Diagnostic Accuracy of Transvaginal Ultrasound and Magnetic Resonance Imaging for the Detection of Myometrial Infiltration in Endometrial Cancer: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategies
2.3. Selection and Data Collection Process
2.4. Data Items
2.5. Study Risk of Bias Assessment
2.6. Synthesis Methods
3. Results
3.1. Search and Selection
3.2. Basic Characteristics and Eligibility Criteria of Included Studies
3.3. Diagnostic Performance of TVS vs. MRI
3.4. Diagnostic Performance of TVS vs. MRI in a Cohort of Patients with Low-Grade Endometrial Cancer
3.5. MRI Performance According to the Sequences Used
3.6. No Myometrial Invasion vs. Invasion of Any Depth
3.7. Risk of Bias Assessment
3.8. Publication Bias and Heterogeneity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gu, B.; Shang, X.; Yan, M.; Li, X.; Wang, W.; Wang, Q.; Zhang, C. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990–2019. Gynecol. Oncol. 2021, 161, 573–580. [Google Scholar] [CrossRef]
- Åkesson, Å.; Wolmesjö, N.; Adok, C.; Milsom, I.; Dahm-Kähler, P. Lymphadenectomy, obesity and open surgery are associated with surgical complications in endometrial cancer. Eur. J. Surg. Oncol. 2021, 47, 2907–2914. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Epstein, E.; Van Holsbeke, C.; Mascilini, F.; Måsbäck, A.; Kannisto, P.; Ameye, L.; Fischerova, D.; Zannoni, G.; Vellone, V.; Timmerman, D.; et al. Gray-scale and color Doppler ultrasound characteristics of endometrial cancer in relation to stage, grade and tumor size. Ultrasound Obstet. Gynecol. 2011, 38, 586–593. [Google Scholar] [CrossRef]
- Verbakel, J.Y.; Mascilini, F.; Wynants, L.; Fischerova, D.; Testa, A.C.; Franchi, D.; Frühauf, F.; Cibula, D.; Lindqvist, P.G.; Fruscio, R.; et al. Validation of ultrasound strategies to assess tumor extension and to predict high-risk endometrial cancer in women from the prospective IETA (International Endometrial Tumor Analysis)-4 cohort. Ultrasound Obstet. Gynecol. 2020, 55, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.; Paglietti, C.; Arcieri, M.; Biasioli, A.; Della Martina, M.; Mariuzzi, L.; Andreetta, C.; Titone, F.; Bogani, G.; Raimondo, D.; et al. Management of Patients Diagnosed with Endometrial Cancer: Comparison of Guidelines. Cancers 2023, 15, 1091. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, J.L.; Gastón, B.; Navarro, B.; Salas, R.; Aranda, J.; Guerriero, S. Transvaginal ultrasound versus magnetic resonance imaging for preoperative assessment of myometrial infiltration in patients with endometrial cancer: A systematic review and meta-analysis. J. Gynecol. Oncol. 2017, 28, e86. [Google Scholar] [CrossRef] [PubMed]
