The Gap of Health Inequalities Amongst Lung Cancer Patients of Different Socioeconomic Status: A Brief Reference to the Greek Reality
Abstract
Simple Summary
Abstract
1. Introduction
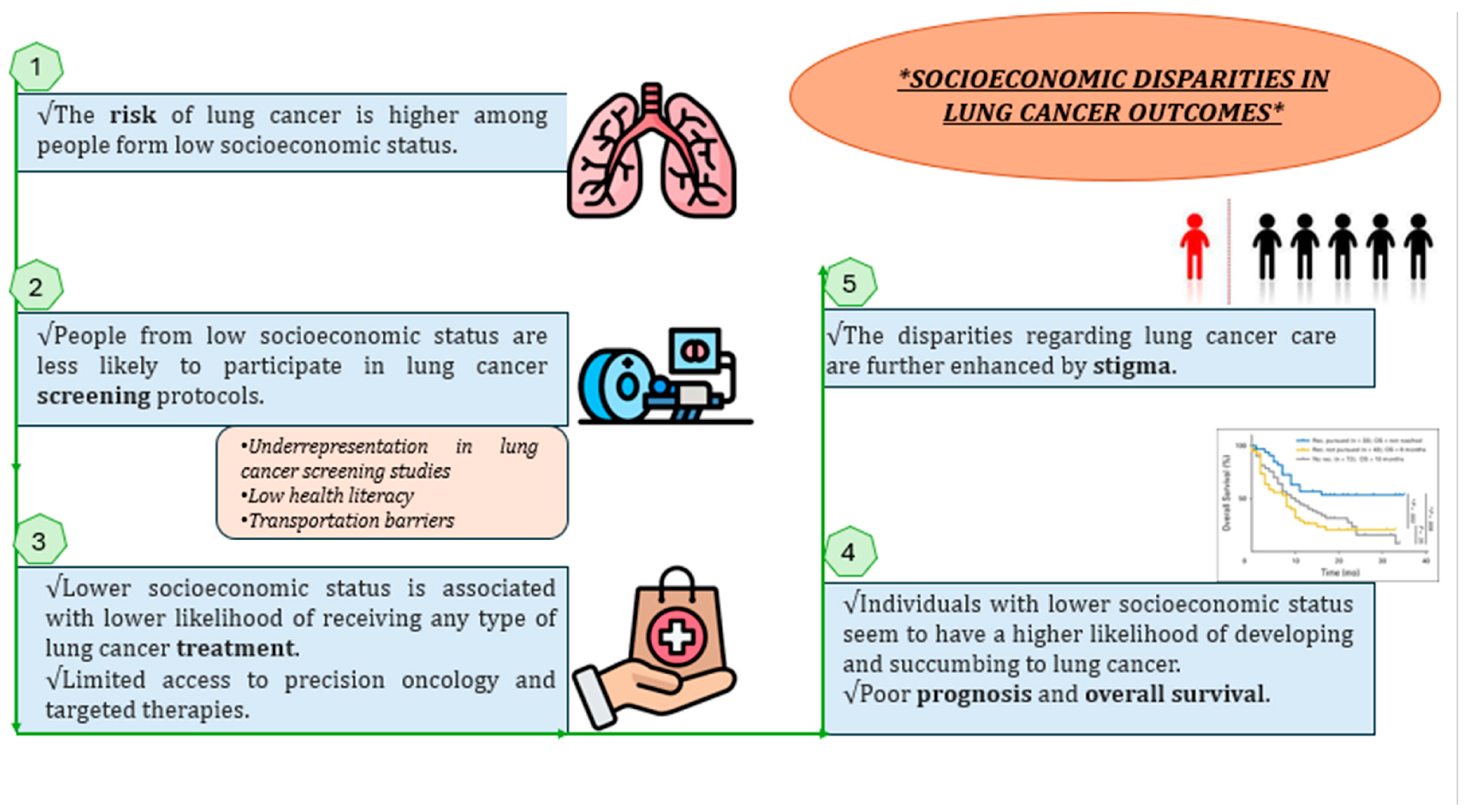
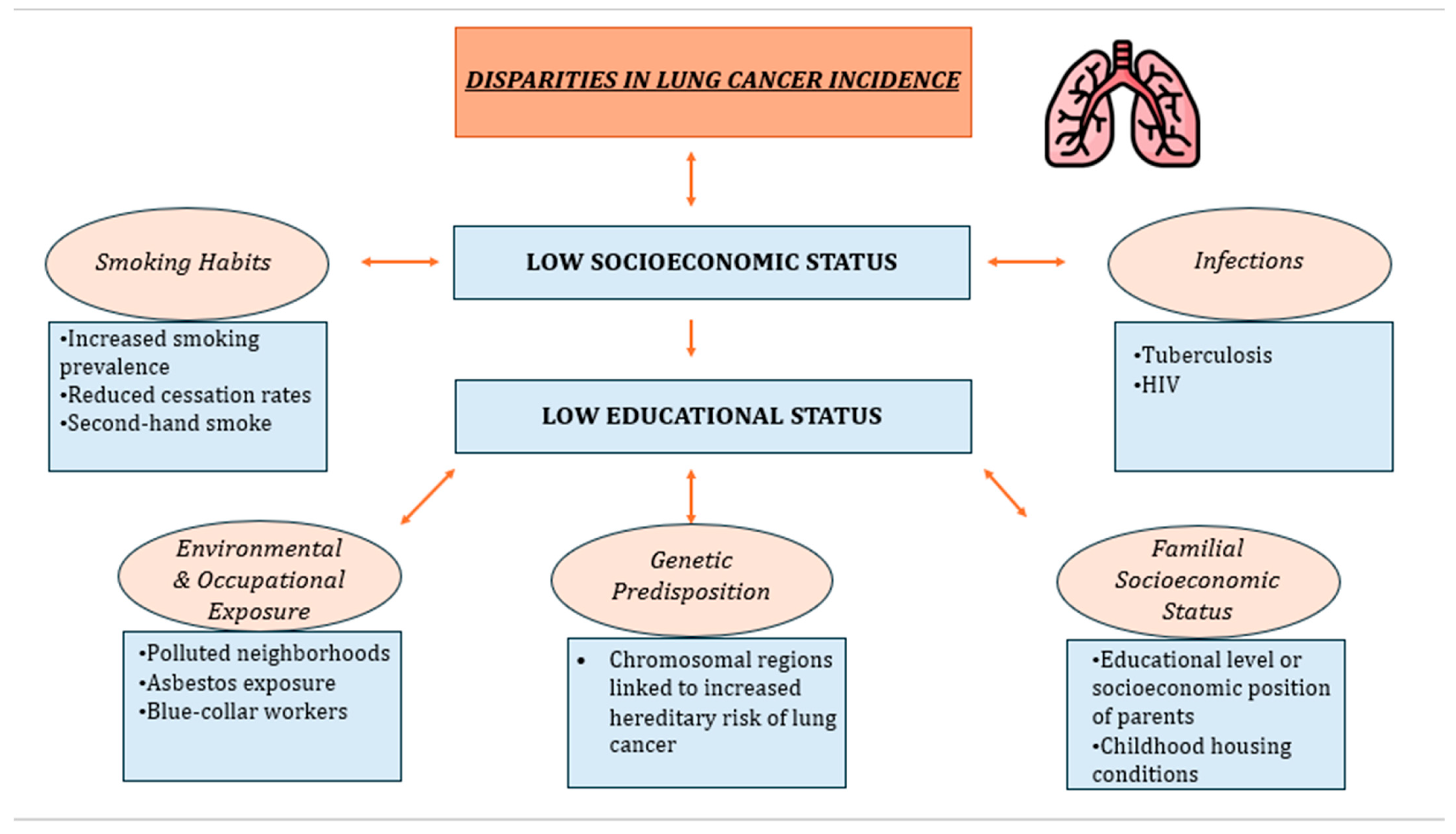
2. Lung Cancer Incidence and SES
2.1. Smoking Habits
2.2. Environmental and Occupational Exposure
2.3. Genetic Predisposition
2.4. Familial Socioeconomic Position
2.5. Infections
3. Relation between Low SES and Poor Lung Cancer Prognosis
3.1. Screening
3.2. Effective Treatment
3.3. Overall Survival and Prognosis
3.4. Stigma
4. Lung Cancer in the Greek Reality
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Agency for Research on Cancer. GLOBOCAN Lung Cancer Facts Sheet 2020; IARC: Lyon, France, 2021. [Google Scholar]
- Herdan, G. The Increase in the Mortality Due to Cancer of the Lung in the Light of the Distribution of the Disease among the Different Social Classes and Occupations. Br. J. Cancer 1958, 12, 492–506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wiley, R.C.; Oliver, A.C.; Snow, M.B.; Bunn, J.Y.; Barrows, A.J.; Tidey, J.W.; Lee, D.C.; Sigmon, S.C.; Gaalema, D.E.; Heil, S.H.; et al. The Impact of the Covid-19 Pandemic on Smoking among Vulnerable Populations. Nicotine Tob. Res. 2023, 25, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Larsen, I.K.; Myklebust, T.Å.; Babigumira, R.; Vinberg, E.; Møller, B.; Ursin, G. Education, Income and Risk of Cancer: Results from a Norwegian Registry-Based Study. Acta Oncol. 2020, 59, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.W.; Cummings, K.M. Tobacco and Lung Cancer: Risks, Trends, and Outcomes in Patients with Cancer. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.M. Tobacco Smoking: The Leading Cause of Preventable Disease Worldwide. Thorac. Surg. Clin. 2013, 23, 103–112. [Google Scholar] [CrossRef]
