Prediction of Patient Outcomes in Locally Advanced Cervical Carcinoma Following Chemoradiotherapy—Comparative Effectiveness of Magnetic Resonance Imaging and 2-Deoxy-2-[18F]fluoro-D-glucose Imaging
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Study Population and Follow-Up
2.3. Concurrent Chemoradiotherapy Regimen
2.4. Image Acquisition
- Non-contrast 2.5 mm thick axial reformats with applied soft tissue kernel, extending from the skull base to the proximal femora.
- Non-contrast 2.5 mm thick axial reformats with applied lung kernel, extending from the lung apices to the upper abdomen.
- Non-attenuation-corrected axial reconstructions of 2-[18F]FDG activity extending from the skull base to the proximal femora.
- Maximum intensity projection (MIP) 3D reconstruction of FDG activity.
- Fused 2-[18F]FDG/CT axial reconstructions with slice thickness of 2.5 mm.
- Fused 2-[18F]FDG/CT coronal reconstructions with slice thickness of 2.5 mm.
- High-resolution small-field-of-view sagittal and axial T2-weighted (T2W) sequences (3 mm slice thickness).
- T2-weighted gradient echo images, including axial, coronal, and oblique reformats (3 mm slice thickness).
- Diffusion-weighted image (DWI) sequences (b0, b150, b500) in axial plane with a corresponding apparent diffusion coefficient (ADC) map (4 mm slice thickness).
- Multi-shot turbo spin echo (TSE) T2 transverse and T1 coronal plane sequences (balanced steady state free precession line acquisition with undersampling, BLADE) (10 mm and 4 mm slice thicknesses, respectively).
2.5. Image Analysis
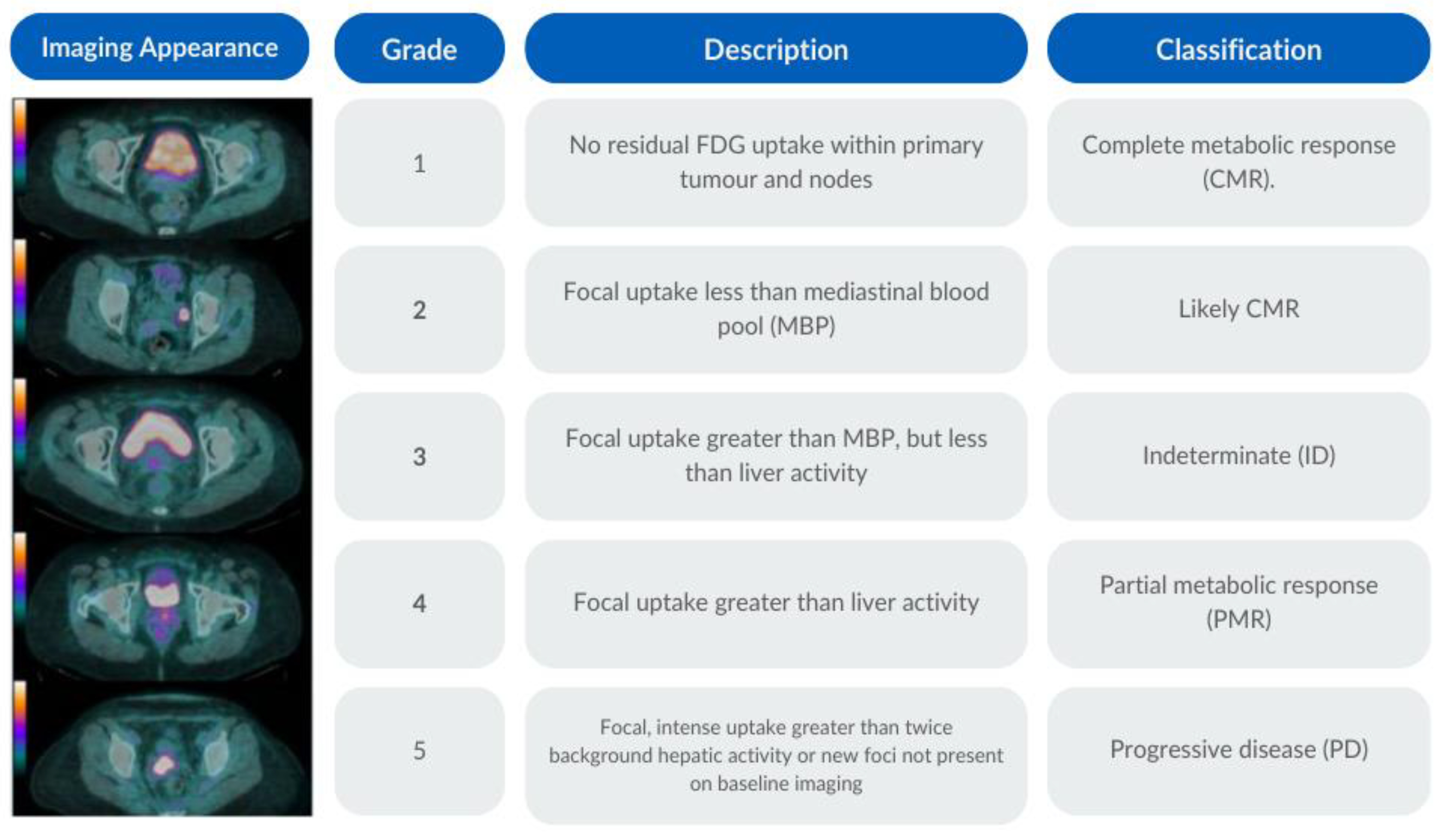
2.6. PET-CT Criteria
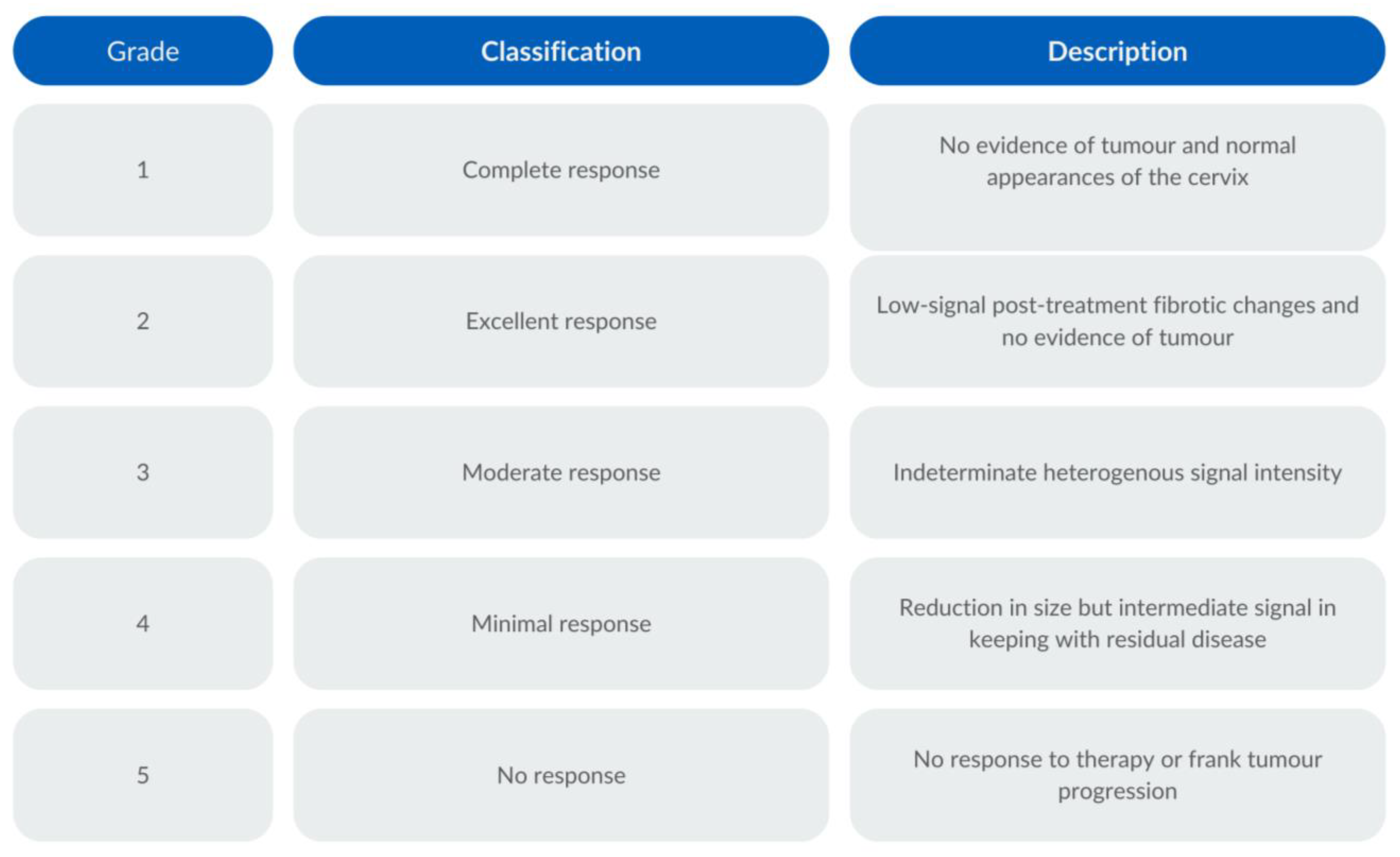
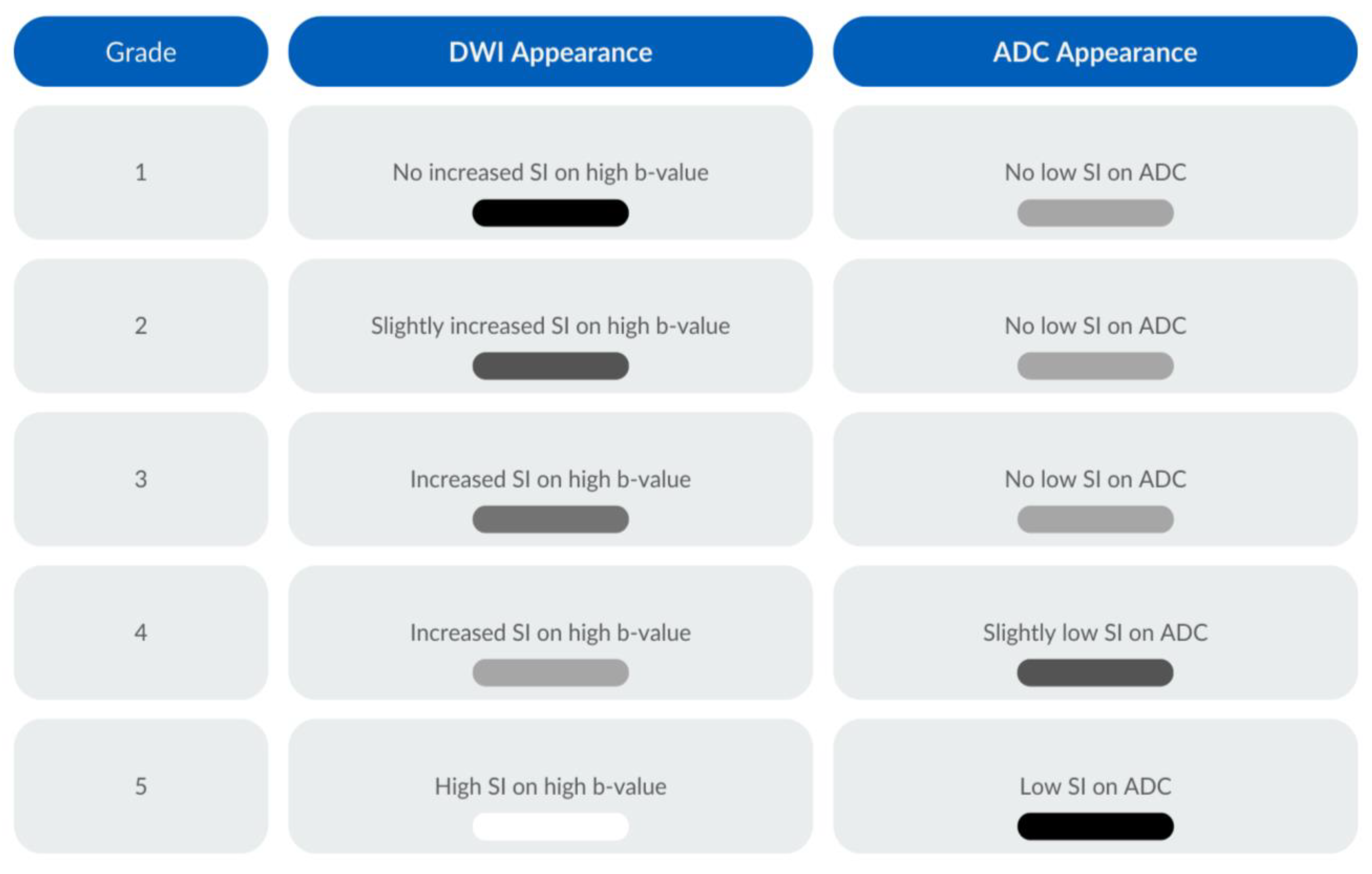
2.7. MRI Criteria
2.8. Statistical Analysis
3. Results
3.1. Study Group
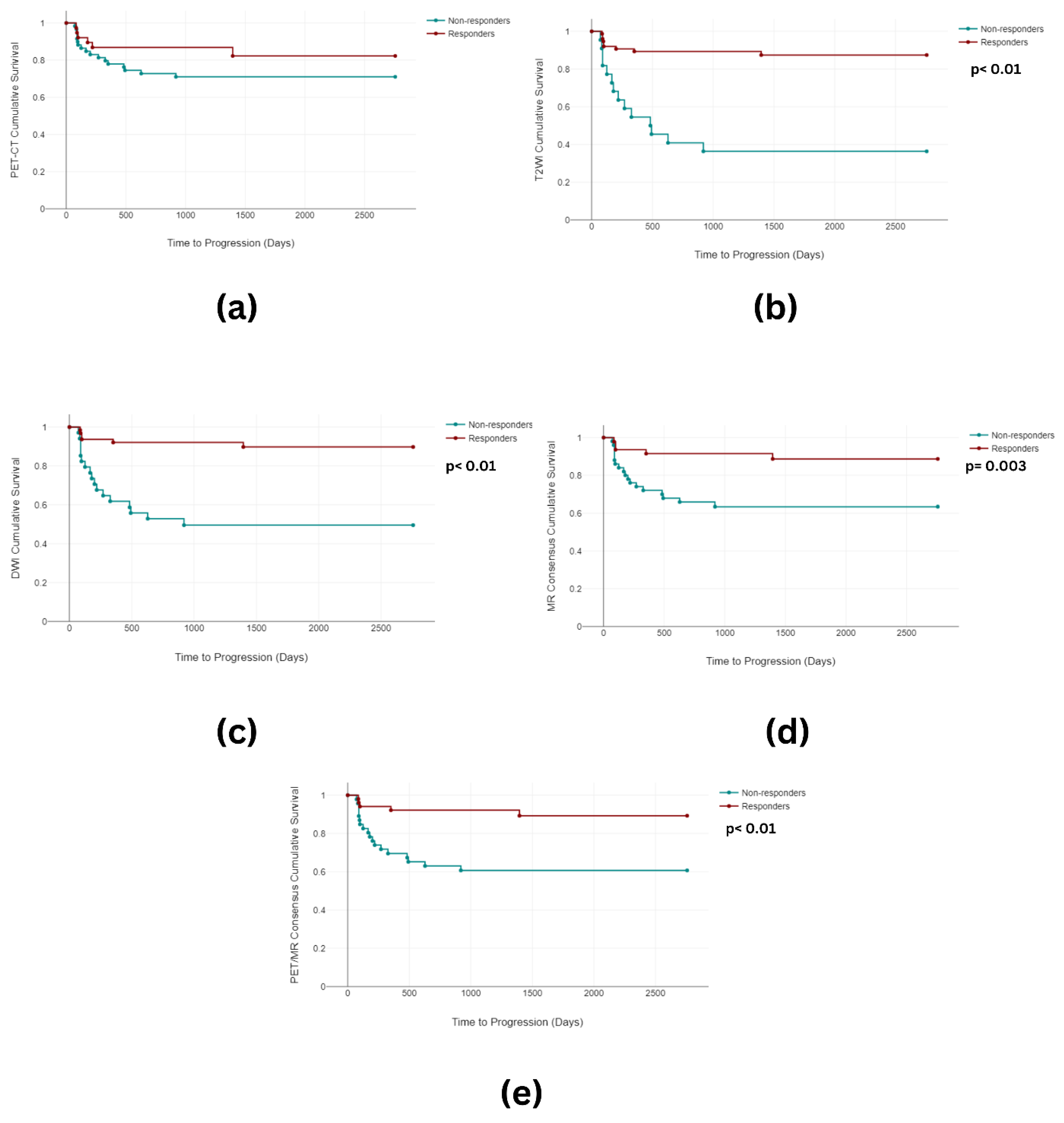
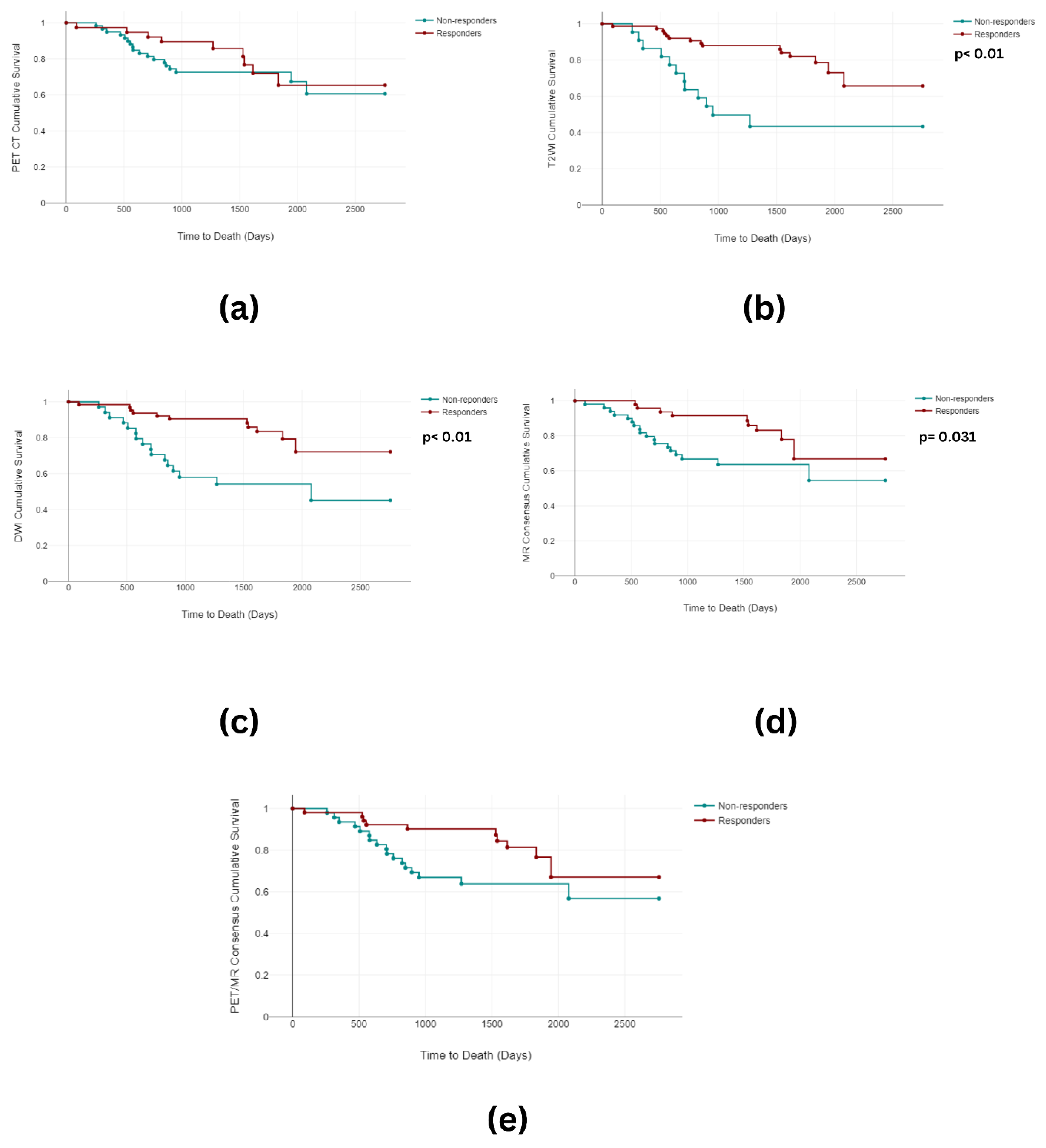
3.2. Overall and Progression-Free Survival
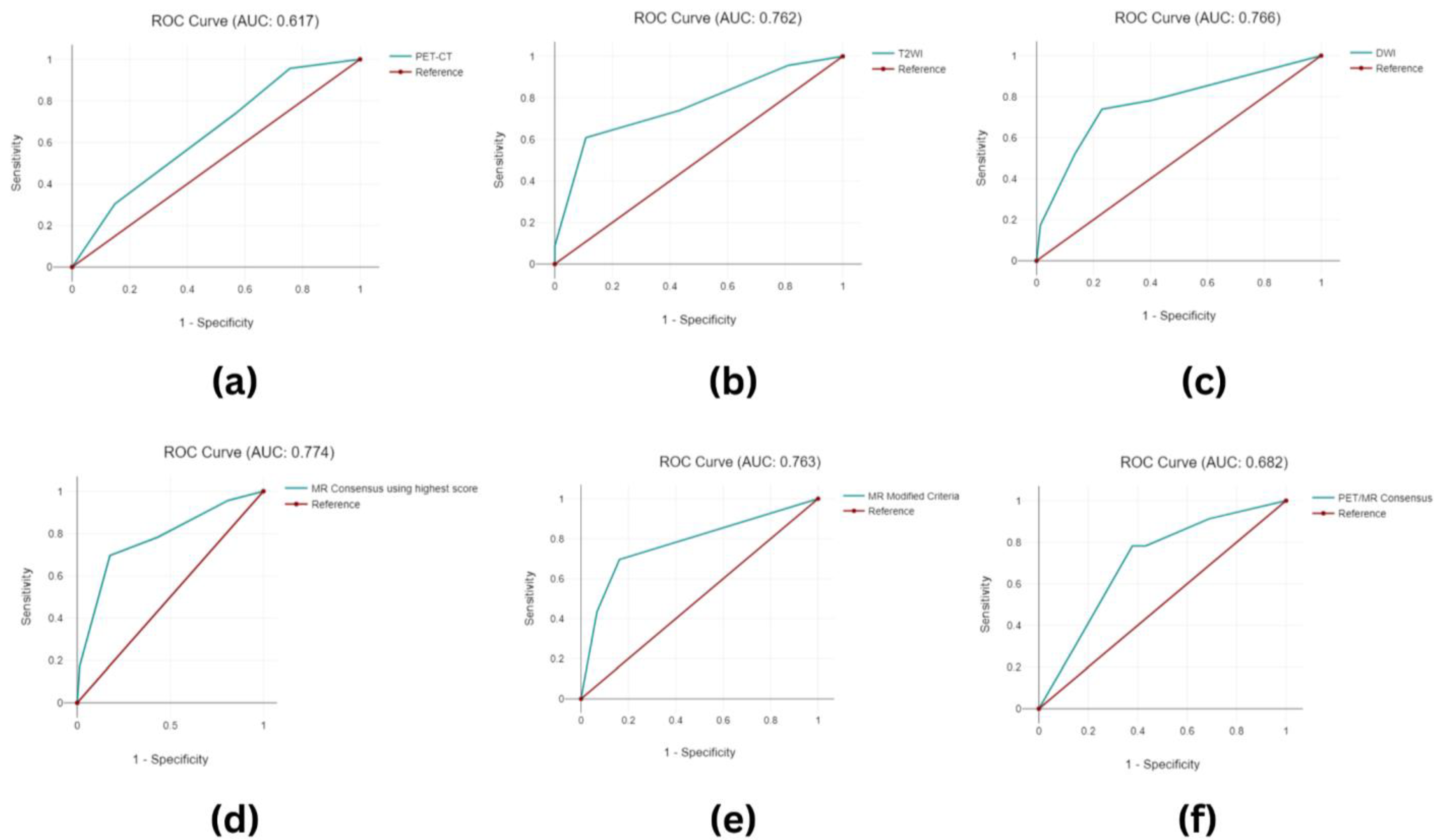
3.3. Imaging Analysis
3.3.1. Image Grouping
3.3.2. Image Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef]
- Ries, L.A.G.; Melbert, D.; Krapcho, M.; Stinchcomb, D.G.; Howlader, N.; Horner, M.J.; Mariotto, A.; Miller, B.A.; Feuer, E.J.; Altekruse, S.F.; et al. SEER Cancer Statistics Review, 1975–2005; National Cancer Institute: Bethesda, MD, USA, 2008. [Google Scholar]
