Towards a Simplified and Cost-Effective Diagnostic Algorithm for the Surveillance of Intraductal Papillary Mucinous Neoplasms (IPMNs): Can We Save Contrast for Later?
Abstract
Simple Summary
Abstract
1. Introduction
2. Pancreatic Cystic Lesions: An Increasing Burden
3. Current Management Protocol for Pancreatic Cystic Lesions
4. Towards Abbreviated MRI Protocols: Is Contrast Media Really Necessary?
5. The Additional Value of Diffusion-Weighted Imaging: Can It Replace the Use of Contrast Agents?
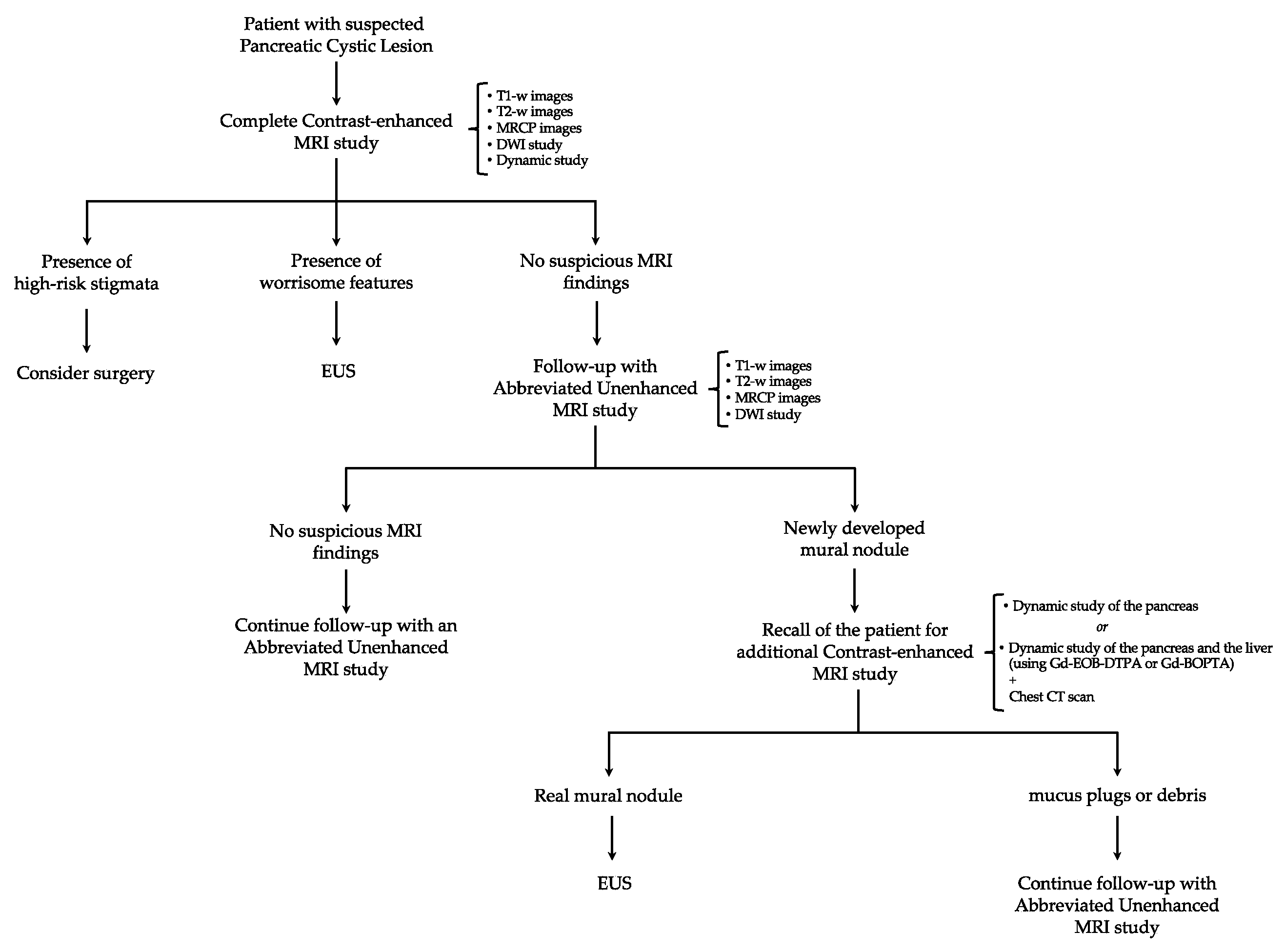
6. Proposal for a New Algorithm
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Pancreas Factsheet. Globocan. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/13-Pancreas-fact-sheet.pdf (accessed on 3 February 2024).
