Comparison of PIV and Other Immune Inflammation Markers of Oncological and Survival Outcomes in Patients Undergoing Radical Cystectomy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Gathering
2.3. Outcomes
2.4. Statistical Methodology
3. Results
3.1. Patient Features
3.2. Oncological Results
3.3. Long-Term Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Giridhar, K.V.; Kohli, M. Management of Muscle-Invasive Urothelial Cancer and the Emerging Role of Immunotherapy in Advanced Urothelial Cancer. Mayo Clin. Proc. 2017, 92, 1564–1582. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Bassi, P.; Ferrante, G.D.; Piazza, N.; Spinadin, R.; Carando, R.; Pappagallo, G.; Pagano, F. Prognostic factors of outcome after radical cystectomy for bladder cancer: A retrospective study of a homogeneous patient cohort. J. Urol. 1999, 161, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.P.; Lieskovsky, G.; Cote, R.; Groshen, S.; Feng, A.C.; Boyd, S.; Skinner, E.; Bochner, B.; Thangathurai, D.; Mikhail, M.; et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1054 patients. J. Clin. Oncol. 2001, 19, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, M.A.; el-Mekresh, M.M.; el-Baz, M.A.; el-Attar, I.A.; Ashamallah, A. Radical cystectomy for carcinoma of the bladder: Critical evaluation of the results in 1026 cases. J. Urol. 1997, 158, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Gao, Y.; Wu, Y.; Lin, H. Prognostic value of systemic immune-inflammation index in patients with urologic cancers: A meta-analysis. Cancer Cell Int. 2020, 20, 499. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, T.; Dai, Y.; Li, J. Preoperative systemic inflammation score (SIS) is superior to neutrophil to lymphocyte ratio (NLR) as a predicting indicator in patients with esophageal squamous cell carcinoma. BMC Cancer 2019, 19, 721. [Google Scholar] [CrossRef]
- Deng, J.P.; Hua, X.; Long, Z.Q.; Zhang, W.W.; Lin, H.X.; He, Z.Y. Erratum to prognostic value of skeletal muscle index and monocyte-to-lymphocyte ratio for lymph node-positive breast cancer patients after mastectomy. Ann. Transl. Med. 2020, 8, 520. [Google Scholar] [CrossRef]
- Marchioni, M.; Primiceri, G.; Ingrosso, M.; Filograna, R.; Castellan, P.; De Francesco, P.; Schips, L. The Clinical Use of the Neutrophil to Lymphocyte Ratio (NLR) in Urothelial Cancer: A Systematic Review. Clin. Genitourin. Cancer 2016, 14, 473–484. [Google Scholar] [CrossRef]
