Exosomes in Glioma: Unraveling Their Roles in Progression, Diagnosis, and Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Exosomes’ Biology and Potentiality for Crossing the BBB
2.1. Production and Structure of Exosomes
2.2. Isolation Methods of Exosomes
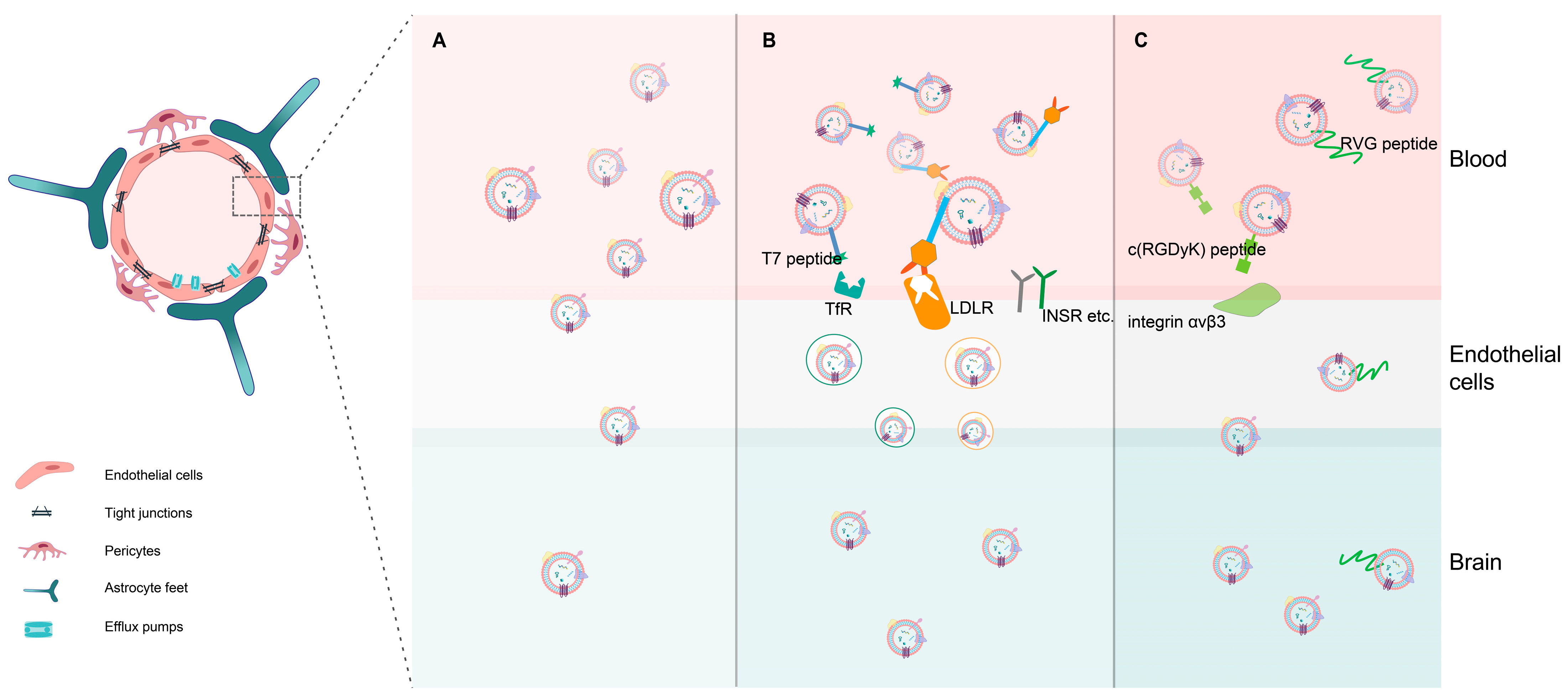
2.3. Potentiality for Crossing the BBB Based on Exosomes
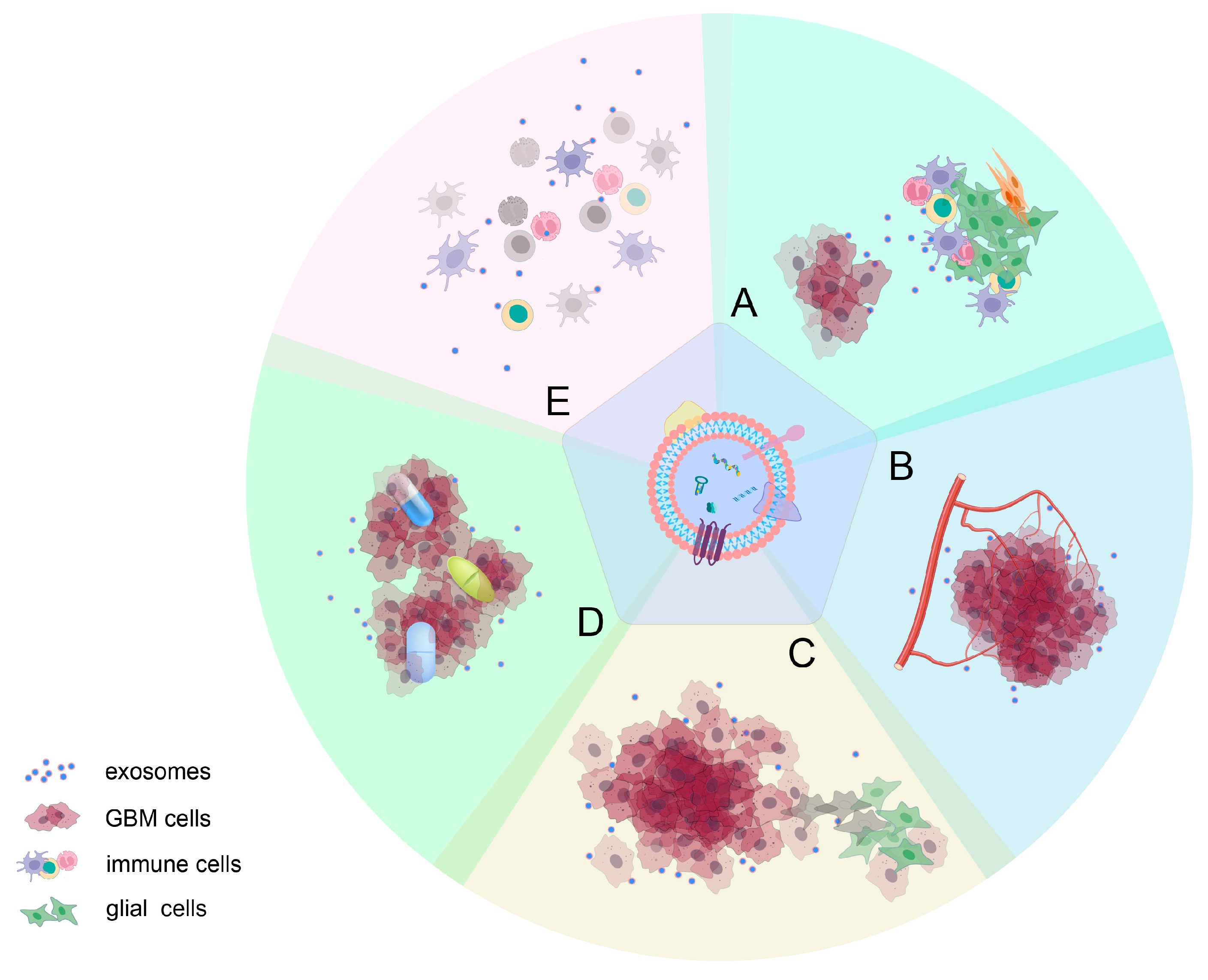
3. Role of Exosomes in Glioma Progression
3.1. Effect on the Microenvironment around Gliomas
3.2. Angiogenesis Mediated by Exosomes
3.3. Role of Exosomes in the Proliferation and Invasiveness of Gliomas
3.4. Role of Exosomes in the Resistance to Glioma Treatment
3.5. Exosomes in Mediating Immune Responses
4. Exosomes as a Promising Strategy for Diagnosis and Prognosis of GBM
4.1. MiRNA
4.2. CircRNA
4.3. LncRNA
4.4. Protein
5. Exosomes in Glioma Treatment
5.1. Utilization of Inherent Characteristics of Exosomes
5.2. Exosomes as Delivery Systems
5.2.1. Chemotherapy Drugs as Exosome Payloads
5.2.2. Nucleotide Drugs as Exosome Payloads
6. Future Directions and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Candidate | Source of Exosomes | Isolation Techniques | Mechanism | Results | Biomarker Potential | Year/Ref. |
|---|---|---|---|---|---|---|
| hTERT transcript | Serum samples | Centrifugation and isolation kit | Telomerase activity is highly upregulated in neoplastic tissues. | Correlated with tumor volume and the disease course in glioblastomas. | A promising biomarker for predicting prognosis of GBM. | 2023 [149] |
| miRNA-19a/19b and PTEN | Serum samples | Centrifugation | Phosphorylation and dephosphorylation of important cell cycle checkpoints. | Analysis showed significant deregulated expression. | Potential diagnostic biomarker in brain tumor. | 2023 [150] |
| miR-155-5p | Blood samples | Centrifugation | Targeting ACOT12 and accelerating mesenchymal transition. | Facilitate the migration and invasion. | High expression was associated with glioma diagnosis and grading. | 2022 [151] |
| exoDNA concentration | Blood samples. | Centrifugation. | DNA transported by exosomes as an excellent source of information. | The amount of circulating exoDNA is directly correlated to the total tumor volume. | A possible role of exoDNAs in the diagnosis of brain glioma. | 2022 [152] |
| CD9 and CD81 | Glioblastoma cell lines. | Centrifugation. | Associated with increased tumor-cell motility and improved tumor growth. | After irradiation, increased in supernatant when glioblastoma cells underwent cell death, particularly apoptosis. | As marker to monitor glioblastoma response to radiotherapy. | 2022 [119] |
| miR-98−5p, miR-183−5p, miR-323−3p, miR-19b-3p | Serum samples. | Unknown (from database). | Negatively regulating MAPK8IP1/FAM175B, OSMR/CASP3, FBXO32 and PTPN2. | Their expression levels in serum exosomes may represent the trend in the tumor cells. | May represent potential diagnostic biomarkers and therapeutic targets for GBM. | 2022 [104] |
| circ_0072083 | Serum samples. | Centrifugation. | Regulating NANOG and ALKBH5 via targeting miR-1252-5p. | Promote TMZ resistance in glioma. | Indicating a lower overall survival in glioma. | 2021 [153] |
| miR-2276-5p | Plasma samples. | Centrifugation. | Targeting RAB1. | Low expression associates with poor prognosis of patients. | A potential diagnostic and prognostic biomarker in glioma. | 2021 [103] |
| circ0055202, circ0074920, circ0043722 | Plasma samples. | Precipitation. | Mediating miRNAs and their target genes, or binding to RNA-binding proteins. | Promote the proliferation of GBM cells. | Potential biomarker for predicting the GBM. | 2021 [105] |
| ldrEXOs-derived circ-METRN | Glioblastoma cells previously treated with low- or high-dose radiation. | Centrifugation. | Via miR-4709-3p/GRB14/PDGFRα pathway. | Enhanced the glioblastoma progression and radio resistance. | Predicting radio resistance and prognosis and assisting MRI diagnosis in early recurrence of glioblastoma. | 2021 [154] |
| miR-210 | Serum samples. | Centrifugation. | Associated with high levels of hypoxia-inducible factor 1a. | The expression levels were increased with ascending pathological grades. | A diagnostic, prognostic, and hypoxic biomarker. | 2020 [155] |
| SOX2 DNA | Glioblastoma tumor masses. | Centrifugation and magnetic beads method. | Mis-regulated in cancer cells by changes in miRNA function, such as SNPs. | Involved in GBM progression and metastasis. | A minimally invasive exosome-based diagnosis. | 2020 [122] |
| miR-9-5p, miR-124-3p, miR-21-5p, miR-138-5p, miR-1-3p and VIM mRNA, | Human GBM cell lines. | Centrifugation. | Regulating the expression of target genes and affecting the expression levels of the corresponding proteins. | Level of expression was associated with grade and survival of GBM patients. | Diagnostic and prognostic biomarkers of GBM cells. | 2020 [123] |
| miR-29b | Serum samples. | Centrifugation. | Targeting MYCN. | Low expression was associated with aggressive clinical findings and shorter survival. | A promising biomarker for predicting prognosis of GBM. | 2019 [156] |
| miR-454-3p | Serum samples. | Isolation reagent. | Suppressing autophagy by targeting ATG12. | Suppress cell proliferation, migration, invasion, and autophagy in glioma. | A diagnostic and prognostic biomarker for glioma. | 2019 [157] |
| PTRF (Cavin1) | Serum samples. | Centrifugation. | Inducing the malignancy of nearby cells. | Overexpression induces cell growth and alters the microenvironment. | A biomarker for GBM diagnosis. | 2018 [158] |
| miR-301a | Serum samples. | Centrifugation and precipitation. | Downregulating PTEN expression in glioma cells. | May reflect the cancer-bearing status and pathological changes in glioma patients. | A novel biomarker for glioma diagnosis and prognosis. | 2018 [101] |
| miR-21, miR-222 and miR-124-3p | Serum samples. | Centrifugation. | Providing information throughout the course of disease. | Higher expression levels in high-grade gliomas and sharply decreased in samples obtained after surgery. | Predicting glioma grading and nonglial metastases. | 2018 [97] |
| lncRNA-HOTAIR | Serum samples. | Precipitation method. | Dysregulated in GBM and required for GBM cell proliferation. | HOTAIR expression was significantly correlated with high-grade brain tumors. | A novel prognostic and diagnostic biomarker for GBM. | 2018 [114] |