- Savelli, L.; Ceccarini, M.; Ludovisi, M.; Fruscella, E.; De Iaco, P.A.; Salizzoni, E.; Mabrouk, M.; Manfredi, R.; Testa, A.C.; Ferrandina, G. Preoperative local staging of endometrial cancer: Transvaginal sonography vs. magnetic resonance imaging. Ultrasound Obstet. Gynecol. 2008, 31, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Savelli, L.; Testa, A.C.; Mabrouk, M.; Zannoni, L.; Ludovisi, M.; Seracchioli, R.; Scambia, G.; De Iaco, P. A prospective blinded comparison of the accuracy of transvaginal sonography and frozen section in the assessment of myometrial invasion in endometrial cancer. Gynecol. Oncol. 2012, 124, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bandyopadhyay, S.; Semaan, A.; Shah, J.P.; Mahdi, H.; Morris, R.; Munkarah, A.; Ali-Fehmi, R. The role of frozen section in surgical staging of low risk endometrial cancer. PLoS ONE 2011, 6, e21912. [Google Scholar] [CrossRef] [PubMed]
- Green, R.W.; Valentin, L.; Alcazar, J.L.; Chiappa, V.; Erdodi, B.; Franchi, D.; Frühauf, F.; Fruscio, R.; Guerriero, S.; Graupera, B.; et al. Endometrial cancer off-line staging using two-dimensional transvaginal ultrasound and three-dimensional volume contrast imaging: Intermethod agreement, interrater reliability and diagnostic accuracy. Gynecol. Oncol. 2018, 150, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, A.; Xydias, E.; Kalantzi, S.; Papageorgouli, D.; Liasidi, P.N.; Lamari, I.; Daponte, A. The diagnostic accuracy of 3D ultrasound compared to 2D ultrasound and MRI in the assessment of deep myometrial invasion in endometrial cancer patients: A systematic review. Taiwan J. Obstet. Gynecol. 2022, 61, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Perniola, G.; Derme, M.; Manganaro, L.; Satta, S.; Palaia, I.; Di Donato, V.; Muzii, L.; Panici, P.B. Correlation between preoperative imaging biomarkers and histological prognostic factors in endometrial cancer: A prospective study. J. Clin. Ultrasound 2022, 50, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Mandelbaum, R.S.; Matsuzaki, S.; Klar, M.; Roman, L.D.; Wright, J.D. Ovarian conservation for young women with early-stage, low-grade endometrial cancer: A 2-step schema. Am. J. Obstet. Gynecol. 2021, 224, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Rodolakis, A.; Scambia, G.; Planchamp, F.; Acien, M.; Di Spiezio Sardo, A.; Farrugia, M.; Grynberg, M.; Pakiz, M.; Pavlakis, K.; Vermeulen, N.; et al. ESGO/ESHRE/ESGE Guidelines for the fertility-sparing treatment of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2023, 33, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Freeman, S.C.; Kerby, C.R.; Patel, A.; Cooper, N.J.; Quinn, T.; Sutton, A.J. Development of an interactive web-based tool to conduct and interrogate meta-analysis of diagnostic test accuracy studies: MetaDTA. BMC Med. Res. Methodol. 2019, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Cole, S.R. Bivariate meta-analysis of sensitivity and specificity with sparse data: A generalized linear mixed model approach. J. Clin. Epidemiol. 2006, 59, 1331–1332, author reply 1332–1333. [Google Scholar] [CrossRef]