- Li, X.; Gu, L.; Chen, Y.; Chong, Y.; Wang, X.; Guo, P.; He, D. Systemic immune-inflammation index is a promising non-invasive biomarker for predicting the survival of urinary system cancers: A systematic review and meta-analysis. Ann. Med. 2021, 53, 1827–1838. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Yang, Y.; Hu, X.; Zhang, R.; Li, X. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: A systematic review and meta-analysis. J. Transl. Med. 2023, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Marino, F.; Rossi, F.; Bizzarri, F.P.; Ragonese, M.; Dibitetto, F.; Filomena, G.B.; Marafon, D.P.; Ciccarese, C.; Iacovelli, R.; et al. Is Systemic Immune-Inflammation Index a Real Non-Invasive Biomarker to Predict Oncological Outcomes in Patients Eligible for Radical Cystectomy? Medicina 2023, 59, 2063. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Chiujdea, S.; Musi, G.; Lucarelli, G.; Del Giudice, F.; Hurle, R.; Damiano, R.; Cantiello, F.; Mari, A.; Minervini, A.; et al. Impact of Age on Outcomes of Patients With Pure Carcinoma In Situ of the Bladder: Multi-Institutional Cohort Analysis. Clin. Genitourin. Cancer 2022, 20, e166–e172. [Google Scholar] [CrossRef] [PubMed]
- Halabi, S.; Li, C.; Luo, S. Developing and Validating Risk Assessment Models of Clinical Outcomes in Modern Oncology. JCO Precis. Oncol. 2019, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kayar, R.; Bastug, Y.; Tokuc, E.; Topaktas, R.; Akyurek, E.A.; Kayar, K.; Artuk, I.; Ozturk, M. Pan-immune-inflammation value as a prognostic tool for overall survival and disease-free survival in non-metastatic muscle-invasive bladder cancer. Int. Urol. Nephrol. 2023, 56, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bae, J.-S. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediat. Inflamm. 2016, 2016, 6058147. [Google Scholar] [CrossRef] [PubMed]
- Lolli, C.; Basso, U.; Derosa, L.; Scarpi, E.; Sava, T.; Santoni, M.; Crabb, S.J.; Massari, F.; Aieta, M.; Conteduca, V.; et al. Systemic immune-inflammation index predicts the clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Oncotarget 2016, 7, 54564–54571. [Google Scholar] [CrossRef]
- Auguste, P.; Fallavollita, L.; Wang, N.; Burnier, J.; Bikfalvi, A.; Brodt, P. The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation. Am. J. Pathol. 2007, 170, 1781–1792. [Google Scholar] [CrossRef]
- Chen, H.-C.; Lin, H.-C.; Liu, C.-Y.; Wang, C.-H.; Hwang, T.; Huang, T.-T.; Lin, C.-H.; Kuo, H.-P. Neutrophil elastase induces IL-8 synthesis by lung epithelial cells via the mitogen-activated protein kinase pathway. J. Biomed. Sci. 2004, 11, 49–58. [Google Scholar] [CrossRef]