- Wardle, J.; Steptoe, A. Socioeconomic Differences in Attitudes and Beliefs about Healthy Lifestyles. J. Epidemiol. Community Health 2003, 57, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Yau, C.; Wallbridge, I.G.; Saini, D.; Prasad, C.P.; Singh, P.; Kaur, J.; Roy, S.; Sinha, P. The Intersection of Tobacco Use, Health Disparities, and Inequalities in Lung Cancer Treatment and Survival. Indian J. Med. Paediatr. Oncol. 2022, 43, 289–293. [Google Scholar] [CrossRef]
- Cao, P.; Jeon, J.; Tam, J.; Fleischer, N.L.; Levy, D.T.; Holford, T.R.; Meza, R. Smoking Disparities by Level of Educational Attainment and Birth Cohort in the US. Am. J. Prev. Med. 2023, 64, S22–S31. [Google Scholar] [CrossRef]
- Fiore, M.C.; Novotny, T.E.; Pierce, J.P.; Hatziandreu, E.J.; Patel, K.M.; Davis, R.M. Trends in Cigarette Smoking in the United States: The Changing Influence of Gender and Race. JAMA 1989, 261, 49–55. [Google Scholar] [CrossRef]
- Barbeau, E.M.; Leavy-Sperounis, A.; Balbach, E.D. Smoking, Social Class, and Gender: What Can Public Health Learn from the Tobacco Industry about Disparities in Smoking? Tob. Control 2004, 13, 115–120. [Google Scholar] [CrossRef]
- Brown-Johnson, C.G.; England, L.J.; Glantz, S.A.; Ling, P.M. Tobacco Industry Marketing to Low Socioeconomic Status Women in the USA. Tob. Control 2014, 23, e139–e146. [Google Scholar] [CrossRef] [PubMed]
- Kotz, D.; West, R. Explaining the Social Gradient in Smoking Cessation: It’s Not in the Trying, but in the Succeeding. Tob. Control 2009, 18, 43–46. [Google Scholar] [CrossRef]