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef]
- Huang, J.; Lok, V.; Ngai, C.H.; Zhang, L.; Yuan, J.; Lao, X.Q.; Ng, K.; Chong, C.; Zheng, Z.J.; Wong, M.C.S. Worldwide Burden of Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology 2021, 160, 744–754. [Google Scholar] [CrossRef]
- Hu, J.X.; Zhao, C.F.; Chen, W.B.; Liu, Q.C.; Li, Q.W.; Lin, Y.Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.M.; Hwang, J.H.; Moayyedi, P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 824–848.e22. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Arcidiacono, P.G.; De Cobelli, F.; Falconi, M. Review of the diagnosis and management of intraductal papillary mucinous neoplasms. United Eur. Gastroenterol. J. 2020, 8, 249–255. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernández-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- Zaheer, A.; Pokharel, S.S.; Wolfgang, C.; Fishman, E.K.; Horton, K.M. Incidentally detected cystic lesions of the pancreas on CT: Review of literature and management suggestions. Abdom. Imaging 2013, 38, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H.; Mortenson, M.M.; Wang, H.; Hwang, R.; Tamm, E.P.; Staerkel, G.; Lee, J.H.; Evans, D.B.; Fleming, J.B. Diagnosis and management of cystic neoplasms of the pancreas: An evidence-based approach. J. Am. Coll. Surg. 2008, 207, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Sahani, D.V.; Kambadakone, A.; Macari, M.; Takahashi, N.; Chari, S.; Fernandez-del Castillo, C. Diagnosis and management of cystic pancreatic lesions. Am. J. Roentgenol. 2013, 200, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Pezzilli, R.; Buscarini, E.; Pollini, T.; Bonamini, D.; Marchegiani, G.; Crippa, S.; Belfiori, G.; Sperti, C.; Moletta, L.; Pozza, G.; et al. Epidemiology, clinical features and diagnostic work-up of cystic neoplasms of the pancreas: Interim analysis of the prospective PANCY survey. Dig. Liver Dis. 2020, 52, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Brugge, W.R.; Lauwers, G.Y.; Sahani, D.; Fernandez-del Castillo, C.; Warshaw, A.L. Cystic neoplasms of the pancreas. N. Engl. J. Med. 2004, 351, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Gao, H.; Ma, Y.; Zhuang, Y.; Yang, Y. Surgical treatment and prognosis of 96 cases of intraductal papillary mucinous neoplasms of the pancreas: A retrospective cohort study. Int. J. Surg. 2015, 13, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Moris, M.; Bridges, M.D.; Pooley, R.A.; Raimondo, M.; Woodward, T.A.; Stauffer, J.A.; Asbun, H.J.; Wallace, M.B. Association Between Advances in High-Resolution Cross-Section Imaging Technologies and Increase in Prevalence of Pancreatic Cysts From 2005 to 2014. Clin. Gastroenterol Hepatol. 2016, 14, 585–593.e3. [Google Scholar] [CrossRef] [PubMed]
- Klibansky, D.A.; Reid-Lombardo, K.M.; Gordon, S.R.; Gardner, T.B. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clin. Gastroenterol. Hepatol. 2012, 10, 555–558. [Google Scholar] [CrossRef]
- Girometti, R.; Intini, S.; Brondani, G.; Como, G.; Londero, F.; Bresadola, F.; Zuiani, C.; Bazzocchi, M. Incidental pancreatic cysts on 3D turbo spin echo magnetic resonance cholangiopancreatography: Prevalence and relation with clinical and imaging features. Abdom. Imaging 2011, 36, 196–205. [Google Scholar] [CrossRef]
- de Jong, K.; Nio, C.Y.; Hermans, J.J.; Dijkgraaf, M.G.; Gouma, D.J.; van Eijck, C.H.; van Heel, E.; Klass, G.; Fockens, P.; Bruno, M.J. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin. Gastroenterol. Hepatol. 2010, 8, 806–811. [Google Scholar] [CrossRef]
- Zerboni, G.; Signoretti, M.; Crippa, S.; Falconi, M.; Arcidiacono, P.G.; Capurso, G. Systematic review and meta-analysis: Prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology 2019, 19, 2–9. [Google Scholar] [CrossRef]
- Kromrey, M.L.; Bülow, R.; Hübner, J.; Paperlein, C.; Lerch, M.M.; Ittermann, T.; Völzke, H.; Mayerle, J.; Kühn, J.P. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut 2018, 67, 138–145. [Google Scholar] [CrossRef]