| Donor Cells | Content | Loading Method | Modification | Model | Mechanism | Result | Years |
|---|---|---|---|---|---|---|---|
| Dendritic-cell-tumor hybrid cell | cGAMP (STING activation) | Electroporation. | - | In vivo (mice models) and in vitro. | Further enhancing tumor-specific T-cell immune response. | Reversing immunosuppressive glioblastoma microenvironments | 2023 [159] |
| Serum | TanIIA-GL nanomicelles | Sonicated and incubated. | CpG oligonucleotides. | In vivo (mice models) and in vitro. | Prolonging blood circulation and maintaining intact structures. | Inducing apoptosis and generating anti-GBM immune responses | 2023 [160] |
| hMSCs | MNP@BQR and siGPX4 | Electroporation. | Angiopep-2 peptide. | In vivo (mice models) and in vitro. | Disrupting DHODH, mediating Fe2+ release, and ferroptosis. | Targeting GBM cells, enhancing ferroptosis in GBM. | 2022 [53] |
| Macrophages | CAT@SiO2, loaded with ICG | Sonication. | AS1411 aptamer modified. | In vivo (mice models) and in vitro. | Producing O2 to relieve tumor hypoxia. | Targeting cancer cells, inhibiting the tumor metastasis of GBM. | 2022 [161] |
| Glioblastoma cells | Selumetinib | Electroporation. | - | In vivo (mice models) and in vitro. | The tropism of GBM-derived exosomes. | Specific antitumor effect and non-toxic to normal brain cells. | 2022 [162] |
| Leukemia monocytic cell | TMZ/O6-benzylguanine | Sonication, freeze and thaw cycles, and incubation. | Angiopep-2 and CD133 RNA aptamers. | In vivo (mice models) and in vitro. | Enhancing BBB penetration and superior tumor accumulation. | Inhibiting proliferation and extending the median survival time. | 2022 [163] |
| Homologous glioma cells | Temozolomide and dihydrotanshinone | Incubation. | - | In vivo (mice models) and in vitro. | Tumor-homing accumulation with homologous effects. | Overcoming TMZ resistance and triggering immune response. | 2022 [164] |
| Human endometrial MSCs | Atorvastatin | Incubation with Tween-20. | - | In vitro 3D culture model. | Suppressing angiogenesis, resulting in decreased VEGF secretion. | Enhancing antitumor effects, resulting in induction of apoptosis. | 2022 [165] |
| Neutrophils | Doxorubicin | Sonication. | - | In vivo (zebrafish and mice models). | Responding to inflammatory stimuli and targeting infiltrating tumor cells. | Efficiently suppressing tumor growth and prolonging survival time. | 2021 [143] |
| HEK-293T | pPolymiR | Transfected. | Viral proteins (Gag/VSVg). | In vivo (mice models) and in vitro. | Resulting in high expression of 3 miRs in GSCs and decreasing GSC proliferation. | Prolonging survival of GSC-bearing mice. | 2023 [166] |
| Umbilical cord mesenchymal stem cells (UCMSCs) | CDA and miR-34a | Co-transfected. | ab139 anti-EGFRvIII antibody. | In vitro. | Targeting delivery of drugs and combination therapy. | Improving apoptosis rate of GBM cells. | 2023 [167] |
| Mesenchymal stem cells | miR-1208 | Transfected. | Combined with focused ultrasound. | In vivo (mice models) and in vitro. | Suppressing NUP214 expression and TGF-β pathway activity. | Resulting in a high-efficiency tumor-suppressive effect. | 2023 [168] |
| Neural stem cells | CpG-STAT3 antisense oligonucleotides | Incubated. | - | In vivo (mice models) and in vitro. | Targeting signal transducer and activator of STAT3 protein into glioma microenvironment. | Enhancing antitumor effects. | 2022 [169] |
| Blood | cPLA2 siRNA/metformin | Electroporation /shaking. | - | In vivo (mice models) and in vitro (PDX). | Impairing the mitochondrial energy metabolism of GBM. | Reducing tumor growth and prolonged survival. | 2022 [170] |
| 293T cells | siYY1 | Electroporation. | T7 peptide. | In vivo (mice models) and in vitro. | Blocking the tumor malignance and reversing the resistance by knockdown of YY1. | Enhancing chemoradiotherapy sensitivity and reversing therapeutic resistance. | 2022 [171] |
| Neural stem cells | miR-124-3p | Electroporation. | - | In vivo (mice models) and in vitro. | Suppressing FLOT2 expression by specifically binding to the 3′ untranslated region of the FLOT2 gene. | Inhibiting glioma cell proliferation, invasion, and migration. | 2022 [172] |
| 293 T cell | AMO-21 | Electroporation. | T7 peptide. | In vivo (mice models) and in vitro. | Reduction of miR-21 induced the expression of PDCD4 and PTEN. | Resulting in reduction of tumor sizes. | 2020 [51] |
| HEK-293T | miR-21-sponge | Freeze and thaw cycles method. | - | In vivo (mice models) and in vitro. | Suppressing miR-21 and upregulating miR-21 target genes, PDCD4, and RECK. | Decline in proliferation, elevation in apoptotic rates, and reduction in the volume of the tumors. | 2019 [173] |
| WJ-MSCs | miR-124 | Incubation. | - | In vitro. | MiR-124 improved neuronal differentiation, suppressed invasion, and migration of GBM cells. | Decreasing cell proliferation and migration and conferring chemosensitivity. | 2018 [174] |
References
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical implications of the 2021 edition of the who classification of central nervous system tumours. Nat. Rev. Neurol. 2022, 18, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Stetson, L.; Virk, S.M.; Barnholtz-Sloan, J.S. Epidemiology of gliomas. Cancer Treat. Res. 2015, 163, 1–14. [Google Scholar] [PubMed]
- Gladson, C.L.; Prayson, R.A.; Liu, W.M. The pathobiology of glioma tumors. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA-Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.B.; Karpova, A.; Gritsenko, M.A.; Kyle, J.E.; Cao, S.; Li, Y.; Rykunov, D.; Colaprico, A.; Rothstein, J.H.; Hong, R.; et al. Proteogenomic and metabolomic characterization of human glioblastoma. Cancer Cell 2021, 39, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.M.; Cloughesy, T.F. Adult glioblastoma. J. Clin. Oncol. 2017, 35, 2402–2409. [Google Scholar] [CrossRef]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef]
- Ahlawat, J.; Guillama, B.G.; Masoudi, A.S.; Alvarado, M.; Armendariz, I.; Bernal, J.; Carabaza, X.; Chavez, S.; Cruz, P.; Escalante, V.; et al. Nanocarriers as potential drug delivery candidates for overcoming the blood-brain barrier: Challenges and possibilities. Acs Omega 2020, 5, 12583–12595. [Google Scholar] [CrossRef]