- Babb, S.; Malarcher, A.; Schauer, G.; Asman, K.; Jamal, A. Quitting Smoking among Adults—United States, 2000–2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 65, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Lebrun-Harris, L.A.; Fiore, M.C.; Tomoyasu, N.; Ngo-Metzger, Q. Cigarette Smoking, Desire to Quit, and Tobacco-Related Counseling among Patients at Adult Health Centers. Am. J. Public Health 2015, 105, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Siahpush, M.; McNeill, A.; Borland, R.; Fong, G.T. Socioeconomic Variations in Nicotine Dependence, Self-Efficacy, and Intention to Quit across Four Countries: Findings from the International Tobacco Control (ITC) Four Country Survey. Tob. Control 2006, 15 (Suppl. S3), iii71–iii75. [Google Scholar] [CrossRef] [PubMed]
- Onwuka, J.U.; Zahed, H.; Feng, X.; Alcala, K.; Johansson, M.; Robbins, H.A.; Consortium, L.C.C. Abstract 1950: Socioeconomic Status and Lung Cancer Incidence: An Analysis of Data from 15 Countries in the Lung Cancer Cohort Consortium. Cancer Res. 2023, 83, 1950. [Google Scholar] [CrossRef]
- WHO. An Estimated 12.6 Million Deaths Each Year ARE Attributable to Unhealthy Environments. 2019. Available online: https://www.who.int/news-room/detail/15-03-2016-an-estimated-12-6-million-deaths-each-year-are-attributable-to-unhealthy-environments (accessed on 20 November 2023).
- International Agency for Research on Cancer. Health Impacts of Chemicals. World Health Organization. 2016. Available online: https://www.who.int/publications/i/item/WHO-FWC-PHE-EPE-16.01-eng (accessed on 20 November 2023).
