A 16-Year Single-Center Series of Trachea Resections for Locally Advanced Thyroid Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
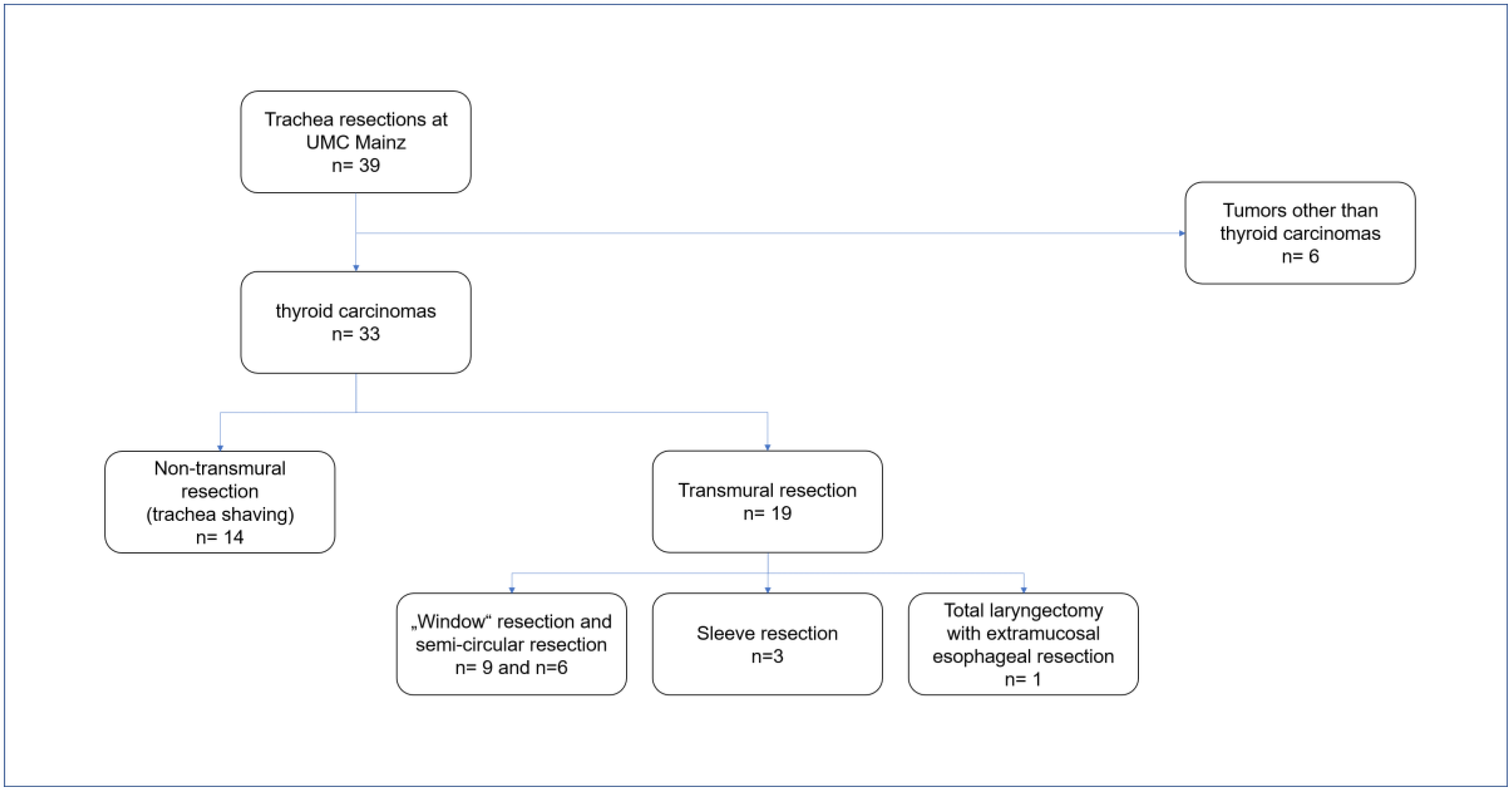
2. Materials and Methods
2.1. Non-Transmural Resection
Trachea Shaving
2.2. Transmural Resection
2.2.1. Partial “Window” and Near-Circular Resections
