The Role of PSMA PET Imaging in the Classification of the Risk of Prostate Cancer Patients: A Systematic Review on the Insights to Guide an Active Surveillance Approach
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
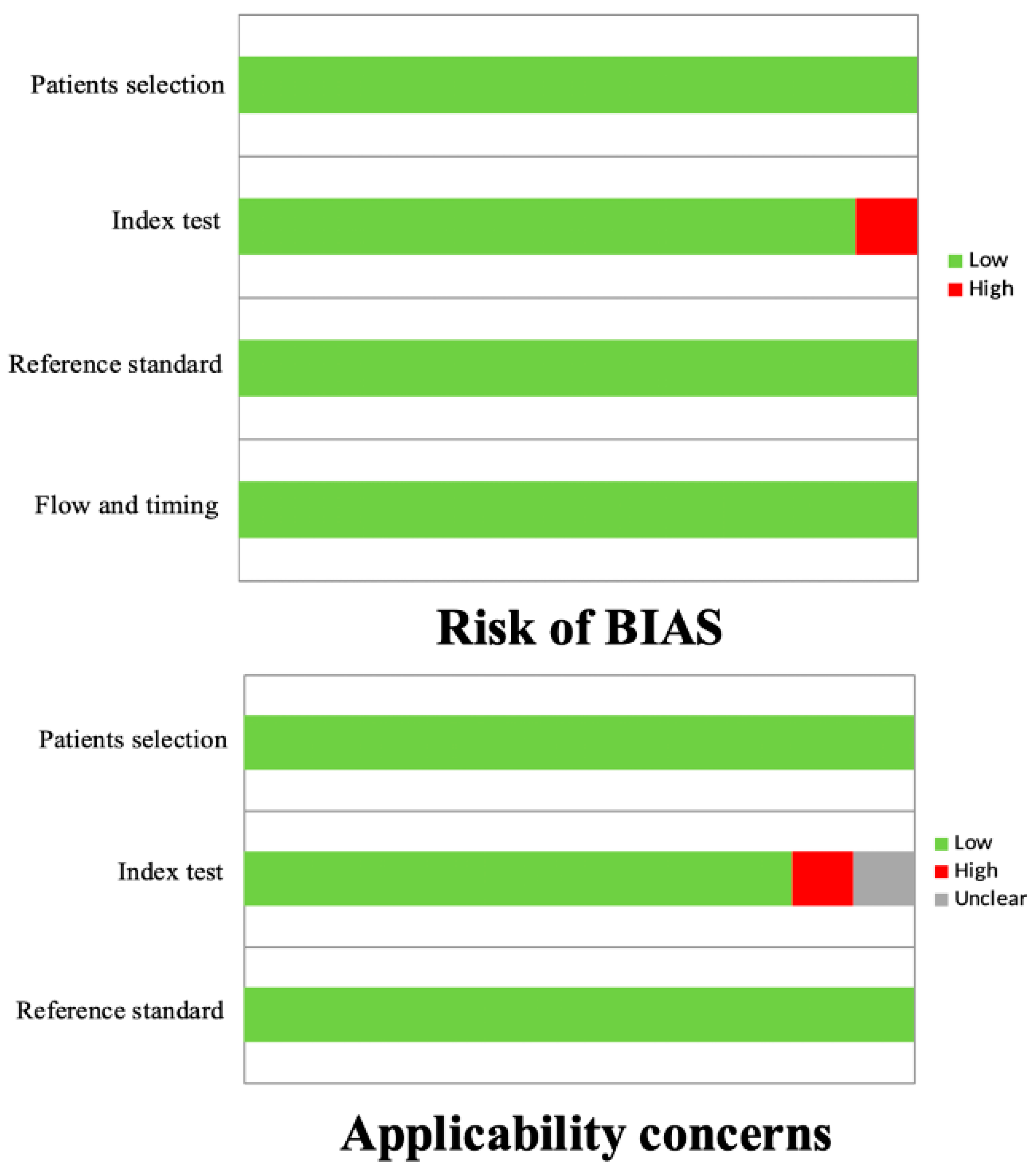
2.3. Quality Assessment
2.4. Data Extraction
3. Results
3.1. Literature Search
3.2. Role of PSMA PET Imaging in Guiding the Risk Classification of PCa and AS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grozescu, T.; Popa, F. Prostate cancer between prognosis and adequate/proper therapy. J. Med. Life 2017, 10, 5–12. [Google Scholar]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.S.; Tan, H.J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Bastian, P.J.; Boorjian, S.A.; Bossi, A.; Briganti, A.; Heidenreich, A.; Freedland, S.J.; Montorsi, F.; Roach, M., 3rd; Schröder, F.; van Poppel, H.; et al. High-risk prostate cancer: From definition to contemporary management. Eur. Urol. 2012, 61, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.J.; Autio, K.A.; Roach, M., 3rd; Scher, H.I. High-risk prostate cancer-classification and therapy. Nat. Rev. Clin. Oncol. 2014, 11, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Caglic, I.; Kovac, V.; Barrett, T. Multiparametric MRI—Local staging of prostate cancer and beyond. Radiol. Oncol. 2019, 53, 159–170. [Google Scholar] [CrossRef]
- Schlemmer, H.P.; Krause, B.J.; Schütz, V.; Bonekamp, D.; Schwarzenböck, S.M.; Hohenfellner, M. Imaging of Prostate Cancer. Dtsch. Arztebl. Int. 2021, 118, 713–719. [Google Scholar] [CrossRef] [PubMed]
- García Garzón, J.R.; de Arcocha Torres, M.; Delgado-Bolton, R.; Ceci, F.; Alvarez Ruiz, S.; Orcajo Rincón, J.; Caresia Aróztegui, A.P.; García Velloso, M.J.; García Vicente, A.M. Oncology Task Force of Spanish Society of Nuclear Medicine and Molecular Imaging. 68Ga-PSMA PET/CT in prostate cancer. Rev. Esp. Med. Nucl. Imagen Mol. 2018, 37, 130–138. [Google Scholar] [CrossRef]
- Dondi, F.; Albano, D.; Bertagna, F.; Treglia, G. Bone Scintigraphy versus PSMA-Targeted PET/CT or PET/MRI in Prostate Cancer: Lessons Learned from Recent Systematic Reviews and Meta-Analyses. Cancers 2022, 14, 4470. [Google Scholar] [CrossRef]
- Roberts, M.J.; Maurer, T.; Perera, M.; Eiber, M.; Hope, T.A.; Ost, P.; Siva, S.; Hofman, M.S.; Murphy, D.G.; Emmett, L.; et al. Using PSMA imaging for prognostication in localized and advanced prostate cancer. Nat. Rev. Urol. 2023, 20, 23–47. [Google Scholar] [CrossRef]
- Chow, K.M.; So, W.Z.; Lee, H.J.; Lee, A.; Yap, D.W.T.; Takwoingi, Y.; Tay, K.J.; Tuan, J.; Thang, S.P.; Lam, W.; et al. Head-to-head Comparison of the Diagnostic Accuracy of Prostate-specific Membrane Antigen Positron Emission Tomography and Conventional Imaging Modalities for Initial Staging of Intermediate- to High-risk Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2023, 84, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Santucci, J.; Woon, D.T.S.; Catterwell, R.; Perera, M.; Murphy, D.G.; Lawrentschuk, N. A