Glutamine Supplementation as an Anticancer Strategy: A Potential Therapeutic Alternative to the Convention
Abstract
Simple Summary
Abstract
1. Introduction
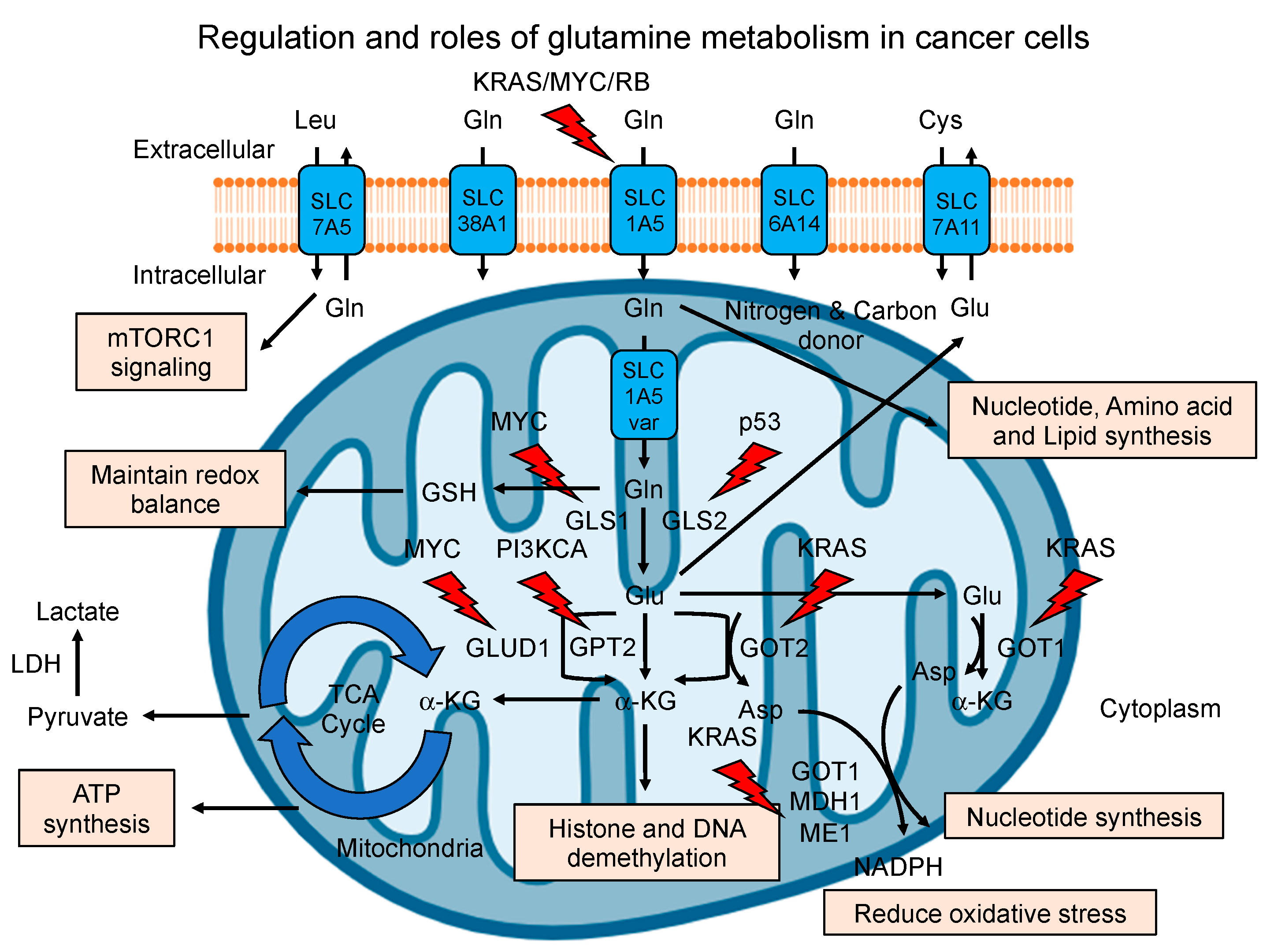
2. Regulation of Glutamine Metabolism in Cancer
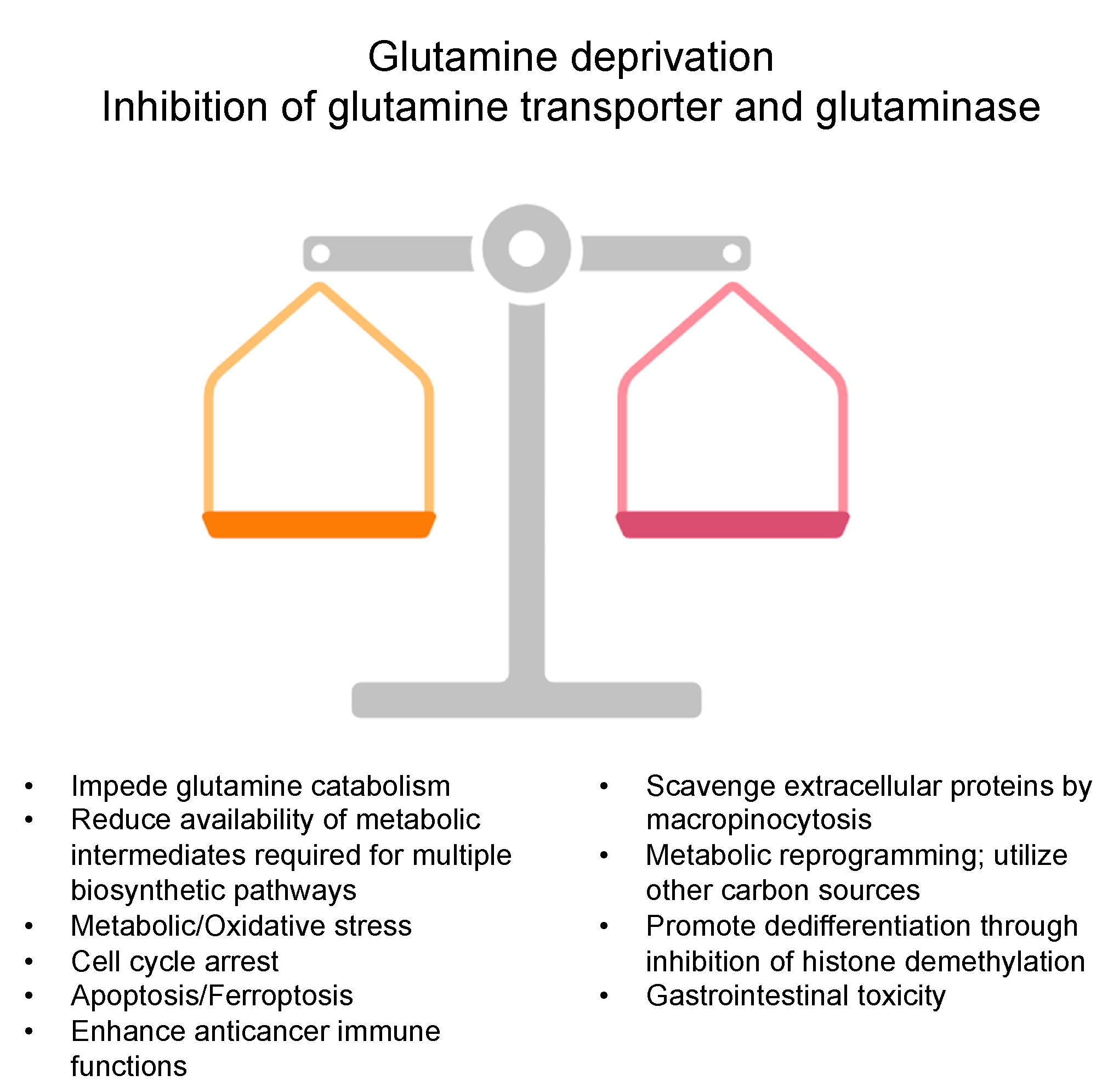
3. Glutamine Deprivation Strategies in Cancer
4. Glutamine Supplementation in Cancer
5. Clinical Evidence of Glutamine Modulation
6. Perspective, Challenges, and Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef]
- Olivares, O.; Mayers, J.R.; Gouirand, V.; Torrence, M.E.; Gicquel, T.; Borge, L.; Lac, S.; Roques, J.; Lavaut, M.-N.; Berthezène, P. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat. Commun. 2017, 8, 16031. [Google Scholar] [CrossRef]
- Sivanand, S.; Vander Heiden, M.G. Emerging roles for branched-chain amino acid metabolism in cancer. Cancer Cell 2020, 37, 147–156. [Google Scholar] [CrossRef]
- Lacey, J.M.; Wilmore, D.W. Is glutamine a conditionally essential amino acid? Nutr. Rev. 1990, 48, 297–309. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 749. [Google Scholar] [CrossRef]
- Yang, L.; Venneti, S.; Nagrath, D. Glutaminolysis: A hallmark of cancer metabolism. Annu. Rev. Biomed. Eng. 2017, 19, 163–194. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- Son, J.; Lyssiotis, C.A.; Ying, H.; Wang, X.; Hua, S.; Ligorio, M.; Perera, R.M.; Ferrone, C.R.; Mullarky, E.; Shyh-Chang, N.; et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013, 496, 101–105. [Google Scholar] [CrossRef]
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J. Clin. Investig. 2013, 123, 3678–3684. [Google Scholar] [CrossRef]
- Halama, A.; Suhre, K. Advancing cancer treatment by targeting glutamine metabolism—A roadmap. Cancers 2022, 14, 553. [Google Scholar] [CrossRef]
- Jin, J.; Byun, J.-K.; Choi, Y.-K.; Park, K.-G. Targeting glutamine metabolism as a therapeutic strategy for cancer. Exp. Mol. Med. 2023, 55, 706–715. [Google Scholar] [CrossRef]
- Biancur, D.E.; Paulo, J.A.; Małachowska, B.; Quiles Del Rey, M.; Sousa, C.M.; Wang, X.; Sohn, A.S.W.; Chu, G.C.; Gygi, S.P.; Harper, J.W.; et al. Compensatory metabolic networks in pancreatic cancers upon perturbation of glutamine metabolism. Nat. Commun. 2017, 8, 15965. [Google Scholar] [CrossRef]
- Shen, Y.A.; Chen, C.L.; Huang, Y.H.; Evans, E.E.; Cheng, C.C.; Chuang, Y.J.; Zhang, C.; Le, A. Inhibition of glutaminolysis in combination with other therapies to improve cancer treatment. Curr. Opin. Chem. Biol. 2021, 62, 64–81. [Google Scholar] [CrossRef]
- Penna, F.; Ballarò, R.; Beltrá, M.; De Lucia, S.; Costelli, P. Modulating metabolism to improve cancer-induced muscle wasting. Oxidative Med. Cell. Longev. 2018, 2018, 7153610. [Google Scholar] [CrossRef]
- Yang, L.; Achreja, A.; Yeung, T.-L.; Mangala, L.S.; Jiang, D.; Han, C.; Baddour, J.; Marini, J.C.; Ni, J.; Nakahara, R. Targeting stromal glutamine synthetase in tumors disrupts tumor microenvironment-regulated cancer cell growth. Cell Metab. 2016, 24, 685–700. [Google Scholar] [CrossRef]
- Peng, T.-R.; Lin, H.-H.; Yang, L.-J.; Wu, T.-W. Effectiveness of glutamine in the management of oral mucositis in cancer patients: A meta-analysis of randomized controlled trials. Support. Care Cancer 2021, 29, 4885–4892. [Google Scholar] [CrossRef]
- Anderson, P.M.; Lalla, R.V. Glutamine for amelioration of radiation and chemotherapy associated mucositis during cancer therapy. Nutrients 2020, 12, 1675. [Google Scholar] [CrossRef]
- Kodama, M.; Oshikawa, K.; Shimizu, H.; Yoshioka, S.; Takahashi, M.; Izumi, Y.; Bamba, T.; Tateishi, C.; Tomonaga, T.; Matsumoto, M. A shift in glutamine nitrogen metabolism contributes to the malignant progression of cancer. Nat. Commun. 2020, 11, 1320. [Google Scholar] [CrossRef]
- Wei, Q.; Qian, Y.; Yu, J.; Wong, C.C. Metabolic rewiring in the promotion of cancer metastasis: Mechanisms and therapeutic implications. Oncogene 2020, 39, 6139–6156. [Google Scholar] [CrossRef]
- Tarrado-Castellarnau, M.; de Atauri, P.; Cascante, M. Oncogenic regulation of tumor metabolic reprogramming. Oncotarget 2016, 7, 62726. [Google Scholar] [CrossRef]