- Lee, K.S.; Sekhar, A.; Rofsky, N.M.; Pedrosa, I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am. J. Gastroenterol. 2010, 105, 2079–2084. [Google Scholar] [CrossRef]
- Giuffrida, P.; Biagiola, D.; Ardiles, V.; Uad, P.; Palavecino, M.; de Santibañes, M.; Clariá, R.S.; Pekolj, J.; de Santibañes, E.; Mazza, O. Long-term follow-up of Branch-Duct Intraductal Papillary Mucinous Neoplasms with negative Sendai Criteria: The therapeutic challenge of patients who convert to positive Sendai Criteria. HPB 2021, 23, 290–300. [Google Scholar] [CrossRef]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P.; Clinical Guidelines Committee; American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 819–822. [Google Scholar] [CrossRef]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Budde, C.; Beyer, G.; Kühn, J.P.; Lerch, M.M.; Mayerle, J. The Clinical and Socio-Economic Relevance of Increased IPMN Detection Rates and Management Choices. Viszeralmedizin 2015, 31, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Weinberg, D.S.; Schünemann, H.; Chak, A. Management of pancreatic cysts in an evidence-based world. Gastroenterology 2015, 148, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Hwang, J.W. Research progress and future directions on intraductal papillary mucinous neoplasm: A bibliometric and visualized analysis of over 30 years of research. Medicine 2023, 102, e33568. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, T.; Fernandez-Del Castillo, C.; Furukawa, T.; Hijioka, S.; Jang, J.Y.; Lennon, A.M.; Miyasaka, Y.; Ohno, E.; Salvia, R.; Wolfgang, C.L.; et al. International evidence-based Kyoto guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas. Pancreatology 2023. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, G.; Salvia, R.; Verona EBM 2020 on IPMN. Guidelines on Pancreatic Cystic Neoplasms: Major Inconsistencies With Available Evidence and Clinical Practice- Results From an International Survey. Gastroenterology 2021, 160, 2234–2238. [Google Scholar] [CrossRef] [PubMed]
- Luk, L.; Hecht, E.M.; Kang, S.; Bhosale, P.R.; Francis, I.R.; Gandhi, N.; Hough, D.M.; Khatri, G.; Megibow, A.; Morgan, D.E.; et al. Society of Abdominal Radiology Disease Focused Panel Survey on Clinical Utilization of Incidental Pancreatic Cyst Management Recommendations and Template Reporting. J. Am. Coll. Radiol. 2021, 18, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Chari, S.; Adsay, V.; Fernandez-del Castillo, C.; Falconi, M.; Shimizu, M.; Yamaguchi, K.; Yamao, K.; Matsuno, S.; International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006, 6, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernández-del Castillo, C.; Adsay, V.; Chari, S.; Falconi, M.; Jang, J.Y.; Kimura, W.; Levy, P.; Pitman, M.B.; Schmidt, C.M. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012, 12, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.W.; Jang, J.Y.; Lee, S.E.; Lim, C.S.; Lee, K.U.; Kim, S.W. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: A 15-year experience at a single academic institution. Langenbecks Arch. Surg. 2012, 397, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.M.; White, P.B.; Waters, J.A.; Yiannoutsos, C.T.; Cummings, O.W.; Baker, M.; Howard, T.J.; Zyromski, N.J.; Nakeeb, A.; DeWitt, J.M.; et al. Intraductal papillary mucinous neoplasms: Predictors of malignant and invasive pathology. Ann. Surg. 2007, 246, 644–651. [Google Scholar] [CrossRef]
- Ridtitid, W.; DeWitt, J.M.; Schmidt, C.M.; Roch, A.; Stuart, J.S.; Sherman, S.; Al-Haddad, M.A. Management of branch-duct intraductal papillary mucinous neoplasms: A large single-center study to assess predictors of malignancy and long-term outcomes. Gastrointest. Endosc. 2016, 84, 436–445. [Google Scholar] [CrossRef]
- Marchegiani, G.; Andrianello, S.; Borin, A.; Dal Borgo, C.; Perri, G.; Pollini, T.; Romanò, G.; D’Onofrio, M.; Gabbrielli, A.; Scarpa, A.; et al. Systematic review, meta-analysis, and a high-volume center experience supporting the new role of mural nodules proposed by the updated 2017 international guidelines on IPMN of the pancreas. Surgery 2018, 163, 1272–1279. [Google Scholar] [CrossRef]