- Noack, A.; Gericke, B.; von Köckritz-Blickwede, M.; Menze, A.; Noack, S.; Gerhauser, I.; Osten, F.; Naim, H.Y.; Löscher, W. Mechanism of drug extrusion by brain endothelial cells via lysosomal drug trapping and disposal by neutrophils. Proc. Natl. Acad. Sci. USA 2018, 115, E9590–E9599. [Google Scholar] [CrossRef]
- Jeong, H.W.; Diéguez-Hurtado, R.; Arf, H.; Song, J.; Park, H.; Kruse, K.; Sorokin, L.; Adams, R.H. Single-cell transcriptomics reveals functionally specialized vascular endothelium in brain. Elife 2022, 11, e57520. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Rodríguez-Camacho, A.; Flores-Vázquez, J.G.; Moscardini-Martelli, J.; Torres-Ríos, J.A.; Olmos-Guzmán, A.; Ortiz-Arce, C.S.; Cid-Sánchez, D.R.; Pérez, S.R.; Macías-González, M.; Hernández-Sánchez, L.C.; et al. Glioblastoma treatment: State-of-the-art and future perspectives. Int. J. Mol. Sci. 2022, 23, 7207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Singh, R.; Souweidane, M.M. Convection-enhanced delivery for diffuse intrinsic pontine glioma treatment. Curr. Neuropharmacol. 2017, 15, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Gorick, C.M.; Breza, V.R.; Nowak, K.M.; Cheng, V.; Fisher, D.G.; Debski, A.C.; Hoch, M.R.; Demir, Z.; Tran, N.M.; Schwartz, M.R.; et al. Applications of focused ultrasound-mediated blood-brain barrier opening. Adv. Drug Deliv. Rev. 2022, 191, 114583. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cho, H.R.; Cha, G.D.; Seo, H.; Lee, S.; Park, C.K.; Kim, J.W.; Qiao, S.; Wang, L.; Kang, D.; et al. Flexible, sticky, and biodegradable wireless device for drug delivery to brain tumors. Nat. Commun. 2019, 10, 5205. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, I.K.; Wood, M.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Zhao, R.; Zhao, L.; Liu, J.; Peng, S.; Fu, X.; Wang, X.; Luo, R.; Wang, R.; et al. Silica nanoparticles: Biomedical applications and toxicity. Biomed. Pharmacother. 2022, 151, 113053. [Google Scholar] [CrossRef]
- Rehman, F.U.; Liu, Y.; Zheng, M.; Shi, B. Exosomes based strategies for brain drug delivery. Biomaterials 2023, 293, 121949. [Google Scholar] [CrossRef]
- Daßler-Plenker, J.; Küttner, V.; Egeblad, M. Communication in tiny packages: Exosomes as means of tumor-stroma communication. Biochim. Biophys. Acta-Rev. Cancer 2020, 1873, 188340. [Google Scholar] [CrossRef]
- Isaac, R.; Reis, F.; Ying, W.; Olefsky, J.M. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021, 33, 1744–1762. [Google Scholar] [CrossRef]
- Kalluri, R.; Lebleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Patel, N.J.; Ashraf, A.; Chung, E.J. Extracellular vesicles as regulators of the extracellular matrix. Bioengineering 2023, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Rak, J.; Strzadala, L. Heterogeneity of extracellular vesicles and particles: Molecular voxels in the blood borne “hologram” of organ function, disfunction and cancer. Arch. Immunol. Ther. Exp. 2023, 71, 5. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of exosome composition. Cell 2019, 177, 428–445. [Google Scholar] [CrossRef] [PubMed]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (misev2018): A position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Parra, D.C.; Motallebnejad, P.; Brocchi, M.; Chen, H.J. Exosomes: Small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact. Mater. 2022, 10, 281–294. [Google Scholar] [CrossRef]
- Paskeh, M.; Entezari, M.; Mirzaei, S.; Zabolian, A.; Saleki, H.; Naghdi, M.J.; Sabet, S.; Khoshbakht, M.A.; Hashemi, M.; Hushmandi, K.; et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J. Hematol. Oncol. 2022, 15, 83. [Google Scholar] [CrossRef]
- Wang, X.; Xia, J.; Yang, L.; Dai, J.; He, L. Recent progress in exosome research: Isolation, characterization and clinical applications. Cancer Gene Ther. 2023, 30, 1051–1065. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation—Efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef]
- Liu, F.; Vermesh, O.; Mani, V.; Ge, T.J.; Madsen, S.J.; Sabour, A.; Hsu, E.C.; Gowrishankar, G.; Kanada, M.; Jokerst, J.V.; et al. The exosome total isolation chip. Acs Nano 2017, 11, 10712–10723. [Google Scholar] [CrossRef]
- He, L.; Zhu, D.; Wang, J.; Wu, X. A highly efficient method for isolating urinary exosomes. Int. J. Mol. Med. 2019, 43, 83–90. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New technologies for analysis of extracellular vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Lai, J.J.; Chau, Z.L.; Chen, S.Y.; Hill, J.J.; Korpany, K.V.; Liang, N.W.; Lin, L.H.; Lin, Y.H.; Liu, J.K.; Liu, Y.C.; et al. Exosome processing and characterization approaches for research and technology development. Adv. Sci. 2022, 9, e2103222. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, S.D.; Wright, J.; Lewis, J.M.; Kim, S.; Ko, S.Y.; Ong, J.; Manouchehri, S.; Vyas, A.; Akers, J.; Chen, C.C.; et al. Rapid isolation and detection of exosomes and associated biomarkers from plasma. Acs Nano 2017, 11, 6641–6651. [Google Scholar] [CrossRef] [PubMed]
- Cumba, G.L.; Peterson, T.E.; Cepeda, M.A.; Johnson, A.J.; Parney, I.F. Isolation and analysis of plasma-derived exosomes in patients with glioma. Front. Oncol. 2019, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of extracellular vesicles across the blood-brain barrier: Brain pharmacokinetics and effects of inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef] [PubMed]
- Mirzaaghasi, A.; Han, Y.; Ahn, S.H.; Choi, C.; Park, J.H. Biodistribution and pharmacokinectics of liposomes and exosomes in a mouse model of sepsis. Pharmaceutics 2021, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Hellwinkel, J.E.; Redzic, J.S.; Harland, T.A.; Gunaydin, D.; Anchordoquy, T.J.; Graner, M.W. Glioma-derived extracellular vesicles selectively suppress immune responses. Neuro-Oncology 2016, 18, 497–506. [Google Scholar] [CrossRef]
- Webb, R.L.; Kaiser, E.E.; Scoville, S.L.; Thompson, T.A.; Fatima, S.; Pandya, C.; Sriram, K.; Swetenburg, R.L.; Vaibhav, K.; Arbab, A.S.; et al. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl. Stroke Res. 2018, 9, 530–539. [Google Scholar] [CrossRef]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V.; Farhoodi, H.P.; Zhang, S.X.; Zimak, J.; Ségaliny, A.; et al. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell. Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and crossing the blood-brain barrier with extracellular vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Pulgar, V.M. Transcytosis to cross the blood brain barrier, new advancements and challenges. Front. Neurosci. 2018, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Azarmi, M.; Maleki, H.; Nikkam, N.; Malekinejad, H. Transcellular brain drug delivery: A review on recent advancements. Int. J. Pharm. 2020, 586, 119582. [Google Scholar] [CrossRef]