- Burke, D.L.; Ensor, J.; Snell, K.I.E.; van der Windt, D.; Riley, R.D. Guidance for deriving and presenting percentage study weights in meta-analysis of test accuracy studies. Res. Synth. Methods 2018, 9, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Stijnen, T.; Hamza, T.H.; Ozdemir, P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat. Med. 2010, 29, 3046–3067. [Google Scholar] [CrossRef]
- Pustejovsky, J.E.; Tipton, E. Meta-analysis with Robust Variance Estimation: Expanding the Range of Working Models. Prev. Sci. 2022, 23, 425–438. [Google Scholar] [CrossRef]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef]
- Angioli, R.; Plotti, F.; Capriglione, S.; Scaletta, G.; Dugo, N.; Aloisi, A.; Piccolo, C.L.; Del Vescovo, R.; Terranova, C.; Zobel, B.B. Preoperative local staging of endometrial cancer: The challenge of imaging techniques and serum biomarkers. Arch. Gynecol. Obstet. 2016, 294, 1291–1298. [Google Scholar] [CrossRef]
- Antonsen, S.L.; Jensen, L.N.; Loft, A.; Berthelsen, A.K.; Costa, J.; Tabor, A.; Qvist, I.; Hansen, M.R.; Fisker, R.; Andersen, E.S.; et al. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer—A multicenter prospective comparative study. Gynecol. Oncol. 2013, 128, 300–308. [Google Scholar] [CrossRef]
- Buhler, J.; Routiot, T.; Polet-Lefebvre, K.; Morel, O. Feedback of ultrasound and RMI in the staging of endometrial carcinoma in early stage. Gynecol. Obstet. Fertil. 2015, 43, 329–331. [Google Scholar] [CrossRef]
- Cagnazzo, G.; D’Addario, V.; Martinelli, G.; Lastilla, G. Depth of myometrial invasion in endometrial cancer: Preoperative assessment by transvaginal ultrasonography and magnetic resonance imaging. Ultrasound Obstet. Gynecol. 1992, 2, 40–43. [Google Scholar] [CrossRef]
- Cerovac, A.; Ljuca, D.; Arnautalic, L.; Habek, D.; Bogdanovic, G.; Mustedanagic-Mujanovic, J.; Grgic, G. Efficacy of transvaginal ultrasound versus magnetic resonance imaging for preoperative assessment of myometrial invasion in patients with endometrioid endometrial cancer: A prospective comparative study. Radiol. Oncol. 2022, 56, 37–45. [Google Scholar] [CrossRef]
- Cubo-Abert, M.; Díaz-Feijoo, B.; Bradbury, M.; Rodríguez-Mías, N.L.; Vera, M.; Pérez-Hoyos, S.; Gómez-Cabeza, J.J.; Gil-Moreno, A. Diagnostic performance of transvaginal ultrasound and magnetic resonance imaging for preoperative evaluation of low-grade endometrioid endometrial carcinoma: Prospective comparative study. Ultrasound Obstet. Gynecol. 2021, 58, 469–475. [Google Scholar] [CrossRef]
- DelMaschio, A.; Vanzulli, A.; Sironi, S.; Spagnolo, D.; Belloni, C.; Garancini, P.; Taccagni, G.L. Estimating the depth of myometrial involvement by endometrial carcinoma: Efficacy of transvaginal sonography vs MR imaging. AJR Am. J. Roentgenol. 1993, 160, 533–538. [Google Scholar] [CrossRef][Green Version]