- Corrales, L.; Rosell, R.; Cardona, A.F.; Martín, C.; Zatarain-Barrón, Z.L.; Arrieta, O. Lung Cancer in Never Smokers: The Role of Different Risk Factors Other than Tobacco Smoking. Crit. Rev. Oncol. Hematol. 2020, 148, 102895. [Google Scholar] [CrossRef]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; Ghissassi, F.E.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. The Carcinogenicity of Outdoor Air Pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The Contribution of Outdoor Air Pollution Sources to Premature Mortality on a Global Scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Swanton, C.; Hill, W.; Lim, E.; Lee, C.; Weeden, C.E.; Augustine, M.; Chen, K.; Kuan, F.-C.; Marongiu, F.; Rodrigues, F.; et al. LBA1 Mechanism of Action and an Actionable Inflammatory Axis for Air Pollution Induced Non-Small Cell Lung Cancer: Towards Molecular Cancer Prevention. Ann. Oncol. 2022, 33, S1413. [Google Scholar] [CrossRef]
- Hajat, A.; MacLehose, R.F.; Rosofsky, A.; Walker, K.D.; Clougherty, J.E. Confounding by Socioeconomic Status in Epidemiological Studies of Air Pollution and Health: Challenges and Opportunities. Environ. Health Perspect. 2021, 129, 65001. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Chen, D.; Buzzelli, M.; Aronson, K.J. Environmental Equity Research: Review with Focus on Outdoor Air Pollution Research Methods and Analytic Tools. Arch. Environ. Occup. Health 2015, 70, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Hajat, A.; Hsia, C.; O’Neill, M.S. Socioeconomic Disparities and Air Pollution Exposure: A Global Review. Curr. Environ. Health Rep. 2015, 2, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Alberg, A.J.; Brock, M.V.; Ford, J.G.; Samet, J.M.; Spivack, S.D. Epidemiology of Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013, 143, e1S–e29S. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.-T.; Hassan, A.; LePage, B.A. Introduction to Environmental Education. In The Living Environmental Education; Springer: Berlin/Heidelberg, Germany, 2023; ISBN 978-981-19-4233-4. [Google Scholar]
- Uguen, M.; Dewitte, J.-D.; Marcorelles, P.; Loddé, B.; Pougnet, R.; Saliou, P.; De Braekeleer, M.; Uguen, A. Asbestos-Related Lung Cancers: A Retrospective Clinical and Pathological Study. Mol. Clin. Oncol. 2017, 7, 135–139. [Google Scholar] [CrossRef]
- Vicari, K.; Ribeiro, I.M.; Aguiar, B.F.; Brey, C.; Boller, S.; Miranda, F.M.D. Occupational Characterization of Workers Exposed to Asbestos: An Integrative Review. Rev. Bras. Med. Trab. 2022, 20, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Lubin, J.H.; Boice, J.D.; Edling, C.; Hornung, R.W.; Howe, G.R.; Kunz, E.; Kusiak, R.A.; Morrison, H.I.; Radford, E.P.; Samet, J.M. Lung Cancer in Radon-Exposed Miners and Estimation of Risk from Indoor Exposure. J. Natl. Cancer Inst. 1995, 87, 817–827. [Google Scholar] [CrossRef]
- Schubauer-Berigan, M.K.; Daniels, R.D.; Pinkerton, L.E. Radon Exposure and Mortality among White and American Indian Uranium Miners: An Update of the Colorado Plateau Cohort. Am. J. Epidemiol. 2009, 169, 718–730. [Google Scholar] [CrossRef]
- Chaitanya Thandra, K.; Barsouk, A.; Saginala, K.; Sukumar Aluru, J.; Barsouk, A. Epidemiology of Lung Cancer. Współczesna Onkol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Counil, E.; Roblin, A.; Ismail, W.; Paris, C.; Luce, D. O-80 Towards Occupational Health Equity Metrics Estimating the Burden of Lung Cancer Attributed to Three Occupational Carcinogens by Socio-Economic Position. Occup. Environ. Med. 2023, 80, A36–A37. [Google Scholar]