- Coussens, L.M.; Tinkle, C.L.; Hanahan, D.; Werb, Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 2000, 103, 481–490. [Google Scholar] [CrossRef]
- Liang, S.; Hoskins, M.; Khanna, P.; Kunz, R.F.; Dong, C. Effects of the Tumor-Leukocyte Microenvironment on Melanoma-Neutrophil Adhesion to the Endothelium in a Shear Flow. Cell. Mol. Bioeng. 2008, 1, 189–200. [Google Scholar] [CrossRef]
- Schumacher, D.; Strilic, B.; Sivaraj, K.K.; Wettschureck, N.; Offermanns, S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 2013, 24, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, Y.; Xia, S.; Lu, L.; Dai, T.; Li, A.; Chen, Y.; Gao, E. Prognostic value of the systemic inflammation response index (SIRI) before and after surgery in operable breast cancer patients. Cancer Biomark. Sect. A Dis. Markers 2020, 28, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Minami, T.; Shimizu, N.; Yamamoto, Y.; De Velasco, M.; Nozawa, M.; Yoshimura, K.; Harashima, N.; Harada, M.; Uemura, H. Identification of Programmed Death Ligand 1-derived Peptides Capable of Inducing Cancer-reactive Cytotoxic T Lymphocytes From HLA-A24+ Patients With Renal Cell Carcinoma. J. Immunother. 2015, 38, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Thommen, D.S.; Koelzer, V.H.; Herzig, P.; Roller, A.; Trefny, M.; Dimeloe, S.; Kiialainen, A.; Hanhart, J.; Schill, C.; Hess, C.; et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 2018, 24, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Ofner, H.; Laukhtina, E.; Hassler, M.R.; Shariat, S.F. Blood-Based Biomarkers as Prognostic Factors of Recurrent Disease after Radical Cystectomy: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 5846. [Google Scholar] [CrossRef]
- Tang, X.; Du, P.; Yang, Y. The clinical use of neutrophil-to-lymphocyte ratio in bladder cancer patients: A systematic review and meta-analysis. Int. J. Clin. Oncol. 2017, 22, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Zhai, E.-T.; Yuan, Y.-J.; Wu, K.-M.; Xu, J.-B.; Peng, J.-J.; Chen, C.-Q.; He, Y.-L.; Cai, S.-R. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J. Gastroenterol. 2017, 23, 6261–6272. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Chen, P.; Xu, W.; Wu, Y.; Che, G. Prognostic value of the pretreatment systemic immune-inflammation index (SII) in patients with non-small cell lung cancer: A meta-analysis. Ann. Transl. Med. 2019, 7, 433. [Google Scholar] [CrossRef]