- Dueholm, M.; Hjorth, I.M.; Dahl, K.; Marinovskij, E.; Ørtoft, G. Preoperative prediction of high-risk endometrial cancer by expert and non-expert transvaginal ultrasonography, magnetic resonance imaging, and endometrial histology. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 263, 181–191. [Google Scholar] [CrossRef]
- Gastón, B.; Muruzábal, J.C.; Lapeña, S.; Modroño, A.; Guarch, R.; García de Eulate, I.; Alcázar, J.L. Transvaginal Ultrasound Versus Magnetic Resonance Imaging for Assessing Myometrial Infiltration in Endometrioid Low Grade Endometrial Cancer: A Prospective Study. J. Ultrasound Med. 2022, 41, 335–342. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.D.; Song, Y.S.; Kang, S.B.; Lee, H.P. Detection of deep myometrial invasion in endometrial carcinoma: Comparison of transvaginal ultrasound, CT, and MRI. J. Comput. Assist. Tomogr. 1995, 19, 766–772. [Google Scholar] [CrossRef]
- Özdemir, S.; Çelik, C.; Emlik, D.; Kiresi, D.; Esen, H. Assessment of myometrial invasion in endometrial cancer by transvaginal sonography, doppler ultrasonography, magnetic resonance imaging and frozen section. Int. J. Gynecol. Cancer 2009, 19, 1085–1090. [Google Scholar] [CrossRef]
- Palmér, M.; Åkesson, Å.; Marcickiewicz, J.; Blank, E.; Hogström, L.; Torle, M.; Mateoiu, C.; Dahm-Kähler, P.; Leonhardt, H. Accuracy of transvaginal ultrasound versus MRI in the PreOperative Diagnostics of low-grade Endometrial Cancer (PODEC) study: A prospective multicentre study. Clin. Radiol. 2023, 78, 70–79. [Google Scholar] [CrossRef]
- Rahmani, M.; Heydari, S.; Mousavi, A.; Ahmadinejad, N.; Azhdeh, S.; Shakiba, M. Accuracy of imaging in preoperative local staging of endometrial cancer: Could imaging predict low risk patients? Int. J. Women’s Health Reprod. Sci. 2018, 6, 363–368. [Google Scholar] [CrossRef]
- Wong, M.; Amin, T.; Thanatsis, N.; Naftalin, J.; Jurkovic, D. A prospective comparison of the diagnostic accuracies of ultrasound and magnetic resonance imaging in preoperative staging of endometrial cancer. J. Gynecol. Oncol. 2022, 33, e22. [Google Scholar] [CrossRef]
- Yahata, T.; Aoki, Y.; Tanaka, K. Prediction of myometrial invasion in patients with endometrial carcinoma: Comparison of magnetic resonance imaging, transvaginal ultrasonography, and gross visual inspection. Eur. J. Gynaecol. Oncol. 2007, 28, 193–195. [Google Scholar]
- Yamashita, Y.; Mizutani, H.; Torashima, M.; Takahashi, M.; Miyazaki, K.; Okamura, H.; Ushijima, H.; Ohtake, H.; Tokunaga, T. Assessment of myometrial invasion by endometrial carcinoma: Transvaginal sonography vs contrast-enhanced MR imaging. AJR Am. J. Roentgenol. 1993, 161, 595–599. [Google Scholar] [CrossRef]