- Maimone, S.; Agrawal, D.; Pollack, M.J.; Wong, R.C.; Willis, J.; Faulx, A.L.; Isenberg, G.A.; Chak, A. Variability in measurements of pancreatic cyst size among EUS, CT, and magnetic resonance imaging modalities. Gastrointest. Endosc. 2010, 71, 945–950. [Google Scholar] [CrossRef]
- Lisotti, A.; Fusaroli, P. Diagnostic accuracy for neoplastic IPMN: Does the contrast make the difference? Abdom. Radiol. 2017, 42, 2698–2699. [Google Scholar] [CrossRef]
- Min, J.H.; Kim, Y.K.; Kim, S.K.; Kim, H.; Ahn, S. Intraductal papillary mucinous neoplasm of the pancreas: Diagnostic performance of the 2017 international consensus guidelines using CT and MRI. Eur. Radiol. 2021, 31, 4774–4784. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, M.; Brandi, N.; Brocchi, S.; Balacchi, C.; Lanza, C.; Pettinari, I.; Stefanini, B.; Carrafiello, G.; Piscaglia, F.; Golfieri, R.; et al. Association between anatomic variations of extrahepatic and intrahepatic bile ducts: Do look up! J. Anat. 2023, 242, 683–694. [Google Scholar] [CrossRef]
- Nougaret, S.; Reinhold, C.; Chong, J.; Escal, L.; Mercier, G.; Fabre, J.M.; Guiu, B.; Molinari, N. Incidental pancreatic cysts: Natural history and diagnostic accuracy of a limited serial pancreatic cyst MRI protocol. Eur. Radiol. 2014, 24, 1020–1029. [Google Scholar] [CrossRef]
- Faccioli, N.; Santi, E.; Foti, G.; D’Onofrio, M. Cost-effectiveness analysis of including contrast-enhanced ultrasound in management of pancreatic cystic neoplasms. Radiol. Med. 2022, 127, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.M.; Heales, C.; Vine, S.J. Radiographer Perspectives on current occurrence and management of claustrophobia in MRI. Radiography 2022, 28, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Oztek, M.A.; Brunnquell, C.L.; Hoff, M.N.; Boulter, D.J.; Mossa-Basha, M.; Beauchamp, L.H.; Haynor, D.L.; Nguyen, X.V. Practical Considerations for Radiologists in Implementing a Patient-friendly MRI Experience. Top. Magn. Reson. Imaging 2020, 29, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, I.; Ohlhues, A.; Madsen, K.H.; Nybing, J.D.; Christensen, H.; Christensen, A. Are Movement Artifacts in Magnetic Resonance Imaging a Real Problem?-A Narrative Review. Front. Neurol. 2017, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Renzulli, M.; La Palombara, C.; Fattori, R. Congenital diseases of the thoracic aorta. Role of MRI and MRA. Eur. Radiol. 2006, 16, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Zivadinov, R.; Bergsland, N.; Hagemeier, J.; Ramasamy, D.P.; Dwyer, M.G.; Schweser, F.; Kolb, C.; Weinstock-Guttman, B.; Hojnacki, D. Cumulative gadodiamide administration leads to brain gadolinium deposition in early MS. Neurology 2019, 93, e611–e623. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Ishii, K.; Kawaguchi, H.; Kitajima, K.; Takenaka, D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: Relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014, 270, 834–841. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; Levine, D.; Weinreb, J.; Kanal, E.; Davenport, M.S.; Ellis, J.H.; Jacobs, P.M.; Lenkinski, R.E.; Maravilla, K.R.; Prince, M.R.; et al. Gadolinium Retention: A Research Roadmap from the 2018 NIH/ACR/RSNA Workshop on Gadolinium Chelates. Radiology 2018, 289, 517–534. [Google Scholar] [CrossRef]