- Zhong, J.-H.; Huang, D.-H.; Chen, Z.-Y. Prognostic role of systemic immune-inflammation index in solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 75381–75388. [Google Scholar] [CrossRef]
- Gorgel, S.N.; Akin, Y.; Koc, E.M.; Kose, O.; Ozcan, S.; Yilmaz, Y. Retrospective study of systemic immune-inflammation index in muscle invasive bladder cancer: Initial results of single centre. Int. Urol. Nephrol. 2020, 52, 469–473. [Google Scholar] [CrossRef]
- Grossmann, N.C.; Schuettfort, V.M.; Pradere, B.; Rajwa, P.; Quhal, F.; Mostafaei, H.; Laukhtina, E.; Mori, K.; Motlagh, R.S.; Aydh, A.; et al. Impact of preoperative systemic immune-inflammation Index on oncologic outcomes in bladder cancer patients treated with radical cystectomy. Urol. Oncol. 2022, 40, 106.e11–106.e19. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, S.; Yang, X.; Wang, R.; Luo, L. Prognostic value of pretreatment systemic immune-inflammation index in patients with gastrointestinal cancers. J. Cell. Physiol. 2019, 234, 5555–5563. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, H. Prognostic prediction of systemic immune-inflammation index for patients with gynecological and breast cancers: A meta-analysis. World J. Surg. Oncol. 2020, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Huang, Y.; Lin, T. Prognostic impact of elevated pre-treatment systemic immune-inflammation index (SII) in hepatocellular carcinoma: A meta-analysis. Medicine 2020, 99, e18571. [Google Scholar] [CrossRef] [PubMed]
- Fucà, G.; Guarini, V.; Antoniotti, C.; Morano, F.; Moretto, R.; Corallo, S.; Marmorino, F.; Lonardi, S.; Rimassa, L.; Sartore-Bianchi, A.; et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer 2020, 123, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Qiao, B.; Song, T.; Huang, D.; Zhang, H.; Liu, Y.; Jin, Q.; Yang, M.; Liu, D. Clinical utility of the pan-immune-inflammation value in breast cancer patients. Front. Oncol. 2023, 13, 1223786. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Lotan, Y.; Vickers, A.; Karakiewicz, P.I.; Schmitz-Dräger, B.J.; Goebell, P.J.; Malats, N. Statistical consideration for clinical biomarker research in bladder cancer. Urol. Oncol. 2010, 28, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Barone, B.; Finati, M.; Cinelli, F.; Fanelli, A.; Del Giudice, F.; De Berardinis, E.; Sciarra, A.; Russo, G.; Mancini, V.; D’altilia, N.; et al. Bladder Cancer and Risk Factors: Data from a Multi-Institutional Long-Term Analysis on Cardiovascular Disease and Cancer Incidence. J. Pers. Med. 2023, 13, 512. [Google Scholar] [CrossRef] [PubMed]
