Latest Progress in Risk-Adapted Surgery for Medullary Thyroid Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Databases and Search Strategy
2.3. Evidence-Based Medicine Grading of the Retrieved Literature
3. Results
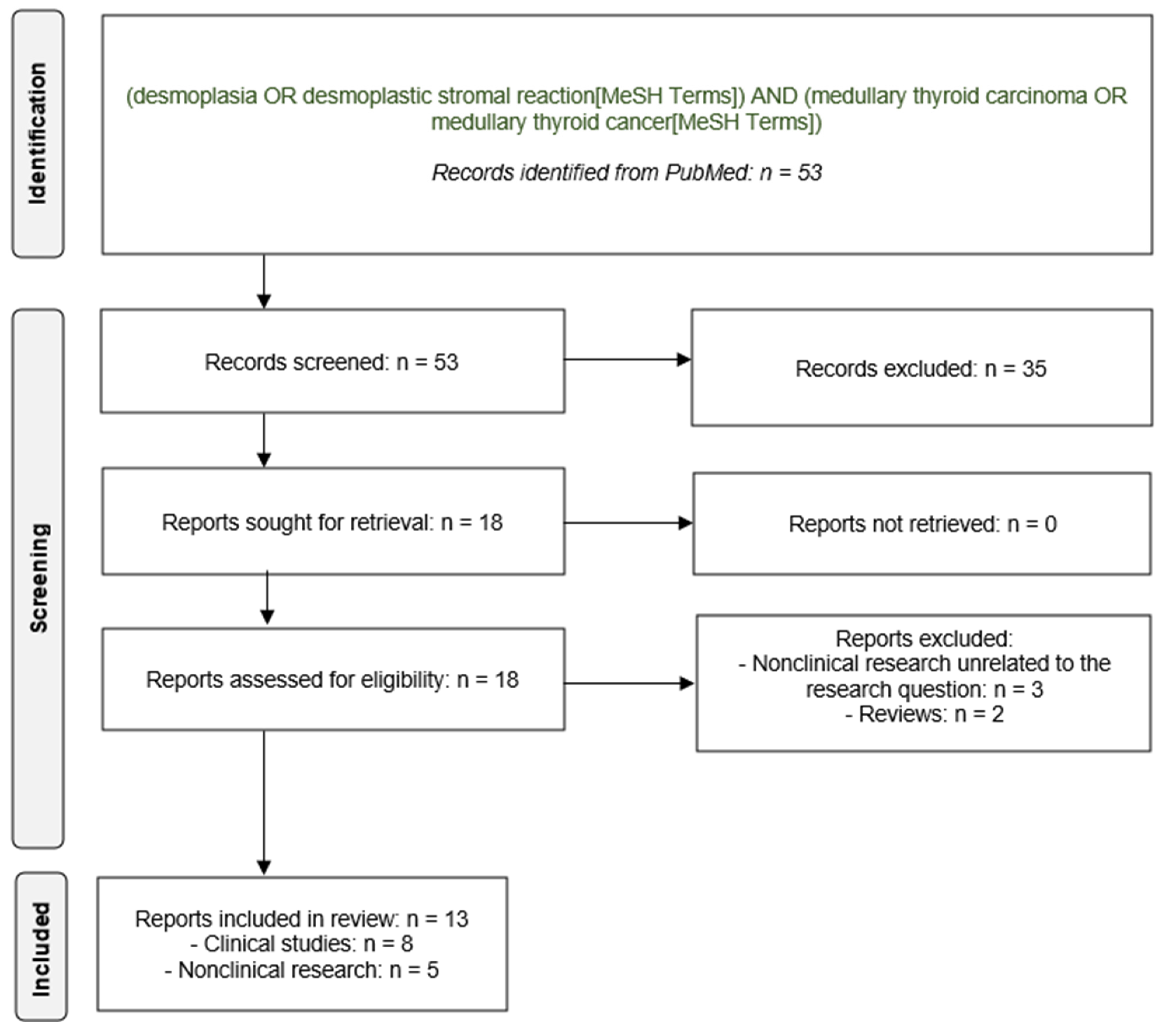
3.1. Results of the Literature Search
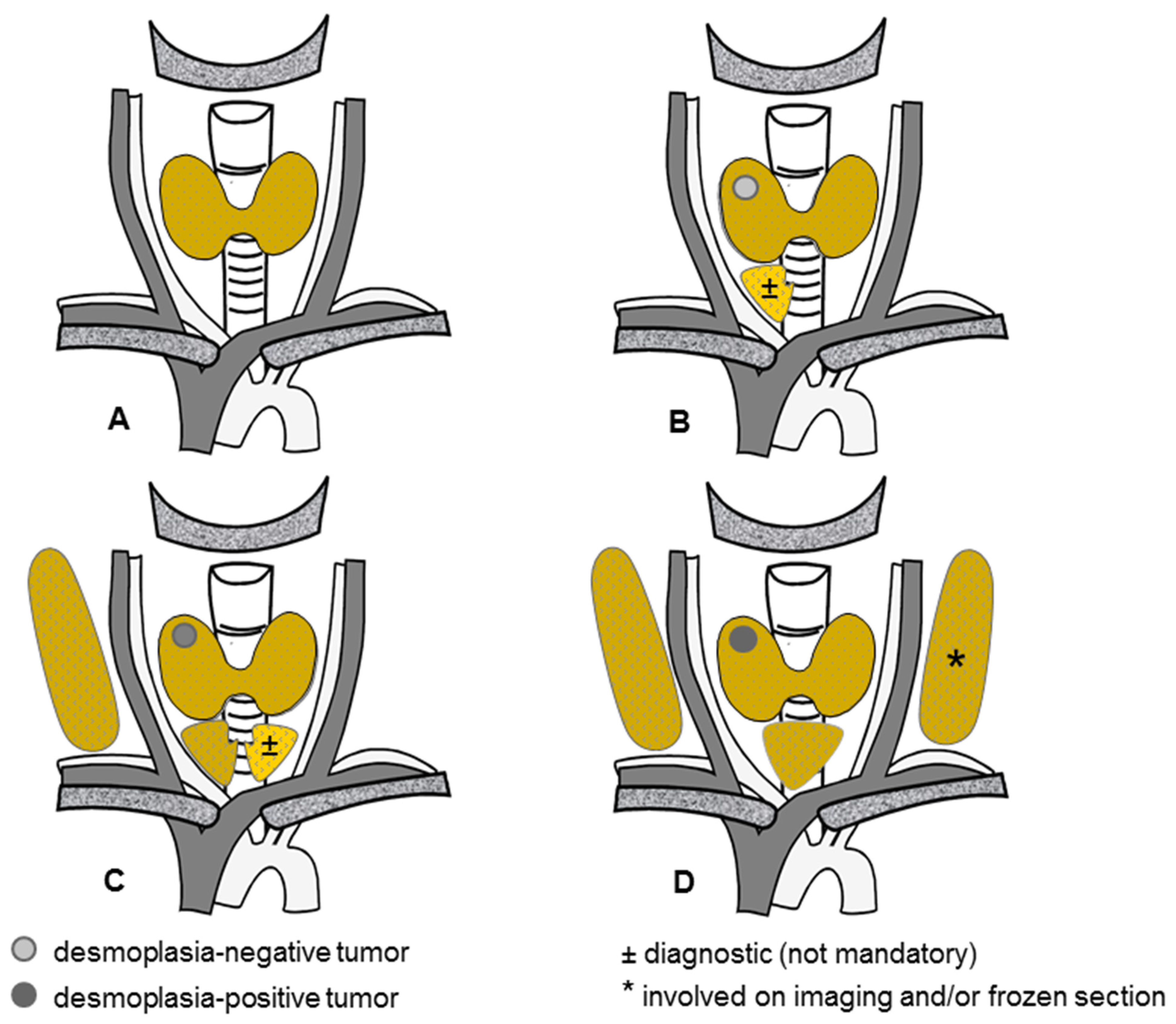
3.2. Experimental Data on Desmoplasia and Medullary Thyroid Cancer
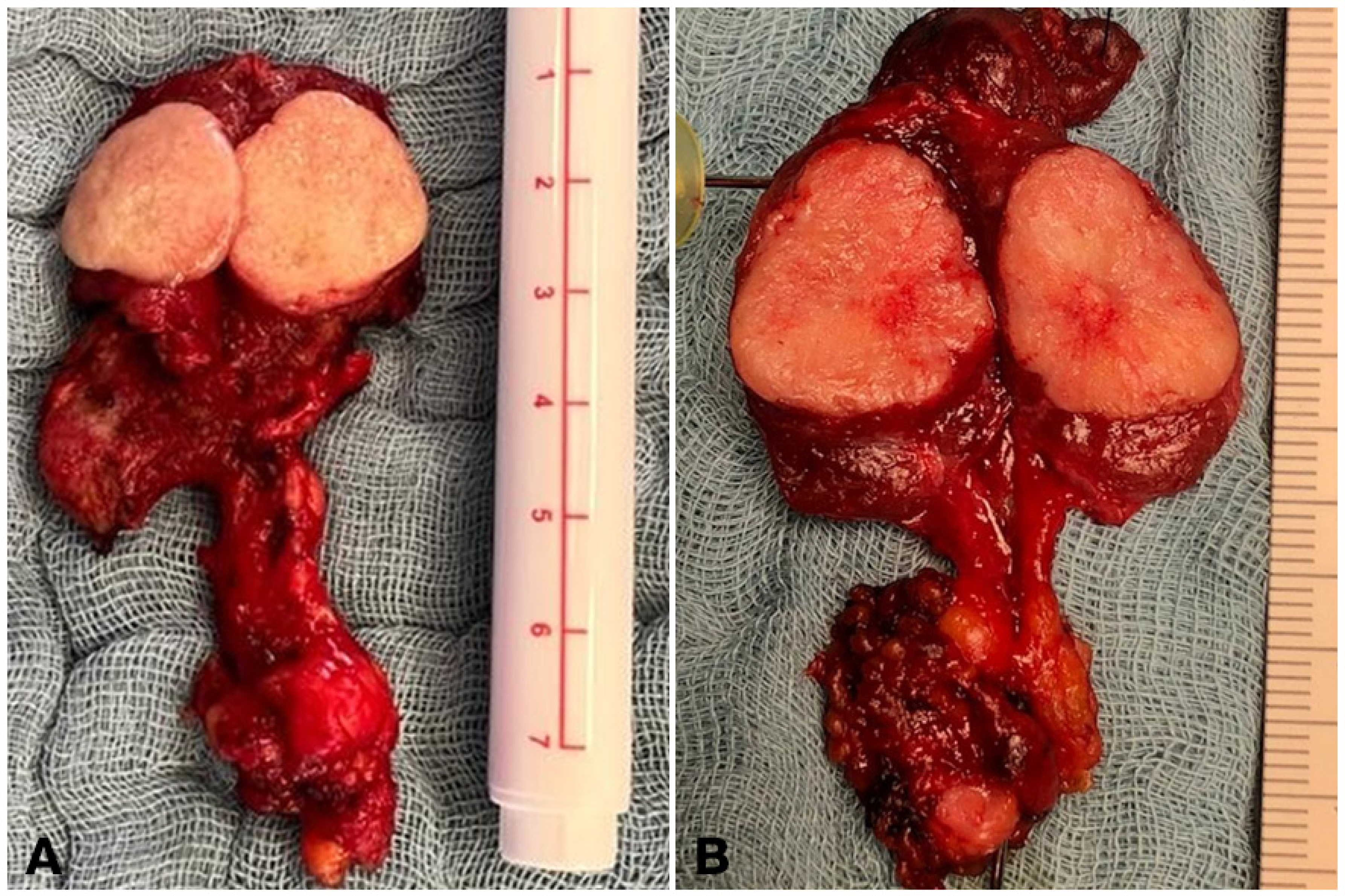
3.3. Primary Tumor Desmoplasia in Sporadic Medullary Thyroid Cancer
3.4. Primary Tumor Desmoplasia in Hereditary Medullary Thyroid Cancer
3.5. Nodal Desmoplasia in Medullary Thyroid Cancer
4. Discussion
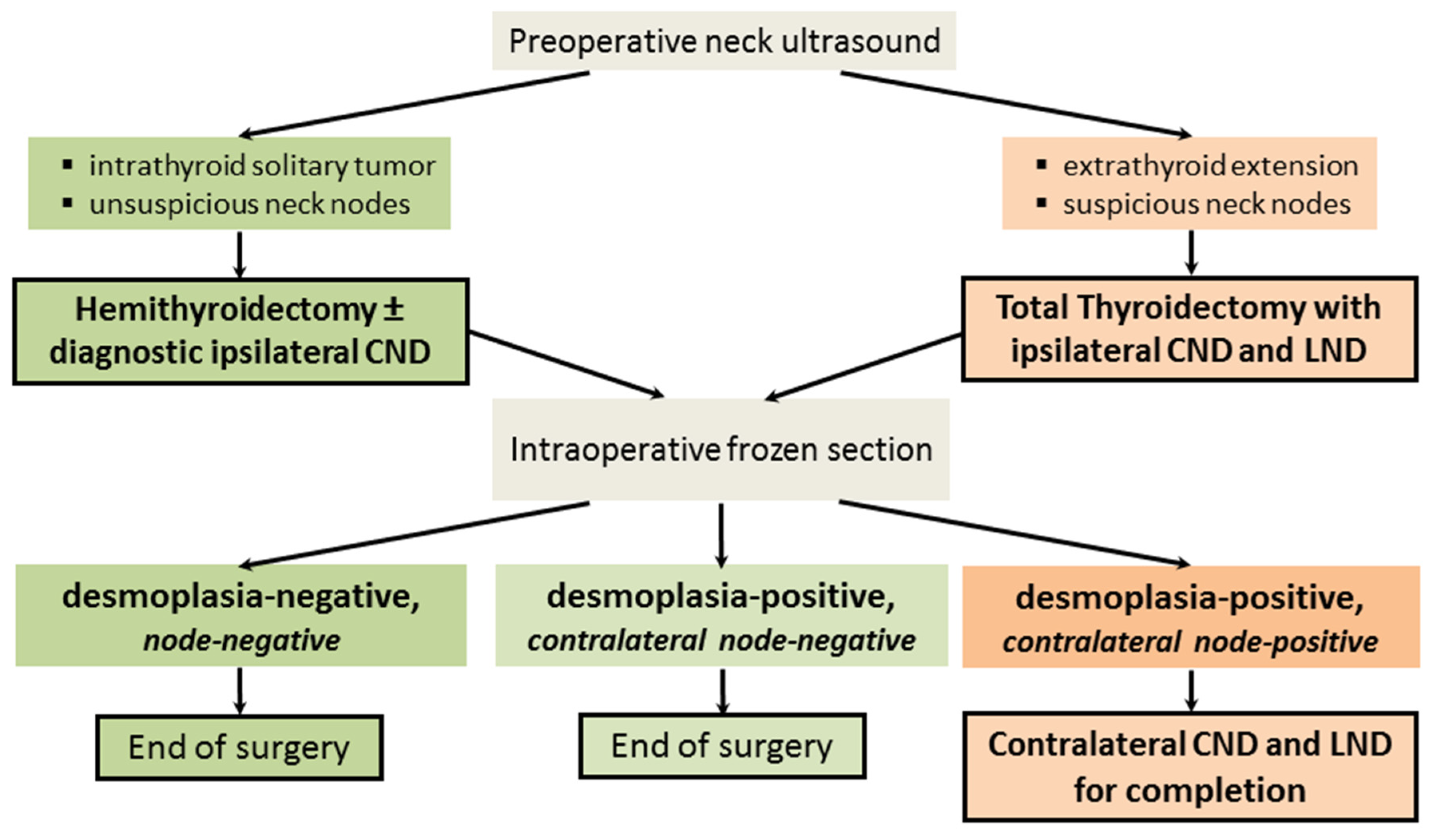
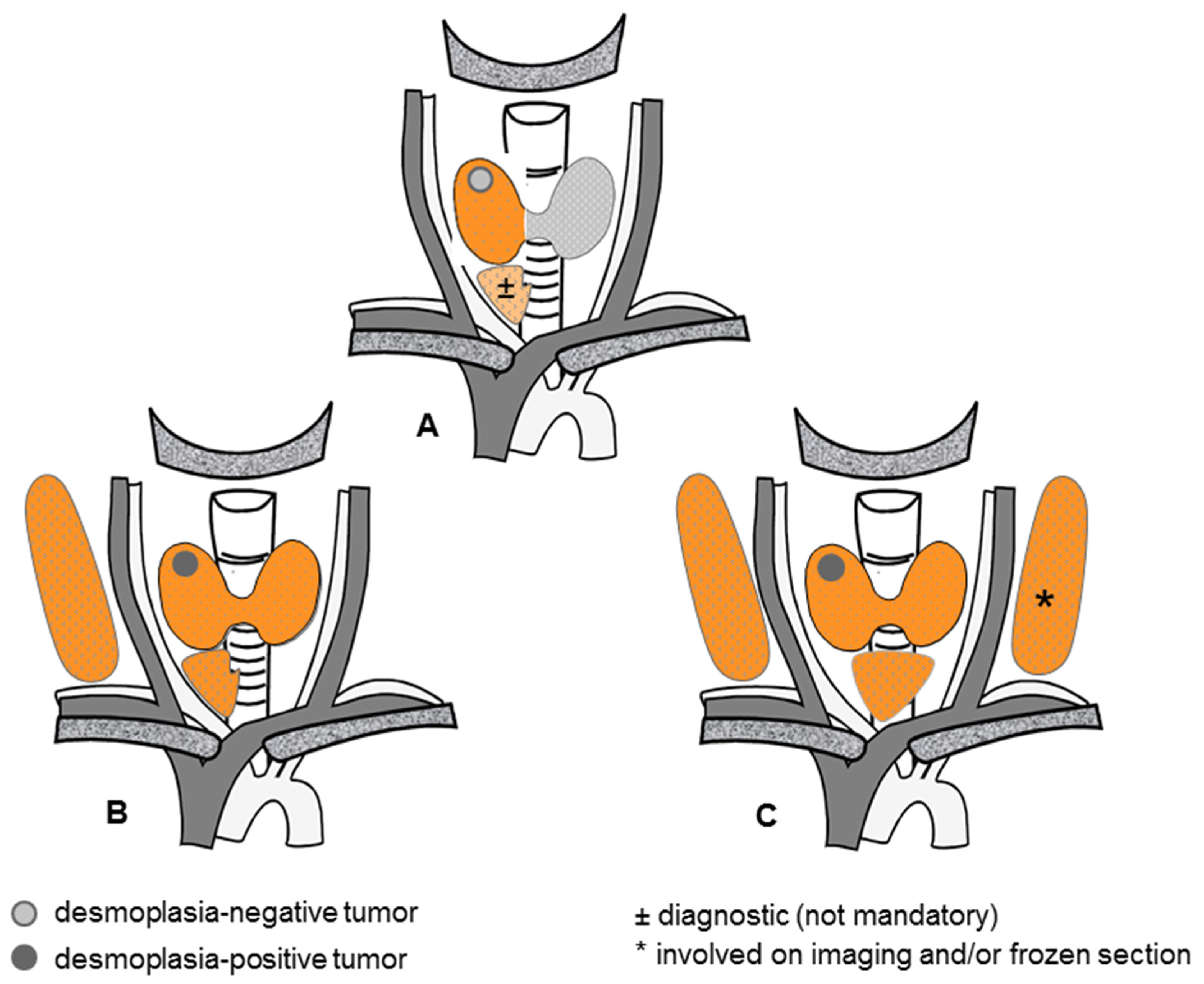
4.1. Key Results
4.2. Interpretation
4.3. Generalizability
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Machens, A.; Dralle, H. Surgical cure rates of sporadic medullary thyroid cancer in the era of calcitonin screening. Eur. J. Endocrinol. 2016, 175, 219–228. [Google Scholar] [CrossRef]
- Randle, R.W.; Balentine, C.J.; Leverson, G.E.; Havlena, J.A.; Sippel, R.S.; Schneider, D.F.; Pitt, S.C. Trends in the presentation, treatment, and survival of patients with medullary thyroid cancer over the past 30 years. Surgery 2017, 161, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Poplawski, A.; Vorländer, C.; Dotzenrath, C.; Ringelband, R.; Schabram, J.; Passler, C.; Zielke, A.; Schlegel, N.; Nies, C.; et al. Preoperative calcitonin testing improves the diagnosis of medullary thyroid carcinoma in female and male patients. Eur. J. Endocrinol. 2022, 186, 223–231. [Google Scholar] [CrossRef]
