Simple Summary
Age-adjusted thyroid cancer incidence is lower in the Hispanic population than in the non-Hispanic White and Asian Pacific Islander populations. However, prior studies have shown an increased prevalence of advanced disease features such as larger tumor sizes and nodal involvement among Hispanics. We sought to characterize the demographic features and tumor characteristics of Hispanic thyroid cancer risk in California. We identified thyroid cancer cases from 2010 to 2020 using the California Cancer Registry. Overall, 56,638 diagnosed thyroid cancer cases were identified, including 16,852 (29.75%) Hispanics. Hispanics had the greatest female-to-male ratio disparity, average annual percentage change in incidence, and advanced disease features at diagnosis, as well as an increased mortality risk. After adjusting for demographic and tumor covariates, Hispanic ethnicity remained a significant independent variable for mortality risk. Consequently, further investigation into other possible factors associated with Hispanic ethnicity in thyroid cancer is needed.
Abstract
Background: Previous studies on Hispanic thyroid cancer cases show sex disparities and an increased prevalence of large tumor sizes and nodal involvement. Here, we characterized Hispanic thyroid cancer cases in California. Methods: We identified thyroid cancer cases from 2010 to 2020 using the California Cancer Registry by sex, race/ethnicity, histology, TNM stage, tumor size, lymph node involvement, and Charlson comorbidity score. The age-adjusted incidence rate (AAIR) and age-adjusted mortality rate (AAMR) for all causes of death were calculated. A Cox proportional hazards regression analysis was performed to evaluate the mortality risk from all causes of death by race. Results: Overall, 56,838 thyroid cancer cases were identified, including 29.75% in Hispanics. Hispanics had the highest female-to-male incidence rate ratio (IRR 3.54) and the highest prevalence of T3/T4 tumor size (28.71%), the highest N1 nodal status (32.69%), and the highest AAMR (0.79 per 100,000 people). After adjusting for demographic and tumor covariates, compared to non-Hispanic White people, Hispanic ethnicity, with an HR of 1.22 (95% CI 1.18–1.25, p < 0.0001), remained a significant independent contributor to mortality risk. Conclusions: Hispanics had the greatest female-to-male IRR ratio, a greater prevalence of advanced disease features at diagnosis, along with the highest AAMR and increased mortality risk despite adjustments for demographic and tumor covariates. Further investigation into other risk factors is needed.
1. Introduction
The incidence of thyroid cancer has risen over much of the last 30 years, with Asian Pacific Islanders and non-Hispanic white people having the highest rates of incidence during this time [1]. Previous studies have attributed this increase in incidence among these ethnic groups to the screening of thyroid nodules and associated this with healthcare access [2].
While Hispanics have had a lower incidence rate of thyroid cancer compared to non-Hispanic White people and Asian Pacific Islanders, previous studies have shown that Hispanics have a higher percentage of metastatic disease upon presentation compared to non-Hispanic White people, along with a greater proportion of larger tumors and lymph node-positive disease at diagnosis, similar to that of Asian Pacific Islanders [3,4,5,6,7,8]. Within California, previous studies using California Cancer Registry (CCR) data from 1998 to 2004 have shown a higher female-to-male ratio of thyroid cancer incidence among Hispanics, along with a higher percentage of Hispanics being diagnosed younger than 55 years of age [9]. Another study from 1999 to 2008 of the CCR on patients with well-differentiated thyroid cancer showed a higher percentage of both metastatic and regional disease at diagnosis among Hispanics compared to non-Hispanic White people, consistent with other previous studies [6].
Furthermore, there have been mixed findings on the survival of Hispanic thyroid cancer patients. One study using CCR data from 1988 to 2010 found worse thyroid-cancer-specific survival among non-Hispanic Black and Hispanic adolescent young adult thyroid cancer patients [10]. In contrast, a Surveillance, Epidemiology, and End Results (SEER) registry analysis from 2010 to 2016 showed that Hispanics and Asian Pacific Islander thyroid cancer patients had significantly better survival than non-Hispanic White patients, while another SEER study from 2007 to 2011 showed that non-Hispanic Black patients had a worse 5-year mortality rate compared to non-Hispanic White patients, but no difference between Hispanic and non-Hispanic White patients was observed [3,11].
However, thyroid cancer incidence has continued to rise, and Hispanics now constitute the largest race/ethnicity in California as of the 2020 U.S. Census [12]. Thus, our study investigates whether these changes in incidence and changing demographics in California from a more recent time period have still correlated with the higher prevalence of advanced disease features in Hispanics and the associated mortality. In addition, we wanted to evaluate the sex disparity gaps suggested in previous studies between women and men. Finally, given the suggestions that thyroid cancer incidence is tied to overdiagnosis and access to healthcare, we evaluated socioeconomic status and healthcare insurance type to see if this plays a role in the incidence change rates and mortality risk between Hispanics and other races/ethnicities.
2. Methods
2.1. Data Source/Study Population
We used the California Cancer Registry (CCR) Research File of July 2023 to identify thyroid cancer cases diagnosed from 2010 to 2020 among California (CA) residents in a SEER*Stat readable format along with statewide mortality data with causes of death information.
We examined demographic characteristics by sex (male, female), age at diagnosis (0–39, 40–49, 50–59, 60–69, 70+ years old), race/ethnicity (Hispanic, non-Hispanic (NH) White, NH Black, and Asian Pacific Islander (API)), and socioeconomic status (lowest, lower middle, middle, upper middle, highest). Socioeconomic status was derived from United States census data based on the patient’s address at the time of initial diagnosis while factoring in the following census variables using a principal components analysis: the proportion of people with a blue collar job, the proportion older than 16 years without a job, the median household income, the proportion of the population living below the 200% Federal Poverty Level, the median gross rent, the median value of owner-occupied houses, and the median education index [13,14]. Socioeconomic status was split into quartiles—lowest, lower middle, middle, upper middle, and highest.
The cases of thyroid cancer from 2010 to 2020 were identified using the International Classification of Disease for Oncology, Third Edition (ICD-O-3), with site code C73.9—thyroid gland. Tumor histotypes included follicular (ICD-O-3 codes 8290, 8330, 8331, 8332, 8333, 8335, 8337, 8339, 8346), papillary (ICD-O-3 codes 8050, 8260, 8261, 8262, 8263, 8340, 8341, 8342, 8343, 8344, 8347), medullary (ICD-O-3 codes 8345, 8510, 8346, 8347), and anaplastic (ICD-O-3 codes 8020, 8021, 8022).