- Donkoh, E.T.; Agyemang-Yeboah, F.; Asmah, R.H.; Wiredu, E.K. Prevalence of cervical cancer and pre-cancerous lesions among unscreened Women in Kumasi, Ghana. Medicine 2019, 98, e14600. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Perrone, A.M.; Dondi, G.; Coe, M.; Ferioli, M.; Telo, S.; Galuppi, A.; De Crescenzo, E.; Tesei, M.; Castellucci, P.; Nanni, C.; et al. Predictive role of MRI and 18F FDG PET response to concurrent chemoradiation in T2b cervical cancer on clinical outcome: A retrospective single center study. Cancers 2020, 12, 659. [Google Scholar] [CrossRef]
- Janicek, M.F.; Averette, H.E. Cervical cancer: Prevention, diagnosis, and therapeutics. CA Cancer J. Clin. 2001, 51, 92–114. [Google Scholar] [CrossRef]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer–Update 2023. Virchows Archiv 2023, 482, 935–966. [Google Scholar] [CrossRef]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef]
- Fagotti, A.; Anchora, L.P.; Conte, C.; Chiantera, V.; Vizza, E.; Tortorella, L.; Surico, D.; De Iaco, P.; Corrado, G.; Fanfani, F.; et al. Beyond sentinel node algorithm. Toward a more tailored surgery for cervical cancer patients. Cancer Med. 2016, 5, 1725–1730. [Google Scholar] [CrossRef]
- Fanfani, F.; Vizza, E.; Landoni, F.; De Iaco, P.; Ferrandina, G.; Corrado, G.; Gallotta, V.; Gambacorta, M.A.; Fagotti, A.; Monterossi, G.; et al. Radical hysterectomy after chemoradiation in FIGO stage III cervical cancer patients versus chemoradiation and brachytherapy: Complications and 3-years survival. Eur. J. Surg. Oncol. 2016, 42, 1519–1525. [Google Scholar] [CrossRef]
- Eifel, P.J.; Winter, K.; Morris, M.; Levenback, C.; Grigsby, P.W.; Cooper, J.; Rotman, M.; Gershenson, D.; Mutch, D.G. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of radiation therapy oncology group trial (RTOG) 90-01. J. Clin. Oncol. 2004, 22, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Georg, P.; Dimopoulos, J.C.; Grimm, M.; Berger, D.; Nesvacil, N.; Georg, D.; Schmid, M.P.; Reinthaller, A.; Sturdza, A.; et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother. Oncol. 2011, 100, 116–123. [Google Scholar] [CrossRef]
- Thomeer, M.G.; Vandecaveye, V.; Braun, L.; Mayer, F.; Franckena-Schouten, M.; de Boer, P.; Stoker, J.; Van Limbergen, E.; Buist, M.; Vergote, I.; et al. Evaluation of T2-W MR imaging and diffusion-weighted imaging for the early post-treatment local response assessment of patients treated conservatively for cervical cancer: A multicentre study. Eur. Radiol. 2019, 29, 309–318. [Google Scholar] [CrossRef]
- Pinho, D.F.; King, B.; Xi, Y.; Albuquerque, K.; Lea, J.; Subramaniam, R.M. Value of intratumoral metabolic heterogeneity and quantitative 18F-FDG PET/CT parameters in predicting prognosis for patients with cervical cancer. Am. J. Roentgenol. 2020, 214, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Dappa, E.; Elger, T.; Hasenburg, A.; Düber, C.; Battista, M.J.; Hötker, A.M. The value of advanced MRI techniques in the assessment of cervical cancer: A review. Insights Imaging 2017, 8, 471–481. [Google Scholar] [CrossRef]