Systematic Review on Prostate-Specific Membrane Antigen Positron Emission Tomography (PSMA PET) Evaluating Localized Low- to Intermediate-Risk Prostate Cancer: A Tool to Improve Risk Stratification for Active Surveillance? Life 2024, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; Brawer, M.K.; Jones, K.M.; Barry, M.J.; Aronson, W.J.; Fox, S.; Gingrich, J.R.; Wei, J.T.; Gilhooly, P.; Grob, B.M.; et al. Prostate Cancer Intervention versus Observation Trial (PIVOT) Study Group. Radical prostatectomy versus observation for localized prostate cancer. N. Engl. J. Med. 2012, 367, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Bill-Axelson, A.; Holmberg, L.; Garmo, H.; Rider, J.R.; Taari, K.; Busch, C.; Nordling, S.; Häggman, M.; Andersson, S.O.; Spångberg, A.; et al. Radical prostatectomy or watchful waiting in early prostate cancer. N. Engl. J. Med. 2014, 370, 932–942. [Google Scholar] [CrossRef]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef] [PubMed]
- Choo, R.; Klotz, L.; Danjoux, C.; Morton, G.C.; DeBoer, G.; Szumacher, E.; Fleshner, N.; Bunting, P.; Hruby, G. Feasibility Study: Watchful Waiting For Localized Low To Intermediate Grade Prostate Carcinoma With Selective Delayed Intervention Based On Prostate Specific Antigen, Histological And/Or Clinical Progression. J. Urol. 2002, 167, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Bergh, R.C.V.D.; Roemeling, S.; Roobol, M.J.; Aus, G.; Hugosson, J.; Rannikko, A.S.; Tammela, T.L.; Bangma, C.H.; Schröder, F.H. Outcomes of Men with Screen-Detected Prostate Cancer Eligible for Active Surveillance Who Were Managed Expectantly. Eur. Urol. 2009, 55, 1–8. [Google Scholar] [CrossRef]
- Costello, A.J. Considering the role of radical prostatectomy in 21st century prostate cancer care. Nat. Rev. Urol. 2020, 17, 177–188. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Grubmüller, B.; Baltzer, P.; Hartenbach, S.; D’Andrea, D.; Helbich, T.H.; Haug, A.R.; Goldner, G.M.; Wadsak, W.; Pfaff, S.; Mitterhauser, M.; et al. PSMA Ligand PET/MRI for Primary Prostate Cancer: Staging Performance and Clinical Impact. Clin. Cancer Res. 2018, 24, 6300–6307. [Google Scholar] [CrossRef]
- Sasikumar, A.; Joy, A.; Pillai, A.M.R.; Oommen, K.E.; Somarajan, S.; Raman, V.K.; Thomas, R.; Dinesh, D. Gallium 68-PSMA PET/CT for lesion characterization in suspected cases of prostate carcinoma. Nucl. Med. Commun. 2018, 39, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhang, M.; Huang, H.; Zhang, C.; Ruan, X.; Lin, W.; Li, B.; Chen, L.; Xu, D. Clinical Utility of 18F-PSMA-1007 Positron Emission Tomography/Magnetic Resonance Imaging in Prostate Cancer: A Single-Center Experience. Front. Oncol. 2021, 10, 612701. [Google Scholar] [CrossRef] [PubMed]