- Macari, M.; Lee, T.; Kim, S.; Jacobs, S.; Megibow, A.J.; Hajdu, C.; Babb, J. Is gadolinium necessary for MRI follow-up evaluation of cystic lesions in the pancreas? Preliminary results. AJR Am. J. Roentgenol. 2009, 192, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Pozzi-Mucelli, R.M.; Rinta-Kiikka, I.; Wünsche, K.; Laukkarinen, J.; Labori, K.J.; Ånonsen, K.; Verbeke, C.; Del Chiaro, M.; Kartalis, N. Pancreatic MRI for the surveillance of cystic neoplasms: Comparison of a short with a comprehensive imaging protocol. Eur. Radiol. 2017, 27, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Lee, D.H.; Lee, J.M.; Yoo, J.; Weiland, E.; Kim, E.; Son, Y. Clinical Feasibility of Abbreviated Magnetic Resonance with Breath-Hold 3-Dimensional Magnetic Resonance Cholangiopancreatography for Surveillance of Pancreatic Intraductal Papillary Mucinous Neoplasm. Investig. Radiol. 2020, 55, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Johansson, K.; Mustonen, H.; Nieminen, H.; Haglund, C.; Lehtimäki, T.E.; Seppänen, H. MRI follow-up for pancreatic intraductal papillary mucinous neoplasm: An ultrashort versus long protocol. Abdom. Radiol. 2022, 47, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Kierans, A.S.; Gavlin, A.; Wehrli, N.; Flisnik, L.M.; Eliades, S.; Pittman, M.E. Utility of gadolinium for identifying the malignant potential of pancreatic cystic lesions. Abdom. Radiol. 2022, 47, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Min, J.H.; Lee, D.H.; Hur, B.Y.; Kim, S.W.; Kim, E. Abbreviated Magnetic Resonance Imaging With Breath-Hold Three-Dimensional Magnetic Resonance Cholangiopancreatography: Assessment of Malignant Risk of Pancreatic Intraductal Papillary Mucinous Neoplasm. J. Magn. Reson. Imaging 2021, 54, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff, K.; Webb, A.N.; Krys, D.; Anderson, D.; Bigam, D.L.; Fung, C.I. Multicentre Analysis of Cost, Uptake and Safety of Canadian Multidisciplinary Pancreatic Cyst Guidelines. J. Can. Assoc. Gastroenterol. 2023, 6, 86–93. [Google Scholar] [CrossRef]
- Ohno, E.; Balduzzi, A.; Hijioka, S.; De Pastena, M.; Marchegiani, G.; Kato, H.; Takenaka, M.; Haba, S.; Salvia, R. Association of high-risk stigmata and worrisome features with advanced neoplasia in intraductal papillary mucinous neoplasms (IPMN): A systematic review. Pancreatology 2024, 24, 48–61. [Google Scholar] [CrossRef]
- Megibow, A.J.; Baker, M.E.; Morgan, D.E.; Kamel, I.R.; Sahani, D.V.; Newman, E.; Brugge, W.R.; Berland, L.L.; Pandharipande, P.V. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J. Am. Coll. Radiol. 2017, 14, 911–923. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, J.H.; Yu, M.H.; Choi, S.Y.; Kang, H.J.; Park, H.J.; Park, Y.S.; Byun, J.H.; Shin, S.S.; Lee, C.H.; et al. Diagnosis and Surveillance of Incidental Pancreatic Cystic Lesions: 2017 Consensus Recommendations of the Korean Society of Abdominal Radiology. Korean J. Radiol. 2019, 20, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Berland, L.L.; Silverman, S.G.; Gore, R.M.; Mayo-Smith, W.W.; Megibow, A.J.; Yee, J.; Brink, J.A.; Baker, M.E.; Federle, M.P.; Foley, W.D. Managing incidental findings on abdominal CT: White paper of the ACR incidental findings committee. J. Am. Coll. Radiol. 2010, 7, 754–773. [Google Scholar] [CrossRef]
- Bassi, C.; Crippa, S.; Salvia, R. Intraductal papillary mucinous neoplasms (IPMNs): Is it time to (sometimes) spare the knife? Gut 2008, 57, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Brounts, L.R.; Lehmann, R.K.; Causey, M.W.; Sebesta, J.A.; Brown, T.A. Natural course and outcome of cystic lesions in the pancreas. Am. J. Surg. 2009, 197, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Shah, P.; Yu, J.; Sebesta, J.A.; Brown, T.A. Cystic pancreatic tumors (CPT): Predictors of malignant behavior. J. Surg. Oncol. 2007, 95, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Shin, C.M.; Park, J.K.; Woo, S.M.; Yoo, J.W.; Ryu, J.K.; Yoon, Y.B.; Kim, Y.T. Outcomes of cystic lesions in the pancreas after extended follow-up. Dig. Dis. Sci. 2007, 52, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Salvia, R.; Crippa, S.; Falconi, M.; Bassi, C.; Guarise, A.; Scarpa, A.; Pederzoli, P. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: To operate or not to operate? Gut 2007, 56, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.A.; Schmidt, C.M.; Pinchot, J.W.; White, P.B.; Cummings, O.W.; Pitt, H.A.; Sandrasegaran, K.; Akisik, F.; Howard, T.J.; Nakeeb, A.; et al. CT vs. MRCP: Optimal classification of IPMN type and extent. J. Gastrointest. Surg. 2008, 12, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Sainani, N.I.; Saokar, A.; Deshpande, V.; Fernández-del Castillo, C.; Hahn, P.; Sahani, D.V. Comparative performance of MDCT and MRI with MR cholangiopancreatography in characterizing small pancreatic cysts. Am. J. Roentgenol. 2009, 193, 722–731. [Google Scholar] [CrossRef]