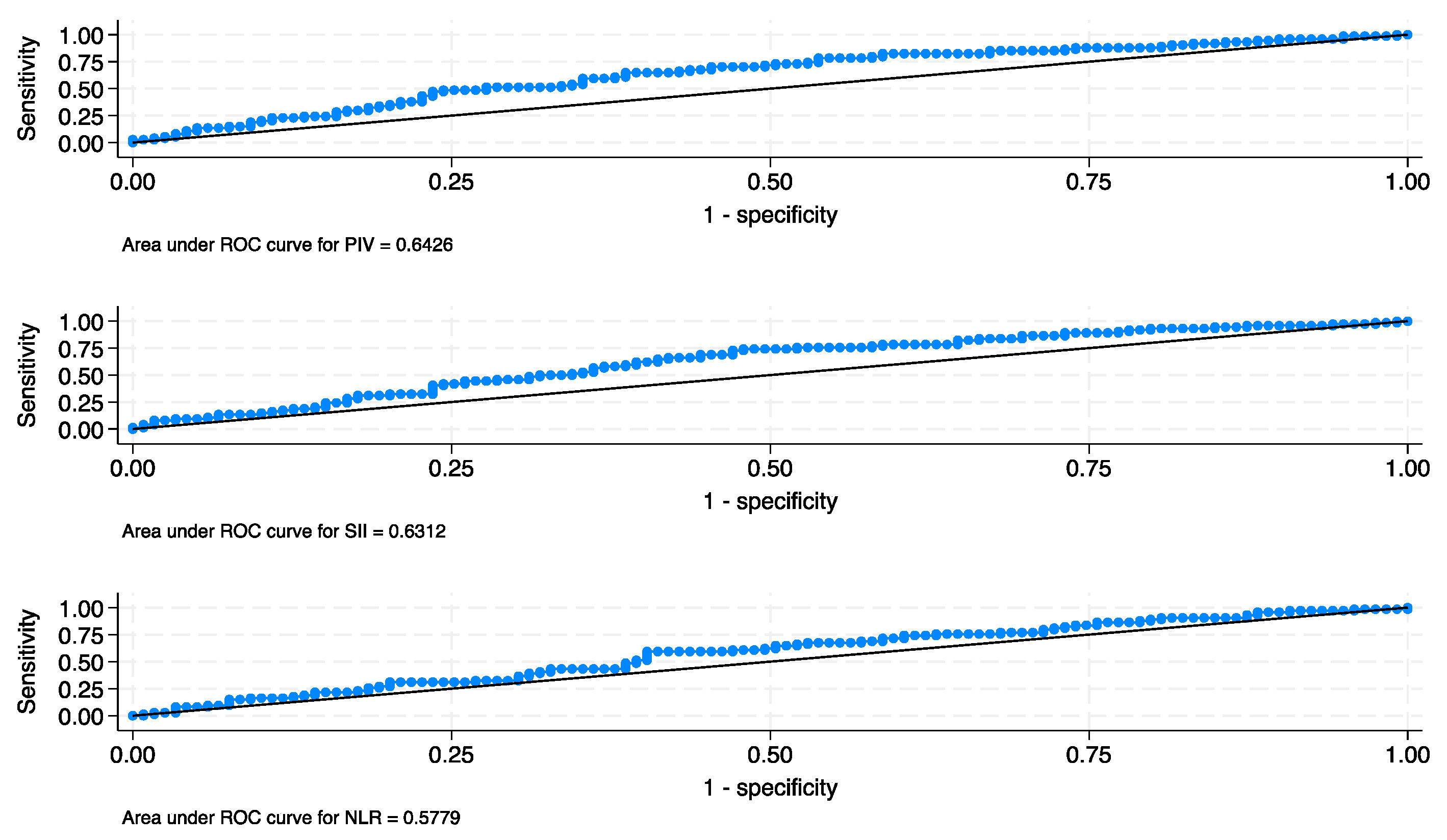
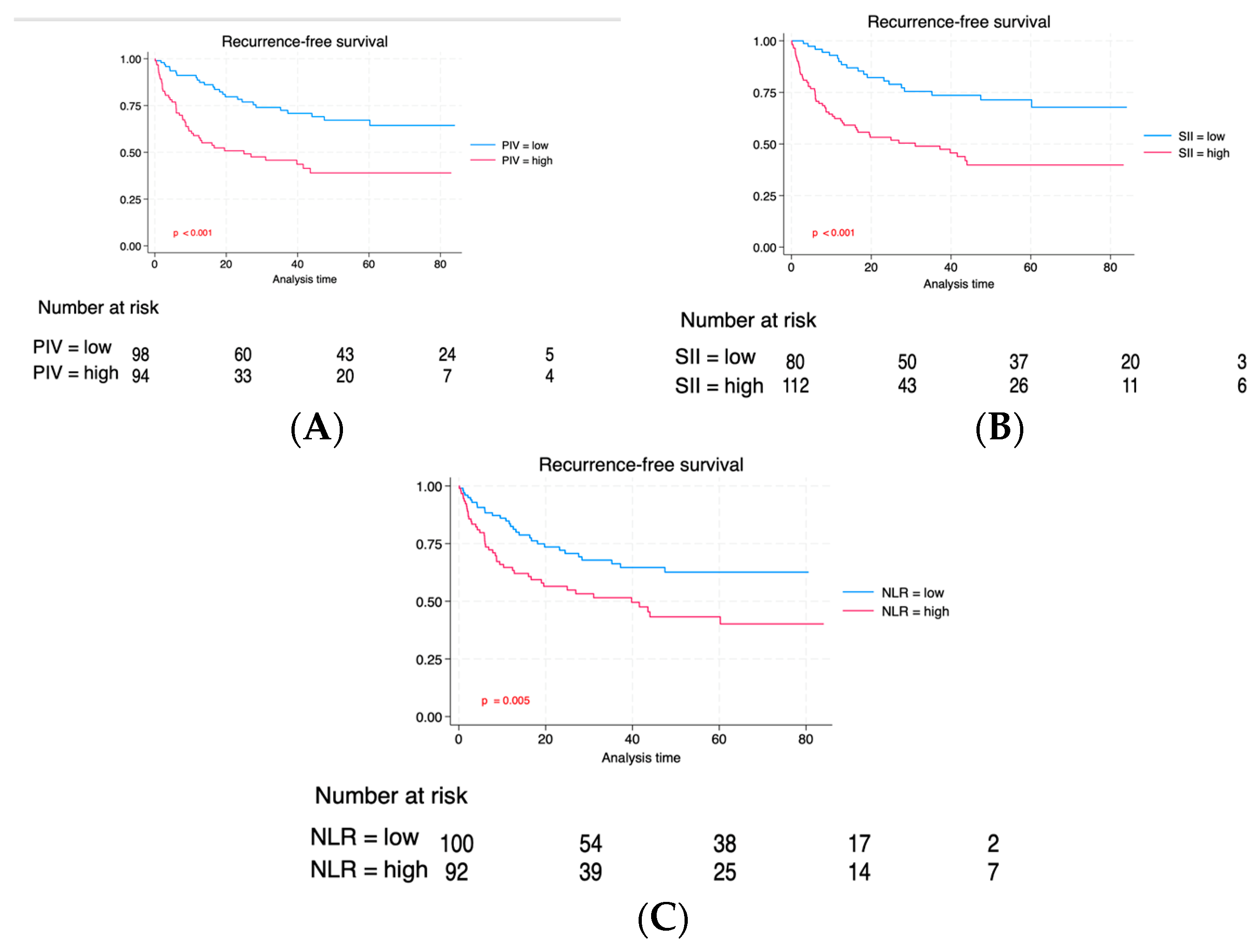
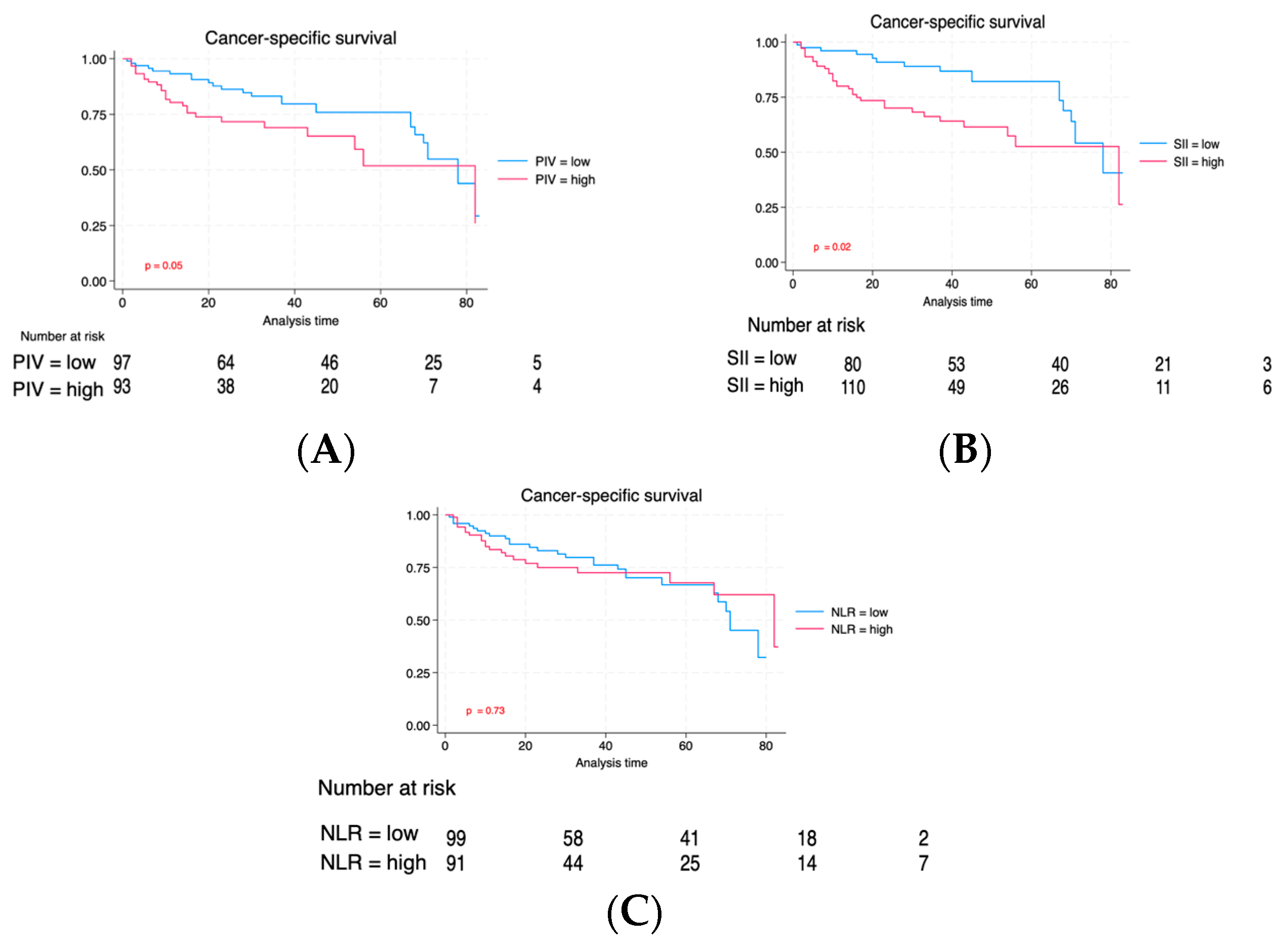
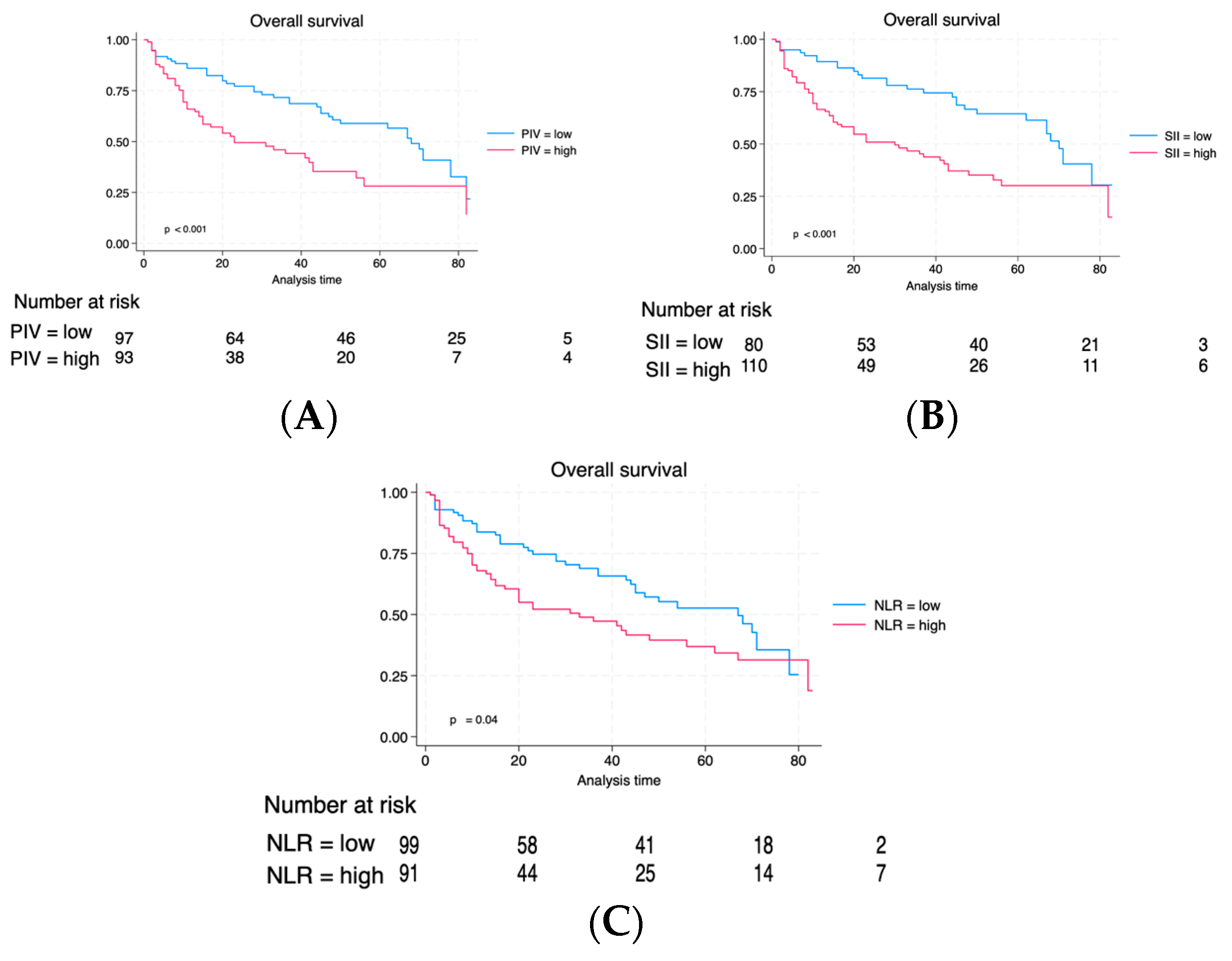
| Characteristic | Total | Low PIV | High PIV | p |
|---|---|---|---|---|
| N = 193 | N = 99 (51.3) | N = 94 (48.7) | ||
| Age, median (IQR) | 78 (73–83) | 78 (73–83) | 79 (73–83) | 0.38 |
| Sex, n (%) | 0.07 | |||
| Male | 154 (79.7) | 84 (84.8) | 70 (74.7) | |
| Female | 39 (20.2) | 15 (15.1) | 24 (25.5) | |
| Smoke, n (%) | 149 (77.2) | 83 (83.8) | 66 (70.2) | 0.02 |
| Diabetes, n (%) | 31 (16.0) | 16 (16.1) | 15 (15.9) | 0.96 |
| Clinical T stage, n (%) | <0.001 | |||
| cTa | 67 (34.7) | 41 (41.4) | 26 (27.6) | |
| cTis | 24 (12.4) | 18 (12.3) | 6 (11.7) | |
| cT1 | 49 (25.3) | 23 (25.1) | 26 (23.9) | |
| cT2 | 29 (15.0) | 14 (14.9) | 15 (14.1) | |
| cT3 | 18 (9.3) | 3 (9.2) | 15 (8.8) | |
| cT4 | 6 (3.1) | 0 (3.1) | 6 (2.9) | |
| BMI, median (IQR) | 26 (24–29) | 26 (24–29) | 26 (24–28) | 0.39 |
| Surgical approach, n (%) | 0.27 | |||
| Open | 186 (96.3) | 94 (94.9) | 92 (97.8) | |
| Robot-assisted | 7 (3.6) | 5 (5.0) | 2 (2.1) | |
| Urinary diversion, n (%) | 0.003 | |||
| Ureterocutaneostomy | 21 (10.8) | 6 (6.0) | 15 (15.9) | |
| Ileal conduit | 148 (76.6) | 74 (78.2) | 74 (78.7) | |
| Orthotopic neobladder | 24 (12.4) | 19 (19.1) | 5 (5.3) | |
| Pathological T stage, n (%) | 0.001 | |||
| pT0 | 14 (7.2) | 10 (10.1) | 4 (4.2) | |
| pTa | 10 (5.1) | (5.0) | 5 (5.3) | |
| pTis | 23 (11.9) | 17 (17.1) | 6 (6.3) | |
| pT1 | 26 (13.4) | 19 (19.1) | 7 (7.4) | |
| pT2a | 30 (15.5) | 16 (16.1) | 14 (14.8) | |
| pT2b | 5 (2.5) | 3 (3.0) | 2 (2.1) | |
| pT3a | 49 (25.3) | 22 (22.2) | 27 (28.7) | |
| pT3b | 8 (4.1) | 4 (3.0) | 5 (5.3) | |
| pT4a | 21 (10.8) | 4 (4.0) | 17 (18.0) | |
| pT4b | 7 (3.6) | 0 (0.0) | 7 (7.4) | |
| Lymph node, n (%) | 37 (19.1) | 11 (11.1) | 26 (27.6) | 0.004 |
| LVI, n (%) | 128 (66.3) | 54 (54.5) | 74 (78.7) | <0.001 |
| Locally advanced disease, n (%) | 102 (52.8) | 37 (37.3) | 65 (69.1) | <0.001 |
| Adjuvant chemotherapy, n (%) | 40 (20.7) | 15 (15.1) | 25 (26.6) | 0.05 |
| Progressive disease, n (%) | 61 (31.6) | 22 (22.2) | 39 (41.4) | 0.004 |
| Cancer-related deaths, n (%) | 96 (49.7) | 44 (44.4) | 52 (55.3) | 0.131 |
| Any-cause deaths, n (%) | 52 (26.9) | 26 (26.2) | 26 (27.6) | 0.827 |