- Sjövall, J.; Bitzén, U.; Kjellén, E.; Nilsson, P.; Wahlberg, P.; Brun, E. Qualitative interpretation of PET scans using a Likert scale to assess neck node response to radiotherapy in head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 609–616. [Google Scholar] [CrossRef]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048. [Google Scholar] [CrossRef]
- Vincens, E.; Balleyguier, C.; Rey, A.; Uzan, C.; Zareski, E.; Gouy, S.; Pautier, P.; Duvillard, P.; Haie-Meder, C.; Morice, P. Accuracy of magnetic resonance imaging in predicting residual disease in patients treated for stage IB2/II cervical carcinoma with chemoradiation therapy: Correlation of radiologic findings with surgicopathologic results. Cancer 2008, 113, 2158–2165. [Google Scholar] [CrossRef]
- Lin, A.; Ma, S.; Dehdashti, F.; Markovina, S.; Schwarz, J.; Siegel, B.; Powell, M.; Grigsby, P. Detection of distant metastatic disease by positron emission tomography with 18F-fluorodeoxyglucose (FDG-PET) at initial staging of cervical carcinoma. Int. J. Gynecol. Cancer 2019, 29, 487–491. [Google Scholar] [CrossRef]
- Palaniswamy, S.S.; Borde, C.R.; Subramanyam, P. 18F-FDG PET/CT in the evaluation of cancer cervix: Where do we stand today? Nucl. Med. Commun. 2018, 39, 583–592. [Google Scholar] [CrossRef]
- Scarsbrook, A.; Vaidyanathan, S.; Chowdhury, F.; Swift, S.; Cooper, R.; Patel, C. Efficacy of qualitative response assessment interpretation criteria at 18F-FDG PET-CT for predicting outcome in locally advanced cervical carcinoma treated with chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.H.; Kim, J.W.; Kang, K.W.; Park, N.-H.; Song, Y.-S.; Chung, J.-K.; Kang, S.-B. Predictive role of post-treatment [18F] FDG PET/CT in patients with uterine cervical cancer. Eur. J. Radiol. 2012, 81, e817–e822. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, H.J.; Jeong, Y.H.; Lee, J.-H.; Cho, A.; Yun, M.; Lee, J.D.; Kim, Y.B.; Kim, Y.T.; Kang, W.J. The role of 18 F-FDG PET/CT in assessing therapy response in cervix cancer after concurrent chemoradiation therapy. Nucl. Med. Mol. Imaging 2014, 48, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Diver, E.; Hinchcliff, E.; Gockley, A.; Melamed, A.; Contrino, L.; Feldman, S.; Growdon, W. Utilization of preoperative PET-CT and pelvic MRI reduces multimodality therapy in the care of women with early-stage cervical carcinoma. Gynecol. Oncol. 2018, 149, 35–36. [Google Scholar] [CrossRef]