- Epstein, E.; Fischerova, D.; Valentin, L.; Testa, A.C.; Franchi, D.; Sladkevicius, P.; Frühauf, F.; Lindqvist, P.G.; Mascilini, F.; Fruscio, R.; et al. Ultrasound characteristics of endometrial cancer as defined by International Endometrial Tumor Analysis (IETA) consensus nomenclature: Prospective multicenter study. Ultrasound Obstet. Gynecol. 2018, 51, 818–828. [Google Scholar] [CrossRef]
- Alcazar, J.L.; Pineda, L.; Martinez-Astorquiza Corral, T.; Orozco, R.; Utrilla-Layna, J.; Juez, L.; Jurado, M. Transvaginal/transrectal ultrasound for assessing myometrial invasion in endometrial cancer: A comparison of six different approaches. J. Gynecol. Oncol. 2015, 26, 201–207. [Google Scholar] [CrossRef]
- Fischerova, D.; Frühauf, F.; Zikan, M.; Pinkavova, I.; Kocián, R.; Dundr, P.; Nemejcova, K.; Dusek, L.; Cibula, D. Factors affecting sonographic preoperative local staging of endometrial cancer. Ultrasound Obstet. Gynecol. 2014, 43, 575–585. [Google Scholar] [CrossRef]
- Tameish, S.; Florez, N.; Vidal, J.R.P.; Chen, H.; Vara, J.; Alcázar, J.L. Transvaginal ultrasound versus magnetic resonance imaging for preoperative assessment of myometrial infiltration in patients with low-grade endometrioid endometrial cancer: A systematic review and head-to-head meta-analysis. J. Clin. Ultrasound 2023, 51, 1188–1197. [Google Scholar] [CrossRef]
- Wynants, L.; Verbakel, J.Y.J.; Valentin, L.; De Cock, B.; Pascual, M.A.; Leone, F.P.G.; Sladkevicius, P.; Heremans, R.; Alcazar, J.L.; Votino, A.; et al. The Risk of Endometrial Malignancy and Other Endometrial Pathology in Women with Abnormal Uterine Bleeding: An Ultrasound-Based Model Development Study by the IETA Group. Gynecol. Obstet. Investig. 2022, 87, 54–61. [Google Scholar] [CrossRef]
- Van Holsbeke, C.; Ameye, L.; Testa, A.C.; Mascilini, F.; Lindqvist, P.; Fischerova, D.; Frühauf, F.; Fransis, S.; de Jonge, E.; Timmerman, D.; et al. Development and external validation of new ultrasound-based mathematical models for preoperative prediction of high-risk endometrial cancer. Ultrasound Obstet. Gynecol. 2014, 43, 586–595. [Google Scholar] [CrossRef]
- Eriksson, L.S.E.; Nastic, D.; Frühauf, F.; Fischerova, D.; Nemejcova, K.; Bono, F.; Franchi, D.; Fruscio, R.; Ghioni, M.; Haak, L.A.; et al. Clinical and Ultrasound Characteristics of the Microcystic Elongated and Fragmented (MELF) Pattern in Endometrial Cancer According to the International Endometrial Tumor Analysis (IETA) criteria. Int. J. Gynecol. Cancer 2019, 29, 119–125. [Google Scholar] [CrossRef]
