1 cm | 80/91 10/91 | 102/130 28/130 | 0.443 |
| Postoperative outcomes | |||
| DVT | 3/91 | 5/132 | 0.846 |
| Pulmonary embolism | 3/91 | 3/132 | 0.642 |
| Infectious diseases | 26/91 | 34/132 | 0.641 |
| Surgical site infection | 7/91 | 17/132 | 0.219 |
| Sepsis | 19/91 | 13/132 | 0.021 |
| Recurrences | 43/91 | 74/132 | 0.196 |
| Deaths | 35/91 | 61/132 | 0.251 |
| Factors Affecting Patient Survival | ||||
|---|---|---|---|---|
| Progression Free Survival | Overall Survival | |||
| Factor | Months (95% CI) | p-Value | Months (95% CI) | p-Value |
| Stage IIIc IV | 40.00 (25.64, 54.35) 24.00 (22.89, 25.17) | 0.384 | 60.00 (11.58, 37.30) 41.00 (26.59, 55.41) | 0.820 |
| Splenic metastases Surface Hilar Parenchymal | 28.13 (17.06, 39.19) 103.33 (73.51, 133.154) 39.33 (24.05, 54.62) | 0.313 | 40.53 (26.80, 54.29) 72.73 (45.87, 99.56) 54.94 (37.62, 72.27) | 0.822 |
| Operation setting PDS IDS | 69.48 (45.25, 93.71) 23.12 (13.88, 32.35) | 0.047 | 78.34 (53.49, 103.19) 37.67 (26.70, 48.63) | 0.047 |
| Tumor resection Complete Optimal (<1 cm) | 58.94 (40.48, 77.40) 49.77 (4.00, 98.67) | 0.259 | 73.80 (52.77, 94.84) 41.92 (5.26, 78.58) | 0.047 |
| Surgical complexity High Intermediate | 42.48 (27.04, 57.92) 66.92 (39.77, 94.06) | 0.413 | 53.41 (36.36, 70.47) 82.34 (53.68, 110.99) | 0.446 |
| Postoperative sepsis Present Absent | 24.00 (16.26, 31.74) 26.00 (7.57, 44.23) | 0.200 | 26.00 (7.74, 52.94) 60.00 (34.06, 85.94) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pergialiotis, V.; Zachariou, E.; Lygizos, V.; Vlachos, D.E.; Stamatakis, E.; Angelou, K.; Daskalakis, G.; Thomakos, N.; Haidopoulos, D. Splenectomy as Part of Maximal-Effort Cytoreductive Surgery in Advanced Epithelial Ovarian Cancer. Cancers 2024, 16, 790. https://doi.org/10.3390/cancers16040790
Pergialiotis V, Zachariou E, Lygizos V, Vlachos DE, Stamatakis E, Angelou K, Daskalakis G, Thomakos N, Haidopoulos D. Splenectomy as Part of Maximal-Effort Cytoreductive Surgery in Advanced Epithelial Ovarian Cancer. Cancers. 2024; 16(4):790. https://doi.org/10.3390/cancers16040790
Chicago/Turabian StylePergialiotis, Vasilios, Eleftherios Zachariou, Vasilios Lygizos, Dimitrios Efthymios Vlachos, Emmanouil Stamatakis, Kyveli Angelou, Georgios Daskalakis, Nikolaos Thomakos, and Dimitrios Haidopoulos. 2024. "Splenectomy as Part of Maximal-Effort Cytoreductive Surgery in Advanced Epithelial Ovarian Cancer" Cancers 16, no. 4: 790. https://doi.org/10.3390/cancers16040790
APA StylePergialiotis, V., Zachariou, E., Lygizos, V., Vlachos, D. E., Stamatakis, E., Angelou, K., Daskalakis, G., Thomakos, N., & Haidopoulos, D. (2024). Splenectomy as Part of Maximal-Effort Cytoreductive Surgery in Advanced Epithelial Ovarian Cancer. Cancers, 16(4), 790. https://doi.org/10.3390/cancers16040790