- Kostakoglu, L.; Agress, H., Jr.; Goldsmith, S.J. Clinical role of FDG PET in evaluation of cancer patients. Radiographics 2003, 23, 315–340, quiz 533. [Google Scholar] [CrossRef]
- Cook, G.J.R.; Maisey, M.N.; Fogelman, I. Normal variants, artefacts and interpretative pitfalls in PET imaging with 18-fluoro-2-deoxyglucose and carbon-11 methionine. Eur. J. Nucl. Med. 1999, 26, 1363–1378. [Google Scholar] [CrossRef] [PubMed]
- Su, T.-P.; Lin, G.; Huang, Y.-T.; Liu, F.-Y.; Wang, C.-C.; Chao, A.; Chou, H.-H.; Yen, T.-C.; Lai, C.-H. Comparison of positron emission tomography/computed tomography and magnetic resonance imaging for posttherapy evaluation in patients with advanced cervical cancer receiving definitive concurrent chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 727–734. [Google Scholar] [CrossRef]
- Kalash, R.; Glaser, S.M.; Rangaswamy, B.; Horne, Z.D.; Kim, H.; Houser, C.; Beriwal, S. Use of functional magnetic resonance imaging in cervical cancer patients with incomplete response on positron emission tomography/computed tomography after image-based high-dose-rate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1008–1013. [Google Scholar] [CrossRef]
- Adusumilli, P.; Elsayed, N.; Theophanous, S.; Samuel, R.; Cooper, R.; Casanova, N.; Tolan, D.J.; Gilbert, A.; Scarsbrook, A.F. Combined PET-CT and MRI for response evaluation in patients with squamous cell anal carcinoma treated with curative-intent chemoradiotherapy. Eur. Radiol. 2022, 32, 5086–5096. [Google Scholar] [CrossRef]
- Kucnerowicz, K.; Pietrzak, A.; Cholewiński, W.; Martenka, P.; Marszałek, A.; Burchardt, E.; Strzesak, E. The quality-adjusted life-years in the oncological patients’ health-related quality of life. Sci. Rep. 2022, 12, 13562. [Google Scholar] [CrossRef]
- ICRU International Commission on Radiation Units and Measurements. Dose and Volume Specifications for Reporting Intracavitary Therapy in Gynecology; ICRU Report 38; ICRU International Commission on Radiation Units and Measurements: Bethesda, MD, USA, 1985. [Google Scholar]
- Haider, M.A.; van der Kwast, T.H.; Tanguay, J.; Evans, A.J.; Hashmi, A.-T.; Lockwood, G.; Trachtenberg, J. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. Am. J. Roentgenol. 2007, 189, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Kim, C.K.; Park, B.K. Prediction of disease progression following concurrent chemoradiotherapy for uterine cervical cancer: Value of post-treatment diffusion-weighted imaging. Eur. Radiol. 2016, 26, 3272–3279. [Google Scholar] [CrossRef] [PubMed]