- Machens, A.; Holzhausen, H.J.; Dralle, H. Contralateral cervical and mediastinal lymph node metastasis in medullary thyroid cancer: Systemic disease? Surgery 2006, 139, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Borget, I.; De Pouvourville, G.; Schlumberger, M. Editorial: Calcitonin determination in patients with nodular thyroid disease. J. Clin. Endocrinol. Metab. 2007, 92, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Oleinikov, K.; Yaakov, E.; Mizrachi, A.; Hirsch, D.; Hirshoren, N.; Bachar, G.; Robenshtok, E.; Benbassat, C.; Atlan, K.; Mizrahi, I.; et al. A comparison of outcomes in medullary thyroid carcinoma patients with and without a preoperative diagnosis: A multicenter retrospective cohort study. Thyroid 2023, 33, 578–585. [Google Scholar] [CrossRef]
- Welch, H.G.; Gorski, D.H.; Albertsen, P.C. Trends in metastatic breast and prostate cancer—Lessons in cancer dynamics. N. Engl. J. Med. 2015, 373, 1685–1687. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Lorenz, K.; Dralle, H. Prediction of biochemical cure in patients with medullary thyroid cancer. Br. J. Surg. 2020, 107, 695–704. [Google Scholar] [CrossRef]
- Krueger, J.E.; Maitra, A.; Albores-Saavedra, J. Inherited medullary microcarcinoma of the thyroid: A study of 11 cases. Am. J. Surg. Pathol. 2000, 24, 853–858. [Google Scholar] [CrossRef]
- Kaserer, K.; Scheuba, C.; Neuhold, N.; Weinhäusel, A.; Haas, O.A.; Vierhapper, H.; Niederle, B. Sporadic versus familial medullary thyroid microcarcinoma: A histopathologic study of 50 consecutive patients. Am. J. Surg. Pathol. 2001, 25, 1245–1251. [Google Scholar] [CrossRef]