- Nakagawa, A.; Yamaguchi, T.; Ohtsuka, M.; Ishihara, T.; Sudo, K.; Nakamura, K.; Hara, T.; Denda, T.; Miyazaki, M. Usefulness of multidetector computed tomography for detecting protruding lesions in intraductal papillary mucinous neoplasm of the pancreas in comparison with single-detector computed tomography and endoscopic ultrasonography. Pancreas 2009, 38, 131–136. [Google Scholar] [CrossRef]
- Kawamoto, S.; Lawler, L.P.; Horton, K.M.; Eng, J.; Hruban, R.H.; Fishman, E.K. MDCT of intraductal papillary mucinous neoplasm of the pancreas: Evaluation of features predictive of invasive carcinoma. Am. J. Roentgenol. 2006, 186, 687–695. [Google Scholar] [CrossRef]
- Ohno, E.; Hirooka, Y.; Itoh, A.; Ishigami, M.; Katano, Y.; Ohmiya, N.; Niwa, Y.; Goto, H. Intraductal papillary mucinous neoplasms of the pancreas: Differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann. Surg. 2009, 249, 628–634. [Google Scholar] [CrossRef]
- Kang, M.J.; Jang, J.Y.; Kim, S.J.; Lee, K.B.; Ryu, J.K.; Kim, Y.T.; Yoon, Y.B.; Kim, S.W. Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clin. Gastroenterol. Hepatol. 2011, 9, 87–93. [Google Scholar] [CrossRef]
- Rautou, P.E.; Lévy, P.; Vullierme, M.P.; O’Toole, D.; Couvelard, A.; Cazals-Hatem, D.; Palazzo, L.; Aubert, A.; Sauvanet, A.; Hammel, P. Morphologic changes in branch duct intraductal papillary mucinous neoplasms of the pancreas: A midterm follow-up study. Clin. Gastroenterol. Hepatol. 2008, 6, 807–814. [Google Scholar] [CrossRef]
- Iwaya, H.; Hijioka, S.; Mizuno, N.; Kuwahara, T.; Okuno, N.; Tajika, M.; Tanaka, T.; Ishihara, M.; Hirayama, Y.; Onishi, S.; et al. Usefulness of septal thickness measurement on endoscopic ultrasound as a predictor of malignancy of branched-duct and mixed-type intraductal papillary mucinous neoplasm of the pancreas. Dig. Endosc. 2019, 31, 672–681. [Google Scholar] [CrossRef]
- Kang, H.J.; Lee, J.M.; Joo, I.; Hur, B.Y.; Jeon, J.H.; Jang, J.Y.; Lee, K.; Ryu, J.K.; Han, J.K.; Choi, B.I. Assessment of Malignant Potential in Intraductal Papillary Mucinous Neoplasms of the Pancreas: Comparison between Multidetector CT and MR Imaging with MR Cholangiopancreatography. Radiology 2016, 279, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Min, J.H.; Kim, J.H.; Park, H.J.; Kim, Y.Y.; Han, Y.E.; Bae, S.H.; Lee, J.H.; Choi, Y.H.; Moon, J.E. Interobserver Variability and Diagnostic Performance in Predicting Malignancy of Pancreatic Intraductal Papillary Mucinous Neoplasm with MRI. Radiology 2023, 308, e222463. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Bogaerts, J.; Ford, R.; Shankar, L.; Therasse, P.; Gwyther, S.; Eisenhauer, E.A. Evaluation of lymph nodes with RECIST 1.1. Eur. J. Cancer 2009, 45, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Hedgire, S.; Harisinghani, M. Radiologic Assessment of Lymph Nodes in Oncologic Patients. Curr. Radiol. Rep. 2014, 2, 36. [Google Scholar] [CrossRef]
- Kobayashi, G.; Fujita, N.; Maguchi, H.; Tanno, S.; Mizuno, N.; Hanada, K.; Hatori, T.; Sadakari, Y.; Yamaguchi, T.; Tobita, K.; et al. Natural history of branch duct intraductal papillary mucinous neoplasm with mural nodules: A Japan Pancreas Society multicenter study. Pancreas 2014, 43, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Nagaike, K.; Chijiiwa, K.; Hiyoshi, M.; Ohuchida, J.; Kataoka, H. Main-duct intraductal papillary mucinous adenoma of the pancreas with a large mural nodule. Int. J. Clin. Oncol. 2007, 12, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Haruki, K.; Wakiyama, S.; Futagawa, Y.; Shiba, H.; Misawa, T.; Yanaga, K. A large mural nodule in branch duct intraductal papillary mucinous adenoma of the pancreas: A case report. Surg. Case Rep. 2015, 1, 20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Renzulli, M.; Braccischi, L.; D’Errico, A.; Pecorelli, A.; Brandi, N.; Golfieri, R.; Albertini, E.; Vasuri, F. State-of-the-art review on the correlations between pathological and magnetic resonance features of cirrhotic nodules. Histol. Histopathol. 2022, 37, 1151–1165. [Google Scholar]