| Lymph Node | Advanced Tumor Grading | Locoregionally Extended State | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value |
| PIV (Reference: low) | |||||||||
| High | 1.84 | 0.76, 4.47 | 0.17 | 2.87 | 1.36, 6.04 | 0.005 | 3.30 | 1.60, 6.77 | 0.001 |
| Age | 1.14 | 0.33, 3.88 | 0.82 | 0.75 | 0.28, 1.99 | 0.576 | 1.00 | 0.39, 2.57 | 0.98 |
| Smoke (Reference: no) | |||||||||
| Smoke | 0.59 | 0.23, 1.50 | 0.27 | 0.93 | 0.37, 2.33 | 0.890 | 1.48 | 0.59, 3.70 | 0.39 |
| Sex (Reference: male) | |||||||||
| Female | 0.56 | 0.19, 1.60 | 0.28 | 0.31 | 0.10, 0.90 | 0.032 | 0.53 | 0.20, 1.42 | 0.21 |
| Clinical tumor stage | |||||||||
| (Reference: cTa/cTis/cT1) | |||||||||
| cT2 | 4.92 | 1.84, 13.13 | 0.001 | 45.10 | 9.46, 215.03 | <0.001 | 29.15 | 6.32, 134.36 | <0.001 |
| cT3/cT4 | 11.16 | 3.86, 32.27 | <0.001 | 23.49 | 4.91, 112.20 | <0.001 | |||
| Goodness-of-fit test | Hosmer–Lemeshow test | 0.20 | 0.68 | 0.91 | |||||
| AUC | |||||||||
| Model with PIV | AUC: 0.78 | AUC: 0.80 | AUC: 0.81 | ||||||
| Model without PIV | AUC: 0.76 (+2%) | AUC: 0.77 (+3%) | AUC: 0.76 (+5%) | ||||||
| (p = difference model) | 0.27 | 0.12 | 0.04 |
| Lymph Node | Advanced Tumor Grading | Locoregionally Extended State | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value |
| NLR (Reference: low) | |||||||||
| High | 1.47 | 0.65, 3.36 | 0.35 | 1.98 | 0.97, 4.05 | 0.06 | 1.60 | 0.80, 3.18 | 0.17 |
| Age | 1.13 | 0.33, 3.82 | 0.84 | 0.76 | 0.29, 1.99 | 0.588 | 1.01 | 0.40, 2.51 | 0.97 |
| Smoke (Reference: no) | |||||||||
| Smoke | 0.54 | 0.21, 1.35 | 0.19 | 0.79 | 0.32, 1.94 | 0.620 | 1.22 | 0.51, 2.93 | 0.65 |
| Sex (Reference: male) | |||||||||
| Female | 0.58 | 0.20, 1.66 | 0.31 | 0.31 | 0.11, 0.92 | 0.035 | 0.57 | 0.21, 1.48 | 0.25 |
| Clinical tumor stage | |||||||||
| (Reference: cTa/cTis/cT1) | |||||||||
| cT2 | 4.99 | 1.88, 13.25 | 0.001 | 44.10 | 9.29, 209.21 | <0.001 | 27.01 | 5.97, 122.05 | <0.001 |
| cT3/cT4 | 13.19 | 4.72, 36.85 | <0.001 | 32.22 | 6.73, 154.29 | <0.001 | |||
| Goodness-of-fit test | Hosmer–Lemeshow test | 0.97 | 0.25 | 0.91 | |||||
| AUC | |||||||||
| Model with NLR | AUC: 0.77 | AUC: 0.78 | AUC: 0.77 | ||||||
| Model without NLR | AUC: 0.76 (+1%) | AUC: 0.77 (+1%) | AUC: 0.76 (+1%) | ||||||
| (p = difference model) | 0.51 | 0.55 | 0.55 |
| Lymph Node | Advanced Tumor Grading | Locoregionally Extended State | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value |
| SII (Reference: low) | |||||||||