- Scheuba, C.; Kaserer, K.; Kaczirek, K.; Asari, R.; Niederle, B. Desmoplastic stromal reaction in medullary thyroid cancer—An intraoperative “marker” for lymph node metastases. World J. Surg. 2006, 30, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Aubert, S.; Berdelou, A.; Gnemmi, V.; Behal, H.; Caiazzo, R.; D’herbomez, M.; Pigny, P.; Wemeau, J.L.; Carnaille, B.; Renaud, F.; et al. Large sporadic thyroid medullary carcinomas: Predictive factors for lymph node involvement. Virchows Arch. 2018, 472, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Kaatzsch, P.; Lorenz, K.; Horn, L.C.; Wickenhauser, C.; Schmid, K.W.; Dralle, H.; Siebolts, U. Abandoning node dissection for desmoplasia-negative encapsulated unifocal sporadic medullary thyroid cancer. Surgery 2022, 171, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Dralle, H.; Brandenburg, T.; Weber, F.; Führer-Sakel, D.; Theurer, S.; Baba, H.A.; Schmid, K.W.; Machens, A. Sporadic noninvasive medullary thyroid neoplasm: A desmoplasia-negative, unifocal, nonmetastatic tumor cured by hemithyroidectomy. Surgery 2023, 174, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, J.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sackett, D.L. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest 1989, 95, 2S–4S. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, S.; Schäfer, M.; Rousson, V.; Clavien, P.A. Evidence-based treatment of acute pancreatitis: A look at established paradigms. Ann. Surg. 2006, 243, 154–168. [Google Scholar] [CrossRef]