We studied tumor behavior by evaluating the TNM stage (I-IV) along with the specific tumor size (T0–T4) and lymph node involvement (N0, N1) in cases from 2010 to 2020 using the American Joint Committee on Cancer (AJCC) staging 7th–8th editions [15,16]. We also looked at the Charlson comorbidity score from 2010 to 2020, categorizing them by 0 for no health comorbidities and 1+ for 1 or more health comorbidities.
The CCR collects limited treatment information within the first 6 months after a cancer diagnosis. Data specific to thyroid surgery (no surgery, lobectomy/local surgery, subtotal or near total thyroidectomy, total thyroidectomy, thyroidectomy/surgery, NOS, and unknown) were available from 2010 to 2020, along with radiation treatment data (no radiation, isotopes, radiation/combination/other, and unknown) from 2010 to 2020.
2.2. Statistical Analysis
To compare cancer risk levels among different groups, we calculated and presented the age-adjusted incidence rates and age-adjusted mortality rates by considering the number of cancer occurrences and cancer-related deaths, respectively, in relation to the size of a group’s at-risk population. The age-adjusted incidence rates (AAIRs) per 100,000 person-years of incidence and histology (papillary, follicular, medullary, anaplastic), along with the average annual percent change (AAPC) from 2010 to 2020, were calculated by sex and race/ethnicity using the CCR SEER*Stat database file of January 2023 [17]. In addition, we calculated the incidence rate ratio (IRR) of females to males. Additionally, age-adjusted mortality rates (AAMRs) for all causes of death were calculated by sex and race, with the AAPC measured from 2010 to 2020 using the California Department of Public Health’s Center for Health Statistics Death Master Files from 1970 to 2020 [18].
To evaluate the mortality risk from all causes of death by race/ethnicity in thyroid cancer cases, we performed a univariate analysis of race/ethnicity (Hispanic, NH White, NH Black, and API) and a multivariate hazard cox ratio regression analysis consisting of race/ethnicity (Hispanic, NH White, NH Black, and API), age (0–39, 40–64, 65+), sex (female, male), socioeconomic status (lowest, lower middle, middle, upper middle, highest), Charlson score (0, 1+), surgery (surgery, no surgery), radiation (isotopes, radiation/combination/other, no radiation), stage (I, II, III, IV), and lymphovascular invasion (present, not present). Given the mixed-race/ethnicity group, we excluded the American Indian/Other/Unknown race/ethnicity group from the univariate and multivariate analyses.
Tests for statistical significance were two-sided and considered statistically significant at p < 0.1. The statistical analysis was performed using SAS software, release 9.4. (SAS Institute, Cary, NC, USA).
3. Results
3.1. Demographics
Hispanics comprised 16,850 of the 56,638 total thyroid cancer cases from 2010 to 2020 in the state of California (Table 1). Hispanics had a higher percentage of cases with diagnoses at an age <40 years (34.42%) compared to the other races/ethnicities, with nearly 60% of Hispanic thyroid cancer cases being diagnosed before the age of 50. Hispanics had the highest female predominance (80.24%) among all the races/ethnicities (Table 1).

Table 1.
Demographics of thyroid cancer cases in California from 2010 to 2020.
3.2. Disease Characteristics
As with all races/ethnicities, the papillary subtype was the most prevalent thyroid cancer subtype among Hispanics, accounting for 89.83%. In addition, 8.04% of thyroid cancer cases among Hispanics were Stage IV at diagnosis, and when considering specific tumor sizes and nodal staging, Hispanics had the highest percentage of the T3 (22.40%) and T4 tumor stages (6.31%), along with the N1 nodal stage (32.69%). Furthermore, 23.04% of Hispanic thyroid cancer patients were found to have a Charlson comorbidity score of one or higher (Table 2).

Table 2.
Disease characteristics of thyroid cancer cases in California from 2010 to 2020 by race/ethnicity. “-” A case count smaller than 11 is withheld to protect patient confidentiality and the stability of the statistical estimation.
3.3. Treatment
In terms of treatment, 77.42% of Hispanic thyroid cancer patients underwent a total thyroidectomy, which was the highest proportion among all the races/ethnicities. In addition, 29.99% of Hispanic thyroid cancer patients had radioactive iodine therapy, and 1.85% received a combination of a thyroidectomy and radiation therapy (Table 3).

Table 3.
Surgery/radiation by race/ethnicity. “-” A case count smaller than 11 is withheld to protect patient confidentiality and the stability of the statistical estimation.
3.4. AAIR
The overall AAIR of thyroid cancer among Hispanics from 2010 to 2020 was the third highest amongst all the races, at 12.11 cases per 100,000 person years, with an AAIR of 5.31 per 100,000 person years in males and 18.81 per 100,000 person years in females. Hispanics had the highest AAPC of 1.0 (95% CI −0.3–2.4, p < 0.1) overall and also when stratified by males, with an AAPC of 3.4 (95% CI 1.4–5.5, p < 0.1), and females, with an AAPC of 0.6 (95% CI −1.2–2.5, p < 0.1). Hispanics had the highest female-to-male incidence rate ratio (IRR) of 3.54 compared to the other races/ethnicities (Figure 1 and Figure 2; Table 4 and Table 5).
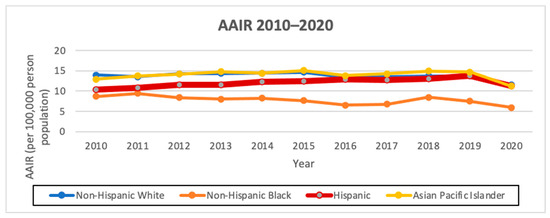
Figure 1.
AAIR of overall thyroid cancer incidence from 2010 to 2020 in California by race/ethnicity (per 100,000 people).