- Wang, Z.V.; Hill, J.A. Protein quality control and metabolism: Bidirectional control in the heart. Cell Metab. 2015, 21, 215–226. [Google Scholar] [CrossRef]
- Reitzer, L.J.; Wice, B.M.; Kennell, D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J. Biol. Chem. 1979, 254, 2669–2676. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.; Li, Y.; Wei, S.; He, G.; Liu, J.; Li, X.; Yang, S.; Li, D.; Lin, W.; et al. Glutamine addiction in tumor cell: Oncogene regulation and clinical treatment. Cell Commun. Signal 2024, 22, 12. [Google Scholar] [CrossRef]
- Bott, A.J.; Shen, J.; Tonelli, C.; Zhan, L.; Sivaram, N.; Jiang, Y.-P.; Yu, X.; Bhatt, V.; Chiles, E.; Zhong, H.; et al. Glutamine Anabolism Plays a Critical Role in Pancreatic Cancer by Coupling Carbon and Nitrogen Metabolism. Cell Rep. 2019, 29, 1287–1298.e6. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Krall, A.S.; Christofk, H.R. Rethinking glutamine addiction. Nat. Cell Biol. 2015, 17, 1515–1517. [Google Scholar] [CrossRef]
- Stine, Z.E.; Walton, Z.E.; Altman, B.J.; Hsieh, A.L.; Dang, C.V. MYC, metabolism, and cancer. Cancer Discov. 2015, 5, 1024–1039. [Google Scholar] [CrossRef]
- Bott, A.J.; Peng, I.-C.; Fan, Y.; Faubert, B.; Zhao, L.; Li, J.; Neidler, S.; Sun, Y.; Jaber, N.; Krokowski, D. Oncogenic Myc induces expression of glutamine synthetase through promoter demethylation. Cell Metab. 2015, 22, 1068–1077. [Google Scholar] [CrossRef]
- Xie, Z.; Li, H.; Zang, J. Knockdown of lysine (K)-specific demethylase 2B KDM2B inhibits glycolysis and induces autophagy in lung squamous cell carcinoma cells by regulating the phosphatidylinositol 3-kinase/AKT/mammalian target of rapamycin pathway. Bioengineered 2021, 12, 12227–12235. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, W. The complexity of p53-mediated metabolic regulation in tumor suppression. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022; pp. 4–32. [Google Scholar]
- Lowman, X.H.; Hanse, E.A.; Yang, Y.; Gabra, M.B.I.; Tran, T.Q.; Li, H.; Kong, M. p53 promotes cancer cell adaptation to glutamine deprivation by upregulating Slc7a3 to increase arginine uptake. Cell Rep. 2019, 26, 3051–3060.e4. [Google Scholar] [CrossRef]
- Ocaña, M.C.; Martínez-Poveda, B.; Quesada, A.R.; Medina, M.Á. Metabolism within the tumor microenvironment and its implication on cancer progression: An ongoing therapeutic target. Med. Res. Rev. 2019, 39, 70–113. [Google Scholar] [CrossRef]
- Reynolds, M.R.; Lane, A.N.; Robertson, B.; Kemp, S.; Liu, Y.; Hill, B.G.; Dean, D.C.; Clem, B.F. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene 2014, 33, 556–566. [Google Scholar] [CrossRef]
- Kamphorst, J.J.; Nofal, M.; Commisso, C.; Hackett, S.R.; Lu, W.; Grabocka, E.; Vander Heiden, M.G.; Miller, G.; Drebin, J.A.; Bar-Sagi, D.; et al. Human Pancreatic Cancer Tumors Are Nutrient Poor and Tumor Cells Actively Scavenge Extracellular Protein. Cancer Res. 2015, 75, 544–553. [Google Scholar] [CrossRef]
- Sun, C.; Li, T.; Song, X.; Huang, L.; Zang, Q.; Xu, J.; Bi, N.; Jiao, G.; Hao, Y.; Chen, Y.; et al. Spatially resolved metabolomics to discover tumor-associated metabolic alterations. Proc. Natl. Acad. Sci. USA 2019, 116, 52–57. [Google Scholar] [CrossRef]
- Jiang, J.; Srivastava, S.; Zhang, J. Starve Cancer Cells of Glutamine: Break the Spell or Make a Hungry Monster? Cancers 2019, 11, 804. [Google Scholar] [CrossRef]
- Commisso, C.; Davidson, S.M.; Soydaner-Azeloglu, R.G.; Parker, S.J.; Kamphorst, J.J.; Hackett, S.; Grabocka, E.; Nofal, M.; Drebin, J.A.; Thompson, C.B.; et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 2013, 497, 633–637. [Google Scholar] [CrossRef]
- Lee, S.-W.; Zhang, Y.; Jung, M.; Cruz, N.; Alas, B.; Commisso, C. EGFR-Pak Signaling Selectively Regulates Glutamine Deprivation-Induced Macropinocytosis. Dev. Cell 2019, 50, 381–392.e5. [Google Scholar] [CrossRef]
- Sun, L.; Suo, C.; Li, S.-t.; Zhang, H.; Gao, P. Metabolic reprogramming for cancer cells and their microenvironment: Beyond the Warburg Effect. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2018, 1870, 51–66. [Google Scholar] [CrossRef]
- Schiliro, C.; Firestein, B.L. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef]