- Kim, G.; Kim, M.; Lee, Y.; Byun, J.W.; Hwang, D.W.; Lee, M. Systemic delivery of microrna-21 antisense oligonucleotides to the brain using t7-peptide decorated exosomes. J. Control. Release 2020, 317, 273–281. [Google Scholar] [CrossRef]
- Han, L.; Huang, R.; Liu, S.; Huang, S.; Jiang, C. Peptide-conjugated pamam for targeted doxorubicin delivery to transferrin receptor overexpressed tumors. Mol. Pharm. 2010, 7, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, X.; Qiu, W.; Zhao, R.; Duan, J.; Zhang, S.; Pan, Z.; Zhao, S.; Guo, Q.; Qi, Y.; et al. Synchronous disintegration of ferroptosis defense axis via engineered exosome-conjugated magnetic nanoparticles for glioblastoma therapy. Adv. Sci. 2022, 9, e2105451. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.X.; He, C.P.; Fan, S.; Zhu, Y.L.; Qi, C.; Huang, N.P.; Xiao, Z.D.; Lu, Z.H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Zhu, Q.; Ling, X.; Yang, Y.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Chen, B.; Li, H.; Wang, Y.; et al. Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Adv. Sci. 2019, 6, 1801899. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Ergün, S.; Karnati, S.; Jha, H.C. Understanding the link between neurotropic viruses, bbb permeability, and ms pathogenesis. J. Neurovirol. 2024. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, S.; Qin, F.; Fu, A.; Fu, C. Application progress of rvg peptides to facilitate the delivery of therapeutic agents into the central nervous system. RSC Adv. 2021, 11, 8505–8515. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.M.; Wiklander, P.B.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic exosomal sirna delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov. Disord. 2014, 29, 1476–1485. [Google Scholar] [CrossRef]
- Tan, S.; Yang, Y.; Yang, W.; Han, Y.; Huang, L.; Yang, R.; Hu, Z.; Tao, Y.; Liu, L.; Li, Y.; et al. Exosomal cargos-mediated metabolic reprogramming in tumor microenvironment. J. Exp. Clin. Cancer Res. 2023, 42, 59. [Google Scholar] [CrossRef]
- Barthel, L.; Hadamitzky, M.; Dammann, P.; Schedlowski, M.; Sure, U.; Thakur, B.K.; Hetze, S. Glioma: Molecular signature and crossroads with tumor microenvironment. Cancer Metastasis Rev. 2022, 41, 53–75. [Google Scholar] [CrossRef]
- Schiffer, D.; Annovazzi, L.; Casalone, C.; Corona, C.; Mellai, M. Glioblastoma: Microenvironment and niche concept. Cancers 2018, 11, 5. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhang, Y.; Wang, H.; Rong, X.; Peng, J.; He, L.; Peng, Y. An mir-340-5p-macrophage feedback loop modulates the progression and tumor microenvironment of glioblastoma multiforme. Oncogene 2019, 38, 7399–7415. [Google Scholar] [CrossRef]
- Li, M.; Xu, H.; Qi, Y.; Pan, Z.; Li, B.; Gao, Z.; Zhao, R.; Xue, H.; Li, G. Tumor-derived exosomes deliver the tumor suppressor mir-3591-3p to induce m2 macrophage polarization and promote glioma progression. Oncogene 2022, 41, 4618–4632. [Google Scholar] [CrossRef]
- Lang, H.L.; Hu, G.W.; Zhang, B.; Kuang, W.; Chen, Y.; Wu, L.; Xu, G.H. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding rna ccat2. Oncol. Rep. 2017, 38, 785–798. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Chen, Z.; Xu, X.; Weng, J.; Zhang, Y.; Mo, Y.; Liu, Y.; Wang, J.; Ke, Y. Circglis3 promotes high-grade glioma invasion via modulating ezrin phosphorylation. Front. Cell. Dev. Biol. 2021, 9, 663207. [Google Scholar] [CrossRef]
- Huo, H.; Yang, S.; Wu, H.; Sun, Y.; Zhao, R.; Ye, R.; Yan, D.; Shi, X.; Yang, J. Brain endothelial cells-derived extracellular vesicles overexpressing ecrg4 inhibit glioma proliferation through suppressing inflammation and angiogenesis. J. Tissue Eng. Regen. Med. 2021, 15, 1162–1171. [Google Scholar] [CrossRef]
- Basu, B.; Ghosh, M.K. Extracellular vesicles in glioma: From diagnosis to therapy. Bioessays 2019, 41, e1800245. [Google Scholar] [CrossRef]
- Cai, Q.; Zhu, A.; Gong, L. Exosomes of glioma cells deliver mir-148a to promote proliferation and metastasis of glioblastoma via targeting cadm1. Bull. Cancer 2018, 105, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xiao, S.; Wang, Y.; Zhu, Z.; Cao, Y.; Yang, S.; Mai, R.; Zheng, Y. Identification of a novel circular rna circznf652/mir-486-5p/serpine1 signaling cascade that regulates cancer aggressiveness in glioblastoma (gbm). Bioengineered 2022, 13, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, J.; Wu, Y.; Luo, H.; Ke, Y. Decrease of circarid1a retards glioblastoma invasion by modulating mir-370-3p/ tgfbr2 pathway. Int. J. Biol. Sci. 2022, 18, 5123–5135. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, M.; Pan, Z.; Zhang, Z.; Gao, Z.; Zhao, R.; Li, B.; Qi, Y.; Qiu, W.; Guo, Q.; et al. Mir-3184-3p enriched in cerebrospinal fluid exosomes contributes to progression of glioma and promotes m2-like macrophage polarization. Cancer Sci. 2022, 113, 2668–2680. [Google Scholar] [CrossRef]
- Chen, X.; Yang, F.; Zhang, T.; Wang, W.; Xi, W.; Li, Y.; Zhang, D.; Huo, Y.; Zhang, J.; Yang, A.; et al. Mir-9 promotes tumorigenesis and angiogenesis and is activated by myc and oct4 in human glioma. J. Exp. Clin. Cancer Res. 2019, 38, 99. [Google Scholar] [CrossRef]
- Yue, X.; Lan, F.; Xia, T. Hypoxic glioma cell-secreted exosomal mir-301a activates wnt/β-catenin signaling and promotes radiation resistance by targeting tceal7. Mol. Ther. 2019, 27, 1939–1949. [Google Scholar] [CrossRef]
- Corcoran, C.; Rani, S.; O’Brien, K.; O’Neill, A.; Prencipe, M.; Sheikh, R.; Webb, G.; Mcdermott, R.; Watson, W.; Crown, J.; et al. Docetaxel-resistance in prostate cancer: Evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS ONE 2012, 7, e50999. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Bao, B.; Sarkar, F.H. Exosomes in cancer development, metastasis, and drug resistance: A comprehensive review. Cancer Metastasis Rev. 2013, 32, 623–642. [Google Scholar] [CrossRef] [PubMed]