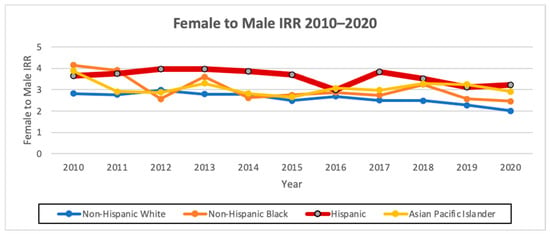
Figure 2.
Female-to-male AAIR ratio from 2010 to 2020 in California by race/ethnicity.

Table 4.
AAIR and IRR from 2010 to 2020 in California by race/ethnicity/sex (per 100,000 people).

Table 5.
AAIR AAPC in California by race/ethnicity/sex from 2010 to 2020.
3.5. AAMR
Hispanics had the highest AAMR from 2010 to 2020 at 0.79 per 100,000 person-years with an AAPC of 1.6 (95% CI −3.2–6.6, p = 1). Stratified by sex, Hispanic males had the highest AAMR from 2010 to 2020 of 0.62 per 100,000 person-years, with an AAPC of 4.2 (95% CI −6.9–16.6, p < 0.1), and Hispanic females also had the highest AAMR from 1988 to 2020 of 0.86, with an AAPC of 0.9 (95% CI −2.3–4.3, p = 1). Hispanics had the second highest female-to-male IRR of 1.38 (Figure 3; Table 6 and Table 7).
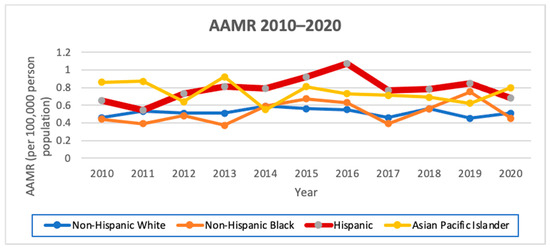
Figure 3.
AAMR from 2010 to 2020 in California by race/ethnicity.

Table 6.
AAMR and IRR overall from 2010 to 2020 in California by race/ethnicity/sex (per 100,000 people).

Table 7.
AAMR by race/ethnicity (per 100,000 people) by year from 2010 to 2020.
3.6. Adjusted Cox Hazard Ratios
The univariate analysis demonstrated that Hispanic ethnicity had the highest risk of mortality among all the races/ethnicities with an HR of 1.22 (95% CI 1.19–1.24, p < 0.0001) in both females, with an HR of 1.24 (95% CI 1.21–1.27, p < 0.0001), and males, with an HR of 1.19 (95% CI 1.14–1.24, p < 0.0001) (Table 8).

Table 8.
Univariate hazard ratio Cox regression analysis 2010–2020.
When addressing disease characteristics and socioeconomic variables (race/ethnicity, sex, age, socioeconomic status, Charlson comorbidity score, AJCC stage, lymphovascular invasion, and treatment), Hispanic ethnicity had a significant mortality risk, with an overall HR of 1.21 (95% CI 1.18–1.24, p < 0.0001), in both females, with an HR of 1.21 (95% CI 1.17–1.24, p < 0.0001), and males, with an HR of 1.20 (95% 1.14–1.27, p < 0.0001). Other significant risk factors included Asian Pacific Islander race (overall HR 1.12, 95% CI 1.09–1.15, p < 0.0001), age 65+ (overall HR 1.14, 95% CI 1.11–1.18, p < 0.0001), male sex (overall HR 1.12, 95% CI 1.09–1.15, p < 0.0001), middle (overall HR 1.06, 95% CI 1.03–1.10), lower middle (overall HR 1.05, 95% CI 1.01–1.09, p = 0.0007), and lowest socioeconomic status (overall HR 1.09, 95% CI 1.04–1.13, p < 0.0001), and stage II (overall HR 1.17, 95% CI 1.13–1.22, p < 0.0001) and stage IV thyroid cancer (overall HR 1.09, 95% CI 1.04–1.15, p < 0.0001) (Table 9).

Table 9.
Multivariate hazard ratio Cox regression analysis with race, age, sex, socioeconomic status, treatment, Charlson score, stage, and lymphovascular invasion data from 2010 to 2020 (excluding missing total n: sex: 8 (0.01%) missing, Charlson score: 13,692 (24.73%) missing, stage: 3284 (5.93%) missing, radiation: 56 (0.10%) unknown/missing, and surgery: 2987 (5.40%) unknown/missing; missing female n: Charlson score: 10,326 (24.83%) missing, stage: 2373 (5.71%) missing, radiation: 47 (0.11%) unknown/missing, and surgery: 2260 (5.44%) unknown/missing; and missing male n: Charlson score: 3361 (24.41%) missing, stage: 911 (6.62%) missing, radiation: 9 (0.07%) unknown/missing, and surgery: 721 (5.24%) unknown/missing).
4. Discussion
In our analysis of Hispanic thyroid cancer patients in California from 2010 to 2020, we show that the more than 3.5:1 ratio of female to male was the largest compared to all the other races/ethnicities, and that Hispanics had the greatest AAIR AAPC, with Hispanic males (3.4; 95% CI 1.4–5.5, p < 0.1) having a significantly positive AAIR AAPC that no other male or female racial/ethnic group demonstrated. Furthermore, a greater proportion of Hispanics were diagnosed with advanced disease features, including larger tumor sizes, positive nodal sizes, and lymphovascular invasion. Hispanic ethnicity had the highest AAMR among both males and females, with Hispanic males having a significantly increased AAPC (4.2; 95% CI −6.9–16.6, p < 0.1). This finding persisted after adjusting for different variables, including both socioeconomic and tumor-related variables; Hispanic ethnicity in California (HR: 1.21, 95% CI 1.18–1.24, p < 0.0001) persistently remained a significantly independent variable in mortality risk in thyroid cancer. Thus, our findings are consistent with studies from past years but also show a significant mortality risk among Hispanics.