- Capozzi, V.A.; Merisio, C.; Rolla, M.; Pugliese, M.; Morganelli, G.; Cianciolo, A.; Gambino, G.; Armano, G.; Sozzi, G.; Riccò, M.; et al. Confounding factors of transvaginal ultrasound accuracy in endometrial cancer. J. Obstet. Gynaecol. 2021, 41, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Moro, F.; Albanese, M.; Boldrini, L.; Chiappa, V.; Lenkowicz, J.; Bertolina, F.; Mascilini, F.; Moroni, R.; Gambacorta, M.A.; Raspagliesi, F.; et al. Developing and validating ultrasound-based radiomics models for predicting high-risk endometrial cancer. Ultrasound Obstet. Gynecol. 2022, 60, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Chiappa, V.; Bogani, G.; Interlenghi, M.; Salvatore, C.; Bertolina, F.; Sarpietro, G.; Signorelli, M.; Castiglioni, I.; Raspagliesi, F. The Adoption of Radiomics and machine learning improves the diagnostic processes of women with Ovarian MAsses (the AROMA pilot study). J. Ultrasound 2021, 24, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Chiappa, V.; Interlenghi, M.; Salvatore, C.; Bertolina, F.; Bogani, G.; Ditto, A.; Martinelli, F.; Castiglioni, I.; Raspagliesi, F. Using rADioMIcs and machine learning with ultrasonography for the differential diagnosis of myometRiAL tumors (the ADMIRAL pilot study). Radiomics and differential diagnosis of myometrial tumors. Gynecol. Oncol. 2021, 161, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Spagnol, G.; Noventa, M.; Bonaldo, G.; Marchetti, M.; Vitagliano, A.; Laganà, A.S.; Cavallin, F.; Scioscia, M.; Saccardi, C.; Tozzi, R. Three-dimensional transvaginal ultrasound vs magnetic resonance imaging for preoperative staging of deep myometrial and cervical invasion in patients with endometrial cancer: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2022, 60, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Costas, T.; Belda, R.; Alcazar, J.L. Transvaginal three-dimensional ultrasound for preoperative assessment of myometrial invasion in patients with endometrial cancer: A systematic review and meta-analysis. Med. Ultrason. 2022, 24, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Frühauf, F.; Zikan, M.; Semeradova, I.; Dundr, P.; Nemejcova, K.; Dusek, L.; Cibula, D.; Fischerova, D. The Diagnostic Accuracy of Ultrasound in Assessment of Myometrial Invasion in Endometrial Cancer: Subjective Assessment versus Objective Techniques. BioMed Res. Int. 2017, 2017, 1318203. [Google Scholar] [CrossRef] [PubMed]
- Sammet, S. Magnetic resonance safety. Abdom. Radiol. 2016, 41, 444–451. [Google Scholar] [CrossRef]
- Murphy, K.J.; Brunberg, J.A. Adult claustrophobia, anxiety and sedation in MRI. Magn. Reson. Imaging 1997, 15, 51–54. [Google Scholar] [CrossRef]
- Soneji, N.D.; Bharwani, N.; Ferri, A.; Stewart, V.; Rockall, A. Pre-operative MRI staging of endometrial cancer in a multicentre cancer network: Can we match single centre study results? Eur. Radiol. 2018, 28, 4725–4734. [Google Scholar] [CrossRef]
- Bi, Q.; Chen, Y.; Wu, K.; Wang, J.; Zhao, Y.; Wang, B.; Du, J. The Diagnostic Value of MRI for Preoperative Staging in Patients with Endometrial Cancer: A Meta-Analysis. Acad. Radiol. 2020, 27, 960–968. [Google Scholar] [CrossRef]