- Dement, J.M.; Ringen, K.; Welch, L.S.; Bingham, E.; Quinn, P. Mortality of Older Construction and Craft Workers Employed at Department of Energy (DOE) Nuclear Sites. Am. J. Ind. Med. 2009, 52, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Stocks, S.J.; McNamee, R.; Carder, M.; Agius, R.M. The Incidence of Medically Reported Work-Related Ill Health in the UK Construction Industry. Occup. Environ. Med. 2010, 67, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Thuret, A.; Geoffroy-Perez, B.; Luce, D.; Goldberg, M.; Imbernon, E. A 26-Year Cohort Mortality Study of French Construction Workers Aged 20 to 64 Years. J. Occup. Environ. Med. 2007, 49, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Vineis, P.; Overvad, K.; Boeing, H.; Bergmann, M.; Trichopoulou, A.; Trichopoulos, D.; Palli, D.; Krogh, V.; Tumino, R.; et al. Occupational Exposures, Environmental Tobacco Smoke, and Lung Cancer. Epidemiol. Camb. Mass 2007, 18, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Collatuzzo, G.; Teglia, F.; Boffetta, P. Role of Occupation in Shaping Cancer Disparities. Cancers 2022, 14, 4259. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.T.; Chatterjee, N.; Yu, K.; Goldin, L.R.; Goldstein, A.M.; Rotunno, M.; Mirabello, L.; Jacobs, K.; Wheeler, W.; Yeager, M.; et al. A Genome-Wide Association Study of Lung Cancer Identifies a Region of Chromosome 5p15 Associated with Risk for Adenocarcinoma. Am. J. Hum. Genet. 2009, 85, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Yokota, J.; Shiraishi, K.; Kohno, T. Genetic Basis for Susceptibility to Lung Cancer: Recent Progress and Future Directions. Adv. Cancer Res. 2010, 109, 51–72. [Google Scholar] [CrossRef]
- Thorgeirsson, T.E.; Geller, F.; Sulem, P.; Rafnar, T.; Wiste, A.; Magnusson, K.P.; Manolescu, A.; Thorleifsson, G.; Stefansson, H.; Ingason, A.; et al. A Variant Associated with Nicotine Dependence, Lung Cancer and Peripheral Arterial Disease. Nature 2008, 452, 638–642. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Liu, J.; Yang, Y.; Fang, W.; Hong, S.; Chen, G.; Zhao, S.; Zhang, Z.; Shen, J.; et al. Education and Lung Cancer: A Mendelian Randomization Study. Int. J. Epidemiol. 2019, 48, 743–750. [Google Scholar] [CrossRef]
- Søndergaard, G.; Mortensen, L.H.; Andersen, A.-M.N.; Andersen, P.K.; Dalton, S.O.; Osler, M. Social Inequality in Breast, Lung and Colorectal Cancers: A Sibling Approach. BMJ Open 2013, 3, e002114. [Google Scholar] [CrossRef]
- Wang, R.; Li, C.; Huo, Z.; Ge, F.; Zhong, R.; Jiang, Y.; Wen, Y.; Su, Z.; Liang, H.; Cheng, B.; et al. Family Socioeconomic Position and Lung Cancer Risk: A Meta-Analysis and a Mendelian Randomization Study. 2020. Available online: https://www.researchsquare.com/article/rs-89906/v1 (accessed on 20 November 2023).
- Brenner, D.R.; McLaughlin, J.R.; Hung, R.J. Previous Lung Diseases and Lung Cancer Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2011, 6, e17479. [Google Scholar] [CrossRef] [PubMed]
- Sigel, K.; Wisnivesky, J.; Gordon, K.; Dubrow, R.; Justice, A.; Brown, S.T.; Goulet, J.; Butt, A.A.; Crystal, S.; Rimland, D.; et al. HIV as an Independent Risk Factor for Incident Lung Cancer. AIDS 2012, 26, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- D’Jaen, G.A.; Pantanowitz, L.; Bower, M.; Buskin, S.; Neil, N.; Greco, E.M.; Cooley, T.P.; Henry, D.; Stem, J.; Dezube, B.J.; et al. Human Immunodeficiency Virus-Associated Primary Lung Cancer in the Era of Highly Active Antiretroviral Therapy: A Multi-Institutional Collaboration. Clin. Lung Cancer 2010, 11, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, M.J.; Lau, B.; Achenbach, C.J.; Jing, Y.; Althoff, K.N.; D’Souza, G.; Engels, E.A.; Hessol, N.A.; Brooks, J.T.; Burchell, A.N.; et al. Cumulative Incidence of Cancer among Persons with HIV in North America: A Cohort Study. Ann. Intern. Med. 2015, 163, 507–518. [Google Scholar] [CrossRef] [PubMed]