In thyroid cancer, estrogen receptor-alpha cells are overexpressed, while estrogen receptor-beta cells reduce tumor growth, and the fact that the ratio of estrogen receptor-alpha is greater than estrogen receptor-beta in thyroid cancer contributes to the high female-to-male ratio. Hispanics have been shown to have similar estrogen levels to non-Hispanic White people but lower levels than non-Hispanic Black people [19,20]. But, obesity appears to be associated with the development of differentiated thyroid cancer, particularly with leptin, a hormone produced primarily by adipose tissue; leptin and its receptor (OB-R) have been implicated to be overexpressed in thyroid carcinomas, particularly papillary thyroid cancer, as multiple studies of PTC tissues have shown an expression of 50–80% of PTC tissue expressing OB-R and leptin [21,22,23,24]. Hispanics (44.8%) do have higher rates of obesity compared to non-Hispanic White people (42.2%) and Asian Pacific Islanders (17.4%), but similar rates of obesity exist between Hispanic males (45.7%) and females (43.7%) [25]. Much of the sex disparity lies in the fact that many of the cases among women were small, early-stage papillary thyroid cancer cases [26]. Yet, in our data, we see that Hispanics have a greater prevalence of large tumor sizes. We note that thyroid cancer is the second most common cancer diagnosis during pregnancy, and there have been links to recent pregnancy and infertility problems causing thyroid cancer [27,28,29]. Compared to Non-Hispanic women, Hispanic women with Medicaid insurance had 22% higher rates of severe pregnancy complications in 2020 and Hispanic women with private insurance had a 28% increase in severe pregnancy complications in 2021 [30]. The sex ratio in thyroid cancer may also be genetic, as six single-nucleotide polymorphisms (SNPs) identified in Colombia have been tied to an increased female-to-male ratio (5:1) along with larger tumor sizes [31]. As such, further investigations are needed to understand the greater sex disparity in thyroid cancer incidence among Hispanics.
Hispanics were also found to have the highest AAIR AAPC (1.0; 95% CI −0.3–2.4, p < 0.1), with Hispanic males having a significantly positive AAIR AAPC (3.4; 95% CI 1.4–5.5, p < 0.1) that no other male or female racial group demonstrated. This could be multifactorial; first, Hispanic presence in California has been increasing, as the percentage of Hispanics has increased from 19.8% in 1980 to 32.4% in 2000 to 37.6% in 2010 to 40.4% in 2020 [12,32]. Furthermore, as mentioned prior, Hispanics have higher percentages of obesity than non-Hispanic White people, which may be contributing to the AAPC [25]. In addition, it has been suggested that metabolic syndrome and its subsequent abnormal metabolism can place the body in a state of chronic inflammation, leading to thyroid-stimulating hormones inducing tumorigenesis [33]. Hispanics have been shown to have a higher prevalence of cardiovascular disease, Type II diabetes, non-alcoholic fatty liver disease, and dyslipidemia, which may indirectly play into the higher increases in thyroid cancer incidence [34].
Our data also demonstrated that Hispanics had a higher prevalence of larger tumor sizes and positive nodal sizes compared to other races/ethnicities, consistent with previous studies [3,4,5,6,7,8]. An SEER analysis from 2017 to 2018 showed that Hispanics had a greater percentage of nodal disease at initial surgery compared to non-Hispanic White people and a greater incidence of recurrent disease, while another SEER analysis showed that Hispanics were more likely to present with distant disease than non-Hispanic White people [5]. Meanwhile, a meta-analysis has shown that Hispanics have the greatest proportion among all races/ethnicities of having tumors > 4 cm in size at diagnosis [4]. Finally, another SEER analysis from 1992 to 2006 showed that Hispanics had the highest rate of anaplastic thyroid cancer diagnosed [35]. This was also seen among papillary thyroid cancer patients in an SEER analysis from 2011 to 2013 receiving adjuvant radiation, in which Hispanics and Asian Pacific Islanders had greater odds of T4, N1, or M1 disease [36].
Socioeconomic status and access to care may factor into the increased mortality risk of Hispanic ethnicity, as middle-to-low socioeconomic status was a significant independent variable in our multivariate analysis. A total of 47% of Hispanic women in Los Angeles with thyroid cancer reported financial hardship, along with 49% reporting low acculturation to the English language [37]. While Hispanics have a lower AAIR of thyroid cancer compared to Asian Pacific Islanders and non-Hispanic White people, uninsured Hispanics had the highest AAIR compared to the other races/ethnicities [1]. Hispanics have the lowest health insurance rate of any racial or ethnic group (34%), including more than twice that of non-Hispanic White people [38,39]. Hispanics also have a 25% lower median income than non-Hispanic White people [39,40]. Subsequently, the limitations of financial resources and access to care may affect Hispanics in terms of having adequate health literacy and limit their means to being able to have a well-balanced diet and places to carry out ample exercise, which are key factors in avoiding some possible triggers to thyroid cancer such as metabolic syndrome and obesity.
Despite factoring disease characteristics and socioeconomic variables into our multivariate analysis, Hispanic ethnicity (HR: 1.21, 95% CI 1.18–1.24, p < 0.05) remains a significant risk factor. Some of this may have to do with genetic changes in Hispanics relative to other races/ethnicities. Hispanics have been shown to have an increased prevalence of BRAF V600E, and this mutation has been linked in the past with increased mortality [41,42]. The BRAF V600E mutation has been shown to be associated with more advanced clinical stages and decreased responsiveness to radioiodine [43]. Even in smaller-size papillary thyroid cancer cases of <1.5 cm, a significantly higher percentage of BRAF V600E cases demonstrate aggressive features such as extrathyroidal extension and lymph node metastasis compared to non-mutated cases [44]. Among Hispanics, poorly differentiated BRAF mutant cases are significantly increased compared to non-Hispanic White people [43,45]. Furthermore, a Colombian study investigating 141 papillary thyroid cancer cases showed that the BRAF V600E and TERT C228T or C250T mutations were associated with large tumors, lymph node metastasis, extra-thyroid extension, and advanced stages, and compared to published data on U.S. white people, Colombian patients had a higher prevalence of both severe pathologic features and double-mutant tumors [46]. It has been thought that some of the key genes triggering this are related to several microRNAs targeting the TGF-beta–SMAD pathway [43]. TERT promoter mutations have been shown in 95% of thyroid tumors that have transformed from papillary carcinoma to a more aggressive anaplastic thyroid carcinoma and so pose a greater risk of transformation to a more aggressive and rare thyroid cancer compared to other mutations [47]. That being said, additional studies with larger sample sizes comparing BRAF V600E-mutant thyroid cancer cases among Hispanics and non-Hispanics are merited to better characterize the effect of BRAF V600E on the prevalence of advanced features and mortality risk.