- André-Grégoire, G.; Gavard, J. Spitting out the demons: Extracellular vesicles in glioblastoma. Cell Adhes. Migr. 2017, 11, 164–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ashley, J.; Cordy, B.; Lucia, D.; Fradkin, L.G.; Budnik, V.; Thomson, T. Retrovirus-like gag protein arc1 binds rna and traffics across synaptic boutons. Cell 2018, 172, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Oldrini, B.; Vaquero-Siguero, N.; Mu, Q.; Kroon, P.; Zhang, Y.; Galán-Ganga, M.; Bao, Z.; Wang, Z.; Liu, H.; Sa, J.K.; et al. Mgmt genomic rearrangements contribute to chemotherapy resistance in gliomas. Nat. Commun. 2020, 11, 3883. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Zhang, Y.; Lin, X.; Zeng, Z.; Hu, J.; Hao, L.; Xu, J.; Wang, X.; Wang, H.; Li, Q. Exosomal circwdr62 promotes temozolomide resistance and malignant progression through regulation of the mir-370-3p/mgmt axis in glioma. Cell Death Dis. 2022, 13, 596. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.T.; Liu, B.Y.; Ji, H.Y.; Lan, Y.F.; Tang, W.H.; Zhou, J.; Zhong, X.Y.; Lian, C.L.; Huang, Q.Z.; Wang, C.Y.; et al. Exosome-mediated transfer of mif confers temozolomide resistance by regulating timp3/pi3k/akt axis in gliomas. Mol. Ther. Oncolytics 2021, 22, 114–128. [Google Scholar] [CrossRef]
- Montemurro, N.; Pahwa, B.; Tayal, A.; Shukla, A.; De Jesus, E.M.; Ramirez, I.; Nurmukhametov, R.; Chavda, V.; De Carlo, A. Macrophages in recurrent glioblastoma as a prognostic factor in the synergistic system of the tumor microenvironment. Neurol. Int. 2023, 15, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Wu, Y.; Wu, Y.; Wang, P.; Vadgama, J.V. Tumor-derived exosomes in tumor-induced immune suppression. Int. J. Mol. Sci. 2022, 23, 1461. [Google Scholar] [CrossRef] [PubMed]
- Graner, M.W.; Alzate, O.; Dechkovskaia, A.M.; Keene, J.D.; Sampson, J.H.; Mitchell, D.A.; Bigner, D.D. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009, 23, 1541–1557. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, R.; Sarkar, S.; Dzikowski, L.; Rawji, K.S.; Khan, L.; Faissner, A.; Bose, P.; Yong, V.W. Brain tumor-initiating cells export tenascin-c associated with exosomes to suppress t cell activity. Oncoimmunology 2018, 7, e1478647. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Wang, S.; Guo, X.; Wang, J.; Zhang, Z.; Qiu, W.; Gao, X.; Chen, Z.; Xu, J.; Zhao, R.; et al. Hypoxic glioma-derived exosomes deliver microrna-1246 to induce m2 macrophage polarization by targeting terf2ip via the stat3 and nf-κb pathways. Oncogene 2020, 39, 428–442. [Google Scholar] [CrossRef]
- Pan, Z.; Zhao, R.; Li, B.; Qi, Y.; Qiu, W.; Guo, Q.; Zhang, S.; Zhao, S.; Xu, H.; Li, M.; et al. Ewsr1-induced circneil3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing igf2bp3. Mol. Cancer 2022, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Yang, G.; Jing, H.; Tan, Y.; Zhao, B.; Zhang, H. Tumor progression and treatment-related changes: Radiological diagnosis challenges for the evaluation of post treated glioma. Cancers 2022, 14, 3771. [Google Scholar] [CrossRef]
- Langen, K.J.; Galldiks, N.; Mauler, J.; Kocher, M.; Filß, C.P.; Stoffels, G.; Régio, B.C.; Stegmayr, C.; Willuweit, A.; Worthoff, W.A.; et al. Hybrid pet/mri in cerebral glioma: Current status and perspectives. Cancers 2023, 15, 3577. [Google Scholar] [CrossRef]
- Jones, J.; Nguyen, H.; Drummond, K.; Morokoff, A. Circulating biomarkers for glioma: A review. Neurosurgery 2021, 88, E221–E230. [Google Scholar] [CrossRef]
- Komatsu, S.; Kitai, H.; Suzuki, H.I. Network regulation of microrna biogenesis and target interaction. Cells 2023, 12, 306. [Google Scholar] [CrossRef]
- Wei, Z.; Batagov, A.O.; Schinelli, S.; Wang, J.; Wang, Y.; El, F.R.; Rabinovsky, R.; Balaj, L.; Chen, C.C.; Hochberg, F.; et al. Coding and noncoding landscape of extracellular rna released by human glioma stem cells. Nat. Commun. 2017, 8, 1145. [Google Scholar] [CrossRef]
- Hanjani, N.A.; Esmaelizad, N.; Zanganeh, S.; Gharavi, A.T.; Heidarizadeh, P.; Radfar, M.; Omidi, F.; Macloughlin, R.; Doroudian, M. Emerging role of exosomes as biomarkers in cancer treatment and diagnosis. Crit. Rev. Oncol./Hematol. 2022, 169, 103565. [Google Scholar] [CrossRef]
- Akers, J.C.; Ramakrishnan, V.; Kim, R.; Skog, J.; Nakano, I.; Pingle, S.; Kalinina, J.; Hua, W.; Kesari, S.; Mao, Y.; et al. Mir-21 in the extracellular vesicles (evs) of cerebrospinal fluid (csf): A platform for glioblastoma biomarker development. PLoS ONE 2013, 8, e78115. [Google Scholar] [CrossRef]
- Li, L.; Li, C.; Wang, S.; Wang, Z.; Jiang, J.; Wang, W.; Li, X.; Chen, J.; Liu, K.; Li, C.; et al. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver mir-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 2016, 76, 1770–1780. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.J.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport rna and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Wang, P.Y.; Li, X.Y.; Chen, J.X.; Li, Y.; Zhang, X.Z.; Zhang, C.G.; Jiang, T.; Li, W.B.; Ding, W.; et al. Exosomal levels of mirna-21 from cerebrospinal fluids associated with poor prognosis and tumor recurrence of glioma patients. Oncotarget 2015, 6, 26971–26981. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, A.; Imbrucè, P.; Gardenghi, B.; Belli, L.; Agushi, R.; Tamanini, A.; Munari, S.; Bossi, A.M.; Scambi, I.; Benati, D.; et al. A microrna signature from serum exosomes of patients with glioma as complementary diagnostic biomarker. J. Neuro-Oncol. 2018, 136, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Olioso, D.; Caccese, M.; Santangelo, A.; Lippi, G.; Zagonel, V.; Cabrini, G.; Lombardi, G.; Dechecchi, M.C. Serum exosomal microrna-21, 222 and 124-3p as noninvasive predictive biomarkers in newly diagnosed high-grade gliomas: A prospective study. Cancers 2021, 13, 3006. [Google Scholar] [CrossRef]