2.2.2. Sleeve Resection
2.2.3. Total Laryngectomy with Extramucosal Esophageal Resection
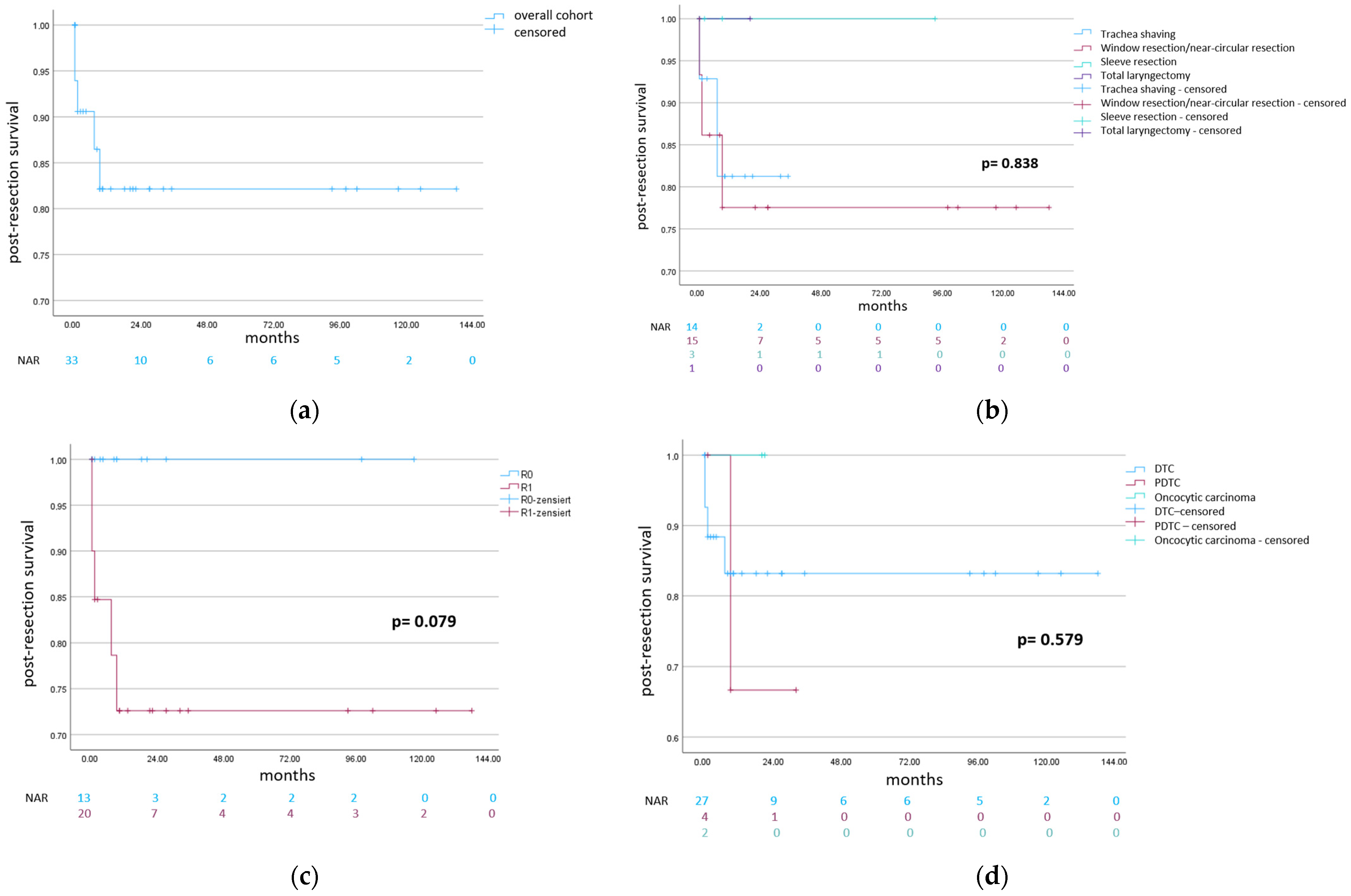
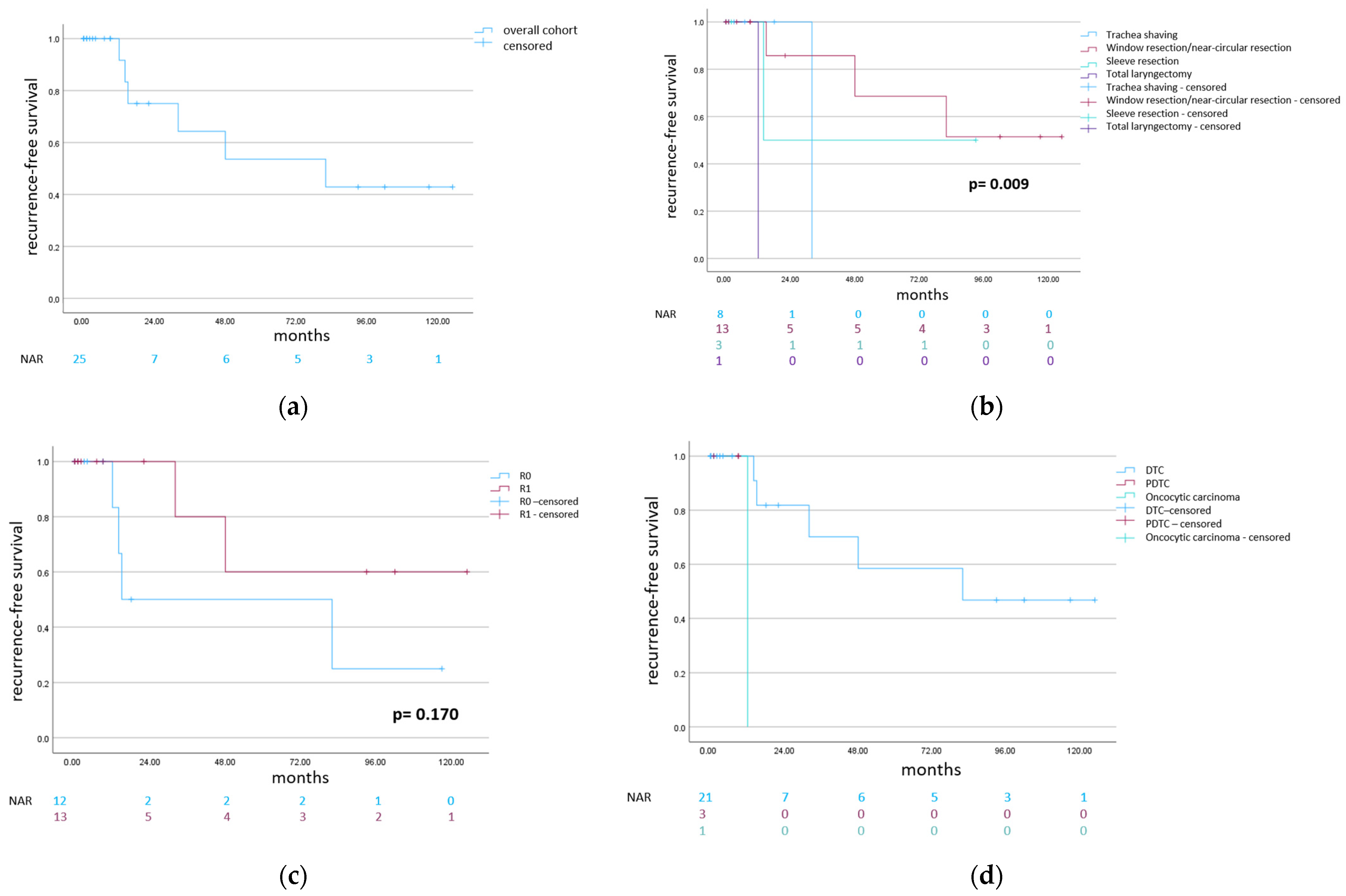
3. Results
3.1. Trachea Shaving
3.2. Partial “Window” and Near-Circular Trachea Resections
3.3. Sleeve Resection
3.4. Total Laryngectomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robert Koch Institute: Cancer in Germany 2017/2018, Chapter Thyroid. Available online: https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2021/kid_2021_c73_schilddruese.pdf?__blob=publicationFile. (accessed on 24 February 2023).