- Pan, M.; Reid, M.A.; Lowman, X.H.; Kulkarni, R.P.; Tran, T.Q.; Liu, X.; Yang, Y.; Hernandez-Davies, J.E.; Rosales, K.K.; Li, H.; et al. Regional glutamine deficiency in tumours promotes dedifferentiation through inhibition of histone demethylation. Nat. Cell Biol. 2016, 18, 1090–1101. [Google Scholar] [CrossRef]
- Reinfeld, B.I.; Madden, M.Z.; Wolf, M.M.; Chytil, A.; Bader, J.E.; Patterson, A.R.; Sugiura, A.; Cohen, A.S.; Ali, A.; Do, B.T.; et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 2021, 593, 282–288. [Google Scholar] [CrossRef]
- Mishra, R.; Haldar, S.; Placencio, V.; Madhav, A.; Rohena-Rivera, K.; Agarwal, P.; Duong, F.; Angara, B.; Tripathi, M.; Liu, Z.; et al. Stromal epigenetic alterations drive metabolic and neuroendocrine prostate cancer reprogramming. J. Clin. Investig. 2018, 128, 4472–4484. [Google Scholar] [CrossRef]
- Zhang, Y.; Recouvreux, M.V.; Jung, M.; Galenkamp, K.M.O.; Li, Y.; Zagnitko, O.; Scott, D.A.; Lowy, A.M.; Commisso, C. Macropinocytosis in Cancer-Associated Fibroblasts Is Dependent on CaMKK2/ARHGEF2 Signaling and Functions to Support Tumor and Stromal Cell Fitness. Cancer Discov. 2021, 11, 1808–1825. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Park, J.H.; Pyun, W.Y.; Park, H.W. Cancer Metabolism: Phenotype, Signaling and Therapeutic Targets. Cells 2020, 9, 2308. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Vander Heiden, M.G.; McCormick, F. The Metabolic Landscape of RAS-Driven Cancers from biology to therapy. Nat. Cancer 2021, 2, 271–283. [Google Scholar] [CrossRef]
- Dong, Y.; Tu, R.; Liu, H.; Qing, G. Regulation of cancer cell metabolism: Oncogenic MYC in the driver’s seat. Signal Transduct. Target. Ther. 2020, 5, 124. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Hu, W.; Feng, Z. Tumor suppressor p53 and metabolism. J. Mol. Cell Biol. 2019, 11, 284–292. [Google Scholar] [CrossRef]
- Yang, W.H.; Qiu, Y.; Stamatatos, O.; Janowitz, T.; Lukey, M.J. Enhancing the Efficacy of Glutamine Metabolism Inhibitors in Cancer Therapy. Trends Cancer 2021, 7, 790–804. [Google Scholar] [CrossRef]
- Song, M.; Kim, S.-H.; Im, C.Y.; Hwang, H.-J. Recent development of small molecule glutaminase inhibitors. Curr. Top. Med. Chem. 2018, 18, 432–443. [Google Scholar] [CrossRef]
- Yi, H.; Talmon, G.; Wang, J. Glutamate in cancers: From metabolism to signaling. J. Biomed. Res. 2020, 34, 260. [Google Scholar] [CrossRef]
- Cluntun, A.A.; Lukey, M.J.; Cerione, R.A.; Locasale, J.W. Glutamine metabolism in cancer: Understanding the heterogeneity. Trends Cancer 2017, 3, 169–180. [Google Scholar] [CrossRef]
- Arnold, P.K.; Finley, L.W. Regulation and function of the mammalian tricarboxylic acid cycle. J. Biol. Chem. 2022, 299, 102838. [Google Scholar] [CrossRef]
- Varghese, S.; Pramanik, S.; Williams, L.J.; Hodges, H.R.; Hudgens, C.W.; Fischer, G.M.; Luo, C.K.; Knighton, B.; Tan, L.; Lorenzi, P.L.; et al. The Glutaminase Inhibitor CB-839 (Telaglenastat) Enhances the Antimelanoma Activity of T-Cell-Mediated Immunotherapies. Mol. Cancer Ther. 2021, 20, 500–511. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Ganapathy, V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2531–2539. [Google Scholar] [CrossRef]
- Pallett, L.J.; Dimeloe, S.; Sinclair, L.V.; Byrne, A.J.; Schurich, A. A glutamine ‘tug-of-war’: Targets to manipulate glutamine metabolism for cancer immunotherapy. Immunother. Adv. 2021, 1, ltab010. [Google Scholar] [CrossRef]
- Lopes, C.; Pereira, C.; Medeiros, R. ASCT2 and LAT1 contribution to the hallmarks of cancer: From a molecular perspective to clinical translation. Cancers 2021, 13, 203. [Google Scholar] [CrossRef]
- Silva, C.; Andrade, N.; Rodrigues, I.; Ferreira, A.C.; Soares, M.L.; Martel, F. The pro-proliferative effect of interferon-γ in breast cancer cell lines is dependent on stimulation of ASCT2-mediated glutamine cellular uptake. Life Sci. 2021, 286, 120054. [Google Scholar] [CrossRef]
- Achmad, A.; Lestari, S.; Holik, H.A.; Rahayu, D.; Bashari, M.H.; Faried, A.; Kartamihardja, A.H.S. Highly specific l-type amino acid transporter 1 inhibition by JPH203 as a potential pan-cancer treatment. Processes 2021, 9, 1170. [Google Scholar] [CrossRef]