There are clinical implications, as dabrafenib, a BRAF-mutated tyrosine kinase inhibitor, has been shown to have efficacy in well-differentiated radioactive-iodine-resistant thyroid cancer patients and, in combination with trametinib, has been shown to improve overall survival in anaplastic thyroid cancer [48,49]. Other important mutations to note that it could be worth investigating the prevalence of among Hispanics given their associations with anaplastic transformation outside of TERT-promoter mutations include subunits of the SWI/SNF complex such as SMARCA4 and alterations of the PIK3CA gene, which were found to be positive in 33% of thyroid tumor samples with a heterogenous pathology of papillary and anaplastic carcinoma components [47]. Mutations in RAS genes occur in 30–45% of follicular thyroid cancer, 30–45% of follicular-variant papillary thyroid cancer, and 20–40% of poorly differentiated thyroid cancer and are also worth investigation among Hispanics [50]. Meanwhile, RET proto-oncogene is found in medullary thyroid carcinoma, and while a small-case analysis of medullary thyroid carcinoma among Mexicans has been performed, larger studies could be conducted to evaluate any possible connection between any particularly Hispanic subgroups and medullary thyroid cancer/multiple endocrine neoplasia type 2 [51,52].
By studying additional molecular alterations in Hispanics relative to other races/ethnicities, this may help mitigate the mortality risk among the Hispanic race/ethnicity. With possible known mutations and associated targeted therapies, the need for equality in obtaining comprehensive genomic profiling of tumors and access to targeted therapy agents is critical. Hispanic ethnicity has been shown to be underrepresented among patients obtaining precision oncology assays [53]. Moreover, Hispanics have had less access to targeted drugs and are severely underrepresented in clinical trials that could provide access to these drugs and help better understand biological differences in response to these drugs [54].
The key limitations of this analysis and important considerations moving forward are that our analysis consists of Hispanics in the state of California in the United States, which means that it is not completely generalizable to Hispanic populations elsewhere. Hispanics in California consist of primarily those with Mexican descent (77%), while Hispanics in Florida consist of those with primarily Caribbean descent, notably Cuban (28%) and Puerto Rican (21%) [55,56]. Further analysis in other sectors of the U.S. may help better elucidate differences in tumor characteristics and socioeconomic challenges among different Hispanic subgroups. In addition, another limitation is the lack of genetic testing available. Comparing molecular testing for key mutations of thyroid cancer, such as BRAF V600E and RET, is important among Hispanics and other races/ethnicities and within Hispanic subgroups.
Another important aspect that is critical in future analysis is comparing differences in thyroid cancer among Hispanic immigrants from different origins and Hispanics who are U.S.-born. U.S.-born Hispanic women younger than 55 have been shown to have a significantly greater incidence of papillary thyroid cancer, while the opposite is true in older Hispanic women [9]. In another study comparing Colombians and U.S. Hispanics, U.S.-born Hispanics had a smaller mean tumor size of 20 mm vs. 27 mm than Colombians and were half as likely to have regional disease (16.7% vs. 31.15%) [31]. In another study comparing Puerto Ricans and U.S. mainland Hispanics, Puerto Rican women had an 83% increased risk of a papillary thyroid cancer diagnosis compared to U.S. Hispanics, and Puerto Rican men had a 2.2-fold increased risk compared to U.S. Hispanic men [57]. This shows that disaggregated data among Hispanics both in the U.S. and outside the U.S. by origin will be important in helping describe mortality risk in thyroid cancer. Finally, 5% of the population in California is made up of undocumented immigrants, with Mexicans and Salvadorans constituting the largest share [58]. Given that some of their data may not be identifiable in the cancer registry as a result, the magnitude of the risks involved in the incidence and mortality of thyroid cancer may be even greater.
Thus, our study highlights key important factors showing that greater attention needs to be paid to Hispanics in thyroid cancer moving forward due to the greater prevalence of large tumor sizes and positive nodal status at presentation, the greater sex disparities in women compared to men, and the increased mortality driven both by tumor characteristics and socioeconomic factors among Hispanics. As much of this could be tied to differences in the genetic makeup of thyroid cancer tumors, access to molecular testing, targeted therapies, clinical trials, and mitigating financial costs is paramount to helping bridge these disparities.
5. Conclusions
The thyroid cancer incidence among Hispanics trails behind Asian Pacific Islanders and non-Hispanic White people, but previous studies have shown that the presentation of thyroid cancer cases among Hispanics has higher incidences of large tumor sizes, nodal involvement, and distant metastasis. There also appear to be possible financial barriers and concerns about undertreatment leading to worse outcomes in thyroid cancer among Hispanics. In our analysis using the California Cancer Registry from 2010 to 2020, we demonstrated that Hispanics have the largest sex disparities, as Hispanic women have an incidence of 3.5-fold that of Hispanic men. While there is no clear reasoning for this, possible risk factors include obesity, infertility, and genetic alterations. Further, we showed that Hispanics had the highest AAPC, which could be tied into not only demographics but other factors such as high obesity rates. Consistent with previous studies, our analysis demonstrated that Hispanics had the largest proportion of T3/T4 tumor sizes and nodal involvement. Ultimately, in our multivariate analysis for thyroid cancer mortality, which included both tumor characteristics and socioeconomic factors, Hispanic race/ethnicity remained a significant risk factor. While we believe that this is multifactorial secondary to both socioeconomic factors and the higher prevalence of high-risk disease features such as large tumor sizes and nodal involvement, the incidence of genetic alterations of mutations such as BRAF V600E and TERT could play a role in such findings. Further investigation to better understand the sex disparities seen and the increased mortality risk among Hispanic thyroid cancer cases will require more analysis of genetic alterations among the Hispanic population, a disaggregation of data within Hispanic subgroups with thyroid cancer, and a study of the differences in thyroid cancer between U.S.-born and foreign-born Hispanic patients.