- International Union Against Cancer (UICC). TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- International Union Against Cancer (UICC). TNM Classification of Malignant Tumours, 7th ed.; Blackwell: Malden, MA, USA, 2010. [Google Scholar]
- Brierley, J.D.; Panzarella, T.; Tsang, R.W.; Gospodarowicz, M.K.; O’Sullivan, B. A comparison of different staging systems predictability of patient outcome. Thyroid carcinoma as an example. Cancer 1997, 79, 2414–2423. [Google Scholar] [CrossRef]
- Czaja, J.M.; McCaffrey, T.V. The surgical management of laryngotracheal invasion by well-differentiated papillary thyroid carcinoma. Arch. Otolaryngol. Head Neck Surg. 1997, 123, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Li, Y.; Yang, X.; Xiao, Z.; Tang, Q.; Wang, Q. A review of the management and prognosis of thyroid carcinoma with tracheal invasion. Eur. Arch. Otorhinolaryngol. 2015, 272, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Price, D.L.; Wong, R.J.; Randolph, G.W. Invasive thyroid cancer: Management of the trachea and esophagus. Otolaryngol. Clin. N. Am. 2008, 41, 1155–1168. [Google Scholar] [CrossRef]
- McCaffrey, T.V.; Bergstralh, E.J.; Hay, I.D. Locally invasive papillary thyroid carcinoma: 1940–1990. Head Neck 1994, 16, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Sireci, F.; Lorusso, F.; Dispenza, F.; Immordino, A.; Gallina, S.; Salvago, P.; Martines, F.; Bonaventura, G.; Uzzo, M.L.; Spatola, G.F. A Prospective Observational Study on the Role of Immunohistochemical Expression of Orphanin in Laryngeal Squamous Cell Carcinoma Recurrence. J. Pers. Med. 2023, 13, 1211. [Google Scholar] [CrossRef] [PubMed]
- Su, S.Y.; Milas, Z.L.; Bhatt, N.; Roberts, D.; Clayman, G.L. Well-differentiated thyroid cancer with aerodigestive tract invasion: Long-term control and functional outcomes. Head Neck 2016, 38, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Mark, E.J.; Suen, H.C.; Grillo, H.C. Pathologic staging of papillary carcinoma of the thyroid with airway invasion based on the anatomic manner of extension to the trachea: A clinicopathologic study based on 22 patients who underwent thyroidectomy and airway resection. Hum. Pathol. 1993, 24, 866–870. [Google Scholar] [CrossRef]
- Dralle, H.; Brauckhoff, M.; Machens, A.; Gimm, O. Surgical management of advanced thyroid cancer invading the aerodigestive tract. In Textbook on Endocrine Surgery; Clark, O.H., Duh, Q.Y., Kebebew, E., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2005; pp. 318–333. [Google Scholar]
- Brauckhoff, M.; Machens, A.; Thanh, P.N.; Lorenz, K.; Schmeil, A.; Stratmann, M.; Sekulla, C.; Brauckhoff, K.; Dralle, H. Impact of extent of resection for thyroid cancer invading the aerodigestive tract on surgical morbidity, local recurrence, and cancer-specific survival. Surgery 2010, 148, 1257–1266. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs; IARC: Lyon, France, 2017; pp. 65–143. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Allen, M.; Spillinger, A.; Arianpour, K.; Johnson, J.; Johnson, A.P.; Folbe, A.J.; Hotaling, J.; Svider, P.F. Tracheal Resection in the Management of Thyroid Cancer: An Evidence-Based Approach. Laryngoscope 2021, 131, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Musholt, T.J.; Musholt, P.B.; Behrend, M.; Raab, R.; Scheumann, G.F.; Klempnauer, J. Invasive differentiated thyroid carcinoma: Tracheal resection and reconstruction procedures in the hands of the endocrine surgeon. Surgery 1999, 126, 1078–1087, discussion 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Piazza, C.; Lancini, D.; Tomasoni, M.; D’Cruz, A.; Hartl, D.M.; Kowalski, L.P.; Randolph, G.W.; Rinaldo, A.; Shah, J.P.; Shaha, A.R.; et al. Tracheal and Cricotracheal Resection With End-to-End Anastomosis for Locally Advanced Thyroid Cancer: A Systematic Review of the Literature on 656 Patients. Front. Endocrinol. 2021, 12, 779999. [Google Scholar] [CrossRef] [PubMed]
- Hay, I.D.; McConahey, W.M.; Goellner, J.R. Managing patients with papillary thyroid carcinoma: Insights gained from the Mayo Clinic’s experience of treating 2512 consecutive patients during 1940 through 2000. Trans. Am. Clin. Climatol. Assoc. 2002, 113, 241–260. [Google Scholar] [PubMed]
- Hotomi, M.; Sugitani, I.; Toda, K.; Kawabata, K.; Fujimoto, Y. A novel definition of extrathyroidal invasion for patients with papillary thyroid carcinoma for predicting prognosis. World J. Surg. 2012, 36, 1231–1240. [Google Scholar] [CrossRef]
- Jung, C.K.; Bychkov, A.; Kakudo, K. Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach. Endocrinol. Metab. 2022, 37, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.S.; Lorch, J.H.; Alexander, E.K.; Marqusee, E.; Cho, N.L.; Nehs, M.A.; Doherty, G.M.; Barletta, J.A. Prognostic Significance of Extent of Invasion in Poorly Differentiated Thyroid Carcinoma. Thyroid 2019, 29, 1255–1261. [Google Scholar] [CrossRef]
- Luster, M.; Aktolun, C.; Amendoeira, I.; Barczyński, M.; Bible, K.C.; Duntas, L.H.; Elisei, R.; Handkiewicz-Junak, D.; Hoffmann, M.; Jarząb, B.; et al. European Perspective on 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: Proceedings of an Interactive International Symposium. Thyroid 2019, 29, 7–26. [Google Scholar] [CrossRef]
- Kim, H.; Jung, H.J.; Lee, S.Y.; Kwon, T.K.; Kim, K.H.; Sung, M.W.; Hun Hah, J. Prognostic factors of locally invasive well-differentiated thyroid carcinoma involving the trachea. Eur. Arch. Otorhinolaryngol. 2016, 273, 1919–1926. [Google Scholar] [CrossRef]
- Tsukahara, K.; Sugitani, I.; Kawabata, K. Surgical management of tracheal shaving for papillary thyroid carcinoma with tracheal invasion. Acta Otolaryngol. 2009, 129, 1498–1502. [Google Scholar] [CrossRef]
- Musholt, T.J. [Resection strategy for locally advanced thyroid carcinoma]. Chirurg 2020, 91, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Rao, C.; Raju, K.; Nemade, H.; Dasu, S.; Jayakarthik, Y.; Shukla, S.; Rao, T.S. Tracheal/Laryngeal Infiltration in Thyroid Cancer: A Single-Centre Experience. Indian J. Surg. Oncol. 2020, 11, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caro, A.; Morcillo, A.; Wins, R.; Molins, L.; Galan, G.; Tarrazona, V. Surgical management of benign tracheal stenosis. Multimed. Man. Cardiothorac. Surg. 2011, 2011, mmcts.2010.004945. [Google Scholar] [CrossRef] [PubMed]
- Mossetti, C.; Palestini, N.; Bruna, M.C.; Camandona, M.; Freddi, M.; Oliaro, A.; Gasparri, G. Segmental tracheal resection for invasive differentiated thyroid carcinoma. Our experience in eight cases. Langenbecks Arch. Surg. 2013, 398, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Scheumann, G.F.W.; Maschek, H.J.; Dralle, H. Arteriotracheal fistula as a fatal complication after tracheal resection and twice-a-day-irradiation for thyroid carcinoma. Acta Chir. Austriaca 1993, 25, 278–280. [Google Scholar] [CrossRef]