- Sprinthall, R.C. Basic Statistical Analysis; Allyn and Bacon: Boston, MA, USA, 2011. [Google Scholar]
- Bland, J.M.; Altman, D.G. The logrank test. BMJ 2004, 328, 1073. [Google Scholar] [CrossRef] [PubMed]
- Breslow, N.E. Analysis of survival data under the proportional hazards model. Int. Stat. Rev./Rev. Int. Stat. 1975, 43, 45–57. [Google Scholar] [CrossRef]
- Freeman, S.J.; Aly, A.M.; Kataoka, M.Y.; Addley, H.C.; Reinhold, C.; Sala, E. The revised FIGO staging system for uterine malignancies: Implications for MR imaging. Radiographics 2012, 32, 1805–1827. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.-C.; Ng, K.-K.; Ma, S.-Y.; Chou, H.-H.; Tsai, C.-S.; Hsueh, S.; Chang, T.-C.; Hong, J.-H.; See, L.-C.; Lin, W.-J.; et al. Value of dual-phase 2-fluoro-2-deoxy-d-glucose positron emission tomography in cervical cancer. J. Clin. Oncol. 2003, 21, 3651–3658. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.M.; Matti, A.; Vara, G.; Dondi, G.; Naselli, N.; De Crescenzo, E.M.; Morganti, A.G.; Perrone, A.M.; De Iaco, P.; Nanni, C.; et al. Prognostic value of posttreatment 18 F-FDG PET/CT and predictors of metabolic response to therapy in patients with locally advanced cervical cancer treated with concomitant chemoradiation therapy: An analysis of intensity-and volume-based PET parameters. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2139–2146. [Google Scholar] [CrossRef]
- Dessole, M.; Petrillo, M.; Lucidi, A.; Naldini, A.; Rossi, M.; De Iaco, P.; Marnitz, S.; Sehouli, J.; Scambia, G.; Chiantera, V. Quality of life in women after pelvic exenteration for gynecological malignancies: A multicentric study. Int. J. Gynecol. Cancer 2018, 28, 267–273. [Google Scholar] [CrossRef]
- Siva, S.; Herschtal, A.; Thomas, J.M.; Bernshaw, D.M.; Gill, S.; Hicks, R.J.; Narayan, K. Impact of post-therapy positron emission tomography on prognostic stratification and surveillance after chemoradiotherapy for cervical cancer. Cancer 2011, 117, 3981–3988. [Google Scholar] [CrossRef]
- Beriwal, S.; Kannan, N.; Sukumvanich, P.; Richard, S.D.; Kelley, J.L.; Edwards, R.P.; Olawaiye, A.; Krivak, T.C. Complete metabolic response after definitive radiation therapy for cervical cancer: Patterns and factors predicting for recurrence. Gynecol. Oncol. 2012, 127, 303–306. [Google Scholar] [CrossRef]