- Muhammad, N.; Lee, H.M.; Kim, J. Oncology therapeutics targeting the metabolism of amino acids. Cells 2020, 9, 1904. [Google Scholar] [CrossRef]
- Zou, J.; Du, K.; Li, S.; Lu, L.; Mei, J.; Lin, W.; Deng, M.; Wei, W.; Guo, R. Glutamine Metabolism Regulators Associated with Cancer Development and the Tumor Microenvironment: A Pan-Cancer Multi-Omics Analysis. Genes 2021, 12, 1305. [Google Scholar] [CrossRef]
- Roux, C.; Riganti, C.; Borgogno, S.F.; Curto, R.; Curcio, C.; Catanzaro, V.; Digilio, G.; Padovan, S.; Puccinelli, M.P.; Isabello, M.; et al. Endogenous glutamine decrease is associated with pancreatic cancer progression. Oncotarget 2017, 8, 95361–95376. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.H.; Jung, W.H.; Koo, J.S. Expression of glutamine metabolism-related proteins according to molecular subtype of breast cancer. Endocr. Relat. Cancer 2013, 20, 339–348. [Google Scholar] [CrossRef]
- Matés, J.M.; Campos-Sandoval, J.A.; de Los Santos-Jiménez, J.; Márquez, J. Dysregulation of glutaminase and glutamine synthetase in cancer. Cancer Lett. 2019, 467, 29–39. [Google Scholar] [CrossRef]
- Kim, G.W.; Lee, D.H.; Jeon, Y.H.; Yoo, J.; Kim, S.Y.; Lee, S.W.; Cho, H.Y.; Kwon, S.H. Glutamine synthetase as a therapeutic target for cancer treatment. Int. J. Mol. Sci. 2021, 22, 1701. [Google Scholar] [CrossRef]
- Tan, B.; Qiu, Y.; Zou, X.; Chen, T.; Xie, G.; Cheng, Y.; Dong, T.; Zhao, L.; Feng, B.; Hu, X.; et al. Metabonomics identifies serum metabolite markers of colorectal cancer. J. Proteome Res. 2013, 12, 3000–3009. [Google Scholar] [CrossRef]
- Ling, H.H.; Pan, Y.P.; Fan, C.W.; Tseng, W.K.; Huang, J.S.; Wu, T.H.; Chou, W.C.; Wang, C.H.; Yeh, K.Y.; Chang, P.H. Clinical Significance of Serum Glutamine Level in Patients with Colorectal Cancer. Nutrients 2019, 11, 898. [Google Scholar] [CrossRef]
- Lemberg, K.M.; Vornov, J.J.; Rais, R.; Slusher, B.S. We’re Not "DON" Yet: Optimal Dosing and Prodrug Delivery of 6-Diazo-5-oxo-L-norleucine. Mol. Cancer Ther. 2018, 17, 1824–1832. [Google Scholar] [CrossRef]
- Leone, R.D.; Zhao, L.; Englert, J.M.; Sun, I.-M.; Oh, M.-H.; Sun, I.-H.; Arwood, M.L.; Bettencourt, I.A.; Patel, C.H.; Wen, J. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 2019, 366, 1013–1021. [Google Scholar] [CrossRef]
- Kuhn, K.S.; Muscaritoli, M.; Wischmeyer, P.; Stehle, P. Glutamine as indispensable nutrient in oncology: Experimental and clinical evidence. Eur. J. Nutr. 2010, 49, 197–210. [Google Scholar] [CrossRef]
- Fahr, M.J.; Kornbluth, J.; Blossom, S.; Schaeffer, R.; Klimberg, V.S.; Harry, M. Vars Research Award. Glutamine enhances immunoregulation of tumor growth. JPEN J. Parenter. Enter. Nutr. 1994, 18, 471–476. [Google Scholar] [CrossRef]
- Guo, C.; You, Z.; Shi, H.; Sun, Y.; Du, X.; Palacios, G.; Guy, C.; Yuan, S.; Chapman, N.M.; Lim, S.A.; et al. SLC38A2 and glutamine signalling in cDC1s dictate anti-tumour immunity. Nature 2023, 620, 200–208. [Google Scholar] [CrossRef]
- Akbarali, H.I.; Muchhala, K.H.; Jessup, D.K.; Cheatham, S. Chemotherapy induced gastrointestinal toxicities. Adv. Cancer Res. 2022, 155, 131–166. [Google Scholar]
- Rao, R.; Samak, G. Role of glutamine in protection of intestinal epithelial tight junctions. J. Epithel. Biol. Pharmacol. 2012, 5, 47. [Google Scholar]
- Perna, S.; Alalwan, T.A.; Alaali, Z.; Alnashaba, T.; Gasparri, C.; Infantino, V.; Hammad, L.; Riva, A.; Petrangolini, G.; Allegrini, P. The role of glutamine in the complex interaction between gut microbiota and health: A narrative review. Int. J. Mol. Sci. 2019, 20, 5232. [Google Scholar] [CrossRef]
- Amores-Sánchez, M.a.I.; Medina, M.Á. Glutamine, as a precursor of glutathione, and oxidative stress. Mol. Genet. Metab. 1999, 67, 100–105. [Google Scholar] [CrossRef]
- Baracos, V.E.; Mazurak, V.C.; Bhullar, A.S. Cancer cachexia is defined by an ongoing loss of skeletal muscle mass. Ann. Palliat. Med. 2019, 8, 3–12. [Google Scholar] [CrossRef]
- Bland, K.A. Evaluating the Role of Exercise as a Management Strategy to Counteract the Burden of Cancer Cachexia. Ph.D. Thesis, Australian Catholic University, Sydney, Australia, 2023. [Google Scholar]
- Taylor, L.; Curthoys, N.P. Glutamine metabolism: Role in acid-base balance. Biochem. Mol. Biol. Educ. 2004, 32, 291–304. [Google Scholar] [CrossRef]