- The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed]
- De Koning, H.J.; Van Der Aalst, C.M.; De Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- National Lung Screening Trial Research Team; Aberle, D.R.; Adams, A.M.; Berg, C.D.; Clapp, J.D.; Clingan, K.L.; Gareen, I.F.; Lynch, D.A.; Marcus, P.M.; Pinsky, P.F. Baseline Characteristics of Participants in the Randomized National Lung Screening Trial. J. Natl. Cancer Inst. 2010, 102, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.D.; Oeffinger, K.C.; Shih, T.Y.; Walter, L.C.; Church, T.R.; Fontham, E.T.H.; Elkin, E.B.; Etzioni, R.D.; Guerra, C.E.; Perkins, R.B.; et al. Screening for Lung Cancer: 2023 Guideline Update from the American Cancer Society. CA Cancer J. Clin. 2023, 74, 50–81. [Google Scholar] [CrossRef]
- Castro, S.; Sosa, E.; Lozano, V.; Akhtar, A.; Love, K.; Duffels, J.; Raz, D.J.; Kim, J.Y.; Sun, V.; Erhunmwunsee, L. The Impact of Income and Education on Lung Cancer Screening Utilization, Eligibility, and Outcomes: A Narrative Review of Socioeconomic Disparities in Lung Cancer Screening. J. Thorac. Dis. 2021, 13, 3745–3757. [Google Scholar] [CrossRef]
- Schütte, S.; Dietrich, D.; Montet, X.; Flahault, A. Participation in Lung Cancer Screening Programs: Are There Gender and Social Differences? A Systematic Review. Public Health Rev. 2018, 39, 23. [Google Scholar] [CrossRef]
- Williams, R.M.; Beck, K.H.; Butler, J.; Lee, S.; Wang, M.Q.; Taylor, K.L.; Knott, C.L. Lung Cancer Screening Decisional Needs among African American Smokers of Lower Socioeconomic Status. Ethn. Health 2022, 27, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Chow, E.; Ten Haaf, K.; Toumazis, I.; Cao, P.; Bastani, M.; Tammemagi, M.; Jeon, J.; Feuer, E.J.; Meza, R.; et al. Disparities of National Lung Cancer Screening Guidelines in the US Population. J. Natl. Cancer Inst. 2020, 112, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Steiling, K.; Loui, T.; Asokan, S.; Nims, S.; Moreira, P.; Rebello, A.; Litle, V.R.; Suzuki, K. Age, Race, and Income Are Associated with Lower Screening Rates at a Safety Net Hospital. Ann. Thorac. Surg. 2020, 109, 1544–1550. [Google Scholar] [CrossRef]
- Rohatgi, K.W.; Marx, C.M.; Lewis-Thames, M.W.; Liu, J.; Colditz, G.A.; James, A.S. Urban-Rural Disparities in Access to Low-Dose Computed Tomography Lung Cancer Screening in Missouri and Illinois. Prev. Chronic. Dis. 2020, 17, E140. [Google Scholar] [CrossRef] [PubMed]
- Sosa, E.; D’Souza, G.; Akhtar, A.; Sur, M.; Love, K.; Duffels, J.; Raz, D.J.; Kim, J.Y.; Sun, V.; Erhunmwunsee, L. Racial and Socioeconomic Disparities in Lung Cancer Screening in the United States: A Systematic Review. CA Cancer J. Clin. 2021, 71, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Wait, S.; Alvarez-Rosete, A.; Osama, T.; Bancroft, D.; Cornelissen, R.; Marušić, A.; Garrido, P.; Adamek, M.; Van Meerbeeck, J.; Snoeckx, A.; et al. Implementing Lung Cancer Screening in Europe: Taking a Systems Approach. JTO Clin. Res. Rep. 2022, 3, 100329. [Google Scholar] [CrossRef] [PubMed]
- Berchet, C.; Dedet, G.; Klazinga, N.; Colombo, F. Inequalities in Cancer Prevention and Care across Europe. Lancet Oncol. 2023, 24, 10–11. [Google Scholar] [CrossRef]
- Forrest, L.F.; Adams, J.; Wareham, H.; Rubin, G.; White, M. Socioeconomic Inequalities in Lung Cancer Treatment: Systematic Review and Meta-Analysis. PLoS Med. 2013, 10, e1001376. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, F.; Ma, X.; Tian, Y.; Cao, D.; Luo, S.; Xuan, Y.; Liu, L.; Wei, Y. Matched-Pair Comparisons of Stereotactic Body Radiotherapy (SBRT) versus Surgery for the Treatment of Early Stage Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014, 112, 250–255. [Google Scholar] [CrossRef]