- Yang, L.; Ma, Y.; Xin, Y.; Han, R.; Li, R.; Hao, X. Role of the microrna 181 family in glioma development. Mol. Med. Rep. 2018, 17, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Stakaitis, R.; Pranckeviciene, A.; Steponaitis, G.; Tamasauskas, A.; Bunevicius, A.; Vaitkiene, P. Unique interplay between molecular mir-181b/d biomarkers and health related quality of life score in the predictive glioma models. Int. J. Mol. Sci. 2020, 21, 7450. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Qing, Q.; Pan, Q.; Hu, M.; Yu, H.; Yue, X. Serum exosomal mir-301a as a potential diagnostic and prognostic biomarker for human glioma. Cell. Oncol. 2018, 41, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, Q.M.; Liu, C.; Wu, S.; Nong, W.X.; Ge, Y.Y.; Lin, L.N.; Li, F.; Xie, X.X.; Luo, B. Microrna-1224-5p is a potential prognostic and therapeutic biomarker in glioblastoma: Integrating bioinformatics and clinical analyses. Curr. Med. Sci. 2022, 42, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, Z.; Gareev, I.; Yan, T.; Chen, X.; Ahmad, A.; Zhang, D.; Zhao, B.; Beylerli, O.; Yang, G.; et al. Exosomal mir-2276-5p in plasma is a potential diagnostic and prognostic biomarker in glioma. Front. Cell. Dev. Biol. 2021, 9, 671202. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wei, B.; Peng, C.; Wang, L.; Li, C. Identification of serum exosomal mir-98-5p, mir-183-5p, mir-323-3p and mir-19b-3p as potential biomarkers for glioblastoma patients and investigation of their mechanisms. Curr. Res. Transl. Med. 2022, 70, 103315. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Gu, X. Plasmatic exosome-derived circrnas panel act as fingerprint for glioblastoma. Aging 2021, 13, 19575–19586. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, M.; Fei, L.; Huang, Z.; Yan, Y. Circfoxm1 promotes the proliferation, migration, invasion, and glutaminolysis of glioblastoma by regulating the mir-577/e2f5 axis. Bosn. J. Basic Med. Sci. 2022, 22, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Wang, Z.; Li, H.; Liu, J.; Liu, Y.; Jiang, Y.; Lou, M. Circular rna circaspm promotes the progression of glioblastoma by acting as a competing endogenous rna to regulate mir-130b-3p/e2f1 axis. J. Cancer 2022, 13, 1664–1678. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Zhou, F.; Lv, K.; Xu, X.; Cao, W. Circndc80 promotes glioblastoma multiforme tumorigenesis via the mir-139-5p/ece1 pathway. J. Transl. Med. 2023, 21, 22. [Google Scholar] [CrossRef]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding rna biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef]
- Gou, Q.; Gao, L.; Nie, X.; Pu, W.; Zhu, J.; Wang, Y.; Liu, X.; Tan, S.; Zhou, J.K.; Gong, Y.; et al. Long noncoding rna ab074169 inhibits cell proliferation via modulation of khsrp-mediated cdkn1a expression in papillary thyroid carcinoma. Cancer Res. 2018, 78, 4163–4174. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional classification and experimental dissection of long noncoding rnas. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, C.; Wu, M. New insights into long noncoding rnas and their roles in glioma. Mol. Cancer 2018, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yin, J.; Lu, C.; Wei, Y.; Zeng, A.; You, Y. Exosomal transfer of long non-coding rna sbf2-as1 enhances chemoresistance to temozolomide in glioblastoma. J. Exp. Clin. Cancer Res. 2019, 38, 166. [Google Scholar] [CrossRef]
- Tan, S.K.; Pastori, C.; Penas, C.; Komotar, R.J.; Ivan, M.E.; Wahlestedt, C.; Ayad, N.G. Serum long noncoding rna hotair as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol. Cancer 2018, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ji, X.; Gao, L.; Guo, X.; Lian, W.; Deng, K.; Xing, B. Comprehensive in silico analysis of a novel serum exosome-derived competitive endogenous rna network for constructing a prognostic model for glioblastoma. Front. Oncol. 2021, 11, 553594. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.; Jo, A.; Giedt, J.; Vinegoni, C.; Yang, K.S.; Peruzzi, P.; Chiocca, E.A.; Breakefield, X.O.; Lee, H.; Weissleder, R. Characterization of single microvesicles in plasma from glioblastoma patients. Neuro-Oncology 2019, 21, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor egfrviii by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Manda, S.V.; Kataria, Y.; Tatireddy, B.R.; Ramakrishnan, B.; Ratnam, B.G.; Lath, R.; Ranjan, A.; Ray, A. Exosomes as a biomarker platform for detecting epidermal growth factor receptor-positive high-grade gliomas. J. Neurosurg. 2018, 128, 1091–1101. [Google Scholar] [CrossRef]
- Jennrich, S.; Pelzer, M.; Tertel, T.; Koska, B.; Vüllings, M.; Thakur, B.K.; Jendrossek, V.; Timmermann, B.; Giebel, B.; Rudner, J. Cd9- and cd81-positive extracellular vesicles provide a marker to monitor glioblastoma cell response to photon-based and proton-based radiotherapy. Front. Oncol. 2022, 12, 947439. [Google Scholar] [CrossRef]
- Hu, P.; Wang, K.; Zhou, D.; Wang, L.; Zhao, M.; Wang, W.; Zhang, Y.; Liu, Y.; Yu, R.; Zhou, X. Golph3 regulates exosome mirna secretion in glioma cells. J. Mol. Neurosci. 2020, 70, 1257–1266. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Zhou, Y.; Dong, L.; Ma, W.; Lv, L.; Zhang, J.; Wang, X. Glioblastoma stem cell-derived exosomes enhance stemness and tumorigenicity of glioma cells by transferring notch1 protein. Cell. Mol. Neurobiol. 2020, 40, 767–784. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, M.; Sugaya, K. Differential sequences and single nucleotide polymorphism of exosomal sox2 dna in cancer. PLoS ONE 2020, 15, e229309. [Google Scholar] [CrossRef] [PubMed]
- Zottel, A.; Aamec, N.; Kump, A.; Raspor, D.L.; Pužar, D.P.; Romih, R.; Hudoklin, S.; Mlakar, J.; Nikitin, D.; Sorokin, M.; et al. Analysis of mir-9-5p, mir-124-3p, mir-21-5p, mir-138-5p, and mir-1-3p in glioblastoma cell lines and extracellular vesicles. Int. J. Mol. Sci. 2020, 21, 8491. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Gourlay, J.; Morokoff, A.P.; Luwor, R.B.; Zhu, H.J.; Kaye, A.H.; Stylli, S.S. The emergent role of exosomes in glioma. J. Clin. Neurosci. 2017, 35, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.C.; Ma, H.; Niu, Z.F.; Sun, S.Y.; Zou, Y.R.; Xia, H.C. Huc-mscs secreted exosomes inhibit the glioma cell progression through ptenp1/mir-10a-5p/pten pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10013–10023. [Google Scholar] [PubMed]
- Parsaei, H.; Moosavifar, M.J.; Eftekharzadeh, M.; Ramezani, R.; Barati, M.; Mirzaei, S.; Nobakht, M. Exosomes to control glioblastoma multiforme: Investigating the effects of mesenchymal stem cell-derived exosomes on c6 cells in vitro. Cell Biol. Int. 2022, 46, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.; Serpe, C.; Chece, G.; Nigro, V.; Sarra, A.; Ruzicka, B.; Relucenti, M.; Familiari, G.; Ruocco, G.; Pascucci, G.R.; et al. Microglia-derived microvesicles affect microglia phenotype in glioma. Front. Cell. Neurosci. 2019, 13, 41. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Guo, K.; Zhang, K.; Liu, Q.; Wang, P.; Wang, X. Ultrasound facilitates naturally equipped exosomes derived from macrophages and blood serum for orthotopic glioma treatment. Acs Appl. Mater. Interfaces 2019, 11, 14576–14587. [Google Scholar] [CrossRef]