Author Contributions
R.C.H.: conceptualization, investigation, supervision, project administration, data curation, methodology, formal analysis, visualization, writing—original draft preparation, writing—review and editing. K.-Y.T.: data curation, methodology, formal analysis, writing—review and editing. D.J.B.: investigation, visualization, writing—original draft preparation, writing—review and editing. K.C.: investigation, visualization, writing—original draft preparation, writing—review and editing. K.Y.W.: writing—review and editing. A.W.L.: writing—review and editing. J.S.T.: writing—review and editing J.J.N.: investigation, supervision, writing—original draft preparation, writing—review and editing. L.L.: conceptualization, investigation, supervision, project administration, formal analysis, writing—original draft preparation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to the California Health and Safety Code, Section 103885; the Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 1NU58DP007156; and the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, the Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors. This work was also funded in part by the USC/Norris Comprehensive Cancer Support Grant P30CA014089 from the National Cancer Institute.
Institutional Board Review Statement
Ethical review and approval were waived for this study because the study was limited to the analysis of deidentified data.
Informed Consent Statement
Patient consent was waived due to all subjects being deidentified for this study.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
R.C.H. is a consultant for Targeted Oncology and Takeda and received honoraria from DAVA Oncology and The Dedham Group. D.J.B. has the following disclosures: Consulting: Seagen. Speakers’ Bureau: Merck. Travel and Accommodations: Merck. J.J.N. reports personal fees from AstraZeneca, Naveris, AADi Biosciences, BioAtla, Mindmed, Genentech, ANP Technologies, Sanofi, and G1 Therapuetics; research support from Genentech and Merck; intellectual property from Cansera; and ownership interests from Cansera, Epic Sciences, Indee Bio, Quantgene, Amgen, Johnson & Johnson, and Novartis. All the other authors do not report any conflicts of interest.
References
- Weeks, K.S.; Kahl, A.R.; Lynch, C.F.; Charlton, M.E. Racial/ethnic differences in thyroid cancer incidence in the United States, 2007–2014. Cancer 2018, 124, 1483–1491. [Google Scholar] [CrossRef]
- Vaccarella, S.; Franceschi, S.; Bray, F.; Wild, C.P.; Plummer, M.; Dal Maso, L. Worldwide Thyroid-Cancer Epidemic? The Increasing Impact of Overdiagnosis. N. Engl. J. Med. 2016, 375, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Nnorom, S.O.; Baig, H.; Akinyemi, O.A.; Tran, J.H.; Harris, J.; Sidhom, F.; Frederick, W.A.; Cornwell, E.E., III; Wilson, L.L. Persistence of Disparity in Thyroid Cancer Survival After Adjustments for Socioeconomic Status and Access. Am. Surg. 2022, 88, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Keane, E.; Francis, E.C.; Catháin, É.Ó.; Rowley, H. The role of race in thyroid cancer: Systematic review. J. Laryngol. Otol. 2017, 131, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Papaleontiou, M.; Evron, J.M.; Esfandiari, N.H.; Reyes-Gastelum, D.; Ward, K.C.; Hamilton, A.S.; Worden, F.; Haymart, M.R. Patient Report of Recurrent and Persistent Thyroid Cancer. Thyroid 2020, 30, 1297–1305. [Google Scholar] [CrossRef]
- Harari, A.; Li, N.; Yeh, M.W. Racial and socioeconomic disparities in presentation and outcomes of well-differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2014, 99, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Goding Sauer, A.; Ortiz, A.P.; Fedewa, S.A.; Pinheiro, P.S.; Tortolero-Luna, G.; Martinez-Tyson, D.; Jemal, A.; Siegel, R.L. Cancer Statistics for Hispanics/Latinos, 2018. CA Cancer J. Clin. 2018, 68, 425–445. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Fedewa, S.A.; Miller, K.D.; Goding-Sauer, A.; Pinheiro, P.S.; Martinez-Tyson, D.; Jemal, A. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J. Clin. 2015, 65, 457–480. [Google Scholar] [CrossRef] [PubMed]
- Horn-Ross, P.L.; Chang, E.T.; Clarke, C.A.; Keegan, T.H.; Rull, R.P.; Quach, T.; Gomez, S.L. Nativity and papillary thyroid cancer incidence rates among Hispanic women in California. Cancer 2012, 118, 216–222. [Google Scholar] [CrossRef]
- Keegan, T.H.; Grogan, R.H.; Parsons, H.M.; Tao, L.; White, M.G.; Onel, K.; Horn-Ross, P.L. Sociodemographic disparities in differentiated thyroid cancer survival among adolescents and young adults in California. Thyroid 2015, 25, 635–648. [Google Scholar] [CrossRef]
- Li, Y.; Huang, D.; Wang, B.; Mao, W.; Chen, X.; Dong, P. Socioeconomic factors are associated with the prognosis of Thyroid Cancer. J. Cancer 2021, 12, 2507–2512. [Google Scholar] [CrossRef] [PubMed]
- U.S. Census Bureau, QuickFacts California; United States. Available online: https://www.census.gov/quickfacts/fact/table/CA,US/PST045223 (accessed on 25 January 2024).
- Liu, L.; Deapen, D.; Bernstein, L. Socioeconomic status and cancers of the female breast and reproductive organs: A comparison across racial/ethnic populations in Los Angeles County, California (United States). Cancer Causes Control 1998, 9, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Yost, K.; Perkins, C.; Cohen, R.; Morris, C.; Wright, W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 2001, 12, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, W.G.; Oh, H.S.; Park, S.; Kwon, H.; Song, D.E.; Kim, T.Y.; Shong, Y.K.; Kim, W.B.; Sung, T.Y.; et al. Comparison of the Seventh and Eighth Editions of the American Joint Committee on Cancer/Union for International Cancer Control Tumor-Node-Metastasis Staging System for Differentiated Thyroid Cancer. Thyroid 2017, 27, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef] [PubMed]
- California Cancer Registry; California Department of Public Health. SEER*Stat Database: Incidence—California, Jan 2023 (1988–2020), 03/12/2023; Benchmarked 1988–1989 DOF Population Estimates, 6/12/2006; NCHS Population Estimates 1990–2020. Available online: www.ccrcal.org (accessed on 17 January 2024).
- California All Cause Mortality 1970–2020, 03/19/2023, California Department of Public Health, Center for Health Statistics Death Master Files 1970–2020. DOF Population Estimates for 1970–1987, Benchmarked DOF Population Estimates for 1988–1989, and NCHS Population Estimates for 1990–2020. Available online: https://data.ca.gov/dataset/statewide-death-profiles (accessed on 17 January 2024).