- Koperek, O.; Scheuba, C.; Puri, C.; Birner, P.; Haslinger, C.; Rettig, W.; Niederle, B.; Kaserer, K.; Garin Chesa, P. Molecular characterization of the desmoplastic tumor stroma in medullary thyroid carcinoma. Int. J. Oncol. 2007, 31, 59–67. [Google Scholar] [CrossRef]
- Koperek, O.; Bergner, O.; Pichlhöfer, B.; Oberndorfer, F.; Hainfellner, J.A.; Kaserer, K.; Horvat, R.; Harris, A.L.; Niederle, B.; Birner, P. Expression of hypoxia-associated proteins in sporadic medullary thyroid cancer is associated with desmoplastic stroma reaction and lymph node metastasis and may indicate somatic mutations in the VHL gene. J. Pathol. 2011, 225, 63–72. [Google Scholar] [CrossRef]
- Vogelstein, B.; Kinzler, K.W. The path to cancer—Three strikes and you’re out. N. Engl. J. Med. 2015, 373, 1895–1898. [Google Scholar] [CrossRef]
- Machens, A.; Dralle, H. Clinical relevance of desmoplasia in medullary thyroid carcinoma. Histopathology 2008, 53, 481. [Google Scholar] [CrossRef]
- Koperek, O.; Scheuba, C.; Cherenko, M.; Neuhold, N.; De Micco, C.; Schmid, K.W.; Niederle, B.; Kaserer, K. Desmoplasia in medullary thyroid carcinoma: A reliable indicator of metastatic potential. Histopathology 2008, 52, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Koperek, O.; Scheuba, C.; Cherenko, M.; Neuhold, N.; De Micco, C.; Schmid, K.W.; Niederle, B.; Kaserer, K. Clinical relevance of desmoplasia in medullary thyroid carcinoma. Histopathology 2008, 53, 482–483. [Google Scholar] [CrossRef]
- Machens, A.; Bensch, C.; Wickenhauser, C.; Dralle, H.; Lorenz, K. Comparing nodal with primary tumor desmoplasia uncovers metastatic patterns in multiple endocrine neoplasia 2B. Eur. J. Endocrinol. 2024, 190, K21–K25. [Google Scholar] [CrossRef] [PubMed]