- Wang, L.J.; Tseng, Y.J.; Wee, N.K.; Low, J.J.H.; Tan, C.H. Diffusion-weighted imaging versus dynamic contrast-enhanced imaging for pre-operative diagnosis of deep myometrial invasion in endometrial cancer: A meta-analysis. Clin. Imaging 2021, 80, 36–42. [Google Scholar] [CrossRef]
- Andreano, A.; Rechichi, G.; Rebora, P.; Sironi, S.; Valsecchi, M.G.; Galimberti, S. MR diffusion imaging for preoperative staging of myometrial invasion in patients with endometrial cancer: A systematic review and meta-analysis. Eur. Radiol. 2014, 24, 1327–1338. [Google Scholar] [CrossRef]
- Di Donato, V.; Kontopantelis, E.; Cuccu, I.; Sgamba, L.; Golia D’Augè, T.; Pernazza, A.; Della Rocca, C.; Manganaro, L.; Catalano, C.; Perniola, G.; et al. Magnetic resonance imaging-radiomics in endometrial cancer: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2023, 33, 1070–1076. [Google Scholar] [CrossRef]
- Chiappa, V.; Bogani, G.; Interlenghi, M.; Vittori Antisari, G.; Salvatore, C.; Zanchi, L.; Ludovisi, M.; Leone Roberti Maggiore, U.; Calareso, G.; Haeusler, E.; et al. Using Radiomics and Machine Learning Applied to MRI to Predict Response to Neoadjuvant Chemotherapy in Locally Advanced Cervical Cancer. Diagnostics 2023, 13, 3139. [Google Scholar] [CrossRef]
- Shrestha, P.; Poudyal, B.; Yadollahi, S.; Wright, D.E.; Gregory, A.V.; Warner, J.D.; Korfiatis, P.; Green, I.C.; Rassier, S.L.; Mariani, A.; et al. A systematic review on the use of artificial intelligence in gynecologic imaging—Background, state of the art, and future directions. Gynecol. Oncol. 2022, 166, 596–605. [Google Scholar] [CrossRef]
- Pesapane, F.; De Marco, P.; Rapino, A.; Lombardo, E.; Nicosia, L.; Tantrige, P.; Rotili, A.; Bozzini, A.C.; Penco, S.; Dominelli, V.; et al. How Radiomics Can Improve Breast Cancer Diagnosis and Treatment. J. Clin. Med. 2023, 12, 1372. [Google Scholar] [CrossRef]
- Hegyi, P.; Erőss, B.; Izbéki, F.; Párniczky, A.; Szentesi, A. Accelerating the translational medicine cycle: The Academia Europaea pilot. Nat. Med. 2021, 27, 1317–1319. [Google Scholar] [CrossRef]
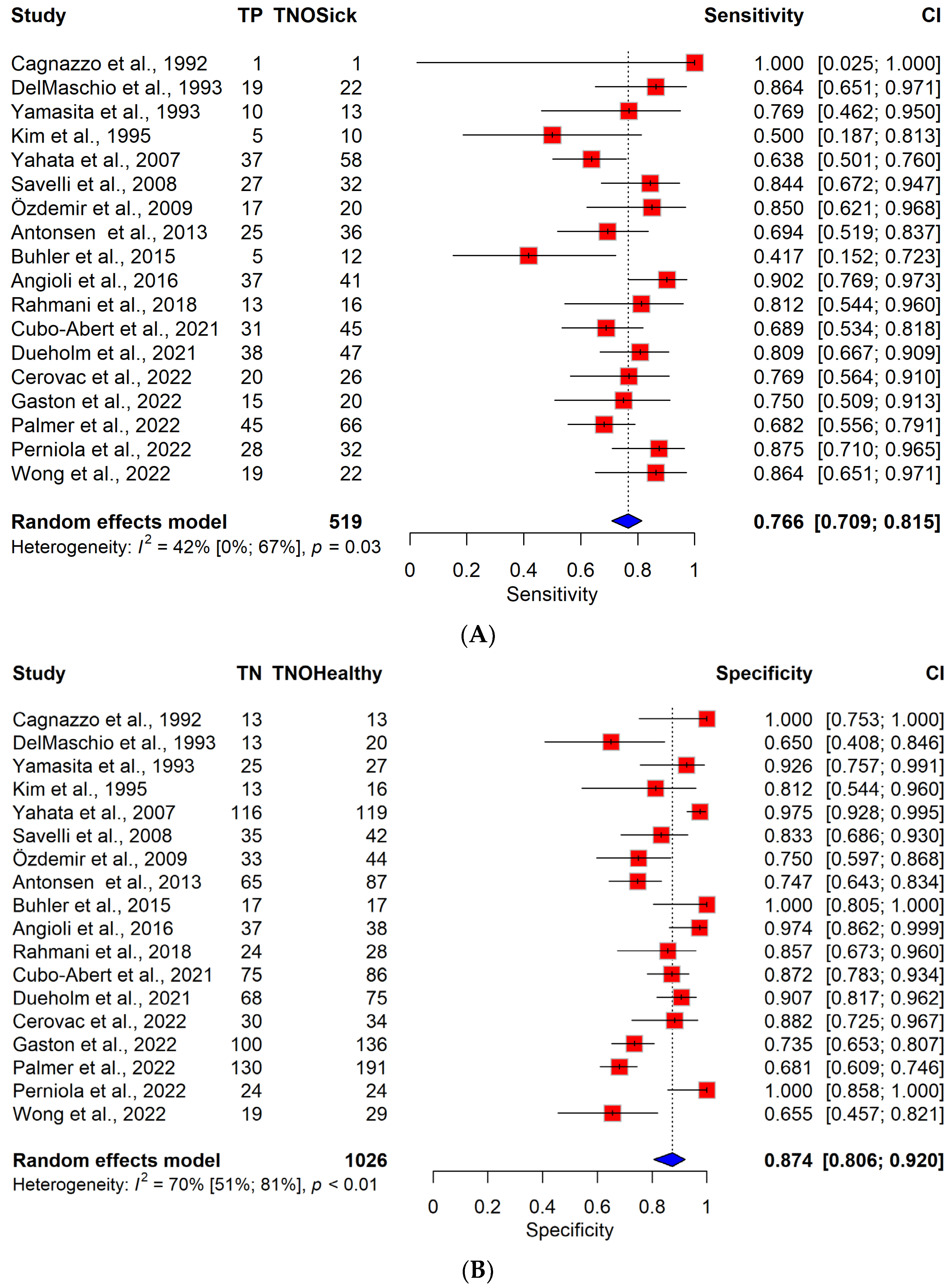
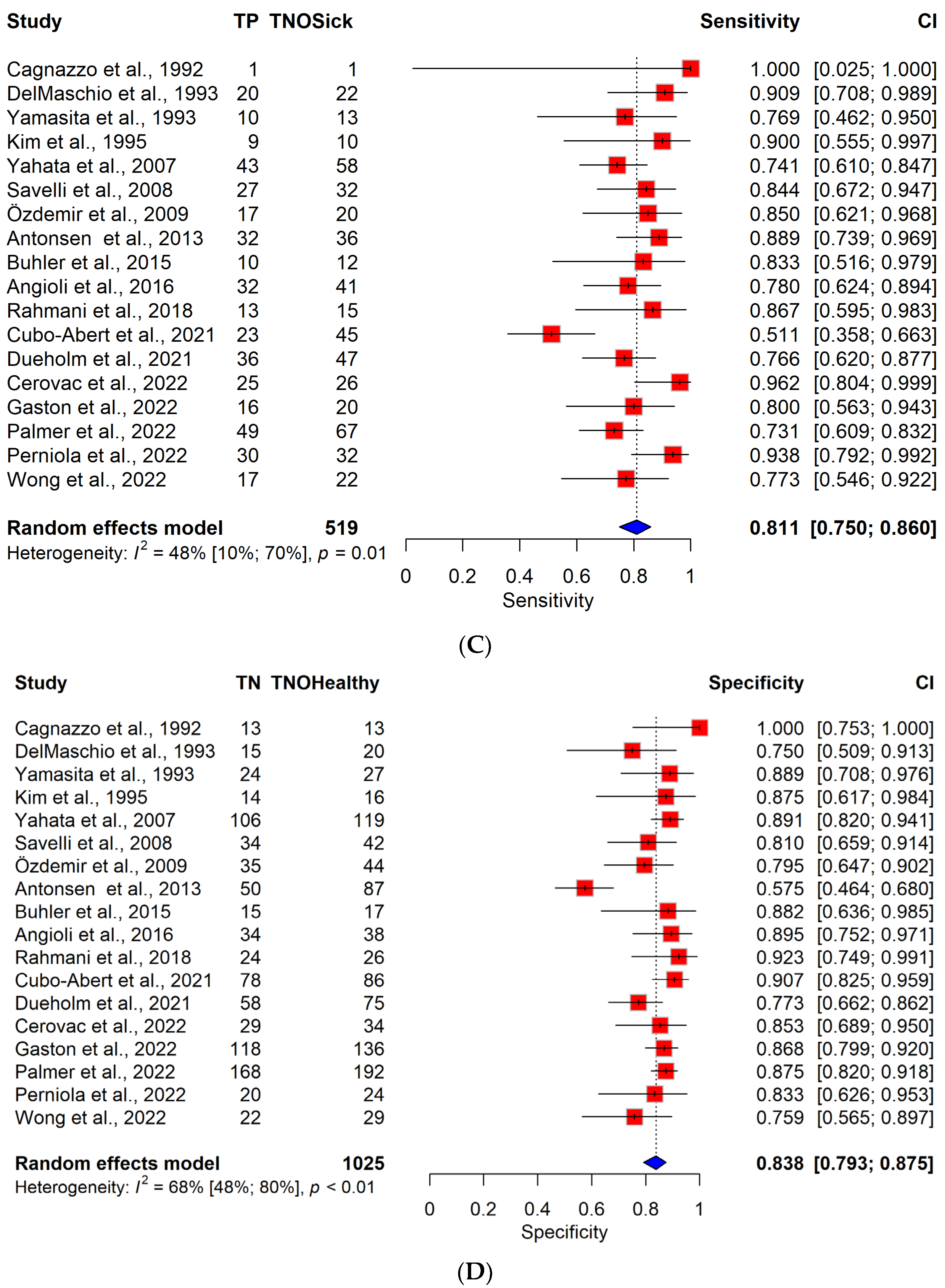


| Author | Study Design | Study Period | Number of Centers | Number of Patients | Number of DMI (≥50% Myometrial Infiltration) | Pathologist Blinded to Imaging | TVS Observer | MRI Observer |
|---|---|---|---|---|---|---|---|---|
| Cubo-Abert, 2021 [33] | prospective | October 2013–July 2018 | single | 131 | 45 | NA | single | multiple |
| Gaston, 2022 [36] | prospective | January 2016–March 2020 | single | 156 | 20 | yes | single | single |
| Palmer, 2023 [39] | prospective | January 2017–June 2019 | multi | 259 | 67 | NA | multiple | multiple |
| Wong, 2022 [41] | prospective | October 2015–October 2018 | single | 51 | 22 | yes | single | single |
| Perniola, 2022 [14] | prospective | March 2019–March 2021 | single | 56 | 32 | NA | single | single |