- Swietach, P.; Boedtkjer, E.; Pedersen, S.F. How protons pave the way to aggressive cancers. Nat. Rev. Cancer 2023, 23, 825–841. [Google Scholar] [CrossRef]
- Rajendram, R.; Preedy, V.R.; Patel, V.B. (Eds.) Glutamine in Clinical Nutrition; Springer: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Gasaly, N.; De Vos, P.; Hermoso, M.A. Impact of bacterial metabolites on gut barrier function and host immunity: A focus on bacterial metabolism and its relevance for intestinal inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Ashaolu, T.; Ashaolu, J.; Adeyeye, S. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef]
- Harris, H.C.; Morrison, D.J.; Edwards, C.A. Impact of the source of fermentable carbohydrate on SCFA production by human gut microbiota in vitro-a systematic scoping review and secondary analysis. Crit. Rev. Food Sci. Nutr. 2021, 61, 3892–3903. [Google Scholar] [CrossRef]
- Ishak Gabra, M.B.; Yang, Y.; Li, H.; Senapati, P.; Hanse, E.A.; Lowman, X.H.; Tran, T.Q.; Zhang, L.; Doan, L.T.; Xu, X. Dietary glutamine supplementation suppresses epigenetically-activated oncogenic pathways to inhibit melanoma tumour growth. Nat. Commun. 2020, 11, 3326. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.; Gu, X.; Jia, R.; Ge, S.; Chai, P.; Zhuang, A.; Fan, X. Crosstalk between metabolic reprogramming and epigenetics in cancer: Updates on mechanisms and therapeutic opportunities. Cancer Commun. 2022, 42, 1049–1082. [Google Scholar] [CrossRef]
- Morrison, A.J. Cancer cell metabolism connects epigenetic modifications to transcriptional regulation. FEBS J. 2022, 289, 1302–1314. [Google Scholar] [CrossRef]
- Scopelliti, A.J.; Font, J.; Vandenberg, R.J.; Boudker, O.; Ryan, R.M. Structural characterisation reveals insights into substrate recognition by the glutamine transporter ASCT2/SLC1A5. Nat. Commun. 2018, 9, 38. [Google Scholar] [CrossRef]
- Torres-Zamorano, V.; Leibach, F.H.; Ganapathy, V. Sodium-dependent homo- and hetero-exchange of neutral amino acids mediated by the amino acid transporter ATB degree. Biochem. Biophys. Res. Commun. 1998, 245, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Muranaka, H.; Billet, S.; Cruz-Hernández, C.; Hoeve, J.T.; Gonzales, G.; Elmadbouh, O.; Zhang, L.; Smith, B.; Tighiouart, M.; You, S.; et al. Supraphysiological glutamine as a means of depleting intracellular amino acids to enhance pancreatic cancer chemosensitivity. Preprints 2023. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Lee, R.J.; Carthon, B.C.; Iliopoulos, O.; Mier, J.W.; Patel, M.R.; Tannir, N.M.; Owonikoko, T.K.; Haas, N.B.; Voss, M.H. CB-839, a Glutaminase Inhibitor, in Combination with Cabozantinib in Patients with Clear Cell and Papillary Metastatic Renal Cell Cancer (mRCC): Results of a PHASE I STUDY; American Society of Clinical Oncology: Alexandria, VA, USA, 2019. [Google Scholar]
- Niihara, Y.; Miller, S.T.; Kanter, J.; Lanzkron, S.; Smith, W.R.; Hsu, L.L.; Gordeuk, V.R.; Viswanathan, K.; Sarnaik, S.; Osunkwo, I.; et al. A Phase 3 Trial of l-Glutamine in Sickle Cell Disease. N. Engl. J. Med. 2018, 379, 226–235. [Google Scholar] [CrossRef]
- Yoshida, S.; Matsui, M.; Shirouzu, Y.; Fujita, H.; Yamana, H.; Shirouzu, K. Effects of glutamine supplements and radiochemotherapy on systemic immune and gut barrier function in patients with advanced esophageal cancer. Ann. Surg. 1998, 227, 485–491. [Google Scholar] [CrossRef]
- Yoshida, S.; Kaibara, A.; Ishibashi, N.; Shirouzu, K. Glutamine supplementation in cancer patients. Nutrition 2001, 17, 766–768. [Google Scholar] [CrossRef]
- Jebb, S.A.; Osborne, R.J.; Maughan, T.S.; Mohideen, N.; Mack, P.; Mort, D.; Shelley, M.D.; Elia, M. 5-fluorouracil and folinic acid-induced mucositis: No effect of oral glutamine supplementation. Br. J. Cancer 1994, 70, 732–735. [Google Scholar] [CrossRef]
- Decker-Baumann, C.; Buhl, K.; Frohmüller, S.; von Herbay, A.; Dueck, M.; Schlag, P.M. Reduction of chemotherapy-induced side-effects by parenteral glutamine supplementation in patients with metastatic colorectal cancer. Eur. J. Cancer 1999, 35, 202–207. [Google Scholar] [CrossRef]