- Liu, J.; Ren, J.; Lv, D.; Wang, J.; Deng, D.; Li, L.; Wang, H.; Chen, F. Simultaneous tracheal and esophageal reconstruction for thyroid cancer involving trachea and esophagus using a free bipaddled posterior tibial artery perforator flap. Head Neck 2019, 41, 3472–3477. [Google Scholar] [CrossRef] [PubMed]
- Gaissert, H.A.; Honings, J.; Grillo, H.C.; Donahue, D.M.; Wain, J.C.; Wright, C.D.; Mathisen, D.J. Segmental laryngotracheal and tracheal resection for invasive thyroid carcinoma. Ann. Thorac. Surg. 2007, 83, 1952–1959. [Google Scholar] [CrossRef]
| Non-Transmural Resection | Transmural Resection | Total | |||
|---|---|---|---|---|---|
| Trachea Shaving n = 14 | “Window” Resection/ Near-Circular Resection n = 15 | Sleeve Resection n = 3 | Total Laryngectomy with Extramucosal Esophageal Resection n = 1 | n = 33 | |
| Age (mean, range, years) | 65.8 (23–88) | 62.9 (17–85) | 69.3 (60–78) | 59.0 (59.0) | 64.6 (17–88) |
| Sex ratio (M: F) | 7:7 | 8:7 | 2:1 | 1:0 | 18:15 |
| Previous thyroid operation history (n, %) | 10 (71.4) | 7 (46.7) | 2 (66.7) | 1 (100.0) | 20 (60.6) |
| Preoperative recurrent laryngeal nerve paresis per patient (n, %) | 11 (78.6) | 8 (53.3) | 1 (33.3) | 1 (100) | 21 (63.6) |
| Operative intention | |||||
| 10 (71.4) | 14 (93.3) | 3 (100) | 1 (100.0) | 28 (84.8) |
| 4 (28.6) | 1 (6.7) | 0 (0) | 0 (0) | 5 (15.2) |
| Further treatment | |||||
| 6 (42.9) | 9 (60.0) | 2 (66.7) | 1 (100.0) | 18 (54.6) |
| 3 (21.4) | 0 (0) | 1 (33.3) | 0 (0) | 4 (12.1) |
| 5 (35.7) | 6 (40.0) | 0 (0) | 0 (0) | 11 (33.3) |
| Mean follow-up (months, range) | 10.8 (0–35) | 45.8 (0–138) | 34.6 (2–93) | 20 (20) | 29.2 (0–138) |
| Histology [14] | |||||
| 8 (57.1) | 9 (60.0) | 2 (33.3) | 0 (0) | 19 (57.6) |
| 3 (21.4) | 4 (26.7) | 1 (33.3) | 0 (0) | 8 (24.2) |
| 2 (14.3) | 2 (13.3) | 0 (0) | 0 (0) | 4 (12.1) |
| 1 (7.1) | 0 (0) | 0 (0) | 1 (100) | 2 (6.1) |
| Tumor persistence after operation (n, %) | 6 (42.9) | 2 (13.3) | 0 (0) | 0 (0) | 8 (24.2) |
| 2 (14.3) | 2 (13.3) | 0 (0) | 0 (0) | 4 (12.1) |
| 3 (21.4) | 0 (0) | 0 (0) | 0 (0) | 3 (9.1) |
| 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | 1 (3.0) |
| Recurrence | 1 (7.1) | 3 (20.0) | 1 (33.3) | 1 (100.0) | 6 (18.2) |
| 0 (0) | 1 (6.7) | 1 (33.3) | 1 (100.0) | 3 (9.1) |
| 1 (7.1) | 1 (6.7) | 0 (0) | 0 (0) | 2 (6.1) |
| 0 (0) | 1 (6.7) | 0 (0) | 0 (0) | 1 (3.0) |
| Non-Transmural Resection | Transmural Resection | Total | |||
|---|---|---|---|---|---|
| Trachea Shaving n = 14 | “Window” Resection/ Near-Circular Resection n = 15 | Sleeve Resection n = 3 | Total Laryngectomy with Extramucosal Esophageal Resection n = 1 | n = 33 | |
| Thyroid resection (n, %) | 6 (42.9) | 9 (60.0) | 2 (66.7) | 0 (0) | 17 (51.5) |
| Lymphadenectomy | |||||
| 10 (71.4) | 11 (73.3) | 3 (100.0) | 0 (0) | 24 (72.7) |
| 0 (0) | 2 (13.3) | 0 (0) | 0 (0) | 2 (6.1) |
| Esophagus resection (n, %) | 10 (71.4) | 3 (20.0) | 0 (0) | 1 (100.0) | 14 (42.4) |
| Vascular resection (n, %) | 4 (28.6) | 1 (6.7) | 0 (0) | 0 (0) | 5 (15.2) |
| Strap muscle resection (n, %) | 10 (71.4) | 5 (33.3) | 2 (66.7) | 0 (0) | 17 (51.5) |