- Raveenthiran, S.; Yaxley, W.J.; Franklin, T.; Coughlin, G.; Roberts, M.; Gianduzzo, T.; Kua, B.; Samaratunga, H.; Delahunt, B.; Egevad, L.; et al. Findings in 1,123 Men with Preoperative 68Ga-Prostate-Specific Membrane Antigen Positron Emission Tomography/Computerized Tomography and Multiparametric Magnetic Resonance Imaging Compared to Totally Embedded Radical Prostatectomy Histopathology: Implications for the Diagnosis and Management of Prostate Cancer. J. Urol. 2022, 207, 573–580. [Google Scholar] [CrossRef]
- Xue, A.L.; Kalapara, A.A.; Ballok, Z.E.; Levy, S.M.; Sivaratnam, D.; Ryan, A.; Ramdave, S.; O’Sullivan, R.; Moon, D.; Grummet, J.P.; et al. 68Ga-Prostate-Specific Membrane Antigen Positron Emission Tomography Maximum Standardized Uptake Value as a Predictor of Gleason Pattern 4 and Pathological Upgrading in Intermediate-Risk Prostate Cancer. J. Urol. 2022, 207, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Heetman, J.G.; Lavalaye, J.; Polm, P.D.; Soeterik, T.F.W.; Wever, L.; Paulino Pereira, L.J.; van der Hoeven, E.J.R.J.; van Melick, H.H.E.; van den Bergh, R.C.N. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography in Active Surveillance for Prostate Cancer Trial (PASPoRT). Eur. Urol. Oncol. 2023, 77, 403–417. [Google Scholar] [CrossRef]
- Jain, A.; Nassour, A.J.; Dean, T.; Patterson, I.; Tarlinton, L.; Kim, L.; Woo, H. Expanding the role of PSMA PET in active surveillance. BMC Urol. 2023, 23, 77. [Google Scholar] [CrossRef]
- Pepe, P.; Pepe, L.; Tamburo, M.; Marletta, G.; Savoca, F.; Pennisi, M.; Fraggetta, F. 68Ga-PSMA PET/CT evaluation in men enrolled in prostate cancer Active Surveillance. Arch. Ital. Urol. Androl. 2023, 95, 11322. [Google Scholar] [CrossRef]
- Dondi, F.; Lazzarato, A.; Gorica, J.; Guglielmo, P.; Borgia, F.; Filice, R.; Vento, A.; Pacella, S.; Camedda, R.; Caracciolo, M.; et al. PET Criteria by Cancer Type from Imaging Interpretation to Treatment Response Assessment: Beyond FDG PET Score. Life 2023, 13, 611. [Google Scholar] [CrossRef]
- Herlemann, A.; Washington, S.L., 3rd; Eapen, R.S.; Cooperberg, M.R. Whom to Treat: Postdiagnostic Risk Assessment with Gleason Score, Risk Models, and Genomic Classifier. Urol. Clin. N. Am. 2017, 44, 547–555. [Google Scholar] [CrossRef]
- van der Sar, E.C.A.; Keusters, W.R.; van Kalmthout, L.W.M.; Braat, A.J.A.T.; de Keizer, B.; Frederix, G.W.J.; Kooistra, A.; Lavalaye, J.; Lam, M.G.E.H.; van Melick, H.H.E. Cost-effectiveness of the implementation of [68Ga]Ga-PSMA-11 PET/CT at initial prostate cancer staging. Insights Imaging 2022, 13, 132. [Google Scholar] [CrossRef]
- Holzgreve, A.; Unterrainer, M.; Calais, J.; Adams, T.; Oprea-Lager, D.E.; Goffin, K.; Lopci, E.; Unterrainer, L.M.; Kramer, K.K.M.; Schmidt-Hegemann, N.S.; et al. Is PSMA PET/CT cost-effective for the primary staging in prostate cancer? First results for European countries and the USA based on the proPSMA trial. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3750–3754. [Google Scholar] [CrossRef] [PubMed]

| First Author | Ref. N. | Year | Country | Study Design | Radiopharmaceuticals | N. Pts. |
|---|---|---|---|---|---|---|
| Grubmüller B | [20] | 2018 | Austria, USA | Prospective | [68Ga]Ga-PSMA-11 | 122 |