- Niederle, M.B.; Riss, P.; Selberherr, A.; Koperek, O.; Kaserer, K.; Niederle, B.; Scheuba, C. Omission of lateral lymph node dissection in medullary thyroid cancer without a desmoplastic stromal reaction. Br. J. Surg. 2021, 108, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramírez, J.; Luengo, P.; Mercader, E.; Quintana, A.; Muñoz de Nova, J.L.; Febrero, B.; Ruz-Caracuel, I.; Rodríguez, J.M. Desmoplastic reaction in medullary thyroid carcinoma predicts presence of lymph node metastasis. Br. J. Surg. 2023, 110, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Shin, S.C.; Lim, Y.S.; Lee, J.C.; Wang, S.G.; Son, S.M.; Kim, I.J.; Lee, B.J. Tumor location-dependent skip lateral cervical lymph node metastasis in papillary thyroid cancer. Head Neck 2014, 36, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Likhterov, I.; Reis, L.L.; Urken, M.L. Central compartment management in patients with papillary thyroid cancer presenting with metastatic disease to the lateral neck: Anatomic pathways of lymphatic spread. Head Neck 2017, 39, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Holzhausen, H.J.; Dralle, H. Skip metastases in thyroid cancer leaping the central lymph node compartment. Arch. Surg. 2004, 139, 43–45. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, T.H.; Ku, Y.H.; Kim, H.I.; Lee, G.H.; Kim, M.J. Central lymph node metastasis in papillary thyroid microcarcinoma can be stratified according to the number, the size of metastatic foci, and the presence of desmoplasia. Surgery 2015, 157, 111–118. [Google Scholar] [CrossRef]