| High | 2.91 | 1.09, 7.72 | 0.03 | 2.09 | 1.00, 4.40 | 0.05 | 2.96 | 1.44, 6.08 | 0.003 |
| Age | 1.10 | 0.33, 3.72 | 0.86 | 0.79 | 0.30, 2.07 | 0.644 | 1.06 | 0.42, 2.70 | 0.89 |
| Smoke (Reference: no) | |||||||||
| Smoke | 0.56 | 0.22, 1.41 | 0.22 | 0.82 | 0.33, 2.02 | 0.680 | 1.30 | 0.53, 3.19 | 0.56 |
| Sex (Reference: male) | |||||||||
| Female | 0.52 | 0.18, 1.51 | 0.23 | 0.31 | 0.11, 0.91 | 0.034 | 0.51 | 0.19, 1.36 | 0.18 |
| Clinical tumor stage | |||||||||
| (Reference: cTa/cTis/cT1) | |||||||||
| cT2 | 5.35 | 1.97, 14.56 | 0.001 | 45.06 | 9.47, 214.31 | <0.001 | 31.41 | 6.73, 146.46 | <0.001 |
| cT3/cT4 | 10.58 | 3.70, 30.19 | <0.001 | 27.08 | 5.66, 129.51 | <0.001 | |||
| Goodness-of-fit test | Hosmer–Lemeshow test | 0.89 | 0.21 | 0.82 | |||||
| AUC | |||||||||
| Model with SII | AUC: 0.79 | AUC: 0.80 | AUC: 0.81 | ||||||
| Model without SII | AUC: 0.76 (+3%) | AUC: 0.77 (+3%) | AUC: 0.76 (+5%) | ||||||
| (p = difference model) | 0.17 | 0.16 | 0.03 |
| Relapse-Free Survival | Cancer-Specific Survival | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| PIV (Reference: low) | |||||||||
| High | 1.89 | 1.12, 3.19 | 0.017 | 1.07 | 0.57, 2.02 | 0.819 | 1.64 | 1.05, 2.56 | 0.029 |
| Age | 0.77 | 0.40, 1.46 | 0.427 | 1.27 | 0.53, 3.04 | 0.579 | 1.11 | 0.61, 2.02 | 0.717 |
| Smoking status (Reference: no) | |||||||||
| Smoke | 0.91 | 0.53, 1.55 | 0.744 | 1.30 | 0.62, 2.73 | 0.478 | 1.25 | 0.74, 2.10 | 0.401 |
| Sex (Reference: male) | |||||||||
| Female | 0.86 | 0.49, 1.52 | 0.624 | 1.47 | 0.78, 2.80 | 0.230 | 1.06 | 0.64, 1.75 | 0.811 |
| Clinical tumor condition | |||||||||
| (Reference: cTa/cTis/cT1) | |||||||||
| cT2 | 1.58 | 0.82, 3.05 | 0.168 | 1.03 | 0.42, 2.53 | 0.938 | 1.25 | 0.69, 2.27 | 0.450 |
| cT3/cT4 | 7.14 | 3.90, 13.06 | <0.001 | 8.77 | 4.13, 18.61 | <0.001 | 4.02 | 2.24, 7.19 | <0.001 |
| Harrel’s index | |||||||||
| Model accuracy | 0.72 | 0.72 | 0.66 | ||||||
| Model accuracy without PIV | 0.68 (+4%) | 0.71 (+1%) | 0.63 (+3%) | ||||||
| (p = difference model) | 0.01 | 0.81 | 0.03 |
| Relapse-Free Survival | Cancer-Specific Survival | Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value |
| PIV (Reference: low) | |||||||||
| High | 1.74 | 1.04, 2.92 | 0.034 | 1.30 | 0.69, 2.43 | 0.412 | 1.58 | 1.00, 2.49 | 0.048 |
| Age | 0.99 | 0.52, 1.88 | 0.994 | 1.46 | 0.61, 3.49 | 0.387 | 1.21 | 0.67, 2.21 | 0.517 |
| Smoking status (Reference: no) | |||||||||
| Smoke | 1.26 | 0.72, 2.19 | 0.403 | 2.13 | 1.02, 4.44 | 0.043 | 1.73 | 1.02, 2.92 | 0.039 |