- Schwarz, J.K.; Siegel, B.A.; Dehdashti, F.; Grigsby, P.W. Association of posttherapy positron emission tomography with tumor response and survival in cervical carcinoma. JAMA 2007, 298, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Chiantera, V.; Rossi, M.; De Iaco, P.; Koehler, C.; Marnitz, S.; Ferrandina, G.; Legge, F.; Parazzini, F.; Scambia, G.; Schneider, A.; et al. Survival after curative pelvic exenteration for primary or recurrent cervical cancer: A retrospective multicentric study of 167 patients. Int. J. Gynecol. Cancer 2014, 24, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.Y.; Kang, H.K.; Chung, T.W.; Seo, J.J.; Park, J.G. Uterine cervical carcinoma after therapy: CT and MR imaging findings. Radiographics 2003, 23, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Cetina, L.; Serrano, A.; Cantú-De-León, D.; Pérez-Montiel, D.; Estrada, E.; Coronel, J.; Hernández-Lucio, M.; Dueñas-González, A. F18-FDG-PET/CT in the evaluation of patients with suspected recurrent or persistent locally advanced cervical carcinoma. Rev. Investig. Clín. 2011, 63, 227–235. [Google Scholar]
- Vandecasteele, K.; Delrue, L.; Lambert, B.; Makar, A.; Lambein, K.; Denys, H.; Tummers, P.; Van den Broecke, R.; Villeirs, G.; De Meerleer, G. Value of magnetic resonance and 18FDG PET-CT in predicting tumor response and resectability of primary locally advanced cervical cancer after treatment with intensity-modulated arc therapy: A prospective pathology-matched study. Int. J. Gynecol. Cancer 2012, 22, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-C.; Chen, S.-W.; Wu, K.-C.; Hsieh, T.-C.; Liang, J.-A.; Hung, Y.-C.; Yeh, L.-S.; Chang, W.-C.; Lin, W.-C.; Yen, K.-Y.; et al. Prediction of local relapse and distant metastasis in patients with definitive chemoradiotherapy-treated cervical cancer by deep learning from [18 F]-fluorodeoxyglucose positron emission tomography/computed tomography. Eur. Radiol. 2019, 29, 6741–6749. [Google Scholar] [CrossRef] [PubMed]
- Gui, B.; Valentini, A.L.; Miccò, M.; D’Agostino, G.R.; Tagliaferri, L.; Zannoni, G.F.; Tummers, P.; Van den Broecke, R.; Villeirs, G.; De Meerleer, G. Cervical cancer response to neoadjuvant chemoradiotherapy: MRI assessment compared with surgery. Acta Radiol. 2016, 57, 1123–1131. [Google Scholar] [CrossRef]
- Atstupėnaitė, V.; Kraujutienė, I.; Nakaitė, R.J.; Bieliūnienė, E.; Basevičius, A. The value of magnetic resonance imaging in the assessment of chemoradiotherapy of cancer of cervix uteri. Radiol. Update 2019, 3, 67–76. [Google Scholar]
- Gong, L.; Yin, R.T.; Li, Q.L.; Bian, C.; Peng, Z.L. Effectiveness of neoadjuvant chemotherapy followed by radical surgery in young patients with locally advanced cervical cancer. Eur. J. Gynaecol. Oncol. 2023, 44, 26–33. [Google Scholar]