- Nakayama, H.; Ohuchida, K.; Yoshida, M.; Miyazaki, T.; Takesue, S.; Abe, T.; Endo, S.; Koikawa, K.; Okumura, T.; Moriyama, T.; et al. Degree of desmoplasia in metastatic lymph node lesions is associated with lesion size and poor prognosis in pancreatic cancer patients. Oncol. Lett. 2017, 14, 3141–3147. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A., Jr.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fuchs, T.L.; Ahmadi, S.; Alghamdi, M.; Alzumaili, B.; Bani, M.A.; Baudin, E.; Chou, A.; De Leo, A.; Fagin, J.A.; et al. International Medullary Thyroid Carcinoma Grading System: A validated grading system for medullary thyroid carcinoma. J. Clin. Oncol. 2022, 40, 96–104. [Google Scholar] [CrossRef]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simões, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.; Barletta, J.A.; Wenig, B.M.; Al Ghuzlan, A.; Kakudo, K.; et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016, 2, 1023–1029. [Google Scholar] [CrossRef]
| Level of Evidence [16] | Type of Trial [16] | Criteria for Classification [17] | Grade of Recommendation |
|---|---|---|---|
| I | Large randomized trials with clear-cut results (and low risk of error). | Sample size calculation provided and fulfilled, study endpoint provided. | A |
| II | Small randomized trials with uncertain results (and moderate-to-high risk of errors). | Matched analysis, sample size calculation not given or not fulfilled, study endpoints not provided, convincing comparative studies. | B |