- Yan, S.X.; Qureshi, M.M.; Dyer, M.; Truong, M.T.; Mak, K.S. Stereotactic Body Radiation Therapy with Higher Biologically Effective Dose Is Associated with Improved Survival in Stage II Non-Small Cell Lung Cancer. Lung Cancer Amst. Neth. 2019, 131, 147–153. [Google Scholar] [CrossRef]
- Yan, S.X.; Qureshi, M.M.; Suzuki, K.; Dyer, M.; Truong, M.T.; Litle, V.; Mak, K.S. Definitive Treatment Patterns and Survival in Stage II Non-Small Cell Lung Cancer. Lung Cancer 2018, 124, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Mak, K.S. Racial and Socioeconomic Disparities in the Use of Stereotactic Body Radiotherapy for Treating Non-Small Cell Lung Cancer: A Narrative Review. J. Thorac. Dis. 2021, 13, 3764–3771. [Google Scholar] [CrossRef] [PubMed]
- Ebner, P.J.; Ding, L.; Kim, A.W.; Atay, S.M.; Yao, M.J.; Toubat, O.; McFadden, P.M.; Balekian, A.A.; David, E.A. The Effect of Socioeconomic Status on Treatment and Mortality in Non-Small Cell Lung Cancer Patients. Ann. Thorac. Surg. 2020, 109, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Varmus, H. A New Initiative on Precision Medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, C.; Doyle, M.A.; Tothill, R.W. Next-Generation Sequencing for Cancer Diagnostics: A Practical Perspective. Clin. Biochem. Rev. 2011, 32, 177–195. [Google Scholar] [PubMed]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Huey, R.W.; Hawk, E.; Offodile, A.C. Mind the Gap: Precision Oncology and Its Potential to Widen Disparities. J. Oncol. Pract. 2019, 15, 301–304. [Google Scholar] [CrossRef]
- Norris, R.P.; Dew, R.; Sharp, L.; Greystoke, A.; Rice, S.; Johnell, K.; Todd, A. Are There Socio-Economic Inequalities in Utilization of Predictive Biomarker Tests and Biological and Precision Therapies for Cancer? A Systematic Review and Meta-Analysis. BMC Med. 2020, 18, 282. [Google Scholar] [CrossRef]
- Riely, G.L. What, When, and How of Biomarker Testing in Non–Small Cell Lung Cancer. J. Natl. Compr. Canc. Netw. 2017, 15, 686–688. [Google Scholar] [CrossRef]
- Palazzo, L.L.; Sheehan, D.F.; Tramontano, A.C.; Kong, C.Y. Disparities and Trends in Genetic Testing and Erlotinib Treatment among Metastatic Non–Small Cell Lung Cancer Patients. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 926–934. [Google Scholar] [CrossRef]
- Gupta, A.; Omeogu, C.; Islam, J.Y.; Joshi, A.; Zhang, D.; Braithwaite, D.; Karanth, S.D.; Tailor, T.D.; Clarke, J.M.; Akinyemiju, T. Socioeconomic Disparities in Immunotherapy Use among Advanced-Stage Non-Small Cell Lung Cancer Patients: Analysis of the National Cancer Database. Sci. Rep. 2023, 13, 8190. [Google Scholar] [CrossRef] [PubMed]
- Hooten, A.; Chu, T.; Silberstein, P. Palliative Care Utilization and Socioeconomic Factors in Patients with Small Cell Lung Cancer: A National Cancer Database Analysis (Sci244). J. Pain Symptom Manag. 2023, 65, e663–e664. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Xia, Q. Role of Gut Microbiome in Cancer Immunotherapy: From Predictive Biomarker to Therapeutic Target. Exp. Hematol. Oncol. 2023, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Bebek, G.; Vu, B.; Ghannoum, M.; Berger, N.A.; Bruno, D.S. Microbiome, Socioeconomic Status (SES), Fiber Intake, and Response to Checkpoint Inhibitor Therapy in Patients with Non-Small Cell Lung Cancer (NSCLC): A Pilot Study. J. Clin. Oncol. 2023, 41, e14710. [Google Scholar] [CrossRef]
- Lung Cancer Early Detection, Diagnosis, and Staging. American Cancer Society. Available online: https://www.cancer.org/cancer/types/lung-cancer/detection-diagnosis-staging.html (accessed on 20 November 2023).