- Subramanya, D.; Grivas, P.D. HPV and cervical cancer: Updates on an established relationship. Postgrad. Med. 2008, 120, 7–13. [Google Scholar] [CrossRef]
- Jamal, M.; Cooper, R.; Dhesi, S.; Scarsbrook, A.; Frood, R. Prediction of outcome post-chemoradiotherapy in locally advanced cervical carcinoma (LACC) using radiomic feature analysis of FDG PET-CT imaging. In Proceedings of the European Congress of Radiology (ECR), Vienna, Austria, 28 February–3 March 2024. [Google Scholar]
| Variables | n-97 | No. |
|---|---|---|
| Age | Median | 47 |
| Range | 24–82 | |
| T stage | T1 | 7 (7%) |
| T2 | 66 (68.0%) | |
| T3 | 19 (20%) | |
| T4 | 5 (5%) | |
| Nodal disease at baseline | Yes | 45 |
| No | 52 | |
| Histological subtype | Squamous cell carcinoma | 77 (79%) |
| Adenocarcinoma | 15 (15%) | |
| Adenosquamous carcinoma | 3 (4%) | |
| Other | 2 (2%) | |
| Metastatic stage at baseline | M0 | 94 |
| M1 | 3 | |
| Primary tumour SUVmax | Median | 15.1 |
| Range | 4.7–52.5 | |
| Deaths | 27 | |
| Progression | 23 | |
| Follow-up period (days) | Median | 1506 |
| Range | 71–2757 | |
| Duration between treatment completion and MRI response assessment (days) | Median | 92 |
| Range | 48–238 | |
| Duration between treatment completion and PET-CT response assessment (days) | Median | 95 |
| Range | 16–246 |
| Grade | T2W- + Diffusion-Weighted Imaging |
|---|---|
| 0, Definitely not cancer | T2W = 1 or DWI = 1 |
| 1, Probably not cancer | T2W ≤ 3 and DWI = 2 or 3 |
| 2, Possible cancer | T2W ≥ 4 and DWI = 2 or 3 or T2W ≤ 3 and DWI = 4 |
| 3, Probably cancer | T2W < 4 and DWI = 4 or 5 |
| 4, Definite cancer | T2W ≥ 4 and DWI = 4 or 5 |
| Response | Clinical Outcome | 3m PET-CT Score | 3m T2W Score | 3m DWI Score | MR Consensus | PET/MR Consensus |
|---|---|---|---|---|---|---|
| Complete Response | 74 | 38 | 75 | 63 | 68 | 51 |
| Incomplete Response | 23 | 59 | 24 | 34 | 25 | 46 |
| False Positive | 42 | 8 | 17 | 13 | 28 | |
| False Negative | 6 | 9 | 6 | 7 | 5 | |
| True Positive | 17 | 14 | 17 | 16 | 18 | |
| True Negative | 32 | 66 | 57 | 61 | 46 | |
| Sensitivity | 73.91% | 60.87% | 73.91% | 69.57% | 78.26% | |
| Specificity | 43.24% | 89.19% | 77.03% | 82.43% | 62.16% | |
| Positive Predictive Value | 28.81% | 63.64% | 50.00% | 55.17% | 39.13% | |
| Negative Predictive Value | 84.21% | 88.00% | 90.48% | 89.71% | 90.20% | |
| Accuracy | 50.52% | 82.47% | 76.29% | 79.38% | 65.98% |
| (a) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chi-Square | df | p-Value | |||||||
| Progression-free survival analysis | |||||||||
| PET-CT | 1.94 | 1 | 0.164 | ||||||
| T2WI | 28.12 | 1 | <0.001 | ||||||