| Sasikumar A | [21] | 2018 | India | Prospective | [68Ga]Ga-PSMA-11 | 118 |
| Liu A | [22] | 2021 | China | Retrospective | [18F]F-PSMA-1007 | 62 |
| Raveenthiran S | [23] | 2022 | Australia, Sweden, New Zealand | Retrospective | [68Ga]Ga-PSMA-11 | 1123 |
| Xue A | [24] | 2022 | Australia | Prospective | [68Ga]Ga-PSMA-11 | 220 |
| Heetman J | [25] | 2023 | The Netherlands | Prospective | ns | 141 |
| Jain A | [26] | 2023 | Australia | Retrospective | [68Ga]Ga-PSMA-11, [18F]F-DCFPyL | 30 |
| Pepe P | [27] | 2023 | Italy | Prospective | [68Ga]Ga-PSMA-11 | 40 |
| First Author | Device | Mean Activity ± SD or Median (Range) (MBq) | PET Analysis | Main Findings |
|---|---|---|---|---|
| Grubmüller B | PET/MRI | ns | Qualitative | PSMA PET/MRI had high diagnostic performances in PCa staging, correctly identifying 97.5% of cancers with an accuracy of 82.5%, 85%, 79%, 94%, and 93% for T, T2, T3a, T3b, and N1 staging, respectively. It also provided important information for the development of a personalized therapeutic strategy with changes in overall treatment strategy in 28.7% of the subjects. In particular, 15.6% of the patients were submitted to AS. |
| Sasikumar A | PET/CT | 100 ± 19 | Qualitative and semiquantitative | PSMA PET/CT could select between suspected PCa patients who should undergo further investigation and those who could be kept on AS. In particular, 43% of the patients had a negative scan and could therefore avoid biopsy and be kept in follow-up or had a negative biopsy. Most of the subjects with a positive scan had a positive biopsy. |
| Liu A | PET/MRI | 263 (164–353) | Qualitative | PSMA PET/MRI was a valuable tool with which to define PCa and tailor the management of the patients. In particular, management changes were reported in 39.3% of the subjects with suspected PCa with an increase in biopsy and a reduction in AS. All subjects with a negative PET/MRI were put on AS and no PCa was reported until the last follow-up. |
| Raveenthiran S | PET/CT | 200 | Qualitative and semiquantitative | Adding PSMA PET/CT to MRI could improve the detection of significant PCa, improving the ability to identify patients suitable for AS. Similar detection rates between the two imaging modalities (82% and 80%, respectively) were reported and, by combining them, a higher accuracy was underlined (92% for GS ≥ 3 + 4). Significant correlation between SUVmax and GS was demonstrated; in addition, for patients suitable for AS with an SUVmax > 11 on PSMA PET/CT, a targeted biopsy of the tracer-avid area should be recommended to exclude high-grade PCa. |
| Xue A | PET/CT | 2/Kg ± 5% and 2/Kg ± 10% | Qualitative and semiquantitative | SUVmax was associated with GP4 and, therefore, PSMA PET/CT could improve risk stratification of favorable intermediate-risk PCa patients. Higher SUVmax for regions with >50% GP4 compared to regions with <50% was reported; the same findings were confirmed using 20% and 10% as reference thresholds. SUVmax was an independent predictor for the presence of GP4, a significant discriminator for upgrading after surgery, and a significant predictor of unfavorable pathology after surgery. |