| III | Nonrandomized, contemporaneous controls. | Noncomparative, prospective. | C |
| IV | Nonrandomized, historical controls. | Retrospective analysis, cohort studies. | – |
| V | No control, case series only; opinion of experts [17]. | Small series, review articles. | – |
| Reference | Scheuba et al. (2006) [11] | Dralle et al. (2023) [14] | |||||
|---|---|---|---|---|---|---|---|
| Primary Tumor Desmoplasia | Definitive Histology | p | Frozen Section * | p | |||
| – | + | – | + | ||||
| No. of Patients | 32 | 88 | 19 | 6 | |||
| Age at thyroidectomy, y | 52.4 ± 11 | 62 ± 12 | 0.0003 | 54 [46; 66] (29–80) | 54 [45; 72] (27–79) | 0.985 | |
| Sex, no. of male patients | 17 (53) | 35 (40) | 0.216 | 6 (32) | 4 (67) | 0.175 | |
| Preoperative basal calcitonin level, pg/mL | – | – | 315 [125; 873] (34.3–49,167) | 280 [194; 1327] (79.8–1406) | 0.975 | ||
| Preoperative carcinoembryonic antigen (CEA) level, ng/ml | 6.2 ± 6.5 (n = 12) | 85.9 ± 224 (n = 50) | 0.08 | 20 [3; 54] (0.5–410) (n = 18) | 10 [1; 21] (0.5–22.6) | 0.140 | |
| No. of patients with | hemithyroidectomy | – | – | >0.999 | 12 (63) | 2 (33) $ | 0.350 |
| total thyroidectomy | 32 (100) | 88 (100) | 7 (37) † | 4 (67) | |||
| No. of patients with central neck dissection | none | 0 | 0 | >0.999 | 2 (11) | 0 (0) | 0.335 |
| ipsilateral | 32 (100) | 88 (100) | 13 (68) | 3 (50) | |||
| contralateral | 4 (21) | 3 (50) | |||||
| No. of patients with lateral neck dissection | ipsilateral | 32 (100) | 88 (100) | >0.999 | 2 (11) | 5 (83) | 0.002 |
| contralateral | 0 (0) | 0 (0) | >0.999 | ||||