- Messenger, S.W.; Woo, S.S.; Sun, Z.; Martin, T. A ca(2+)-stimulated exosome release pathway in cancer cells is regulated by munc13-4. J. Cell Biol. 2018, 217, 2877–2890. [Google Scholar] [CrossRef]
- Cen, X.; Chen, Q.; Wang, B.; Xu, H.; Wang, X.; Ling, Y.; Zhang, X.; Qin, D. Ube2o ubiquitinates ptrf/cavin1 and inhibits the secretion of exosome-related ptrf/cavin1. Cell Commun. Signal. 2022, 20, 191. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Iguchi, H.; Hagiwara, K.; Yoshioka, Y.; Takeshita, F.; Ochiya, T. Neutral sphingomyelinase 2 (nsmase2)-dependent exosomal transfer of angiogenic micrornas regulate cancer cell metastasis. J. Biol. Chem. 2013, 288, 10849–10859. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.W.; Zhao, F.; Wang, J.Y.; Wang, H.Y.; Ge, S.H.; Wang, X.; Zhang, L.; Liu, R.; Ba, Y.; Li, H.L.; et al. Tumor microenvironment interruption: A novel anti-cancer mechanism of proton-pump inhibitor in gastric cancer by suppressing the release of microrna-carrying exosomes. Am. J. Cancer Res. 2017, 7, 1913–1925. [Google Scholar] [PubMed]
- Qin, S.; Cao, J.; Ma, X. Function and clinical application of exosome-how to improve tumor immunotherapy? Front. Cell. Dev. Biol. 2023, 11, 1228624. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, L.; Jia, M.; Liao, Q.; Peng, G.; Luo, G.; Zhou, Y. Dendritic cell vaccines improve the glioma microenvironment: Influence, challenges, and future directions. Cancer Med. 2023, 12, 7207–7221. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Miao, Y.; Wang, X.; Huang, X.; Dai, J. Recent progress of dendritic cell-derived exosomes (dex) as an anti-cancer nanovaccine. Biomed. Pharmacother. 2022, 152, 113250. [Google Scholar] [CrossRef] [PubMed]
- Bu, N.; Wu, H.; Zhang, G.; Zhan, S.; Zhang, R.; Sun, H.; Du, Y.; Yao, L.; Wang, H. Exosomes from dendritic cells loaded with chaperone-rich cell lysates elicit a potent t cell immune response against intracranial glioma in mice. J. Mol. Neurosci. 2015, 56, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, L.; Liu, J.; Meng, H.; Zhang, R.; Ma, L.; Wu, L.; Yu, S.; Shi, F.; Li, Y.; et al. Co-delivery of tumor-derived exosomes with alpha-galactosylceramide on dendritic cell-based immunotherapy for glioblastoma. Cancer Lett. 2017, 411, 182–190. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, K.; Wang, Z.; Wang, D.; Yin, X.; Liu, Y.; Yu, F.; Zhao, W. An efficient and safe muc1-dendritic cell-derived exosome conjugate vaccine elicits potent cellular and humoral immunity and tumor inhibition in vivo. Acta Biomater. 2022, 138, 491–504. [Google Scholar] [CrossRef]
- Ghorbaninezhad, F.; Alemohammad, H.; Najafzadeh, B.; Masoumi, J.; Shadbad, M.A.; Shahpouri, M.; Saeedi, H.; Rahbarfarzam, O.; Baradaran, B. Dendritic cell-derived exosomes: A new horizon in personalized cancer immunotherapy? Cancer Lett. 2023, 562, 216168. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in danio rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Zhang, C.; Song, J.; Lou, L.; Qi, X.; Zhao, L.; Fan, B.; Sun, G.; Lv, Z.; Fan, Z.; Jiao, B.; et al. Doxorubicin-loaded nanoparticle coated with endothelial cells-derived exosomes for immunogenic chemotherapy of glioblastoma. Bioeng. Transl. Med. 2021, 6, e10203. [Google Scholar] [CrossRef]
- Wang, J.; Tang, W.; Yang, M.; Yin, Y.; Li, H.; Hu, F.; Tang, L.; Ma, X.; Zhang, Y.; Wang, Y. Inflammatory tumor microenvironment responsive neutrophil exosomes-based drug delivery system for targeted glioma therapy. Biomaterials 2021, 273, 120784. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. Nrp-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, X.; Zhao, H.; Li, N.; Li, J.; Zhang, H.; Di, L. Targeted delivery of hybrid nanovesicles for enhanced brain penetration to achieve synergistic therapy of glioma. J. Control. Release 2023, 365, 331–347. [Google Scholar] [CrossRef]
- Lang, F.M.; Hossain, A.; Gumin, J.; Momin, E.N.; Shimizu, Y.; Ledbetter, D.; Shahar, T.; Yamashita, S.; Parker, K.B.; Fueyo, J.; et al. Mesenchymal stem cells as natural biofactories for exosomes carrying mir-124a in the treatment of gliomas. Neuro-Oncology 2018, 20, 380–390. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, X.; Guo, X.; Yu, R.; Qian, M.; Wang, S.; Gao, X.; Qiu, W.; Guo, Q.; Xu, J.; et al. Microrna-29a-3p delivery via exosomes derived from engineered human mesenchymal stem cells exerts tumour suppressive effects by inhibiting migration and vasculogenic mimicry in glioma. Aging 2021, 13, 5055–5068. [Google Scholar] [CrossRef]
- Duong, A.; Parmar, G.; Kirkham, A.M.; Burger, D.; Allan, D.S. Registered clinical trials investigating treatment with cell-derived extracellular vesicles: A scoping review. Cytotherapy 2023, 25, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Uziel, O.; Kanner, A.A.; Beery, E.; Lev, S.; Lahav, M.; Horn-Fichman, S.; Nof, S.H.; Laviv, Y.; Katz, S.Y.; Amiel, A.; et al. Is serum-derived exosomal htert transcript a marker of oncogenic activity in primary brain tumors? An exploratory study. Cancer Med. 2023, 13, e6784. [Google Scholar] [CrossRef]
- Ashraf, N.S.; Mahjabeen, I.; Hussain, M.Z.; Rizwan, M.; Arshad, M.; Mehmood, A.; Haris, M.S.; Kayani, M.A. Role of exosomal mirna-19a/19b and pten in brain tumor diagnosis. Future Oncol. 2023, 19, 1563–1576. [Google Scholar] [CrossRef]
- Bao, Z.; Zhang, N.; Niu, W.; Mu, M.; Zhang, X.; Hu, S.; Niu, C. Exosomal mir-155-5p derived from glioma stem-like cells promotes mesenchymal transition via targeting acot12. Cell Death Dis. 2022, 13, 725. [Google Scholar] [CrossRef]
- Piazza, A.; Rosa, P.; Ricciardi, L.; Mangraviti, A.; Pacini, L.; Calogero, A.; Raco, A.; Miscusi, M. Circulating exosomal-dna in glioma patients: A quantitative study and histopathological correlations-a preliminary study. Brain Sci. 2022, 12, 500. [Google Scholar] [CrossRef]