| Heetman J | PET/CT | 1.5/Kg | ns | PSMA PET/CT could improve PCa risk stratification and allow a better selection of AS patients. In particular, 32% of the subjects had an additional biopsy due to PET/CT findings and in 9% of the subjects, upgrading was detected. PET/CT and biopsies yielded upgrading frequently in MRI-negative patients. |
| Jain A | PET/CT | ns | Qualitative and semiquantitative | PSMA PET/CT could influence the management of patients with a new diagnosis of low or favorable intermediate-risk PCa before their consideration for AS. The presence of concerning features at PET/CT imaging or high SUVmax values were correlated to an upgrade of GG or to the presence of adverse pathological features after radical prostatectomy. Based on PET/CT findings, 37% of the men were submitted to AS. |
| Pepe P | PET/CT | 144 ± 12 | Qualitative and semiquantitative | PSMA PET/CT did not improve the detection rate of clinically significant PCa on biopsy; however, it demonstrated better diagnostic accuracy compared to mpMRI. In particular, mpMRI and PSMA PET/CT revealed that 45% and 22.5% of lesions were suspicious for PCa, respectively. PSMA-target biopsy diagnosed 66.6% of Pca compared to 66.6% and 100% of mpMRI-guided biopsy and transperineal saturation biopsy, respectively. mpMRI demonstrated 40% and 33.33% of FP and of FN results, respectively, while PET/CT revealed 17.5% of FP and 33.3% of FN findings, respectively. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dondi, F.; Antonelli, A.; Suardi, N.; Treglia, G.; Bertagna, F. The Role of PSMA PET Imaging in the Classification of the Risk of Prostate Cancer Patients: A Systematic Review on the Insights to Guide an Active Surveillance Approach. Cancers 2024, 16, 1122. https://doi.org/10.3390/cancers16061122
Dondi F, Antonelli A, Suardi N, Treglia G, Bertagna F. The Role of PSMA PET Imaging in the Classification of the Risk of Prostate Cancer Patients: A Systematic Review on the Insights to Guide an Active Surveillance Approach. Cancers. 2024; 16(6):1122. https://doi.org/10.3390/cancers16061122
Chicago/Turabian StyleDondi, Francesco, Alessandro Antonelli, Nazareno Suardi, Giorgio Treglia, and Francesco Bertagna. 2024. "The Role of PSMA PET Imaging in the Classification of the Risk of Prostate Cancer Patients: A Systematic Review on the Insights to Guide an Active Surveillance Approach" Cancers 16, no. 6: 1122. https://doi.org/10.3390/cancers16061122
APA StyleDondi, F., Antonelli, A., Suardi, N., Treglia, G., & Bertagna, F. (2024). The Role of PSMA PET Imaging in the Classification of the Risk of Prostate Cancer Patients: A Systematic Review on the Insights to Guide an Active Surveillance Approach. Cancers, 16(6), 1122. https://doi.org/10.3390/cancers16061122