| Cerovac, 2022 [32] | prospective | July 2019–April 2021 | single | 60 | 26 | NA | single | single |
| Rahmani, 2018 [40] | prospective | October 2009–December 2012 | single | 45 | 16 | NA | single | single |
| Dueholm, 2021 [35] | prospective | November 2013–December 2015 | single | 122 | 47 | NA | multiple | multiple |
| Cagnazzo, 1992 [31] | NA | NA | NA | 14 | 1 | NA | NA | NA |
| DelMaschio, 1993 [34] | prospective | NA | NA | 42 | 22 | yes | single | multiple |
| Yamasita, 1993 [43] | prospective | NA | NA | 40 | 13 | yes | single | multiple |
| Kim, 1995 [37] | prospective | January 1991–April 1994 | NA | 26 | 10 | NA | multiple | multiple |
| Yahata, 2007 [42] | retrospective | January 1995–April 2004 | NA | 177 | 58 | NA | NA | NA |
| Savelli, 2008 [9] | prospective | February 2000–May 2004 | multi | 74 | 32 | NA | multiple | multiple |
| Özdemir, 2009 [38] | prospective | January 2007–November2008 | single | 64 | 20 | NA | single | multiple |
| Antonsen, 2013 [29] | prospective | September 2009–January 2012 | multi | 123 | 36 | NA | multiple | multiple |
| Buhler, 2015 [30] | retrospective | July 2012–July 2014 | single | 29 | 12 | NA | multiple | multiple |
| Angioli, 2016 [28] | prospective | January 2012–February 2015 | single | 79 | 41 | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madár, I.; Szabó, A.; Vleskó, G.; Hegyi, P.; Ács, N.; Fehérvári, P.; Kói, T.; Kálovics, E.; Szabó, G. Diagnostic Accuracy of Transvaginal Ultrasound and Magnetic Resonance Imaging for the Detection of Myometrial Infiltration in Endometrial Cancer: A Systematic Review and Meta-Analysis. Cancers 2024, 16, 907. https://doi.org/10.3390/cancers16050907
Madár I, Szabó A, Vleskó G, Hegyi P, Ács N, Fehérvári P, Kói T, Kálovics E, Szabó G. Diagnostic Accuracy of Transvaginal Ultrasound and Magnetic Resonance Imaging for the Detection of Myometrial Infiltration in Endometrial Cancer: A Systematic Review and Meta-Analysis. Cancers. 2024; 16(5):907. https://doi.org/10.3390/cancers16050907
Chicago/Turabian StyleMadár, István, Anett Szabó, Gábor Vleskó, Péter Hegyi, Nándor Ács, Péter Fehérvári, Tamás Kói, Emma Kálovics, and Gábor Szabó. 2024. "Diagnostic Accuracy of Transvaginal Ultrasound and Magnetic Resonance Imaging for the Detection of Myometrial Infiltration in Endometrial Cancer: A Systematic Review and Meta-Analysis" Cancers 16, no. 5: 907. https://doi.org/10.3390/cancers16050907
APA StyleMadár, I., Szabó, A., Vleskó, G., Hegyi, P., Ács, N., Fehérvári, P., Kói, T., Kálovics, E., & Szabó, G. (2024). Diagnostic Accuracy of Transvaginal Ultrasound and Magnetic Resonance Imaging for the Detection of Myometrial Infiltration in Endometrial Cancer: A Systematic Review and Meta-Analysis. Cancers, 16(5), 907. https://doi.org/10.3390/cancers16050907