- Ding, C.; Yi, X.; Chen, X.; Wu, Z.; You, H.; Chen, X.; Zhang, G.; Sun, Y.; Bu, X.; Wu, X.; et al. Warburg effect-promoted exosomal circ_0072083 releasing up-regulates nango expression through multiple pathways and enhances temozolomide resistance in glioma. J. Exp. Clin. Cancer Res. 2021, 40, 164. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Q.; Shi, Y.; Wu, X.; Mi, Y.; Liu, K.; Kan, Q.; Fan, R.; Liu, Z.; Zhang, M. Identification of low-dose radiation-induced exosomal circ-metrn and mir-4709-3p/grb14/pdgfrα pathway as a key regulatory mechanism in glioblastoma progression and radioresistance: Functional validation and clinical theranostic significance. Int. J. Biol. Sci. 2021, 17, 1061–1078. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Yue, X.; Xia, T. Exosomal microrna-210 is a potentially non-invasive biomarker for the diagnosis and prognosis of glioma. Oncol. Lett. 2020, 19, 1967–1974. [Google Scholar] [CrossRef]
- Zhong, F.; Huang, T.; Leng, J. Serum mir-29b as a novel biomarker for glioblastoma diagnosis and prognosis. Int. J. Clin. Exp. Pathol. 2019, 12, 4106–4112. [Google Scholar] [PubMed]
- Shao, N.; Xue, L.; Wang, R.; Luo, K.; Zhi, F.; Lan, Q. Mir-454-3p is an exosomal biomarker and functions as a tumor suppressor in glioma. Mol. Cancer Ther. 2019, 18, 459–469. [Google Scholar] [CrossRef]
- Huang, K.; Fang, C.; Yi, K.; Liu, X.; Qi, H.; Tan, Y.; Zhou, J.; Li, Y.; Liu, M.; Zhang, Y.; et al. The role of ptrf/cavin1 as a biomarker in both glioma and serum exosomes. Theranostics 2018, 8, 1540–1557. [Google Scholar] [CrossRef]
- Bao, P.; Gu, H.Y.; Ye, J.J.; He, J.L.; Zhong, Z.; Yu, A.X.; Zhang, X.Z. Chimeric exosomes functionalized with sting activation for personalized glioblastoma immunotherapy. Adv. Sci. 2023, 11, e2306336. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, X.; Li, J.; Zhu, A.; Du, Y.; Zeng, W.; Guo, Y.; Di, L.; Wang, R. Immune exosomes loading self-assembled nanomicelles traverse the blood-brain barrier for chemo-immunotherapy against glioblastoma. Acs Nano 2023, 17, 1464–1484. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Cao, Y.; Liu, Z. Engineering macrophage exosome disguised biodegradable nanoplatform for enhanced sonodynamic therapy of glioblastoma. Adv. Mater. 2022, 34, e2110364. [Google Scholar] [CrossRef]
- Lee, H.; Bae, K.; Baek, A.R.; Kwon, E.B.; Kim, Y.H.; Nam, S.W.; Lee, G.H.; Chang, Y. Glioblastoma-derived exosomes as nanopharmaceutics for improved glioma treatment. Pharmaceutics 2022, 14, 1002. [Google Scholar] [CrossRef]
- Liang, S.; Xu, H.; Ye, B.C. Membrane-decorated exosomes for combination drug delivery and improved glioma therapy. Langmuir 2022, 38, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liang, Q.; Zhang, X.; Di, Z.; Wang, X.; Di, L. Tumor-derived exosomes reversing tmz resistance by synergistic drug delivery for glioma-targeting treatment. Colloid. Surf. B-Biointerfaces 2022, 215, 112505. [Google Scholar] [CrossRef] [PubMed]
- Valipour, E.; Ranjbar, F.E.; Mousavi, M.; Ai, J.; Malekshahi, Z.V.; Mokhberian, N.; Taghdiri-Nooshabadi, Z.; Khanmohammadi, M.; Nooshabadi, V.T. The anti-angiogenic effect of atorvastatin loaded exosomes on glioblastoma tumor cells: An in vitro 3d culture model. Microvasc. Res. 2022, 143, 104385. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, M.F.; Hossain, A.; Momin, E.; Hasan, I.; Singh, S.; Adachi, S.; Gumin, J.; Ledbetter, D.; Yang, J.; Long, L.; et al. Tumor-specific polycistronic mirna delivered by engineered exosomes for the treatment of glioblastoma. Neuro-Oncology 2023, 26, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, R.; Kiani, J.; Tong, W.Y.; Soleimani, M.; Voelcker, N.H.; Arefian, E. Engineered anti-egfrviii targeted exosomes induce apoptosis in glioblastoma multiforme. J. Drug Target 2023, 31, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Song, Y.; Qiao, W.; Sun, L.; Wang, X.; Yi, B.; Yang, X.; Ji, L.; Su, P.; Zhao, W.; et al. Focused ultrasound combined with mir-1208-equipped exosomes inhibits malignant progression of glioma. Br. J. Cancer 2023, 129, 1083–1094. [Google Scholar] [CrossRef]
- Adamus, T.; Hung, C.Y.; Yu, C.; Kang, E.; Hammad, M.; Flores, L.; Nechaev, S.; Zhang, Q.; Gonzaga, J.M.; Muthaiyah, K.; et al. Glioma-targeted delivery of exosome-encapsulated antisense oligonucleotides using neural stem cells. Mol. Ther. Nucl. Acids 2022, 27, 611–620. [Google Scholar] [CrossRef]
- Zhan, Q.; Yi, K.; Cui, X.; Li, X.; Yang, S.; Wang, Q.; Fang, C.; Tan, Y.; Li, L.; Xu, C.; et al. Blood exosomes-based targeted delivery of cpla2 sirna and metformin to modulate glioblastoma energy metabolism for tailoring personalized therapy. Neuro-Oncology 2022, 24, 1871–1883. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Z.; Liu, N.; Gao, G.; Du, M.; Wang, Y.; Cheng, B.; Zhu, M.; Jia, B.; Pan, L.; et al. Kill two birds with one stone: Engineered exosome-mediated delivery of cholesterol modified yy1-sirna enhances chemoradiotherapy sensitivity of glioblastoma. Front. Pharmacol. 2022, 13, 975291. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Wang, Y.; Ji, Y.; Chen, D.; Wang, C.; Zhang, G.; Wang, Y. Neural stem cell-derived exosomes transfer mir-124-3p into cells to inhibit glioma growth by targeting flot2. Int. J. Oncol. 2022, 61, 115. [Google Scholar] [CrossRef] [PubMed]
- Monfared, H.; Jahangard, Y.; Nikkhah, M.; Mirnajafi-Zadeh, J.; Mowla, S.J. Potential therapeutic effects of exosomes packed with a mir-21-sponge construct in a rat model of glioblastoma. Front. Oncol. 2019, 9, 782. [Google Scholar] [CrossRef]
- Sharif, S.; Ghahremani, M.H.; Soleimani, M. Delivery of exogenous mir-124 to glioblastoma multiform cells by wharton’s jelly mesenchymal stem cells decreases cell proliferation and migration, and confers chemosensitivity. Stem Cell Rev. Rep. 2018, 14, 236–246. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Sun, Y.; Liu, W.; Zhang, Y.; Sun, G.; Xiang, B.; Yang, J. Exosomes in Glioma: Unraveling Their Roles in Progression, Diagnosis, and Therapy. Cancers 2024, 16, 823. https://doi.org/10.3390/cancers16040823
Yang S, Sun Y, Liu W, Zhang Y, Sun G, Xiang B, Yang J. Exosomes in Glioma: Unraveling Their Roles in Progression, Diagnosis, and Therapy. Cancers. 2024; 16(4):823. https://doi.org/10.3390/cancers16040823
Chicago/Turabian StyleYang, Song, Yumeng Sun, Wei Liu, Yi Zhang, Guozhu Sun, Bai Xiang, and Jiankai Yang. 2024. "Exosomes in Glioma: Unraveling Their Roles in Progression, Diagnosis, and Therapy" Cancers 16, no. 4: 823. https://doi.org/10.3390/cancers16040823
APA StyleYang, S., Sun, Y., Liu, W., Zhang, Y., Sun, G., Xiang, B., & Yang, J. (2024). Exosomes in Glioma: Unraveling Their Roles in Progression, Diagnosis, and Therapy. Cancers, 16(4), 823. https://doi.org/10.3390/cancers16040823