| Laryngeal muscle resection (n, %) | 3 (21.5) | 1 (6.7) | 0 (0) | 0 (0) | 4 (12.2) |
| Laryngectomy | |||||
| 0 (0) | 0 (0) | 0 (0) | 1 (100.0) | 1 (3.0) |
| 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 1 (3.0) |
| Resection of recurrent laryngeal nerve (n, %) | 11 (78.6) | 7 (46.7) | 1 (33.3) | 0 (0) | 19 (57.6) |
| Thymectomy (n, %) | 7 (50.0) | 8 (53.3) | 1 (33.3) | 0 (0) | 16 (48.5) |
| Protective tracheostomy (n, %) | 0 (0) | 8 (53.3) | 3 (100.0) | 0 (0) | 11 (33.3) |
| Permanent tracheostomy (n, %) | 2 (14.3) | 4 (26.7) | 0 (0) | 1 (100.0) | 7 (21.2) |
| Reconstruction with muscle flap (n, %) | 2 (14.3) | 14 (93.3) | 1 (33.3) | 0 (0) | 17 (51.5) |
| 2 (14.3) | 11 (73.3) | 0 (0) | 0 (0) | 13 (39.4) |
| 0 (0) | 1 (6.7) | 1 (33.3) | 0 (0) | 2 (6.1) |
| 0 (0) | 1 (6.7) | 0 (0) | 0 (0) | 1 (3.0) |
| 0 (0) | 1 (6.7) | 0 (0) | 0 (0) | 1 (3.0) |
| Non-Transmural Resection | Transmural Resection | Total | |||
|---|---|---|---|---|---|
| Trachea Shaving n = 14 | “Window” Resection/ Near-Circular Resection n = 15 | Sleeve Resection n = 3 | Total Laryngectomy with Extramucosal Esophageal Resection n = 1 | n = 33 | |
| Median intensive care unit stay (days, range) | 3 (0–30) | 9 (0–35) | 14(0–24) | 2 (2) | 7 (0–35) |
| Complications (n, %) [15] | 7 (50.0) | 5 (33.3) | 2 (66.7) | 1 (100.0) | 15 (45.5) |
| 4 (28.6) | 2 (13.3) | 2 (66.7) | 1 (100.0) | 9 (27.3) |
| 1 (7.1) | 1 (6.7) | 0 (0) | 0 (0) | 2 (6.1) |
| 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | 1 (3.0) |
| 1 (7.1) | 2 (13.3) | 0 (0) | 0 (0) | 3 (9.1) |
| Registered time of death | |||||
| 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1 (7.1) | 2 (13.3) | 0 (0) | 0 (0) | 3 (9.1) |
| 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Reason for death | |||||
| 1 1 | 1 2 | 0 | 0 | 2 |
| 0 | 1 3 | 0 | 0 | 1 |
| Median follow-up after surgery (months, range) | 9 (0–35) | 22 (0–138) | 9 (2–93) | 20 (20) | 10 (0–138) |
| Resection margin | |||||
| 4 (28.6) | 7 (46.7) | 1 (33.3) | 1 (100) | 13 (39.4) |
| 10 (71.4) | 8 (53.3) | 2 (66.7) | 0 (0) | 20 (60.6) |
| Permanent vocal cord paresis per patient (n, % *) | 12 (92.3) | 8 (61.5) | 1 (50.0) | 1 (100.0) | 22 (75.9) |
| Parathormone below 15 pg/mL in long-term follow-up (n, % **) | 1 (7.7) | 1 (6.7) | 2 (66.7) | 1 (100.0) | 5 (16.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staubitz-Vernazza, J.I.; Schwind, S.; Lozan, O.; Musholt, T.J. A 16-Year Single-Center Series of Trachea Resections for Locally Advanced Thyroid Carcinoma. Cancers 2024, 16, 163. https://doi.org/10.3390/cancers16010163
Staubitz-Vernazza JI, Schwind S, Lozan O, Musholt TJ. A 16-Year Single-Center Series of Trachea Resections for Locally Advanced Thyroid Carcinoma. Cancers. 2024; 16(1):163. https://doi.org/10.3390/cancers16010163
Chicago/Turabian StyleStaubitz-Vernazza, Julia I., Sina Schwind, Oana Lozan, and Thomas J. Musholt. 2024. "A 16-Year Single-Center Series of Trachea Resections for Locally Advanced Thyroid Carcinoma" Cancers 16, no. 1: 163. https://doi.org/10.3390/cancers16010163
APA StyleStaubitz-Vernazza, J. I., Schwind, S., Lozan, O., & Musholt, T. J. (2024). A 16-Year Single-Center Series of Trachea Resections for Locally Advanced Thyroid Carcinoma. Cancers, 16(1), 163. https://doi.org/10.3390/cancers16010163