| Sex (Reference: male) | |||||||||
| Female | 1.42 | 0.77, 2.61 | 0.258 | 1.78 | 0.92, 3.45 | 0.084 | 1.30 | 0.77, 2.18 | 0.314 |
| Tumor grading | |||||||||
| (Reference: pT0/pTa/pTispT1) | |||||||||
| pT2 | 1.67 | 0.65, 4.28 | 0.283 | 0.98 | 0.31, 3.11 | 0.981 | 1.54 | 0.70, 3.40 | 0.276 |
| pT3/pT4 | 2.84 | 1.14, 7.09 | 0.025 | 2.88 | 1.17, 7.11 | 0.021 | 3.23 | 1.59, 6.53 | 0.001 |
| Lymphovascular invasion | 1.03 | 0.41, 2.54 | 0.94 | 0.97 | 0.38, 2.44 | 0.957 | 0.90 | 0.44, 1.81 | 0.774 |
| Lymph node invasion | 3.85 | 2.12, 6.99 | <0.001 | 4.26 | 1.89, 9.61 | <0.001 | 3.12 | 1.74, 5.60 | <0.001 |
| Adjuvant chemotherapy | 1.31 | 0.73, 2.34 | 0.349 | 0.44 | 0.19, 1.00 | 0.05 | 0.61 | 0.34, 1.08 | 0.092 |
| C-index | |||||||||
| Model with PIV | 0.78 | 0.76 | 0.73 | ||||||
| Model without PIV | 0.77 (+1%) | 0.76 | 0.72 (1%) | ||||||
| (p = difference model) | 0.03 | 0.67 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, P.; Palermo, G.; Iacovelli, R.; Ragonese, M.; Ciccarese, C.; Maioriello, G.; Fantasia, F.; Bizzarri, F.P.; Marino, F.; Moosavi, K.; et al. Comparison of PIV and Other Immune Inflammation Markers of Oncological and Survival Outcomes in Patients Undergoing Radical Cystectomy. Cancers 2024, 16, 651. https://doi.org/10.3390/cancers16030651
Russo P, Palermo G, Iacovelli R, Ragonese M, Ciccarese C, Maioriello G, Fantasia F, Bizzarri FP, Marino F, Moosavi K, et al. Comparison of PIV and Other Immune Inflammation Markers of Oncological and Survival Outcomes in Patients Undergoing Radical Cystectomy. Cancers. 2024; 16(3):651. https://doi.org/10.3390/cancers16030651
Chicago/Turabian StyleRusso, Pierluigi, Giuseppe Palermo, Roberto Iacovelli, Mauro Ragonese, Chiara Ciccarese, Giuseppe Maioriello, Fabrizio Fantasia, Francesco Pio Bizzarri, Filippo Marino, Koosha Moosavi, and et al. 2024. "Comparison of PIV and Other Immune Inflammation Markers of Oncological and Survival Outcomes in Patients Undergoing Radical Cystectomy" Cancers 16, no. 3: 651. https://doi.org/10.3390/cancers16030651
APA StyleRusso, P., Palermo, G., Iacovelli, R., Ragonese, M., Ciccarese, C., Maioriello, G., Fantasia, F., Bizzarri, F. P., Marino, F., Moosavi, K., Nigro, D., Filomena, G. B., Gavi, F., Rossi, F., Pinto, F., Racioppi, M., & Foschi, N. (2024). Comparison of PIV and Other Immune Inflammation Markers of Oncological and Survival Outcomes in Patients Undergoing Radical Cystectomy. Cancers, 16(3), 651. https://doi.org/10.3390/cancers16030651