- Huang, E.Y.; Leung, S.W.; Wang, C.J.; Chen, H.C.; Sun, L.M.; Fang, F.M.; Yeh, S.A.; Hsu, H.C.; Hsiung, C.Y. Oral glutamine to alleviate radiation-induced oral mucositis: A pilot randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 535–539. [Google Scholar] [CrossRef]
- Peterson, D.E.; Jones, J.B.; Petit, R.G., 2nd. Randomized, placebo-controlled trial of Saforis for prevention and treatment of oral mucositis in breast cancer patients receiving anthracycline-based chemotherapy. Cancer 2007, 109, 322–331. [Google Scholar] [CrossRef]
- Wang, W.S.; Lin, J.K.; Lin, T.C.; Chen, W.S.; Jiang, J.K.; Wang, H.S.; Chiou, T.J.; Liu, J.H.; Yen, C.C.; Chen, P.M. Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist 2007, 12, 312–319. [Google Scholar] [CrossRef]
- Skubitz, K.M.; Anderson, P.M. Oral glutamine to prevent chemotherapy induced stomatitis: A pilot study. J. Lab. Clin. Med. 1996, 127, 223–228. [Google Scholar] [CrossRef]
- Anderson, P.M.; Schroeder, G.; Skubitz, K.M. Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer 1998, 83, 1433–1439. [Google Scholar] [CrossRef]
- Bozzetti, F.; Biganzoli, L.; Gavazzi, C.; Cappuzzo, F.; Carnaghi, C.; Buzzoni, R.; Dibartolomeo, M.; Baietta, E. Glutamine supplementation in cancer patients receiving chemotherapy: A double-blind randomized study. Nutrition 1997, 13, 748–751. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Liu, F.; Tan, L.; Wu, B.; Li, J. Oral glutamine ameliorates chemotherapy-induced changes of intestinal permeability and does not interfere with the antitumor effect of chemotherapy in patients with breast cancer: A prospective randomized trial. Tumori 2006, 92, 396–401. [Google Scholar] [CrossRef]
- Kucuktulu, E.; Guner, A.; Kahraman, I.; Topbas, M.; Kucuktulu, U. The protective effects of glutamine on radiation-induced diarrhea. Support. Care Cancer 2013, 21, 1071–1075. [Google Scholar] [CrossRef]
- Daniele, B.; Perrone, F.; Gallo, C.; Pignata, S.; De Martino, S.; De Vivo, R.; Barletta, E.; Tambaro, R.; Abbiati, R.; D’Agostino, L. Oral glutamine in the prevention of fluorouracil induced intestinal toxicity: A double blind, placebo controlled, randomised trial. Gut 2001, 48, 28–33. [Google Scholar] [CrossRef]
- Jiang, H.P.; Liu, C.A. Protective effect of glutamine on intestinal barrier function in patients receiving chemotherapy. Zhonghua Wei Chang Wai Ke Za Zhi 2006, 9, 59–61. [Google Scholar]
- Choi, K.; Lee, S.S.; Oh, S.J.; Lim, S.Y.; Lim, S.Y.; Jeon, W.K.; Oh, T.Y.; Kim, J.W. The effect of oral glutamine on 5-fluorouracil/leucovorin-induced mucositis/stomatitis assessed by intestinal permeability test. Clin. Nutr. 2007, 26, 57–62. [Google Scholar] [CrossRef] [PubMed]
- May, P.E.; Barber, A.; D’Olimpio, J.T.; Hourihane, A.; Abumrad, N.N. Reversal of cancer-related wasting using oral supplementation with a combination of β-hydroxy-β-methylbutyrate, arginine, and glutamine. Am. J. Surg. 2002, 183, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Benes, P.; Pytlik, R.; Chocenska, E.; Pat’orková, M.; Klepetar, J.; Prochazka, B.; Gregora, E.; Kozak, T.; Andĕl, M. Parenteral glutamine does not improve the nutritional status in patients during high-dose chemotherapy and autologous peripheral stem cell transplantation. Vnitr. Lek. 2002, 48, 1039–1048. [Google Scholar]
- Azman, M.; Mohd Yunus, M.R.; Sulaiman, S.; Syed Omar, S.N. Enteral glutamine supplementation in surgical patients with head and neck malignancy: A randomized controlled trial. Head Neck 2015, 37, 1799–1807. [Google Scholar] [CrossRef]
- Chang, S.-C.; Lai, Y.-C.; Hung, J.-C.; Chang, C.-Y. Oral glutamine supplements reduce concurrent chemoradiotherapy-induced esophagitis in patients with advanced non-small cell lung cancer. Medicine 2019, 98, e14463. [Google Scholar] [CrossRef]
- Wu, J.-M.; Ho, T.-W.; Lai, I.-R.; Chen, C.-N.; Lin, M.-T. Parenteral glutamine supplementation improves serum albumin values in surgical cancer patients. Clin. Nutr. 2021, 40, 645–650. [Google Scholar] [CrossRef]