- Rohrmann, S.; Nelson, W.G.; Rifai, N.; Brown, T.R.; Dobs, A.; Kanarek, N.; Yager, J.D.; Platz, E.A. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J. Clin. Endocrinol. Metab. 2007, 92, 2519–2525. [Google Scholar] [CrossRef]
- Liu, J.; Xu, T.; Ma, L.; Chang, W. Signal Pathway of Estrogen and Estrogen Receptor in the Development of Thyroid Cancer. Front. Oncol. 2021, 11, 593479. [Google Scholar] [CrossRef]
- Zhang, G.-A.; Hou, S.; Han, S.; Zhou, J.; Wang, X.; Cui, W. Clinicopathological implications of leptin and leptin receptor expression in papillary thyroid cancer. Oncol. Lett. 2013, 5, 797–800. [Google Scholar] [CrossRef]
- Uddin, S.; Bavi, P.; Siraj, A.K.; Ahmed, M.; Al-Rasheed, M.; Hussain, A.R.; Ahmed, M.; Amin, T.; Alzahrani, A.; Al-Dayel, F.; et al. Leptin-R and its association with PI3K/AKT signaling pathway in papillary thyroid carcinoma. Endocr. Relat. Cancer 2010, 17, 191–202. [Google Scholar] [CrossRef]
- Cheng, S.P.; Chi, C.W.; Tzen, C.Y.; Yang, T.L.; Lee, J.J.; Liu, T.P.; Liu, C.L. Clinicopathologic significance of leptin and leptin receptor expressions in papillary thyroid carcinoma. Surgery 2010, 147, 847–853. [Google Scholar] [CrossRef]
- Franchini, F.; Palatucci, G.; Colao, A.; Ungaro, P.; Macchia, P.E.; Nettore, I.C. Obesity and Thyroid Cancer Risk: An Update. Int. J. Environ. Res. Public Health 2022, 19, 1116. [Google Scholar] [CrossRef]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018. NCHS Data Brief 2020, 360, 1–8. [Google Scholar]
- LeClair, K.; Bell, K.J.L.; Furuya-Kanamori, L.; Doi, S.A.; Francis, D.O.; Davies, L. Evaluation of Gender Inequity in Thyroid Cancer Diagnosis: Differences by Sex in US Thyroid Cancer Incidence Compared With a Meta-analysis of Subclinical Thyroid Cancer Rates at Autopsy. JAMA Intern. Med. 2021, 181, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Rinaldi, S.; Biessy, C.; Tjønneland, A.; Halkjaer, J.; Fournier, A.; Boutron-Ruault, M.C.; Mesrine, S.; Tikk, K.; Fortner, R.T.; et al. Reproductive and menstrual factors and risk of differentiated thyroid carcinoma: The EPIC study. Int. J. Cancer 2015, 136, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Chen, W.; Wang, J.-H.; Lin, S.-Z.; Sung, F.-C. Thyroid cancer risk in women with infertility and association with fertility medications in Taiwan. Cancer 2019, 125, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, Z.; Gu, J.; Hu, F.; Qi, Y.; Yin, Q.; Sun, Q.; Li, G.; Quan, B. Reproductive Factors but Not Hormonal Factors Associated with Thyroid Cancer Risk: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2015, 2015, 103515. [Google Scholar] [CrossRef] [PubMed]
- Blue Cross Blue Shield. Racial and Ethnic Disparities in Maternal Health. 2022. Available online: https://www.bcbs.com/the-health-of-america/reports/racial-and-ethnic-disparities-maternal-health (accessed on 8 January 2024).
- Estrada-Florez, A.P.; Bohórquez, M.E.; Sahasrabudhe, R.; Prieto, R.; Lott, P.; Duque, C.S.; Donado, J.; Mateus, G.; Bolaños, F.; Vélez, A.; et al. Clinical features of Hispanic thyroid cancer cases and the role of known genetic variants on disease risk. Medicine 2016, 95, e4148. [Google Scholar] [CrossRef] [PubMed]
- Wikipedia Contributors. (2023, November 6). Hispanics and Latinos in California. In Wikipedia, The Free Encyclopedia. Available online: https://en.wikipedia.org/w/index.php?title=Hispanics_and_Latinos_in_California&oldid=1183802731 (accessed on 26 January 2024).
- Li, L.-R.; Song, J.-L.; Liu, H.-Q.; Chen, C. Metabolic syndrome and thyroid Cancer: Risk, prognosis, and mechanism. Discov. Oncol. 2023, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Alemán, J.O.; Almandoz, J.P.; Frias, J.P.; Galindo, R.J. Obesity among Latinx people in the United States: A review. Obesity 2023, 31, 329–337. [Google Scholar] [CrossRef]
- Aschebrook-Kilfoy, B.; Ward, M.H.; Sabra, M.M.; Devesa, S.S. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid 2011, 21, 125–134. [Google Scholar] [CrossRef]
- Moten, A.S.; Zhao, H.; Intenzo, C.M.; Willis, A.I. Disparity in the use of adjuvant radioactive iodine ablation among high-risk papillary thyroid cancer patients. Eur. J. Surg. Oncol. 2019, 45, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.W.; Reyes-Gastelum, D.; Veenstra, C.M.; Hamilton, A.S.; Banerjee, M.; Haymart, M.R. Financial Hardship among Hispanic Women with Thyroid Cancer. Thyroid 2021, 31, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Monnat, S.M. The New Destination Disadvantage: Disparities in Hispanic Health Insurance Coverage Rates in Metropolitan and Nonmetropolitan New and Established Destinations. Rural Sociol. 2017, 82, 3–43. [Google Scholar] [CrossRef]
- Kronenfeld, J.P.; Graves, K.D.; Penedo, F.J.; Yanez, B. Overcoming Disparities in Cancer: A Need for Meaningful Reform for Hispanic and Latino Cancer Survivors. Oncologist 2021, 26, 443–452. [Google Scholar] [CrossRef]
- Real Median Household Income by Race and Hispanic Origin: 1967 to 2017. U.S. Department of Commerce. Available online: https://www.census.gov/content/dam/Census/library/visualizations/2018/demo/p60-263/figure1.pdf.2018 (accessed on 25 February 2024).