- Redondo-Sánchez, D.; Petrova, D.; Rodríguez-Barranco, M.; Fernández-Navarro, P.; Jiménez-Moleón, J.J.; Sánchez, M.-J. Socio-Economic Inequalities in Lung Cancer Outcomes: An Overview of Systematic Reviews. Cancers 2022, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Omeogu, C.H.; Islam, J.Y.; Joshi, A.R.; Akinyemiju, T.F. Association of Area-level Socioeconomic Status and Non–Small Cell Lung Cancer Stage by Race/Ethnicity and Health Care–Level Factors: Analysis of the National Cancer Database. Cancer 2022, 128, 3099–3108. [Google Scholar] [CrossRef]
- Klassen, A.; Hsieh, S.; Pankiewicz, A.; Kabbe, A.; Hayes, J.; Curriero, F. The Association of Neighborhood-Level Social Classand Tobacco Consumption with Adverse Lung Cancercharacteristics in Maryland. Tob. Induc. Dis. 2019, 17, 6. [Google Scholar] [CrossRef]
- Verplancke, K.B.; Keirns, D.L.; McMahon, K.; Creech, Z.A.; Truong, G.T.; Silberstein, P.T.; Dahl, M.-B. Association between Demographic and Socioeconomic Factors and Diagnosis of Advanced Non-Small Cell Lung Cancer: An Analysis of the National Cancer Database. Cureus 2023, 15, e44351. [Google Scholar] [CrossRef]
- Zhou, K.; Shi, H.; Chen, R.; Cochuyt, J.J.; Hodge, D.O.; Manochakian, R.; Zhao, Y.; Ailawadhi, S.; Lou, Y. Association of Race, Socioeconomic Factors, and Treatment Characteristics with Overall Survival in Patients with Limited-Stage Small Cell Lung Cancer. JAMA Netw. Open 2021, 4, e2032276. [Google Scholar] [CrossRef]
- Pezzi, T.A.; Schwartz, D.L.; Pisters, K.M.W.; Mohamed, A.S.R.; Welsh, J.W.; Chang, J.Y.; Liao, Z.; Gandhi, S.J.; Byers, L.A.; Minsky, B.D.; et al. Association of Medicaid Insurance with Survival among Patients with Small Cell Lung Cancer. JAMA Netw. Open 2020, 3, e203277. [Google Scholar] [CrossRef] [PubMed]
- Canadian Medical Association. CMA’s Recommendations for Effective Poverty Reduction Strategies; Canadian Medical Association: Ottawa, ON, Canada, 2017. [Google Scholar]
- GLOBOCAN 2020: New Global Cancer Data. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 24 November 2021).
- Stafylis, C.; Rachiotis, G.; Katsioulis, A.; Mouchtouri, V.; Hadjichristodoulou, C. Prevalence and Determinants of Smoking and Secondhand Smoke Exposure in a Rural Population of Central Greece: A Cross-Sectional Study. Rural Remote Health 2018, 18, 4218. [Google Scholar] [CrossRef] [PubMed]
- Souliotis, K.; Golna, C.; Golnas, P.; Markakis, I.-A.; Linardou, H.; Sifaki-Pistolla, D.; Hatziandreou, E. Lung Cancer Screening in Greece: A Modelling Study to Estimate the Impact on Lung Cancer Life Years. Cancers 2022, 14, 5484. [Google Scholar] [CrossRef] [PubMed]
- Mountzios, G.; Gkiozos, I.; Stratakos, G.; Pissakas, G.; Charpidou, A.; Toukfetzian, L.; Vamvakaris, I.; Syrigos, K. Lung Cancer in Greece. J. Thorac. Oncol. 2021, 16, 1058–1066. [Google Scholar] [CrossRef]
- Hillas, G.; Bakakos, P.; Trichas; Vlastos, F. The Disparity of Health Facilities in an Urban Area Discourages Proposed Treatment Application in Inoperable Lung Cancer Patients. Cancer Manag. Res. 2010, 287, 287–291. [Google Scholar] [CrossRef][Green Version]
- Galiti, D.; Linardou, H.; Agelaki, S.; Karampeazis, A.; Tsoukalas, N.; Psyrri, A.; Karamouzis, M.; Syrigos, K.N.; Ardavanis, A.; Athanasiadis, I.; et al. Exploring the Use of a Digital Platform for Cancer Patients to Report Their Demographics, Disease and Therapy Characteristics, Age, and Educational Disparities: An Early-Stage Feasibility Study. Curr. Oncol. 2023, 30, 7608–7619. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sofianidi, A.; Karadimou, A.; Charpidou, A.; Syrigos, K.N. The Gap of Health Inequalities Amongst Lung Cancer Patients of Different Socioeconomic Status: A Brief Reference to the Greek Reality. Cancers 2024, 16, 906. https://doi.org/10.3390/cancers16050906
Sofianidi A, Karadimou A, Charpidou A, Syrigos KN. The Gap of Health Inequalities Amongst Lung Cancer Patients of Different Socioeconomic Status: A Brief Reference to the Greek Reality. Cancers. 2024; 16(5):906. https://doi.org/10.3390/cancers16050906
Chicago/Turabian StyleSofianidi, Amalia, Alexandra Karadimou, Andriani Charpidou, and Konstantinos N. Syrigos. 2024. "The Gap of Health Inequalities Amongst Lung Cancer Patients of Different Socioeconomic Status: A Brief Reference to the Greek Reality" Cancers 16, no. 5: 906. https://doi.org/10.3390/cancers16050906
APA StyleSofianidi, A., Karadimou, A., Charpidou, A., & Syrigos, K. N. (2024). The Gap of Health Inequalities Amongst Lung Cancer Patients of Different Socioeconomic Status: A Brief Reference to the Greek Reality. Cancers, 16(5), 906. https://doi.org/10.3390/cancers16050906