- Bozgeyik, Z.; Onur, M.R.; Poyraz, A.K. The role of diffusion weighted magnetic resonance imaging in oncologic settings. Quant. Imaging Med. Surg. 2013, 3, 269–278. [Google Scholar] [PubMed]
- Padhani, A.R.; Liu, G.; Koh, D.M.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef]
- Hao, J.G.; Wang, J.P.; Gu, Y.L.; Lu, M.L. Importance of b value in diffusion weighted imaging for the diagnosis of pancreatic cancer. World J. Gastroenterol. 2013, 19, 6651–6655. [Google Scholar] [CrossRef]
- Ichikawa, T.; Haradome, H.; Hachiya, J.; Nitatori, T.; Araki, T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: Detection and characterization of focal hepatic lesions. Am. J. Roentgenol. 1998, 170, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhao, L.; Zhong, S.; Wu, X.; Guo, J.; Zhuang, X.; Han, H. A comparison of high b-value vs standard b-value diffusion-weighted magnetic resonance imaging at 3.0 T for medulloblastomas. Br. J. Radiol. 2015, 88, 20150220. [Google Scholar] [CrossRef]
- Ogawa, T.; Horaguchi, J.; Fujita, N.; Noda, Y.; Kobayashi, G.; Ito, K.; Koshita, S.; Kanno, Y.; Masu, K.; Sugita, R. Diffusion-weighted magnetic resonance imaging for evaluating the histological degree of malignancy in patients with intraductal papillary mucinous neoplasm. J. Hepatobiliary Pancreat. Sci. 2014, 21, 801–808. [Google Scholar] [CrossRef]
- Sandrasegaran, K.; Akisik, F.M.; Patel, A.A.; Rydberg, M.; Cramer, H.M.; Agaram, N.P.; Schmidt, C.M. Diffusion-weighted imaging in characterization of cystic pancreatic lesions. Clin. Radiol. 2011, 66, 808–814. [Google Scholar] [CrossRef]
- Kang, K.M.; Lee, J.M.; Shin, C.I.; Baek, J.H.; Kim, S.H.; Yoon, J.H.; Han, J.K.; Choi, B.I. Added value of diffusion-weighted imaging to MR cholangiopancreatography with unenhanced mr imaging for predicting malignancy or invasiveness of intraductal papillary mucinous neoplasm of the pancreas. J. Magn. Reson. Imaging 2013, 38, 555–563. [Google Scholar] [CrossRef]
- Kim, M.; Mi Jang, K.; Kim, S.H.; Doo Song, K.; Jeong, W.K.; Kang, T.W.; Kim, Y.K.; Cha, D.I.; Kim, K.; Yoo, H. Diagnostic accuracy of diffusion restriction in intraductal papillary mucinous neoplasm of the pancreas in comparison with “high-risk stigmata” of the 2012 international consensus guidelines for prediction of the malignancy and invasiveness. Acta Radiol. 2017, 58, 1157–1166. [Google Scholar] [CrossRef]
- Xu, F.; Liang, Y.; Guo, W.; Liang, Z.; Li, L.; Xiong, Y.; Ye, G.; Zeng, X. Diagnostic Performance of Diffusion-Weighted Imaging for Differentiating Malignant From Benign Intraductal Papillary Mucinous Neoplasms of the Pancreas: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 637681. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cui, Y.; Shao, J.; Shao, Z.; Su, F.; Li, Y. The diagnostic role of CT, MRI/MRCP, PET/CT, EUS and DWI in the differentiation of benign and malignant IPMN: A meta-analysis. Clin. Imaging 2021, 72, 183–193. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, M.; Tedesco, G.; Cardobi, N.; De Robertis, R.; Sarno, A.; Capelli, P.; Martini, P.T.; Giannotti, G.; Beleù, A.; Marchegiani, G.; et al. Magnetic resonance (MR) for mural nodule detection studying Intraductal papillary mucinous neoplasms (IPMN) of pancreas: Imaging-pathologic correlation. Pancreatology 2021, 21, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Fukasawa, M.; Shimizu, T.; Ichikawa, S.; Sato, T.; Takano, S.; Kadokura, M.; Shindo, H.; Takahashi, E.; Hirose, S. Diffusion-weighted image improves detectability of magnetic resonance cholangiopancreatography for pancreatic ductal adenocarcinoma concomitant with intraductal papillary mucinous neoplasm. Medicine 2019, 98, e18039. [Google Scholar] [CrossRef] [PubMed]