- Li, J.; Zhang, S.; Zheng, S.; Zhang, D.; Qiu, X. The BRAF V600E mutation predicts poor survival outcome in patients with papillary thyroid carcinoma: A meta analysis. Int. J. Clin. Exp. Med. 2015, 8, 22246–22253. [Google Scholar]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, R.M.; et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013, 309, 1493–1501. [Google Scholar] [CrossRef]
- Boucai, L.; Seshan, V.; Williams, M.; Knauf, J.A.; Saqcena, M.; Ghossein, R.A.; Fagin, J.A. Characterization of Subtypes of BRAF-Mutant Papillary Thyroid Cancer Defined by Their Thyroid Differentiation Score. J. Clin. Endocrinol. Metab. 2022, 107, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.A.; Bogatchenko, M.; Pusztaszeri, M.; Forest, V.I.; Hier, M.P.; Yang, J.W.; Tamilia, M.; Payne, R.J. BRAF V600E mutation is associated with aggressive features in papillary thyroid carcinomas ≤1.5 cm. J. Otolaryngol. Head Neck Surg. 2021, 50, 63. [Google Scholar] [CrossRef]
- Nikita, M.E.; Jiang, W.; Cheng, S.M.; Hantash, F.M.; McPhaul, M.J.; Newbury, R.O.; Phillips, S.A.; Reitz, R.E.; Waldman, F.M.; Newfield, R.S. Mutational Analysis in Pediatric Thyroid Cancer and Correlations with Age, Ethnicity, and Clinical Presentation. Thyroid 2016, 26, 227–234. [Google Scholar] [CrossRef]
- Estrada-Flórez, A.P.; Bohórquez, M.E.; Vélez, A.; Duque, C.S.; Donado, J.H.; Mateus, G.; Panqueba-Tarazona, C.; Polanco-Echeverry, G.; Sahasrabudhe, R.; Echeverry, M.; et al. BRAF and TERT mutations in papillary thyroid cancer patients of Latino ancestry. Endocr. Connect. 2019, 8, 1310–1317. [Google Scholar] [CrossRef]
- Oishi, N.; Kondo, T.; Ebina, A.; Sato, Y.; Akaishi, J.; Hino, R.; Yamamoto, N.; Mochizuki, K.; Nakazawa, T.; Yokomichi, H.; et al. Molecular alterations of coexisting thyroid papillary carcinoma and anaplastic carcinoma: Identification of TERT mutation as an independent risk factor for transformation. Mod. Pathol. 2017, 30, 1527–1537. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.; Soria, J.C.; Wen, P.Y.; Zielinski, C.C.; Cabanillas, M.E.; Boran, A.; et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: Updated analysis from the phase II ROAR basket study. Ann. Oncol. 2022, 33, 406–415. [Google Scholar] [CrossRef]
- Falchook, G.S.; Millward, M.; Hong, D.; Naing, A.; Piha-Paul, S.; Waguespack, S.G.; Cabanillas, M.E.; Sherman, S.I.; Ma, B.; Curtis, M.; et al. BRAF inhibitor dabrafenib in patients with metastatic BRAF-mutant thyroid cancer. Thyroid 2015, 25, 71–77. [Google Scholar] [CrossRef]
- Xing, M. Clinical utility of RAS mutations in thyroid cancer: A blurred picture now emerging clearer. BMC Med. 2016, 14, 12. [Google Scholar] [CrossRef]
- González, B.; Salcedo, M.; Medrano, M.E.; Mantilla, A.; Quiñónez, G.; Benítez-Bribiesca, L.; Rodríguez-Cuevas, S.; Cabrera, L.; de León, B.; Altamirano, N.; et al. RET oncogene mutations in medullary thyroid carcinoma in Mexican families. Arch. Med. Res. 2003, 34, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Garcia, E.; Vidal-Millan, S.; Lopez-Yañez, A.; Torres, J.A.; Guadarrama-Orozco, J.A.; Lino-Silva, L.S.; Meneses-Garcia, A.; Astudillo-de la Vega, H.; Garcia, M.G. Search of the p.M918T Mutation in the RET Oncogene in Mexican Adult Patients with Medullary Thyroid Carcinoma. Exp. Clin. Endocrinol. Diabetes 2017, 125, 218–222. [Google Scholar] [CrossRef]
- Mata, D.A.; Rotenstein, L.S.; Ramos, M.A.; Jena, A.B. Disparities According to Genetic Ancestry in the Use of Precision Oncology Assays. N. Engl. J. Med. 2023, 388, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Gutarra, M.R.; Duma, N.; Aristizabal, P.; Segarra-Vazquez, B.; Borno, H.; Halbert, C.H.; Simon, M.A.; Velazquez, A.I. The Problem of Hispanic/Latinx Under-Representation in Cancer Clinical Trials. JCO Oncol. Pract. 2022, 18, 380–384. [Google Scholar] [CrossRef]
- UCLA Latino Policy & Politics Institute. 15 Facts About Latino Well-Being in Florida. Available online: https://latino.ucla.edu/research/15-facts-latinos-florida/ (accessed on 8 January 2024).
- Public Policy Institute of California. California’s Hispanic Community. Available online: https://www.ppic.org/blog/californias-hispanic-community/#:~:text=%E2%80%9CHispanic%E2%80%9D%20is%20a%20label%20that,77%25%20from%20Mexico%20alone (accessed on 8 January 2024).
- Tortolero-Luna, G.; Torres-Cintrón, C.R.; Alvarado-Ortiz, M.; Ortiz-Ortiz, K.J.; Zavala-Zegarra, D.E.; Mora-Piñero, E. Incidence of thyroid cancer in Puerto Rico and the US by racial/ethnic group, 2011–2015. BMC Cancer 2019, 19, 637. [Google Scholar] [CrossRef] [PubMed]
- Passel, J.S.; Krogstad, J.M. What We Know about Unauthorized Immigrants Living in the U.S; Pew Research Center: Washington, DC, USA, 2023; Available online: https://www.pewresearch.org/short-reads/2023/11/16/what-we-know-about-unauthorized-immigrants-living-in-the-us/ (accessed on 8 January 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).