- Pascoe, J.; Jackson, A.; Gaskell, C.; Gaunt, C.; Thompson, J.; Billingham, L.; Steven, N. Beta-hydroxy beta-methylbutyrate/arginine/glutamine (HMB/Arg/Gln) supplementation to improve the management of cachexia in patients with advanced lung cancer: An open-label, multicentre, randomised, controlled phase II trial (NOURISH). BMC Cancer 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Muranaka, H.; Hendifar, A.; Osipov, A.; Moshayedi, N.; Placencio-Hickok, V.; Tatonetti, N.; Stotland, A.; Parker, S.; Van Eyk, J.; Pandol, S.J.; et al. Plasma Metabolomics Predicts Chemotherapy Response in Advanced Pancreatic Cancer. Cancers 2023, 15, 3020. [Google Scholar] [CrossRef] [PubMed]
- Garlick, P.J. Assessment of the safety of glutamine and other amino acids. J. Nutr. 2001, 131, 2556s–2561s. [Google Scholar] [CrossRef]
- Holecek, M. Side effects of long-term glutamine supplementation. JPEN J. Parenter. Enter. Nutr. 2013, 37, 607–616. [Google Scholar] [CrossRef]
- Hatami, B.; Saffaei, A.; Jamali, F.; Abbasinazari, M. Glutamine powder-induced hepatotoxicity: It is time to understand the side effects of sports nutritional supplements. Gastroenterol. Hepatol. Bed Bench 2020, 13, 86–89. [Google Scholar]
- Topkan, E.; Parlak, C.; Topuk, S.; Pehlivan, B. Influence of oral glutamine supplementation on survival outcomes of patients treated with concurrent chemoradiotherapy for locally advanced non-small cell lung cancer. BMC Cancer 2012, 12, 502. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Yamamoto, Y.; Wasa, M.; Takenaka, Y.; Nakahara, S.; Takagi, T.; Tsugane, M.; Hayashi, N.; Maeda, K.; Inohara, H.; et al. L-glutamine decreases the severity of mucositis induced by chemoradiotherapy in patients with locally advanced head and neck cancer: A double-blind, randomized, placebo-controlled trial. Oncol. Rep. 2015, 33, 33–39. [Google Scholar] [CrossRef]
- Gong, J.; Osipov, A.; Lorber, J.; Tighiouart, M.; Kwan, A.K.; Muranaka, H.; Akinsola, R.; Billet, S.; Levi, A.; Abbas, A.; et al. Combination L-Glutamine with Gemcitabine and Nab-Paclitaxel in Treatment-Naïve Advanced Pancreatic Cancer: The Phase I GlutaPanc Study Protocol. Biomedicines 2023, 11, 1392. [Google Scholar] [CrossRef]
- Valencia, E.; Marin, A.; Hardy, G. Impact of oral l-glutamine on glutathione, glutamine, and glutamate blood levels in volunteers. Nutrition 2002, 18, 367–370. [Google Scholar] [CrossRef]
- Smedberg, M.; Wernerman, J. Is the glutamine story over? Crit. Care 2016, 20, 361. [Google Scholar] [CrossRef]
- Stehle, P.; Ellger, B.; Kojic, D.; Feuersenger, A.; Schneid, C.; Stover, J.; Scheiner, D.; Westphal, M. Glutamine dipeptide-supplemented parenteral nutrition improves the clinical outcomes of critically ill patients: A systematic evaluation of randomised controlled trials. Clin. Nutr. ESPEN 2017, 17, 75–85. [Google Scholar] [CrossRef]
- Grau, T.; Bonet, A.; Miñambres, E.; Piñeiro, L.; Irles, J.A.; Robles, A.; Acosta, J.; Herrero, I.; Palacios, V.; Lopez, J.; et al. The effect of L-alanyl-L-glutamine dipeptide supplemented total parenteral nutrition on infectious morbidity and insulin sensitivity in critically ill patients. Crit. Care Med. 2011, 39, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Estívariz, C.F.; Griffith, D.P.; Luo, M.; Szeszycki, E.E.; Bazargan, N.; Dave, N.; Daignault, N.M.; Bergman, G.F.; McNally, T.; Battey, C.H.; et al. Efficacy of parenteral nutrition supplemented with glutamine dipeptide to decrease hospital infections in critically ill surgical patients. JPEN J. Parenter. Enter. Nutr. 2008, 32, 389–402. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.M.; Nolan, M.T.; Jiang, H.; Han, H.R.; Yu, K.; Li, H.L.; Jie, B.; Liang, X.K. The impact of glutamine dipeptide-supplemented parenteral nutrition on outcomes of surgical patients: A meta-analysis of randomized clinical trials. JPEN J. Parenter. Enter. Nutr. 2010, 34, 521–529. [Google Scholar] [CrossRef]
- Bollhalder, L.; Pfeil, A.M.; Tomonaga, Y.; Schwenkglenks, M. A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin. Nutr. 2013, 32, 213–223. [Google Scholar] [CrossRef]
- Déchelotte, P.; Hasselmann, M.; Cynober, L.; Allaouchiche, B.; Coëffier, M.; Hecketsweiler, B.; Merle, V.; Mazerolles, M.; Samba, D.; Guillou, Y.M.; et al. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: The French controlled, randomized, double-blind, multicenter study. Crit. Care Med. 2006, 34, 598–604. [Google Scholar] [CrossRef]