| DWI | 21.19 | 1 | <0.001 | ||||||
| MR Consensus | 8.85 | 1 | 0.003 | ||||||
| PET/MR Consensus | 11.36 | 1 | 0.001 | ||||||
| Overall Survival Analysis | |||||||||
| PET-CT | 0.48 | 1 | 0.489 | ||||||
| T2WI | 13.91 | 1 | <0.001 | ||||||
| DWI | 11.08 | 1 | 0.001 | ||||||
| MR Consensus | 4.67 | 1 | 0.031 | ||||||
| PET/MR Consensus | 3.84 | 1 | 0.05 | ||||||
| (b) | |||||||||
| Hazard Ratio | Lower 95% Confidence Interval | Upper 95% Confidence Interval | p-Value | ||||||
| Progression-free survival analysis | |||||||||
| PET-CT | −0.02 | −1.34 | 0.89 | 0.696 | |||||
| T2WI | 1.17 | −0.02 | 2.37 | 0.054 | |||||
| DWI | 1.5 | −0.63 | 3.63 | 0.169 | |||||
| MR Consensus | −1.03 | −3.36 | 1.3 | 0.385 | |||||
| PET/MR Consensus | 0.74 | −0.84 | 2.32 | 0.358 | |||||
| Overall Survival Analysis | |||||||||
| PET-CT | 0.05 | −0.90 | 1 | 0.913 | |||||
| T2WI | 0.82 | −0.27 | 1.91 | 0.14 | |||||
| DWI | 0.98 | −0.65 | 2.61 | 0.238 | |||||
| MR Consensus | −0.35 | −2.03 | 1.34 | 0.683 | |||||
| PET/MR Consensus | 0.01 | −1.28 | 1.3 | 0.986 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhesi, S.S.; Frood, R.; Swift, S.; Cooper, R.; Muzumdar, S.; Jamal, M.; Scarsbrook, A. Prediction of Patient Outcomes in Locally Advanced Cervical Carcinoma Following Chemoradiotherapy—Comparative Effectiveness of Magnetic Resonance Imaging and 2-Deoxy-2-[18F]fluoro-D-glucose Imaging. Cancers 2024, 16, 476. https://doi.org/10.3390/cancers16030476
Dhesi SS, Frood R, Swift S, Cooper R, Muzumdar S, Jamal M, Scarsbrook A. Prediction of Patient Outcomes in Locally Advanced Cervical Carcinoma Following Chemoradiotherapy—Comparative Effectiveness of Magnetic Resonance Imaging and 2-Deoxy-2-[18F]fluoro-D-glucose Imaging. Cancers. 2024; 16(3):476. https://doi.org/10.3390/cancers16030476
Chicago/Turabian StyleDhesi, Simran Singh, Russell Frood, Sarah Swift, Rachel Cooper, Siddhant Muzumdar, Mehvish Jamal, and Andrew Scarsbrook. 2024. "Prediction of Patient Outcomes in Locally Advanced Cervical Carcinoma Following Chemoradiotherapy—Comparative Effectiveness of Magnetic Resonance Imaging and 2-Deoxy-2-[18F]fluoro-D-glucose Imaging" Cancers 16, no. 3: 476. https://doi.org/10.3390/cancers16030476
APA StyleDhesi, S. S., Frood, R., Swift, S., Cooper, R., Muzumdar, S., Jamal, M., & Scarsbrook, A. (2024). Prediction of Patient Outcomes in Locally Advanced Cervical Carcinoma Following Chemoradiotherapy—Comparative Effectiveness of Magnetic Resonance Imaging and 2-Deoxy-2-[18F]fluoro-D-glucose Imaging. Cancers, 16(3), 476. https://doi.org/10.3390/cancers16030476