| Primary tumor diameter, mm, median [IQR] (range) | – | – | 17 [9; 25] (5–90) | 15 [8; 20] (6–21) | 0.427 | ||
| No. of patients with multifocal growth | – | – | 0 (0) | 2 (33) ‡ | 0.050 | ||
| No. of patients with extrathyroid extension | 0 (0) | 11 (14) | 0.035 | 0 (0) | 0 (0) | >0.999 | |
| No. of patients with node metastases | 0 (0) | 31 (35) | <0.001 | 0 (0) | 6 (100) | <0.001 | |
| No. of node metastases removed | – | – | 0 [0; 0] (0–0) (n = 17) | 3.5 [3; 11] (1–14) | <0.001 | ||
| No. of nodes removed | – | – | 6 [4.5; 15.5] (1–25) (n = 17) | 31 [22; 43] (20–53) | <0.001 | ||
| No. of patients with biochemical cure | 32 (100) | 66 (75) | <0.001 | 19 (100) | 5 (83) | 0.240 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machens, A.; Lorenz, K.; Brandenburg, T.; Führer, D.; Weber, F.; Dralle, H. Latest Progress in Risk-Adapted Surgery for Medullary Thyroid Cancer. Cancers 2024, 16, 917. https://doi.org/10.3390/cancers16050917
Machens A, Lorenz K, Brandenburg T, Führer D, Weber F, Dralle H. Latest Progress in Risk-Adapted Surgery for Medullary Thyroid Cancer. Cancers. 2024; 16(5):917. https://doi.org/10.3390/cancers16050917
Chicago/Turabian StyleMachens, Andreas, Kerstin Lorenz, Tim Brandenburg, Dagmar Führer, Frank Weber, and Henning Dralle. 2024. "Latest Progress in Risk-Adapted Surgery for Medullary Thyroid Cancer" Cancers 16, no. 5: 917. https://doi.org/10.3390/cancers16050917
APA StyleMachens, A., Lorenz, K., Brandenburg, T., Führer, D., Weber, F., & Dralle, H. (2024). Latest Progress in Risk-Adapted Surgery for Medullary Thyroid Cancer. Cancers, 16(5), 917. https://doi.org/10.3390/cancers16050917