- Weitzel, L.R.; Wischmeyer, P.E. Glutamine in critical illness: The time has come, the time is now. Crit. Care Clin. 2010, 26, 515–525. [Google Scholar] [CrossRef]
- Klassen, P.; Mazariegos, M.; Solomons, N.W.; Fürst, P. The pharmacokinetic responses of humans to 20 g of alanyl-glutamine dipeptide differ with the dosing protocol but not with gastric acidity or in patients with acute Dengue fever. J. Nutr. 2000, 130, 177–182. [Google Scholar] [CrossRef]


| Patients (Cancer Types) | n | Therapy | Gln Supplementation | Outcome | Ref. |
|---|---|---|---|---|---|
| Esophageal cancer | 13 | Radiochemotherapy; cisplatin and 5-FU *1 | 30 g/day Gln over 28 days |
| [97] |
| Esophageal cancer | 13 | Radiochemotherapy | 30 g/day Gln over 4 weeks |
| [98] |
| Gastrointestinal cancer | 28 | Chemotherapy; 5-FU and FA *2 | 16 g/day Gln for 8 days |
| [99] |
| Metastatic colorectal cancer | 24 | Chemotherapy; 5-FU and CF *3 | 0.4 g/day i.v. Gly-Gln for 5 days |
| [100] |
| Head and neck cancer | 17 | Radiation therapy | 2 g/m2 Gln swish therapy, 4×/day |
| [101] |
| Breast cancer | 326 | Chemotherapy; cyclophosphamide *4, doxorubicin and 5-FU | 2.5 g Gln (Saforis), 3×/day for 14 days |
| [102] |
| Metastatic colorectal cancer | 86 | Chemotherapy; oxaliplatin | 15 g Gln, 2×/day for 7 days, every 2 weeks during chemotherapy |
| [103] |
| Soft tissue sarcoma, osteosarcoma, Kaposi’s sarcoma, and breast cancer | 14 | Chemotherapy; doxorubicin, dacarbazine, CP, etc. | 4 g Gln swish and swallow 2×/day |
| [104] |
| Sarcoma and neuroblastoma | 24 | Chemotherapy; 5-FU and leucovorin; carboplatin and etoposide, methotrexate | 4 g/day Gln for at least 14 days |
| [105] |
| Breast cancer | 65 | Doxifluridine and leucovorin | 30 g/day Gln for 8 days |
| [106] |
| Breast cancer | 60 | Chemotherapy | Gln ≥ 12 days |
| [107] |
| Rectal, bladder, prostate, and gynecologic cancers and pelvic soft tissue sarcomas | 36 | Radiotherapy | 15 g Gln, 3×/day for 2 weeks |
| [108] |
| Advanced/metastatic colorectal cancer | 70 | Chemotherapy; 5-FU and FA | 18 g/day Gln for 15 days |
| [109] |
| Gastrointestinal cancer | 39 | Chemotherapy; CF and 5-FU | 30 g/day Gln for 7 days |
| [110] |
| Advanced/metastatic cancer | 51 | Chemotherapy; 5-FU and leucovorin | 30 g/day Gln for 15 days |
| [111] |
| Breast cancer | 32 | HMB *5 | 14 g/day of Gln for 24 weeks |
| [112] |
| Hematological, solid cancer, and multiple sclerosis | 40 | High-dose chemotherapy with autologous stem cell transplantation | 30 g/day Gln for 14 days |
| [113] |
| Neck and head malignancy | 44 | Surgery | 0.3 g/day Gln for 4 weeks |
| [114] |
| NSCLC *6 | 60 | Concurrent radiotherapy | 10 g/8 h Gln for 12 months |
| [115] |
| Gastric adenocarcinoma | 1950 | Gastrectomy | 0.05–0.49 g/kg/day Gln |
| [116] |
| Lung cancer (SCLC*7 or NSCLC) | 96 | - | 2.4 g HMB, 14 g Arg *8, Gln 14 g Gln/day for 12 weeks |
| [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muranaka, H.; Akinsola, R.; Billet, S.; Pandol, S.J.; Hendifar, A.E.; Bhowmick, N.A.; Gong, J. Glutamine Supplementation as an Anticancer Strategy: A Potential Therapeutic Alternative to the Convention. Cancers 2024, 16, 1057. https://doi.org/10.3390/cancers16051057
Muranaka H, Akinsola R, Billet S, Pandol SJ, Hendifar AE, Bhowmick NA, Gong J. Glutamine Supplementation as an Anticancer Strategy: A Potential Therapeutic Alternative to the Convention. Cancers. 2024; 16(5):1057. https://doi.org/10.3390/cancers16051057
Chicago/Turabian StyleMuranaka, Hayato, Rasaq Akinsola, Sandrine Billet, Stephen J. Pandol, Andrew E. Hendifar, Neil A. Bhowmick, and Jun Gong. 2024. "Glutamine Supplementation as an Anticancer Strategy: A Potential Therapeutic Alternative to the Convention" Cancers 16, no. 5: 1057. https://doi.org/10.3390/cancers16051057
APA StyleMuranaka, H., Akinsola, R., Billet, S., Pandol, S. J., Hendifar, A. E., Bhowmick, N. A., & Gong, J. (2024). Glutamine Supplementation as an Anticancer Strategy: A Potential Therapeutic Alternative to the Convention. Cancers, 16(5), 1057. https://doi.org/10.3390/cancers16051057