- Fukukura, Y.; Takumi, K.; Kamimura, K.; Shindo, T.; Kumagae, Y.; Tateyama, A.; Nakajo, M. Pancreatic adenocarcinoma: Variability of diffusion-weighted MR imaging findings. Radiology 2012, 263, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, O.; Biffar, A.; Baur-Melnyk, A.; Reiser, M.F. Technical aspects of MR diffusion imaging of the body. Eur. J. Radiol. 2010, 76, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Pandharipande, P.V.; Herts, B.R.; Gore, R.M.; Mayo-Smith, W.W.; Harvey, H.B.; Megibow, A.J.; Berland, L.L. Rethinking Normal: Benefits and Risks of Not Reporting Harmless Incidental Findings. J. Am. Coll. Radiol. 2016, 13, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, M.; Caretti, D.; Pettinari, I.; Biselli, M.; Brocchi, S.; Sergenti, A.; Brandi, N.; Golfieri, R. Optimization of pineapple juice amount used as a negative oral contrast agent in magnetic resonance cholangiopancreatography. Sci. Rep. 2022, 12, 531. [Google Scholar] [CrossRef]
- Vasuri, F.; Golfieri, R.; Fiorentino, M.; Capizzi, E.; Renzulli, M.; Pinna, A.D.; Grigioni, W.F.; D’Errico-Grigioni, A. OATP 1B1/1B3 expression in hepatocellular carcinomas treated with orthotopic liver transplantation. Virchows Arch. 2011, 459, 141–146. [Google Scholar] [CrossRef]
- Guarino, M.; Viganò, L.; Ponziani, F.R.; Giannini, E.G.; Lai, Q.; Morisco, F.; Special Interest Group on “Hepatocellular Carcinoma and New Anti-HCV Therapies” of the Italian Association for the Study of the Liver. Recurrence of hepatocellular carcinoma after direct acting antiviral treatment for hepatitis C virus infection: Literature review and risk analysis. Dig. Liver Dis. 2018, 50, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Putzer, D.; Jaschke, W. Radiological evaluation of focal pancreatic lesions. Dig. Dis. 2015, 33, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C.; Fontham, E.T.; Etzioni, R.; Herzig, A.; Michaelson, J.S.; Shih, Y.C.; Walter, L.C.; Church, T.R.; Flowers, C.R.; LaMonte, S.J. Breast Cancer Screening for Women at Average Risk: 2015 Guideline Update From the American Cancer Society. JAMA 2015, 314, 1599–1614. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
| MRI Magnetic Field | A-MRI Protocol Sequences | Time Reduction Compared to Standard Contrast-Enhanced MRI Protocol | Cost Reduction Compared to Standard Contrast-Enhanced MRI Protocol | |
|---|---|---|---|---|
| Macari et al., 2009 [52] | 1.5 T |
| - | - |
| Nougaret et al., 2014 [43] | 1.5 T |
| 15–20 min. vs. 25–30 min. | - |
| Pozzi-Mucelli et al., 2017 [53] | 1.5 T |
| 7–8 min. vs. 30–35 min. | EUR 260 vs. 1043 (75%) |
| Kang et al., 2020 [54] | 3 T |
| 5.5 ± 2.1 min. vs. 32.7 ± 8 min. | - |
| Johansson et al., 2022 [55] | 1.5 T |
| 7 min. vs. 23 min. | EUR 201 vs. 514 (61%) |
| Kierans et al., 2022 [56] | 1.5 T and 3 T |
| - | - |
| Yoo et al., 2022 [57] | 3 T |
| 5–7 min. vs. 35 min. | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandi, N.; Renzulli, M. Towards a Simplified and Cost-Effective Diagnostic Algorithm for the Surveillance of Intraductal Papillary Mucinous Neoplasms (IPMNs): Can We Save Contrast for Later? Cancers 2024, 16, 905. https://doi.org/10.3390/cancers16050905
Brandi N, Renzulli M. Towards a Simplified and Cost-Effective Diagnostic Algorithm for the Surveillance of Intraductal Papillary Mucinous Neoplasms (IPMNs): Can We Save Contrast for Later? Cancers. 2024; 16(5):905. https://doi.org/10.3390/cancers16050905
Chicago/Turabian StyleBrandi, Nicolò, and Matteo Renzulli. 2024. "Towards a Simplified and Cost-Effective Diagnostic Algorithm for the Surveillance of Intraductal Papillary Mucinous Neoplasms (IPMNs): Can We Save Contrast for Later?" Cancers 16, no. 5: 905. https://doi.org/10.3390/cancers16050905
APA StyleBrandi, N., & Renzulli, M. (2024). Towards a Simplified and Cost-Effective Diagnostic Algorithm for the Surveillance of Intraductal Papillary Mucinous Neoplasms (IPMNs): Can We Save Contrast for Later? Cancers, 16(5), 905. https://doi.org/10.3390/cancers16050905








